Abstract
Objective
To study the clinical and laboratory profile of children with progressive familial intrahepatic cholestasis (PFIC) and evaluate their outcome.
Methods
The study is a retrospective review of all cases diagnosed with PFIC between January 2011 and July 2015. All children underwent histopathological examination and immunostaining. Management was done as per institute's protocol.
Results
There were a total of 24 PFIC cases (PFIC 1—2, PFIC 2—19, PFIC 3—3). Eleven presented as neonatal cholestasis, whereas 13 others presented after 6 months of life. Median age of presentation in PFIC 2 was 5.5 months with a time lag of 13 months in diagnosis. PFIC 1 and 2 presented in infancy, whereas PFIC 3 presented late. Familial clustering was seen in 12 of 24 cases. Pruritus resolved with medical management in two-thirds of cases, 3 cases required biliary diversion (BD) with dramatic improvement. One child improved after liver transplantation.
Conclusions
PFIC accounts for 8% of neonatal cholestasis and 34% of cholestasis in older children with PFIC 2 being the commonest subtype. Medical therapy is successful in majority. Partial internal BD should be offered to non-cirrhotic low gamma glutamyl transferase PFIC with intractable pruritus. Progression to cirrhosis may be prevented or delayed by early diagnosis and timely intervention.
Abbreviations: BD, biliary diversion; BSEP, bile salt export pump; DDLT, deceased donor liver transplantation; ESLD, end stage liver disease; GGT, gamma glutamyl transferase; HE, hepatic encephalopathy; ICP, intrahepatic cholestasis of pregnancy; LFT, liver functions test; LT, liver transplantation; MDR3, multi drug resistant protein 3; NCS, neonatal cholestasis syndrome; PEBD, partial external biliary diversion; PIBD, partial internal biliary diversion; PFIC, progressive familial intrahepatic cholestasis; UDCA, ursodeoxycholic acid
Keywords: biliary diversion, gamma-glutamyl transferase, immunostaining, neonatal cholestasis, pruritus
Progressive familial intrahepatic cholestasis (PFIC) is a heterogeneous group of liver disorders with autosomal recessive inheritance, presenting in infancy or early childhood.1, 2 The disease has been classified into three types (types 1, 2 and 3) based on the genetic defect involved in bile transport. PFIC accounts for 3–13% cases of neonatal cholestasis syndrome (NCS) and accounts for 10–15% of children requiring liver transplantation (LT).3, 4, 5, 6, 7 It has an estimated prevalence of 1 per 50,000 to 1 per 100,000 births although the exact prevalence is unknown.1, 8 PFIC 1 and 2 present in infancy or early childhood, whereas PFIC 3 can present at any age from infancy to adolescence. PFIC 2 is the most rapidly progressive subtype. Cholestasis is the characteristic clinical presentation in all subtypes, either in the form of pruritus, or jaundice or both. Extrahepatic manifestations may be seen in PFIC 1, most frequently diarrhoea, recurrent wheeze, sensorineural hearing defect and pancreatitis. Medical therapy is the first line of treatment with an objective to provide relief from pruritus, improve the nutritional status, correct vitamin deficiencies and treat complications of advanced liver disease. LT is usually reserved for children with crippling pruritus, growth failure, uncontrolled portal hypertension and poor quality of life. More recently biliary diversion (BD) procedures have been considered for relief of pruritus in children with preserved synthetic liver function.9, 10
The published data on PFIC from India is in the form of case reports11, 12 and small case series.13 Understanding the disease spectrum in Indian children is limited by lack of diagnostic facilities (genetic analysis and immunostaining). The study was planned with the objective to describe the clinical and laboratory profile of PFIC in Indian children and evaluate their outcome on a protocol based management.
