Abstract
Hepatitis C virus (HCV) is a globally prevalent pathogen and is a major cause of healthcare burden in India. HCV poses a significant problem in the state of Punjab, India owing to the higher prevalence of risk factors like unsafe medical practices (including unsafe injections and dental procedures) and intravenous drug use. The reported prevalence of HCV in this part of the country was 5.2% in 2012, while a recent study has shown the prevalence to be 3.2% in 2016. Similar to the other geographic belts in India, genotype 3 predominates in the state of Punjab. Control of HCV infection in Punjab requires focusing on several strategies. There is a need to formulate a health educational curriculum targeting not only the high-risk population but also the general population regarding the transmission of HCV. Training of family physicians who form the first link to patients in the community is imperative in the success of healthcare programmes. Adopting the dual approach of treating the old cases (decreasing the reservoir pool of HCV) and decreasing the incidence of new ones would help curtail the disease and decrease liver related mortality. A commendable initiative has been launched by the Punjab state government to eliminate HCV from Punjab. However, besides the initiative by the government, a concerted effort by all other stakeholders in managing the HCV burden in India, namely the doctors, the drug companies and the non-government organizations is required for control of HCV.
Abbreviations: BPL, below poverty line; DNDi, drugs for neglected diseases initiative; ECHO, Extension for Community Healthcare Outcomes; HD, hemodialysis; HCW, health care worker; HBV, hepatitis B virus; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; INASL, Indian National Association for study of the Liver; IVDU, intravenous drug user; MMPHCRF, Mukh Mantri Punjab Hepatitis-C Relief Fund; NAT, nucleic acid testing; NGO, non-government organization; PSACS, Punjab State AIDS Control Society
Keywords: hepatitis C, Punjab, prevalence, control
Hepatitis C virus (HCV) is a single stranded RNA virus belonging to the family Flaviviridae. It has six major genotypes 1–6 with genotype 1 being the most prevalent genotype globally (46%), followed by genotype 3 in 22% and genotype 2 and 4 in 13% each.1 There is a significant genotypic variation across various geographic regions globally. While genotype 1 predominates in Europe, North America, and Australia, genotype 3 is more prevalent in Asian countries namely India, Pakistan, Bangladesh, etc.2, 3
HCV is known to cause both acute and chronic infection in humans and unlike hepatitis B virus (HBV), 80% of acute HCV infections in adults can lead onto chronic viremia. Of these persons with chronic infection, 30% will develop progressive liver disease culminating in cirrhosis and/or hepatocellular carcinoma (HCC).4
Global Prevalence of HCV
Globally, HCV is the predominant cause for post-transfusion hepatitis. A systematic review in 2013 concluded that about 185 million persons globally had evidence of HCV infection, of which an estimated 170 million may be chronically infected.5 However, recent data estimated that 110 million people are HCV-antibody positive and 80 million have chronic infection.1 This lower estimate can be explained by a combination of decreasing incidence and improved diagnostic tests for HCV infection leading to fewer false-positive results. The estimated prevalence of HCV infection by Global Burden of Disease regions has been depicted in Table 1.1
Table 1.
Estimated Prevalence of HCV Infection by Global Burden of Disease Regions.1
| Regions | Anti-HCV prevalence (CI) | Viremic HCV prevalence (CI) |
|---|---|---|
| Asia, Central | 5.4% (3.5–6.8%) | 2.3% (1.5–3.0%) |
| Asia, East | 1.2% (0.4–1.8%) | 0.7% (0.3–1.1%) |
| Asia, South | 1.1% (0.7–1.5%) | 0.9% (0.5–1.2%) |
| Asia, Southeast | 1.0% (0.8–1.8%) | 0.7% (0.5–1.1%) |
| Australasia | 1.4% (1.0–1.5%) | 1.0% (0.8–1.1%) |
| Caribbean | 0.8% (0.2–1.3%) | 0.6% (0.1–0.9%) |
| Europe, Central | 1.3% (1.1–1.6%) | 1.0% (0.9–1.2%) |
| North Africa/Middle East | 3.1% (2.5–3.9%) | 2.1% (1.7–2.6%) |
| Sub-Saharan Africa, Central | 4.2% (2.4–9.2%) | 2.6% (1.5–5.5%) |
HCV, hepatitis C virus; CI, confidence interval.
Prevalence of HCV in India
The estimated prevalence of HCV infection in India is about 0.5–1.5%.6 Despite the low prevalence of HCV, India with its large population accounts for a significant proportion of the global HCV burden.7 Approximately 12–18 million people are thought to be infected with HCV in India.8 An estimated viremic rate of about 80%, corresponds to a viremic prevalence of about 0.68%.9 A significant variation in prevalence has been noted across various geographical regions in India. The availability and quality of epidemiological data from India is, however, not optimal.7
There is paucity of population-based studies of HCV from India. A population-based study from West Bengal, which included 2973 individuals, found the prevalence of HCV to be 0.87%. The highest anti-HCV seroprevalence was in those who were over 60 years and no difference in prevalence was observed among men and women.10 Sachdeva and Mehta11 screened 150,000 residents of Fatehabad district in Haryana for anti-HCV positivity and found a population prevalence of 1%. However, they also carried out a screening of select group 7114 persons who were at high risk for acquiring HCV infection (high risk behavior, history of jaundice), in whom a prevalence of 21% was noted. In another study carried out on 22,666 trainees of Indian Armed Forces, the point prevalence of anti-HCV seropositivity was noted to be 0.44%. The possible explanation of this low prevalence was exclusion of those who may be at risk for HCV infection from recruitment as military trainees.12 Few studies done in the tribal population of India have depicted prevalence of anti-HCV positivity (7.89% in Lisu community in Changland district of Arunachal Pradesh and 2.02% in Lambada tribe in Andhra Pradesh).13, 14
Data from screening of blood donors in India show an anti-HCV prevalence of 0.29–1.85% in northern states, 0.08–1.4% in southern states, 0.27–1.17% in northeastern states, and 0.31–1.09% in eastern states. In contrast, data from the western Indian states show a lower prevalence of 0–0.9% except for a few reports with higher prevalence.6 Owing to the bias of self-selection, screening blood donors usually underestimates the real prevalence of HCV in a community.
