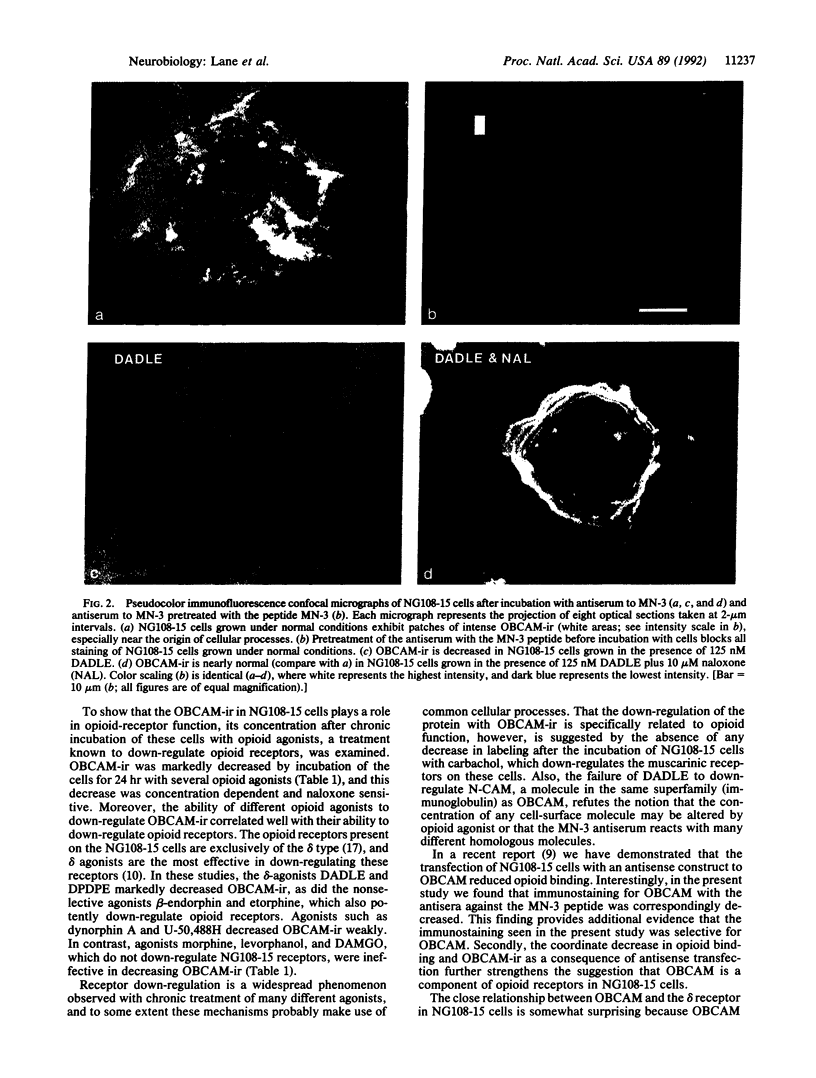
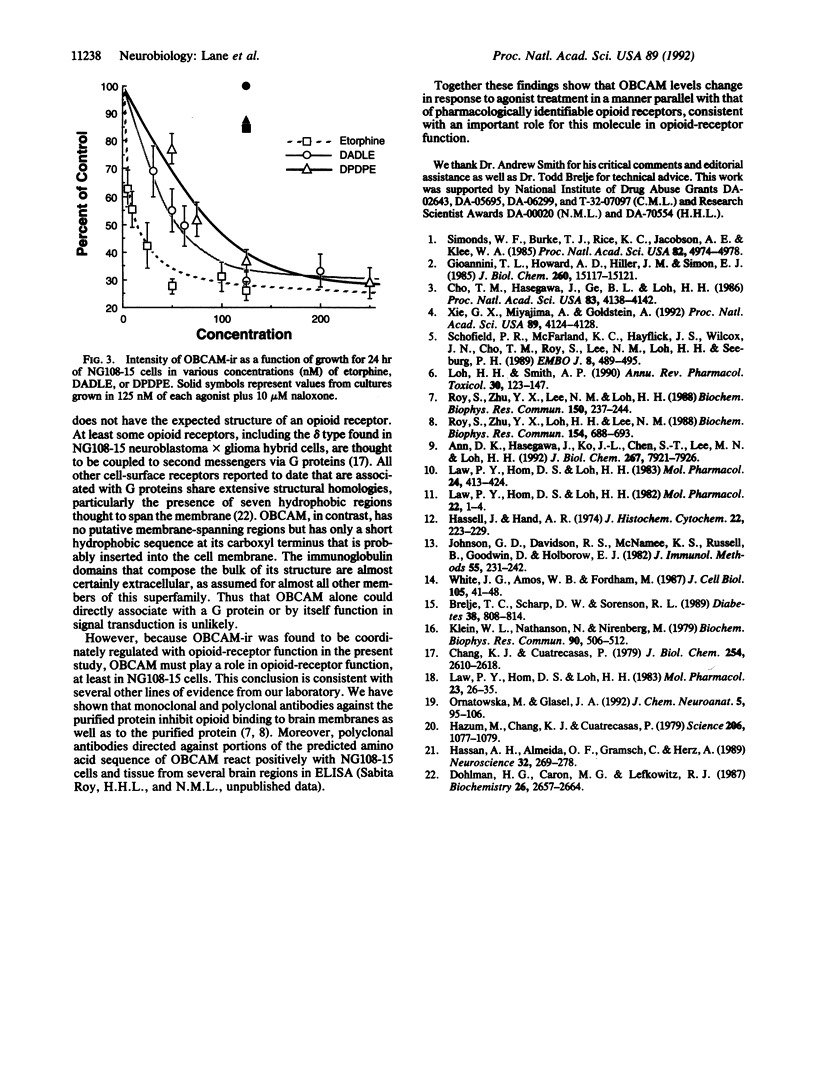
Abstract
An opioid-binding protein has recently been purified from bovine brain and cloned, and its cDNA sequence has been obtained. Indirect evidence suggests that this protein has a role in opioid-receptor function. However, because direct testing of its function by expression of its cDNA has not yet been possible and because its structure bears no resemblance to G protein-coupled receptors, the role of this protein in opioid-receptor activity is still in question. An antibody raised to a portion of the predicted amino acid sequence of opioid-binding cell-adhesion molecule (OBCAM) specifically labeled the surface of NG108-15 cells, as visualized by immunofluorescence with confocal microscopy. Furthermore, chronic treatment of these cells with opioid agonist, which down-regulates opioid receptors, reduced OBCAM immunoreactivity (ir). Down-regulation of both opioid receptors and OBCAM-ir was greatest after chronic treatment of NG108-15 cells with delta-opioid agonists, as well as with nonselective agonists such as etorphine, whereas other agonists including [D-Ala2-N-MePhe4-Gly-ol]enkephalin, morphine, levorphanol, dynorphin A-(1-13), and U-50,488H were less effective or ineffective. Chronic treatment of NG108-15 cells with muscarinic agonists had no effect on OBCAM-ir. Furthermore, NG108-15 cells transfected with an antisense construct to OBCAM have a reduced density of opioid-binding sites as well as reduced OBCAM-ir. Taken together, these results strongly suggest that OBCAM has a role in opioid-receptor function in NG108-15 cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ann D. K., Hasegawa J., Ko J. L., Chen S. T., Lee N. M., Loh H. H. Specific reduction of delta-opioid receptor binding in transfected NG108-15 cells. J Biol Chem. 1992 Apr 15;267(11):7921–7926. [PubMed] [Google Scholar]
- Brelje T. C., Scharp D. W., Sorenson R. L. Three-dimensional imaging of intact isolated islets of Langerhans with confocal microscopy. Diabetes. 1989 Jun;38(6):808–814. doi: 10.2337/diab.38.6.808. [DOI] [PubMed] [Google Scholar]
- Chang K. J., Cuatrecasas P. Multiple opiate receptors. Enkephalins and morphine bind to receptors of different specificity. J Biol Chem. 1979 Apr 25;254(8):2610–2618. [PubMed] [Google Scholar]