Subject and Methods
A retrospective review of 24 children and adolescents under 18 years of age, diagnosed as PFIC between January 2011 and July 2015 was carried out. The study was conducted in Department of Pediatric Hepatology, Institute of Liver and Biliary Sciences, New Delhi. For all infantile and childhood cholestasis with/without pruritus, the confirmation of diagnosis of PFIC was made on histopathological features and immunostaining. Anti-BSEP antibody (bile salt export protein) was used for immunostaining in normal or low gamma glutamyl transferase (GGT) cholestasis and anti-MDR3 antibody (multi drug resistant protein-3) staining was done in high GGT cholestasis. Cases with reduced or absent BSEP staining on canalicular membrane on histopathology were labelled as PFIC 2. Cases with reduced or absent MDR-3 immunostaining on liver biopsy labelled as PFIC 3. Cases with low GGT cholestasis, positive BSEP staining and bland cholestasis on liver histopathology with or without extrahepatic manifestations were labelled as PFIC 1. Genetic analysis was not done in our cases for diagnosing PFIC. Pruritus grading was based on 5D score,14 especially for those undergoing BD surgery, to assess response. Upper GI endoscopy was done in cases with clinical evidence of portal hypertension. Fasting serum bile acid analysis was done in 10 patients who did not improve on medical management. Serum bile acid analysis was done by enzymatic cycling assay using DXC600 autoanalyzer. For those on ursodeoxycholic acid (UDCA), therapy was temporarily stopped for 7 days before estimation of serum total bile acid. z score below 2 on weight for height/length or height for age WHO growth charts was used to define growth failure.
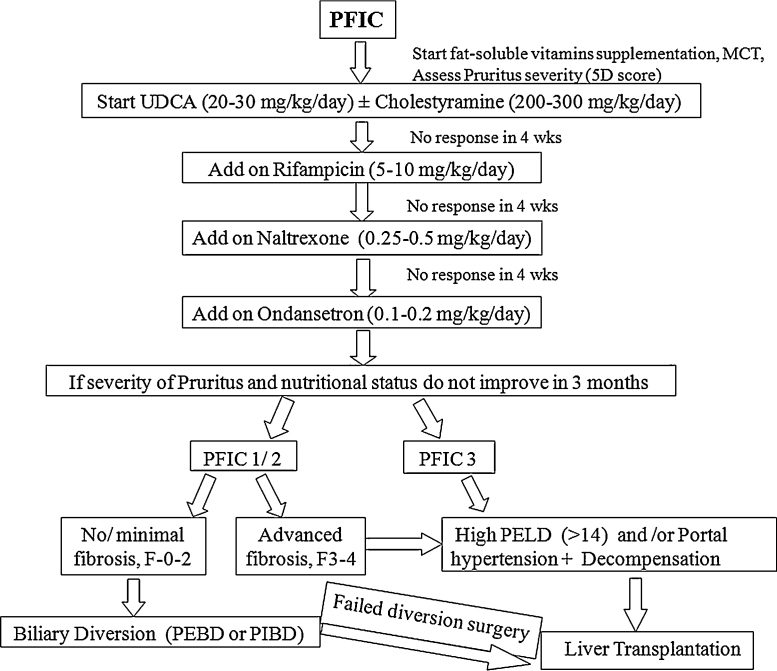
The management of these cases was done as per protocol depicted in Figure 1. It involved medical and/or surgical treatment along with nutritional rehabilitation, multivitamin supplementation and treatment of co-existing fat soluble vitamin deficiency. Nutritional intervention in form of high calorie intake 180–200 cal/kg/day and high protein 3–4 g/kg/day was given for children with failure to thrive. Fat soluble vitamin supplementation was done orally: vitamin D 3000–5000 IU/d, vitamin A 2500 IU/d, vitamin E 50–400 IU/d, vitamin K 10 mg/week. Vitamin D measurement was done at admission in cases admitted after June 2013. Medium chain triglycerides were supplemented at dose of 1–2 ml/kg/day in 4 divided doses. Tube feeding was given as per guidelines for management of severely malnourished children15 or to those with poor oral intake. As soon as the oral intake improved, child was shifted to oral feeds.
Figure 1.
Flowchart for management of pruritus in children with PFIC.