Prevalence of HCV antibodies in pregnant women has ranged from 0.6% to 1.4% in various studies.6 The prevalence of HCV infection in high risk group of patients is expected to be more when compared to the general population, which include patients undergoing hemodialysis (HD), repeated blood transfusions (e.g. thalassemia major), intravenous drug users (IVDU's), and health care workers (HCW's). Multi-transfused thalassemic patients have a high prevalence of HCV infection. However, the prevalence rate has declined from 22.5% among those who received transfusions prior to 2001, when mandatory testing for anti-HCV was introduced in blood banks, to 13.6% among those who received transfusions only thereafter.6 In a study from north India, 54 patients with anti-HCV positivity were enrolled and it was noted that iatrogenic procedures was responsible for the transmission of HCV infection in 83.3%, with 67% being due to blood transfusion alone.15 In a study from New Delhi, 119 patients undergoing HD were enrolled and the prevalence of HCV RNA positivity was found to be as high as 27.7%.16 The prevalence of HCV infection in renal transplant recipients in India has been reported to be as high as 26.2–55.9%.17 The prevalence of HCV infection in HCWs has ranged from 0% to 4%, and is notably higher among staff working in HD units.18, 19 Mehta et al.20 in their study, recruited 1158 IVDU's and noted an extremely high prevalence of anti-HCV positivity (55%).
In published studies, genotype 3 is reported as the most common genotype in India, accounting for 54–80% of cases.21, 22 Studies from northern, western, and eastern parts of the country have uniformly shown predominance of genotype 3; however, in southern India, both genotype 1 and 3 HCV are found to be prevalent.23 Genotype 4 HCV has been identified in some cases from southern and western India.3, 24, 25
Scenario in Punjab, India
General Prevalence
The prevalence of HCV infection in the general population of Punjab was studied by Sood et al.26 in the year 2012, where a house-to-house survey was conducted in a defined population of 26,273 subjects and 5258 subjects were screened. The prevalence of HCV was 5.2% with the highest prevalence being noted in the age group of 41–60 years and prevalence was no different among men and women. The authors also assessed the risk factors associated with the acquisition of HCV and reported prior history of dental treatment, surgery, and unsafe sexual practices as the leading causes for HCV infection. Patients who were anti-HCV positive were more frequently diabetic and had a higher prevalence of HCV infection in the family when compared to subjects who were anti-HCV negative (29% vs. 9%). This study was an eye-opener regarding the major healthcare burden that HCV was posing in the state of Punjab, India. A higher prevalence of HCV infection has also been noted in the adjoining Punjab province of the neighboring country, Pakistan.27 This is labeled as the ‘hepatitis C belt’ occupied by Punjabis on both sides of the border and a possible relation to common cultural, social, and patterns of medical practice has been postulated. However, a genetic predisposition of the Punjabi community to acquire HCV infection cannot be completely negated and further studies are needed to confirm this notion.26
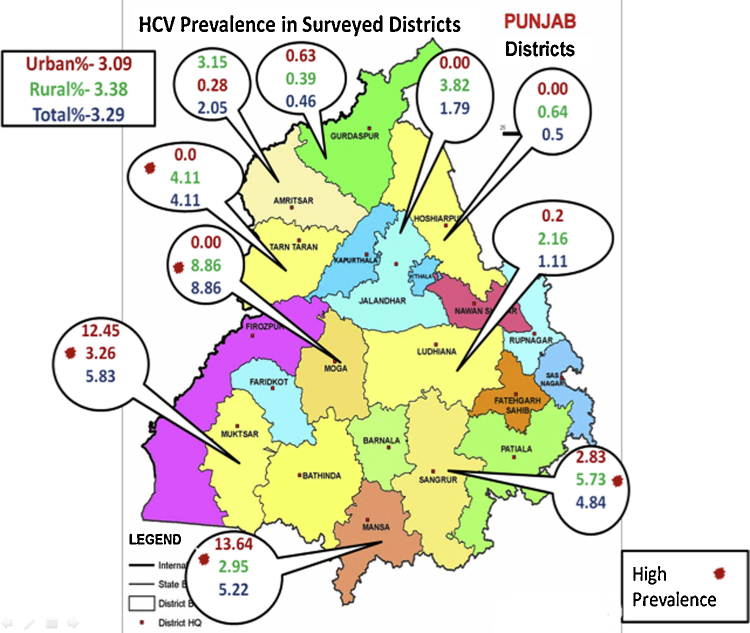
In a recent study conducted by the same group this year, 5526 subjects were screened and a prevalence of 3.29% was demonstrated, with the highest prevalence being noted in the age group of 46–60 years. The prevalence of HCV seropositivity was very high in certain districts of Punjab such as Mansa (13.6%), Muktsar (12.4%), Moga (8.8%), and in Sangroor (5.7%); Figure 1 depicts the prevalence of HCV and difference among rural and urban population in the surveyed districts of Punjab in this study (Professor Ajit Sood, 2016, personal communication). The Punjab State AIDS Control Society (PSACS) has issued its report on the prevalence of HCV seropositivity across various districts of Punjab (Table 2). As agriculture forms a vital occupational backbone in Punjab, a study was conducted by Garg et al.28 to evaluate the seroprevalence of HCV among the farmers in the Malwa belt of Punjab. A total of 1219 farmers were screened and the prevalence of HCV was noted be as high as 5%.