- Cho T. M., Hasegawa J., Ge B. L., Loh H. H. Purification to apparent homogeneity of a mu-type opioid receptor from rat brain. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4138–4142. doi: 10.1073/pnas.83.12.4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohlman H. G., Caron M. G., Lefkowitz R. J. A family of receptors coupled to guanine nucleotide regulatory proteins. Biochemistry. 1987 May 19;26(10):2657–2664. doi: 10.1021/bi00384a001. [DOI] [PubMed] [Google Scholar]
- Gioannini T. L., Howard A. D., Hiller J. M., Simon E. J. Purification of an active opioid-binding protein from bovine striatum. J Biol Chem. 1985 Dec 5;260(28):15117–15121. [PubMed] [Google Scholar]
- Hassan A. H., Almeida O. F., Gramsch C., Herz A. Immunocytochemical demonstration of opioid receptors in selected rat brain areas and neuroblastoma x glioma hybrid (NG108-15) cells using a monoclonal anti-idiotypic antibody. Neuroscience. 1989;32(1):269–278. doi: 10.1016/0306-4522(89)90126-7. [DOI] [PubMed] [Google Scholar]
- Hassell J., Hand A. R. Tissue fixation with diimidoesters as an alternative to aldehydes. I. Comparison of cross-linking and ultrastructure obtained with dimethylsuberimidate and glutaraldehyde. J Histochem Cytochem. 1974 Apr;22(4):223–229. doi: 10.1177/22.4.223. [DOI] [PubMed] [Google Scholar]
- Hazum E., Chang K. J., Cuatrecasas P. Opiate (Enkephalin) receptors of neuroblastoma cells: occurrence in clusters on the cell surface. Science. 1979 Nov 30;206(4422):1077–1079. doi: 10.1126/science.227058. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Davidson R. S., McNamee K. C., Russell G., Goodwin D., Holborow E. J. Fading of immunofluorescence during microscopy: a study of the phenomenon and its remedy. J Immunol Methods. 1982 Dec 17;55(2):231–242. doi: 10.1016/0022-1759(82)90035-7. [DOI] [PubMed] [Google Scholar]
- Klein W. L., Nathanson N., Nirenberg M. Muscarinic acetylcholine receptor regulation by accelerated rate of receptor loss. Biochem Biophys Res Commun. 1979 Sep 27;90(2):506–512. doi: 10.1016/0006-291x(79)91264-6. [DOI] [PubMed] [Google Scholar]
- Law P. Y., Hom D. S., Loh H. H. Loss of opiate receptor activity in neuroblastoma X glioma NG108-15 hybrid cells after chronic opiate treatment. A multiple-step process. Mol Pharmacol. 1982 Jul;22(1):1–4. [PubMed] [Google Scholar]
- Law P. Y., Hom D. S., Loh H. H. Opiate receptor down-regulation and desensitization in neuroblastoma X glioma NG108-15 hybrid cells are two separate cellular adaptation processes. Mol Pharmacol. 1983 Nov;24(3):413–424. [PubMed] [Google Scholar]