All children with refractory pruritus (no response to medical therapy) without advanced fibrosis on liver biopsy were offered BD surgery. Those who failed to improve were listed for LT. The decision of partial external biliary diversion (PEBD) versus partial internal biliary diversion (PIBD) was based on parental preferences after explaining the benefits and risks associated with both. PIBD was done using an isolated jejunal loop as a conduit from gall bladder to mid ascending colon. During follow-up patients were assessed by 5D pruritus score, nutritional status, liver function test (LFT), transient elastography and serum total bile acid levels. Serum bile acid levels were checked at 2 months post-op, 6 months post-op and 6 monthly thereafter. Transient elastography was done at yearly intervals post-operatively. No liver biopsy was obtained during surgery and follow-up. Children with intractable pruritus and advanced fibrosis on liver biopsy were directly listed for LT as BD is not useful in cases with advanced fibrosis. Patients who had growth failure, uncontrolled portal hypertension or end stage liver disease (ESLD) were listed for LT.
Results
During the study period, we diagnosed PFIC in 24 (14 boys, 58%) children. PFIC accounted for 11 out of 135 (8.2%) cases of NCS and 13 out of 38 (34%) older children with cholestasis. PFIC 2 accounted for 19/24 (79%) children, while 2/24 (8%) and 3/24 (12.5%) were PFIC 1 and PFIC 3 respectively. The median age at onset of symptoms was 3 months (range 1–5 months) for PFIC 1 and 5.5 months (range 1–24 months) for PFIC 2. The age at onset of symptoms for the 2 male siblings were 1 and 2 years, whereas the third child was 11 years old when the symptoms started. Median time lag from onset of symptoms to diagnosis was 4 months in PFIC 1, 13 months in PFIC 2 and 8 years in PFIC 3. Cholestasis in form of jaundice and/or pruritus was the presentation in all. Jaundice or pruritus alone was the presenting symptom in 1/3rd cases each, whereas remaining 1/3rd presented with both jaundice as well as pruritus. Extra-hepatic manifestations were not seen in any of our 2 cases of PFIC 1. Only 1 child with PFIC 2 presented with decompensation (ascites/HE) during infancy. Two out of 3 children with PFIC 3 presented with decompensation (ascites). The age at diagnosis was 9, 10 and 12 years respectively. While, 2 of them had significant cholestasis with pruritus, the third child presented with uncontrolled portal hypertension manifesting as upper GI bleed. Both the male siblings with PFIC 3 decompensated within an year of presentation. Antenatal history of pruritus was evident in 6/19 (32%) PFIC 2 and 3/3 (100%) PFIC 3 cases, while family history of cholestasis was present in 7/19 (39%) PFIC 2 and 2/3 (66%) PFIC 3 cases. Family history of cholestasis was present in first order relatives in 4 cases and second order relatives in 3 cases of PFIC 2. In case of male siblings with PFIC 3, intrahepatic cholestasis of pregnancy (ICP) was present in mother and aunt. All children were born at term with birth weight above 2.5 kg. Growth failure was present in 18 children. Weight for height z score was less than −2 (wasting) in 15 children, height for age z score less than −2 (stunting) in 15 children and both wasting and stunting in 13 children. Clinical or laboratory evidence of fat soluble vitamin deficiency was present in 14/24 (58%) children. Three (12.5%) children had xerophthalmia and 4 (16.6%) had clinical evidence of rickets. Mean 25-OH vitamin D3 levels was 11.5 ± 8 ng/ml.
Upper GI endoscopy was done in 14 cases with clinical or sonographic evidence of portal hypertension. Three of the 11 children with PFIC 2 were found to have grade 2 oesophageal varices without red colour signs. Two of the 3 children with PFIC 3 were found to have grade 2 oesophageal varices without red colour signs. The third child with PFIC 3 presented with haematemesis with large varices and was put on variceal ligation sessions.
Laboratory values of all PFIC subtypes at presentation are shown in Table 1. Liver biopsy showed advanced fibrosis (F 3/4) in 9/19 (47%) PFIC 2 and 3/3 (100%) PFIC 3 cases. Liver biopsy and immunostaining findings of different PFIC subtypes are shown in Table 2. BSEP staining was positive in the 2 PFIC 1 cases. BSEP staining was reduced in 8 and absent in 11 PFIC 2 cases. MDR-3 immunostaining was absent in all three PFIC 3 cases.
Table 1.