Figure 1.
Prevalence of HCV seropositivity across surveyed districts in Punjab.
(Sood et al., 2016, personal communication).
Table 2.
District Wise Seropositivity of Hepatitis C as per Data of Punjab State AIDS Control Society (PSACS).
| District-wise seroreactivity/seropositivity (%) | ||||
|---|---|---|---|---|
| S. no. | District | Hepatitis C |
||
| 2013–14 | 2014–15 | 2015–16 (till December) | ||
| 1 | Moga | 2.4 | 2.9 | 3.5 |
| 2 | Tarn-Taran | 1.8 | 2.3 | 2.9 |
| 3 | Nawanshahr | 2.1 | 2 | 2.3 |
| 4 | Amritsar | 1.1 | 1.4 | 1.9 |
| 5 | Mansa | 1.8 | 2.1 | 1.9 |
| 6 | Faridkot | 2.1 | 2.2 | 1.7 |
| 7 | Sangrur | 1.8 | 1.4 | 1.6 |
| 8 | Kapurthala | 0.7 | 0.8 | 1.4 |
| 9 | Ludhiana | 1.1 | 1.1 | 1.4 |
| 10 | Mohali | 1.4 | 1.5 | 1.4 |
| 11 | Firozpur | 1.0 | 1.3 | 1.3 |
| 12 | Muktsar | 1.4 | 0.9 | 1.3 |
| 13 | Barnala | 1.8 | 1.6 | 1.2 |
| 14 | Bathinda | 0.8 | 0.9 | 1.2 |
| 15 | Gurdaspur | 0.5 | 0.7 | 1.1 |
| 16 | Hoshiarpur | 0.8 | 0.8 | 1.1 |
| 17 | Jalandhar | 0.6 | 0.9 | 1 |
| 18 | Fatehgarh Sahib | 1.0 | 0.5 | 0.9 |
| 19 | Patiala | 0.8 | 0.9 | 0.7 |
| 20 | Rupnagar | 0.6 | 0.4 | 0.6 |
| 21 | Fazilka | 0.7 | 0.5 | 0.5 |
| 22 | Pathankot | 0.2 | 0.2 | 0.2 |
Prevalence Among Blood Donors
As mentioned earlier, blood transfusion forms a major portal for HCV transmission which is why seropositivity of HCV was assessed among blood donors by Gupta et al.29 in a study from Ludhiana. A total of 156,695 blood donors were screened for anti-HCV antibodies and the prevalence of HCV was found to be 1.45%. A study from Amritsar including 35,793 blood donors demonstrated an anti-HCV seropositivity in 1.38%. They also noted a declining trend from 2.03% in 2005 to 0.87% in 2011.30 A subsequent study from Amritsar which enrolled 5000 blood donors revealed HCV seropositivity in 0.98% of donors, which was significantly higher in replacement (1.37%) than in voluntary donors (0.23%). They noted rural blood donors to have a higher seropositivity than urban donors and the majority of seropositivity was depicted in the 30–39 years of age group.31
Prevalence of HCV Among Pregnant Women
A study carried out in Faridkot by Goyal et al.32 screened 1412 pregnant women and found HCV seropositivity in 2.8%. The risk factors to acquire HCV infection identified from this study were a history of previous surgery and blood transfusion. This study depicts the higher seropositivity of HCV among pregnant women in the state of Punjab when compared to the prevalence reported by other studies done elsewhere in India (1.03%).33
Rural Predominance of HCV Infection in Punjab
Singh et al.34 in their study from Faridkot district enrolled 516 patients with HCV infection and noted a predominant rural distribution of patients (67.3%), with maximum cases being reported from Ludhiana district (30.4%). Majority of the patients were males (72.8%) in the age group of 41–60 years and 44.8% of patients were detected positive for HCV on routine screening.
Prevalence in High Risk Groups
The high-risk groups are constituted by those patients who are multitransfused (e.g. thalassemia major), patients on HD, IVDU, and organ transplant recipients. Various studies done in Punjab depicting the prevalence of HCV infection across various high-risk groups have been shown in Table 3. The prevalence of HIV and HCV seropositivity was assessed in 1155 IVDU's across five cities of Punjab (Amritsar, Taran-Taran, Batala, Ludhiana, and Jalandhar) and the prevalence of HIV, HCV, and co-infection with HIV and HCV was noted in 29%, 49%, and 33% respectively.35 A study from Faridkot including 262 patients on maintenance HD showed a very high prevalence of anti-HCV seropositivity in 33.5%. The highest prevalence was found in the age group of 41–60 years and among the various districts in Punjab, highest frequency distribution of HCV seropositivity was noted in Faridkot (32.9%) followed by Firozpur (23.8%), Moga (19.3%) and lowest was observed in Zira district (1.13%).36
Table 3.