- Law P. Y., Hom D. S., Loh H. H. Opiate regulation of adenosine 3':5'-cyclic monophosphate level in neuroblastoma X glioma NG108-15 hybrid cells. Relationship between receptor occupancy and effect. Mol Pharmacol. 1983 Jan;23(1):26–35. [PubMed] [Google Scholar]
- Loh H. H., Smith A. P. Molecular characterization of opioid receptors. Annu Rev Pharmacol Toxicol. 1990;30:123–147. doi: 10.1146/annurev.pa.30.040190.001011. [DOI] [PubMed] [Google Scholar]
- Ornatowska M., Glasel J. A. Two- and three-dimensional distributions of opioid receptors on NG108-15 cells visualized with the aid of fluorescence confocal microscopy and anti-idiotypic antibodies. J Chem Neuroanat. 1992 Mar-Apr;5(2):95–106. doi: 10.1016/0891-0618(92)90037-q. [DOI] [PubMed] [Google Scholar]
- Roy S., Zhu Y. X., Lee N. M., Loh H. H. Different molecular weight forms of opioid receptors revealed by polyclonal antibodies. Biochem Biophys Res Commun. 1988 Jan 15;150(1):237–244. doi: 10.1016/0006-291x(88)90511-6. [DOI] [PubMed] [Google Scholar]
- Roy S., Zhu Y. X., Loh H. H., Lee N. M. A monoclonal antibody that inhibits opioid binding to rat brain membranes. Biochem Biophys Res Commun. 1988 Jul 29;154(2):688–693. doi: 10.1016/0006-291x(88)90194-5. [DOI] [PubMed] [Google Scholar]
- Schofield P. R., McFarland K. C., Hayflick J. S., Wilcox J. N., Cho T. M., Roy S., Lee N. M., Loh H. H., Seeburg P. H. Molecular characterization of a new immunoglobulin superfamily protein with potential roles in opioid binding and cell contact. EMBO J. 1989 Feb;8(2):489–495. doi: 10.1002/j.1460-2075.1989.tb03402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds W. F., Burke T. R., Jr, Rice K. C., Jacobson A. E., Klee W. A. Purification of the opiate receptor of NG108-15 neuroblastoma-glioma hybrid cells. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4974–4978. doi: 10.1073/pnas.82.15.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G., Amos W. B., Fordham M. An evaluation of confocal versus conventional imaging of biological structures by fluorescence light microscopy. J Cell Biol. 1987 Jul;105(1):41–48. doi: 10.1083/jcb.105.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G. X., Miyajima A., Goldstein A. Expression cloning of cDNA encoding a seven-helix receptor from human placenta with affinity for opioid ligands. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4124–4128. doi: 10.1073/pnas.89.9.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]