Laboratory Values at Presentation of PFIC Subtypes.
| Normal | PFIC 1b (n = 2) | PFIC 2a (n = 19) | PFIC 3b (n = 3) | |
|---|---|---|---|---|
| Bilirubin total (mg/dl) | 0.3–1.2 | 5.5, 13.3 | 2.7 (0.6–17.8) | 0.6, 2.1, 3.6 |
| Bilirubin direct (mg/dl) | 0–0.2 | 3.1, 9.5 | 1.8 (0.2–8.9) | 0.4, 1.3, 2.4 |
| AST (IU/L) | 5–40 | 48, 64 | 97 (42–403) | 60, 107, 143 |
| ALT (IU/L) | 10–40 | 24, 44 | 65 (29–579) | 70, 147, 165 |
| SAP (IU/L) | 32–92 | 581, 776 | 384 (132–667) | 226, 342, 628 |
| GGT (IU/L) | 7–64 | 38, 40 | 21 (5–158) | 112, 158, 225 |
| Albumin (g/dl) | 3.5–5.5 | 2.8, 3 | 3.1 (2.7–4.1) | 2.7, 3.6, 4.1 |
| Haemoglobin (g/dl) | 11–15 | 9.9, 11 | 10 (6.8–14) | 8.4, 13.6, 14 |
| Total count (per mm3) | 4000–11,000 | 11,800, 16,000 | 13,500 (4700–29,000) | 1300, 7200, 7900 |
| Platelet (per mm3) | 180–400 | 399, 646 | 238 (164–709) | 55, 164, 266 |
| Triglyceride (mg/dl) | 35–150 | 178, 352 | 189 (55–578) | 114, 152, 205 |
| Cholesterol (mg/dl) | <200 | 150, 164 | 148 (72–211) | 128, 136, 214 |
| Low 25-OH vitamin D3 (<30 ng/ml) | – | 7/8 (87%) | 3/3 (100%) | |
| Serum bile acid (μmol/L) | <10 | – | 100 (52.7–362)c | – |
Value in median (range).
Absolute values given for patients with PFIC 1 and 3.
Serum bile acid level measured in 10 cases of PFIC 2 only.
Table 2.
Liver Biopsy Finding in PFIC Subtypes.
| PFIC-1 (N = 2) | PFIC-2 (N = 19) | PFIC-3 (N = 3) | |
|---|---|---|---|
| Liver biopsy | |||
| (1) Giant cell transformation | – | 16 | 0 |
| (2) Bile ductular proliferation | – | 0 | 3 |
| (3) Cholestasis | 2 | 19 | 2 |
| (4) Fibrosis grading (F) | F0—2 | F0—2, F1—3, F2—6, F3—3, F4—5 | F3—1, F4—2 |
| Immunostaining | BSEP+ | BSEP absent: 11 | MDR-3 absent in all 3 cases |
| BSEP reduced: 8 | |||
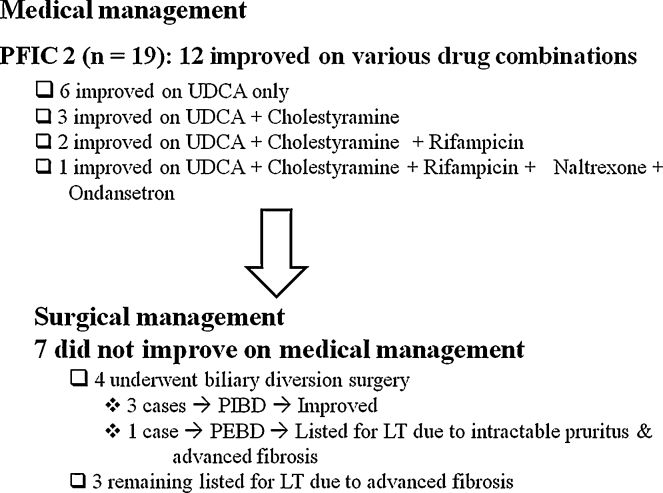
Pruritus was managed using a step-up algorithm depicted in Figure 1. Pruritus was well controlled in both the PFIC 1 cases with medical management. Out of 19 PFIC 2 cases, 12 improved on medical management, 4 underwent BD surgery (3 at our centre) and 3 were listed for LT (Figure 2). No adverse effects with antipruritic medication were seen. All children who underwent BD surgery at our centre had a high pre-operative serum total bile acid level with no evidence of portal hypertension or advanced fibrosis. Post-operatively, they received prophylactic oral antibiotic for 1 month and were continued on UDCA after surgery. No post-operative complication occurred in any case. Post-operative significant resolution of pruritus and decrease in serum bile acids were seen (Table 3). In all three cases of BD, serum bile acids declined within 2 months post-op (serum bile acid at baseline: 195, 