Prevalence of HCV Across Various High Risk Groups: Data From Punjab.
| High risk groups | Author | Study location | Number of subjects | Prevalence |
|---|---|---|---|---|
| Patients on HD | Soin et al.36 | Faridkot | 262 | 33.5% |
| Patients with thalassemia major | Grewal and Sobti50 | Ludhiana | 116 | 59.4% |
| IVDU's | Panda et al.35 | Amritsar, Taran-Taran, Batala, Ludhiana, and Jalandhar | 1155 | 49% |
HD, hemodialysis; IVDU's, intravenous drug users.
Risk Factors to Acquire HCV Infection in Punjab
The predominant modes of transmission of HCV in India are likely to be unsafe therapeutic injections and blood transfusions.6 In a study from Faridkot, the authors screened 12,490 patients attending inpatient and outpatient departments at their hospital and noted 839 (6.71%) patients to be anti-HCV positive. The most common risk factors noted were unsafe medical procedures (47.6%), which included history of therapeutic injections and major/minor surgeries followed by blood transfusion in 30.2% and dental procedures in 22.2%.37
IVDU's pose a significant risk to acquire HCV infection and the problem estimate in Punjab, Haryana, and Chandigarh was assessed by Ambekar and Tripathi in their study by using a novel method to estimate the burden of IVDU's. They used respondent driven sampling for reaching out to IVDU's and multiplier technique to estimate the number of IVDU's. The study was conducted in seven districts of Punjab including Gurdaspur, Faridkot-Moga, Ludhiana, Patiala, Ropar (including Mohali), seven districts in Haryana including Ambala, Jind, Kurukshetra-Kaithal, Narnaul-Rewari, Sonepat-Kharkoda, and Chandigarh. They attempted to project the estimates of IVDU's population in these districts to entire Punjab and Haryana. The study revealed most IVDU's to be in the age group of 18–30 years and most (50–90%) were frequent injectors (daily). A concerning finding of this study was that 34–94% of IVDU's reported sharing their injecting equipments.38 Estimated number of IDVU's identified are depicted in Table 4.38
Table 4.
Estimated Number of IVDU's in Punjab, Haryana, and Chandigarh.
| Estimated number of IVDU's | ||
|---|---|---|
| State/UT | Lower limit | Upper limit |
| Punjab | 2600 | 18,148 |
| Chandigarh–Mohali–Panchkula | 762 | 1170 |
| Haryana | 2265 | 15,858 |
IVDU's, intravenous drug users.
Genotype Distribution in Punjab
The genotypic distribution of HCV in the state of Punjab matches that of the rest of India as demonstrated by a study done recently Jindal et al.37 from Faridkot. Genotype 3 was found to be most common (55.6%) followed by 1 (42.8%) and 4 (1.6%). Genotype 3 was also noted to be the most common genotype among all the risk groups mentioned in the study. Similar results were also obtained by a recent study done by Sood et al. in 2016, wherein genotype 3 was the commonest genotype (55.7%) followed by 1 (28.2%) and 4 (10.1%) (Professor Ajit Sood, 2016, personal communication).
Hot Spots
Clustering is a broad term which refers to ‘usual aggregation of events’. A hotspot is referred to clustering of patients across a limited geographic area. In a recent study by Sood et al. in 2016, the hotspots identified in Punjab are depicted in Figure 2 (Professor Ajit Sood, 2016, personal communication).
Figure 2.
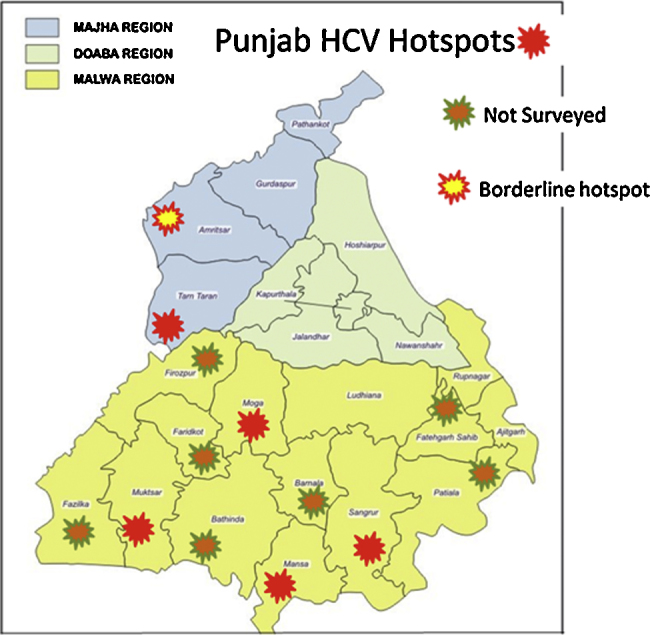
Hotspots of HCV in Punjab.
(Sood et al., 2016, personal communication).