52 and 362 μmol/L; At 2 months post-op: 73, 35 and 52 μmol/L respectively). The serum bile acid levels for the 3 cases at 2-year follow-up are mentioned in table III. All cases were asymptomatic at a median follow-up of 2 years. Fibrosis did not progress post-surgery (transient elastography decreased post-op in 2; mild increase in 1). Liver biopsy was not repeated. One case of PFIC 2 in whom PEBD was done at another hospital earlier, presented to us with spontaneously closed stoma and was listed for LT. Two siblings with PFIC 3 underwent LT. Post-LT, graft function was normal with latest follow-up at 2 and 9 months.
Figure 2.
Outcome on medical and surgical management.
Table 3.
Pre- and Post-biliary Diversion Surgery Profile in 3 PFIC 2 Cases.
| PFIC | CASE 1 (7 years female) |
CASE 2 (16 years female) |
CASE 3 (15 years female) |
|||
|---|---|---|---|---|---|---|
| Pre-op | Post-op (2 yrs) | Pre-op | Post-op (2 yrs) | Pre-op | Post-op (1 yrs) | |
| Pruritus grade (5D score) | 18 | 7 | 19 | 5 | 21 | 5 |
| Weight in kg (z score) | 17 (−1.46) | 21.6 (−1.1) | 36 (−1.2) | 41.6 (−1.3) | 32 (−1.3) | 43 (−0.73) |
| Height in cm (z score) | 108 (−2.4) | 119 (−2.0) | 140 (−3.1) | 142 (−2.85) | 139 (−3.6) | 147 (−2.1) |
| BMI (z score) | 14.65 (−0.55) | 15.31 (−0.35) | 18.36 (−1.21) | 20.8 (0) | 16.58 (−2) | 19.9 (−0.29) |
| Bilirubin (mg/dl) | 0.5 | 0.7 | 0.9 | 0.4 | 1.9 | 0.5 |
| ALT (mg/dl) | 40 | 57 | 111 | 71 | 49 | 36 |
| Serum albumin (g/dl) | 3.9 | 4 | 3.6 | 3.8 | 3.4 | 3.6 |
| Total serum bile acid (μM/L) | 195 | 25.3 | 52 | 24.40 | 362 | 10 |
| Transient elastography (kPa) | 5.9 | 4.9 | 8.5 | 6 | 6.5 | 7.6 |
| Oesophageal varix | No | No | – | No | – | |
| Liver biopsy-fibrosis | F0 | – | F1 | – | F1 | – |
Discussion
The present analysis of the clinical course and management options provides new insights into this not so rare systemic disorder. Contrary to the previous Indian studies,3, 16 prevalence rate of PFIC in NCS (8%) and older children (34%) was higher in the present study. The higher number of PFIC cases in our study can be attributed to the use of immunostaining as a diagnostic modality for the first time in India. There could also be an element of referral bias. Comparable to the present study, disease onset in PFIC 1 and 2 has been reported by 3 months of age.17, 18 As also seen in the present study, PFIC 3 presents relatively late with cholestatic symptoms developing in late infancy (1/3rd) to adolescent age group.6 Median time lag in diagnosing PFIC was 13 months to 8 years which could be attributable to poor awareness of PFIC disease spectrum among paediatricians, late referral and diagnostic difficulty.