Awareness Among Physicians
Family physicians form the first link to patients in the community and thus the awareness regarding HCV infection among 936 family physicians in Punjab was assessed by Sood et al.39 in 2002. Though 76% doctors were aware about the parenteral transmission of HCV, 18% still continued reusing syringes and needles. Only 72% doctors knew about the relevant investigations needed to diagnose HCV and 58% considered HCV as a cause of acute viral hepatitis. This study demonstrates the importance of providing apt education to family physicians to ensure adequate diagnosis and treatment of HCV at the community level.
Strategies to Control HCV Infection in Punjab, India
The relatively high prevalence of HCV in the state of Punjab calls for an urgent need to implement strategies to control HCV.
The various strategies to control HCV have been enumerated below.
-
1)
Improving awareness in the community and healthcare provider education
Lack of public awareness is a significant barrier to prevention of spread of the disease and early detection. Moreover, poor knowledge in healthcare providers adds to the delay in early diagnosis and appropriate therapy. This can be addressed by health education programmes for the public and by programmes to improve the knowledge of healthcare providers to ensure maximal benefit. The public health education programme should target not only the high-risk population but also the general population. The educational curriculum for the healthcare providers should incorporate all the aspects of prevention, care and treatment which can be used by multiple health care providers including specialists, family physicians and paramedical staff.
-
2)
Improving HCV testing, care and management
This can be attained by making standardized recommendation to guide HCV testing and referral to care. The quality of test kits used for anti-HCV testing may be a reason for concern, since some test kits used in limited-resource countries including India has been found to be unsatisfactory.40, 41 Once a patient is diagnosed with HCV infection, proper ongoing care and facilities for prompt treatment should instituted. One of the significant barriers to providing optimal care is the cost of investigations and therapy. The cost of therapy is not reimbursed by health insurance and in most states of India, the costs of therapy are not covered by the state governments. It has been estimated that only 0.2% of the HCV infected population in India received therapy.42
National guidelines should address the issue of large-scale public healthcare delivery to HCV-infected populations. The latest 2016 guidance for anti-viral therapy against HCV issued by the Indian National Association for study of the Liver (INASL) has taken a step in this direction. Some of the steps recommended include simplified testing and treatment regimen. For example, the need for viral load testing, genotype testing and fibrosis assessment may not be mandatory in non-cirrhotic treatment naïve patients. HCV RNA testing has been recommended only at baseline and end-of-treatment. These measures have been implemented in the HCV elimination project launched by the Punjab state government which has been alluded to later in this article. The results of the impact of these measures in Punjab are eagerly awaited before they are considered for implementation in the rest of the country.
-
3)
Strengthening the public based HCV surveillance programs
It is important to have accurate population based data to guide implementation of HCV control therapy. While limited epidemiological data are available from Punjab, it is important to have a larger scale reliable data from all districts of Punjab so that the local and state based programmes can actively address the issue.
-
4)
Screening of high risk groups
The high risk group of subjects mentioned earlier should be carefully screened and offer prompt treatment. Treating patients actively would decrease the reservoir pool of HCV and would help in curtailing the transmission of the virus. Providing access to effective therapy in HCV-infected population is required to reduce the prevalence of HCV.43
-
5)
Provision of safe blood and blood products
A strict audit of blood banking practices is required to prevent transmission of the disease. Use of nucleic acid testing (NAT) has been proposed for preventing transmission of blood borne pathogens in Indian blood donors. However, such a strategy would add to the cost of blood screening and is therefore not routinely recommended.
-
6)
Avoiding unsafe injection practices
In Punjab, as in many areas of the rest of India, injections are overprescribed and unsafe injection practices are common. The annual frequency of injections is estimated as 2.9 per person, almost double of that in developed countries. Of the nearly 3.0 billion injections are administered annually in India, 1.89 billion are estimated to be unsafe due to inadequate sterilization, use of faulty techniques or unsatisfactory injection waste disposal.44
Safe injection practices have to be undertaken by HCW's which include the usage of aseptic technique, not to reuse syringes or fluid infusion sets for multiple patients, and usage of proper precautions when multiple-dose vials are used. The auto-disable syringe has been shown to be a cost-effective alternative to the reuse of syringes in India.45
-
7)
Prevention in IVDUs
Health education programmes in IVDUs need focus about mechanisms of HCV transmission and the role of sharing of needles and syringes. There is insufficient evidence that interventions such as provision of sterile injecting equipment are effective in reducing transmission.46 There should, therefore, be a greater stress on education of persons with IVDU about transmission of infection and to avoid sharing of needles and syringes.
-
8)
Development of HCV vaccine
As HCV genome demonstrates high level of heterogeneity, generating an effective vaccine against HCV has remained an unsolved matter. Newer vaccine candidates including recombinant protein, peptide and vector based vaccines have shown promise and have lately entered phase I/II clinical trials.47
-
9)
Dual approach
In India, it was shown that prevention of HCV decreased the overall prevalence but it did not impact the short-term liver-related mortality or development of HCC. Thus a dual approach of decreasing the incidence of new cases and treatment of old cases was likely a play a vital role in bringing down the burden of the disease.48
-
10)
Initiative by the government
Punjab government has been a flag-bearer for HCV treatment in India. Back in 2013, the government added HCV in the list of diseases covered under the Punjab Nirogi Scheme; through which patients suffering from Hepatitis-C who were below poverty line (BPL) could avail treatment for up to INR 1.5 lakh. The drugs (pegylated interferon α and ribavirin) were made available at subsidized rates at the Jan Aushadhi stores after a rate contract with the pharmaceutical companies in 2013–14.