PFIC 2 was the commonest subtype as also seen before.19 Jaundice and/or pruritus are the predominant initial presenting symptoms in the present study. Similar findings have been reported earlier.16, 17 Growth faltering is prominent in PFIC and in part related to diminished luminal absorption of long-chain fatty acids. In 2 previous series,17, 18 growth failure was present in 71% and clinical evidence of vitamin D and K deficiency was found in 8–9%. More than half of our cases reported with rickets and/or coagulopathy. PFIC cases need careful attention to growth and nutrition which is critical in the early management of this disorder.
History suggestive of ICP in the mother was common in all subtypes. ICP has been described in heterozygous mothers of affected children with PFIC.20 Implicating family screening, 40% of the patients of PFIC had an affected sibling or family member. Previous studies17, 18 have reported affected sibling in 15–25% of PFIC. Similar to the present study, another series19 of 5 cases have reported all cases born at term with normal birth weight. Whereas, premature birth was observed in 5–10% of PFIC with gender-specified birth-weights below the median in another study.21
Medical management is usually considered to be ineffective in interrupting the progression of disease and in alleviating the pruritus in the majority. In the largest series18 of 62 low-GGT PFIC cases (diagnosis based on genetic analysis), only 30% cases responded to medical management. In the present study, pruritus was well controlled on protocol based antipruritic management in 60% cases. Prior to 1990s, LT was the only effective therapy for PFIC. In the last 10 years, BD has become an effective alternative in treatment of low-GGT PFIC. We have demonstrated a successful outcome in all 3 cases undergoing PIBD with resolution of pruritus, cessation of progression of fibrosis (based on transient elastography), improvement of laboratory parameters (LFT, bile acid) and normal growth. Serum bile acids showed a rapid decline within 2 months post-operatively. There is paucity of literature from our country addressing the management options employed in PFIC and their outcome. In a series of 7 cases from India, only one responded to medical therapy, 2 required BD (1 underwent PEBD and 1 PIBD) and three of the four subjects with decompensated cirrhosis underwent LT.13 In another study,10 pruritus resolved in 8 out of 10 PFIC (9 PFIC 1 and 1 PFIC 2) cases that underwent PIBD, with significant reduction of serum bile acids. The reported complications of PIBD including intestinal obstruction, osmotic diarrhoea and ascending cholangitis were not seen in the present series.22 Parents preferred to go for PIBD as compared to PEBD due to better cosmetic results. Recently, laparascopic button cholecystostomy has been described as an alternative to PEBD using a button instead of a bowel conduit, thus avoiding an enteric anastomosis.23
LT is curative in PFIC at an advanced stage with established cirrhosis. In various series, 10–50% of these cases require LT.6, 7, 18 LT improves cholestasis and its symptoms in 75–100% patients, irrespective of PFIC subtype over a short term follow-up of 3–5 years.24 In our study, LT was done in two cases of PFIC 3. The first child received graft from deceased donor and the second child received liver from his mother who had ICP. The second child had been continued post-transplant on UDCA. Both these children are doing well at 9 months and 2 months post-LT respectively. Four cases with advanced liver disease are on the waiting list for LT. In a previous series of 13 patients undergoing LT for PFIC, authors have reported a 2 year survival of 85%.25 LT should be offered after thoughtful consideration in PFIC 1 as extrahepatic manifestations like diarrhoea and liver steatosis do not improve or even worsen after LT.26 Chronic diarrhoea may become intractable after restoration of bile acid secretion post-LT in some patients.27 Alloimmunization of the recipient against the affected protein (FIC1, BSEP or MDR3) is a possibility post-LT, especially in patients with severe mutations leading to absence of the protein.28 Concerns of increased risk of immunosuppression related cholestasis/cholelithiasis in the post-transplant period due to the heterozygous state of donor liver (from father/mother) has not been proven to be true as yet. The feasibility of living donor LT with donor being first order relatives (probably heterozygous state) is an interesting area for future research.