However, with the growing cognizance of the HCV burden in the state, the government of Punjab has launched a public health program under the Mukh Mantri Punjab Hepatitis-C Relief Fund (MMPHCRF) that offers free HCV treatment to all residents of Punjab through a highly decentralized network of 22 District Hospitals and 3 Government Medical Colleges.49
MMPHCRF: INR 100 crores have been approved for the Mukh Mantri Punjab Hepatitis-C Relief Fund; of which INR 20 crores have been released so far for procurement of drugs for treatment of HCV.
Goal: The goal of MMPHCRF is to eliminate Hepatitis C from Punjab in approximately 10 years. This will result in reduction in all-cause mortality (deaths) and liver-related deaths, reduction in risk of developing HCC and reduction in risk of developing end-stage liver disease and need for liver transplantation. Thus eliminating HCV from Punjab would save thousands of lives.
Capacity building of medical specialists: In order to effectively decentralize HCV care in the state, 250 medical specialists from state government hospitals have been identified for training on diagnosis and treatment of chronic HCV. Of these 250 medical specialists, roughly half of them have already been trained through a 4-h long training session followed by regular INASL-Punjab Government-Extension for Community Healthcare Outcomes (ECHO) Clinic, where difficult patient cases are presented and consulted with senior hepatologists from PGIMER, Chandigarh using an online interface.
-
11)
Role of non-government organizations (NGO's) and pharmaceuticals
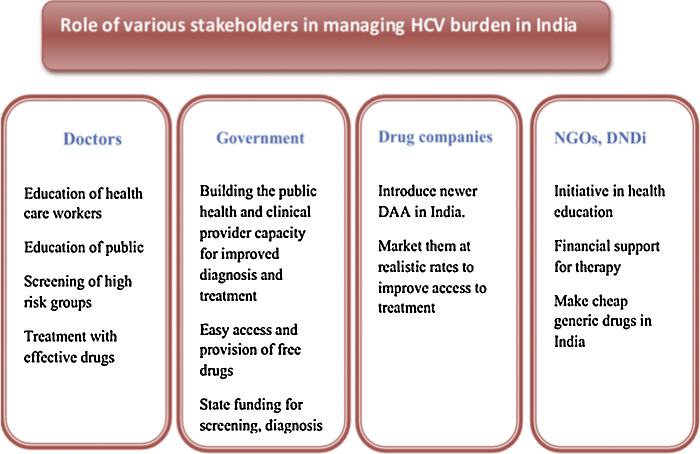
Project ECHO is a collaborative model of medical education and care management that empowers clinicians everywhere to provide better care to more people. It empowers front-line primary care providers to do more, for more patients. Pharmaceutical companies can help the existing scenario by launching new drugs at affordable prices in India so that maximal number of patients can be cured effectively without imposing a major financial burden on the family. The strategy to tackle the burden of HCV in India has been depicted in Figure 3.
Figure 3.
Methods to tackle HCV infection in India. NGOs, non-governmental organizations; DNDi, drugs for neglected diseases initiative.
Conclusion
HCV infection poses a major healthcare burden in India and the relatively higher prevalence in the state of Punjab makes it absolutely vital to embark upon urgent solutions to tackle this problem. Studies have depicted a higher occurrence of risk factors like IVDU in Punjab and thus it is imperative to target this issue at the community level to bring down the incidence of new cases. Finally, the dual approach of treating old cases in order to decrease the reservoir pool of HCV and reducing the incidence of new ones would help curtail the growing burden of HCV in the state of Punjab.
Conflicts of Interest
The authors have none to declare.
Acknowledgement
The authors are thankful to Professor Ajit Sood for providing data of their recent survey of viral hepatitis in the state of Punjab.
References
- 1.Gower E., Estes C., Blach S., Razavi-Shearer K., Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61(1 suppl):S45–S57. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 2.Hissar S.S., Goyal A., Kumar M. Hepatitis C virus genotype 3 predominates in North and Central India and is associated with significant histopathologic liver disease. J Med Virol. 2006;78(4):452–458. doi: 10.1002/jmv.20561. [DOI] [PubMed] [Google Scholar]
- 3.Raghuraman S., Shaji R.V., Sridharan G. Distribution of the different genotypes of HCV among patients attending a tertiary care hospital in south India. J Clin Virol. 2003;26(1):61–69. doi: 10.1016/s1386-6532(02)00025-2. [DOI] [PubMed] [Google Scholar]
- 4.Alter H.J., Seeff L.B. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20(1):17–35. doi: 10.1055/s-2000-9505. [DOI] [PubMed] [Google Scholar]
- 5.Mohd Hanafiah K., Groeger J., Flaxman A.D., Wiersma S.T. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 6.Puri P., Anand A.C., Saraswat V.A. Consensus statement of HCV Task Force of the Indian National Association for Study of the Liver (INASL). Part I: status report of HCV infection in India. J Clin Exp Hepatol. 2014;4(2):106–116. doi: 10.1016/j.jceh.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sievert W., Altraif I., Razavi H.A. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 2011;31(suppl 2):61–80. doi: 10.1111/j.1478-3231.2011.02540.x. [DOI] [PubMed] [Google Scholar]