The lacunae of this study are the absence of mutation analysis and short-term follow-up for most of the cases. Mutational analysis by whole genome sequencing would be ideal as the mutations leading to PFIC in India population might be different than those in western world. We conclude that prevalence rate of PFIC in NCS and the older children with cholestatic liver disease is 8% and 34% respectively with PFIC 2 being the commonest subtype. Pruritus is well managed by using step-up algorithmic approach of antipruritic drug therapy. Non-transplant surgical management (PIBD) has a good outcome in non-cirrhotic low-GGT PFIC children and should be offered in cases with intractable pruritus as an alternative to LT. Early diagnosis leading to “prompt institution of medical therapy” and “BD surgery” in cases with intractable pruritus are interventions which can delay the progression of fibrosis, especially in PFIC 2. Long-term follow-up with larger sample size is required for better understanding of the natural course of disease as well as to see the effectiveness of medical and surgical management.
Conflicts of Interest
The authors have none to declare.
References
- 1.Davit-Spraul A., Gonzales E., Baussan C., Jacquemin E. Progressive familial intrahepatic cholestasis. Orphanet J Rare Dis. 2009;4:1. doi: 10.1186/1750-1172-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clayton R.J., Iber F.I., Ruebner B.H., McKusick V.A. Byler disease: fatal familial intrahepatic cholestasis in an Amish kindred. Am J Dis Child. 1969;117(1):112–124. [PubMed] [Google Scholar]
- 3.Arora N., Arora S., Ahuja A. Alpha 1 antitrypsin deficiency in children with chronic liver disease in North India. Indian Pediatr. 2010;47(12):1015–1023. doi: 10.1007/s13312-010-0174-3. [DOI] [PubMed] [Google Scholar]
- 4.Fischler B., Papadogiannakis N., Nemeth A. Aetiological factors in neonatal cholestasis. Acta Paediatr. 2001;90:88–92. doi: 10.1080/080352501750064932. [DOI] [PubMed] [Google Scholar]
- 5.Poddar U., Thapa B.R., Das A., Bhattacharya A., Rao K.L.N., Singh K. Neonatal cholestasis: differentiation of biliary atresia from neonatal hepatitis in a developing country. Acta Paediatr. 2009;98:1260–1264. doi: 10.1111/j.1651-2227.2009.01338.x. [DOI] [PubMed] [Google Scholar]
- 6.Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol. 2012;36(suppl 1):S26–S35. doi: 10.1016/S2210-7401(12)70018-9. [DOI] [PubMed] [Google Scholar]
- 7.Hori T., Nguyen J.H., Uemoto S. Progressive familial intrahepatic cholestasis. Hepatobiliary Pancreat Dis Int. 2010;9:570–578. [PubMed] [Google Scholar]
- 8.Bull L.N., Carlton V.E., Stricker N.L. Genetic and morphological findings in progressive familial intrahepatic cholestasis (Byler disease [PFIC 1] and Byler syndrome): evidence for heterogenicity. Hepatology. 1997;26:155–164. doi: 10.1002/hep.510260121. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava A. Progressive familial intrahepatic cholestasis. J Clin Exp Hepatol. 2014;4:25–36. doi: 10.1016/j.jceh.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramachandran P., Shanmugam N., Sinani S. Outcome of partial internal biliary diversion for intractable pruritus in children with cholestatic liver disease. Pediatr Surg Int. 2014;30:1045–1049. doi: 10.1007/s00383-014-3559-x. [DOI] [PubMed] [Google Scholar]
- 11.Ganesh R., Suresh N., Sathiyasekeran M., Ramachandran P. Partial internal biliary diversion: a solution for intractable pruritus in progressive familial intrahepatic cholestasis type 1. Saudi J Gastroenterol. 2011;17:212–214. doi: 10.4103/1319-3767.80387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma D., Shah U.H., Sibal A., Chowdhary S.K. Cholecystoappendicostomy for progressive familial intrahepatic cholestasis. Indian Pediatr. 2010;47:626–628. doi: 10.1007/s13312-010-0122-2. [DOI] [PubMed] [Google Scholar]
- 13.Kaur S., Sharma D., Wadhwa N., Gupta S., Chowdhary S.K., Sibal A. Therapeutic interventions in progressive familial intrahepatic cholestasis: experience from a tertiary care centre in north India. Indian J Pediatr. 2012;79:270–273. doi: 10.1007/s12098-011-0516-8. [DOI] [PubMed] [Google Scholar]
- 14.Elman S., Hynan L.S., Gabriel V., Mayo M.J. The 5-D itch scale: a new measure of pruritus. Br J Dermatol. 2010;162(3):587–593. doi: 10.1111/j.1365-2133.2009.09586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO . World Health Organization; Geneva: 2013. Guideline Updates on the Management of Severe Acute Malnutrition in Infants and Children.http://www.who.int/nutrition/publications/guidelines/updates_management_SAM_infantandchildren/en/ [PubMed] [Google Scholar]
- 16.Consensus report on neonatal cholestasis syndrome. Pediatric Gastroenterology Subspecialty Chapter of Indian Academy of Pediatrics. Indian Pediatr. 2000;37:845–851. [PubMed] [Google Scholar]
- 17.Pawlikowska L., Strautnieks S., Jankowska I. Differences in presentation and progression between severe FIC1 and BSEP deficiencies. J Hepatol. 2010;53:170–178. doi: 10.1016/j.jhep.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davit-Spraul A., Fabre M., Branchereau S. ATP8B1 and ABCB11 analysis in 62 children with normal GGT progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology. 2010;51:1645–1655. doi: 10.1002/hep.23539. [DOI] [PubMed] [Google Scholar]
- 19.Baussan C., Cresteil D., Gonzales E. Genetic cholestatic liver diseases: the example of progressive familial intrahepatic cholestasis and related disorders. Acta Gastroenterol Belg. 2004;64(3):179–183. [PubMed] [Google Scholar]
- 20.Savander M., Ropponen A., Avela K. Genetic evidence of heterogeneity in intrahepatic cholestasis of pregnancy. Gut. 2003;52(7):1025–1029. doi: 10.1136/gut.52.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee W.S., Chai P.F., Looi L.M. Progressive familial intrahepatic cholestasis in Malaysian patients—a report of five cases. Med J Malaysia. 2009;64(3):216–219. [PubMed] [Google Scholar]
- 22.Davis A.R., Rosenthal P., Newman T.B. Non-transplant surgical interventions in progressive familial intrahepatic cholestasis. J Pediatr Surg. 2009;44:821–827. doi: 10.1016/j.jpedsurg.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Schukfeh N., Gerner P., Paul A., Kathemann S., Metzelder M. Laparoscopic button cholecystostomy for progressive familial intrahepatic cholestasis in two children. Eur J Pediatr Surg. 2014;24:433–436. doi: 10.1055/s-0033-1360457. [DOI] [PubMed] [Google Scholar]
- 24.Aydogdu S., Cakir M., Arikan C. Liver transplantation for progressive familial intrahepatic cholestasis: clinical and histopathological findings, outcome and impact on growth. Pediatr Transplant. 2007;11:634–640. doi: 10.1111/j.1399-3046.2007.00722.x. [DOI] [PubMed] [Google Scholar]
- 25.Bassas A., Chehab M., Hebby H. Living related liver transplantation in 13 cases of progressive familial intrahepatic cholestasis. Transplant Proc. 2003;35:3003–3005. doi: 10.1016/j.transproceed.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 26.Lykavieris P., van Mil S., Cresteil D. Progressive familial intrahepatic cholestasis type 1 and extrahepatic features: no catch-up of stature growth, exacerbation of diarrhea, and appearance of liver steatosis after liver transplantation. J Hepatol. 2003;39:447–452. doi: 10.1016/s0168-8278(03)00286-1. [DOI] [PubMed] [Google Scholar]
- 27.Egawa H., Yorifuji T., Sumazaki R., Kimura A., Hasegawa M., Tanaka K. Intractable diarrhoea after liver transplantation for Byler's disease: successful treatment with bile adsorptive resin. Liver Transplant. 2002;8:714–716. doi: 10.1053/jlts.2002.34384. [DOI] [PubMed] [Google Scholar]
- 28.Maggiore G., Gonzales E., Sciveres M. Relapsing features of bile salt export pump deficiency after liver transplantation in two patients with progressive familial intrahepatic cholestasis type 2. J Hepatol. 2010;53:981–986. doi: 10.1016/j.jhep.2010.05.025. [DOI] [PubMed] [Google Scholar]