- 8.Dhiman R.K. Future of therapy for hepatitis C in India: a matter of accessibility and affordability? J Clin Exp Hepatol. 2014;4(2):85–86. doi: 10.1016/j.jceh.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saraswat V., Norris S., de Knegt R.J. Historical epidemiology of hepatitis C virus (HCV) in select countries—volume 2. J Viral Hepat. 2015;22(suppl 1):6–25. doi: 10.1111/jvh.12350. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhury A., Santra A., Chaudhuri S. Hepatitis C virus infection in the general population: a community-based study in West Bengal, India. Hepatology. 2003;37(4):802–809. doi: 10.1053/jhep.2003.50157. [DOI] [PubMed] [Google Scholar]
- 11.Sachdeva S., Mehta B. Population-based hepatitis C survey in a rural block. N Am J Med Sci. 2012;4(11):591–592. doi: 10.4103/1947-2714.103325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh M., Kotwal A., Gupta R.M., Adhya S., Chatterjee K., Jayaram J. Sero-epidemiological and behavioural survey of HIV, HBV and HCV amongst Indian armed forces trainees. Med J Armed Forces India. 2010;66(1):50–54. doi: 10.1016/S0377-1237(10)80093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phukan A.C., Sharma S.K., Das H.K., Mahanta J. HCV activity in an isolated community in north east India. Indian J Pathol Microbiol. 2001;44(4):403–405. [PubMed] [Google Scholar]
- 14.Chandra M., Khaja M.N., Farees N. Prevalence, risk factors and genotype distribution of HCV and HBV infection in the tribal population: a community based study in south India. Trop Gastroenterol. 2003;24(4):193–195. [PubMed] [Google Scholar]
- 15.Chakravarti A., Ashraf A., Malik S. A study of changing trends of prevalence and genotypic distribution of hepatitis C virus among high risk groups in North India. Indian J Med Microbiol. 2013;31(4):354–359. doi: 10.4103/0255-0857.118877. [DOI] [PubMed] [Google Scholar]
- 16.Jasuja S., Gupta A.K., Choudhry R. Prevalence and associations of hepatitis C viremia in hemodialysis patients at a tertiary care hospital. Indian J Nephrol. 2009;19(2):62–67. doi: 10.4103/0971-4065.53324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radhakrishnan S., Abraham P., Raghuraman S. Role of molecular techniques in the detection of HBV DNA & HCV RNA among renal transplant recipients in India. Indian J Med Res. 2000;111:204–211. [PubMed] [Google Scholar]
- 18.Ganju S.A., Goel A. Prevalence of HBV and HCV infection among health care workers (HCWs) J Commun Dis. 2000;32(3):228–230. [PubMed] [Google Scholar]
- 19.Duseja A., Arora L., Masih B. Hepatitis B and C virus—prevalence and prevention in health care workers. Trop Gastroenterol. 2002;23(3):125–126. [PubMed] [Google Scholar]
- 20.Mehta S.H., Vogt S.L., Srikrishnan A.K. Epidemiology of hepatitis C virus infection & liver disease among injection drug users (IDUs) in Chennai, India. Indian J Med Res. 2010;132:706–714. [PMC free article] [PubMed] [Google Scholar]
- 21.Verma V., Chakravarti A., Kar P. Genotypic characterization of hepatitis C virus and its significance in patients with chronic liver disease from Northern India. Diagn Microbiol Infect Dis. 2008;61(4):408–414. doi: 10.1016/j.diagmicrobio.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Narahari S., Juwle A., Basak S., Saranath D. Prevalence and geographic distribution of Hepatitis C Virus genotypes in Indian patient cohort. Infect Genet Evol. 2009;9(4):643–645. doi: 10.1016/j.meegid.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Chandra M., Thippavuzzula R., Ramachandra Rao V.V. Genotyping of Hepatitis C virus (HCV) in infected patients from South India. Infect Genet Evol. 2007;7(6):724–730. doi: 10.1016/j.meegid.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Das B.R., Kundu B., Khandapkar R., Sahni S. Geographical distribution of hepatitis C virus genotypes in India. Indian J Pathol Microbiol. 2002;45(3):323–328. [PubMed] [Google Scholar]
- 25.Raghuraman S., Abraham P., Sridharan G., Daniel H.D., Ramakrishna B.S., Shaji R.V. HCV genotype 4—an emerging threat as a cause of chronic liver disease in Indian (south) patients. J Clin Virol. 2004;31(4):253–258. doi: 10.1016/j.jcv.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Sood A., Sarin S.K., Midha V. Prevalence of hepatitis C virus in a selected geographical area of northern India: a population based survey. Indian J Gastroenterol. 2012;31(5):232–236. doi: 10.1007/s12664-012-0251-8. [DOI] [PubMed] [Google Scholar]
- 27.Ali S.A., Donahue R.M., Qureshi H., Vermund S.H. Hepatitis B and hepatitis C in Pakistan: prevalence and risk factors. Int J Infect Dis. 2009;13(1):9–19. doi: 10.1016/j.ijid.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garg R., Kaur S., Aseri R. Hepatitis B & C among farmers—a seroprevalence study. J Clin Diagn Res. 2014;8(11):MC07–MC09. doi: 10.7860/JCDR/2014/9514.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta S., Kumar R., Kaur A. Prevalence of hepatitis C virus seropositivity among blood donors in a tertiary care hospital. Int Res J Med Sci. 2015;3(2):22–24. [Google Scholar]
- 30.Kaur H., Manjari M., Thaman R.G., Singh G. Prevalence of markers of hepatitis C virus among the blood donors. J Clin Diagn Res. 2012;6(6):959–962. [Google Scholar]
- 31.Sharma A., Kaur S. Seropositivity of hepatitis C infection among voluntary and replacement blood donors in a tertiary-care hospital in Punjab. Int J Med Sci Public Health. 2014;3(12):1535–1539. [Google Scholar]
- 32.Goyal L.D., Kaur S., Jindal N., Kaur H. HCV and pregnancy: prevalence, risk factors, and pregnancy outcome in north Indian population: a case–control study. J Obstet Gynaecol India. 2014;64(5):332–336. doi: 10.1007/s13224-014-0548-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar A., Sharma K.A., Gupta R.K., Kar P., Chakravarti A. Prevalence & risk factors for hepatitis C virus among pregnant women. Indian J Med Res. 2007;126(3):211–215. [PubMed] [Google Scholar]
- 34.Singh P., Kaur R., Kaur A. Frequency distribution of hepatitis C virus in different geographical regions of Punjab: retrospective study from a tertiary care centre in North India. J Nat Sci Biol Med. 2014;5(1):56–58. doi: 10.4103/0976-9668.127288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panda S., Roy T., Pahari S. Alarming epidemics of human immunodeficiency virus and hepatitis C virus among injection drug users in the northwestern bordering state of Punjab, India: prevalence and correlates. Int J STD AIDS. 2014;25(8):596–606. doi: 10.1177/0956462413515659. [DOI] [PubMed] [Google Scholar]
- 36.Soin D., Grover P., Malhotra R. Hepatitis C virus infection in dialysis patients: a retrospective study from a tertiary care hospital of North India. Int J Res Dev Pharm Life Sci. 2015;4(3):1529–1532. [Google Scholar]
- 37.Jindal N., Bansal R., Grover P., Malhotra R. Risk factors and genotypes of HCV infected patients attending tertiary care hospital in North India. Indian J Med Microbiol. 2015;33:189–190. doi: 10.4103/0255-0857.148440. [DOI] [PubMed] [Google Scholar]
- 38.Ambekar A., Tripathi B.M. UNAIDS India; 2008. Size Estimation of Injecting Drug Use in Punjab and Haryana. [Google Scholar]
- 39.Sood A., Midha V., Awasthi G. Hepatitis C—knowledge & practices among the family physicians. Trop Gastroenterol. 2002;23(4):198–201. [PubMed] [Google Scholar]
- 40.Scheiblauer H., El-Nageh M., Nick S., Fields H., Prince A., Diaz S. Evaluation of the performance of 44 assays used in countries with limited resources for the detection of antibodies to hepatitis C virus. Transfusion. 2006;46(5):708–718. doi: 10.1111/j.1537-2995.2006.00789.x. [DOI] [PubMed] [Google Scholar]
- 41.Mammone A., Pezzotti P., Angeletti C. HIV incidence estimate combining HIV/AIDS surveillance, testing history information and HIV test to identify recent infections in Lazio, Italy. BMC Infect Dis. 2012;12:65. doi: 10.1186/1471-2334-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wedemeyer H., Dore G.J., Ward J.W. Estimates on HCV disease burden worldwide—filling the gaps. J Viral Hepat. 2015;22(suppl 1):1–5. doi: 10.1111/jvh.12371. [DOI] [PubMed] [Google Scholar]
- 43.Gane E., Kershenobich D., Seguin-Devaux C. Strategies to manage hepatitis C virus (HCV) infection disease burden—volume 2. J Viral Hepat. 2015;22(suppl 1):46–73. doi: 10.1111/jvh.12352. [DOI] [PubMed] [Google Scholar]
- 44.IPEN Study Group Injection practices in India. WHO South-East Asia J Public Health. 2012;1:189–200. doi: 10.4103/2224-3151.206931. [DOI] [PubMed] [Google Scholar]
- 45.Reid S. Estimating the burden of disease from unsafe injections in India: a cost-benefit assessment of the auto-disable syringe in a country with low blood-borne virus prevalence. Indian J Community Med. 2012;37(2):89–94. doi: 10.4103/0970-0218.96093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmateer N., Kimber J., Hickman M., Hutchinson S., Rhodes T., Goldberg D. Evidence for the effectiveness of sterile injecting equipment provision in preventing hepatitis C and human immunodeficiency virus transmission among injecting drug users: a review of reviews. Addiction. 2010;105(5):844–859. doi: 10.1111/j.1360-0443.2009.02888.x. [DOI] [PubMed] [Google Scholar]
- 47.Naderi M., Gholipour N., Zolfaghari M.R., Moradi Binabaj M., Yegane Moghadam A., Motalleb G. Hepatitis C virus and vaccine development. Int J Mol Cell Med. 2014;3(4):207–215. [PMC free article] [PubMed] [Google Scholar]
- 48.Puri P., Anand A.C., Saraswat V.A. AASLD. 2014. Disease burden of chronic hepatitis C virus (HCV) infection in India. Abstract. [Google Scholar]
- 49.http://pbhealth.gov.in/mmphcrf.htm [accessed 31.08.16].
- 50.Grewal A., Sobti P.C. Prevalence of hepatitis B and C in thalassemic children in Punjab. Riv Ital Med Adolesc. 2007;5(3) [Google Scholar]