Abstract
Background
Rare ginsenosides in Panax quinquefolius L. have strong bioactivities. The fact that it is hard to obtain large amounts of rare ginsenosides seriously restricts further research on these compounds. An easy, fast, and efficient method to obtain different kinds of rare ginsenosides simultaneously and to quantify each one precisely is urgently needed.
Methods
Microwave-assisted extraction (MAE) was used to extract nine kinds of rare ginsenosides from P. quinquefolius L. In this article, rare ginsenosides [20(S)-Rh1, 20(R)-Rh1, Rg6, F4, Rk3, 20(S)-Rg3, 20(R)-Rg3, Rk1, and Rg5] were identified by high performance liquid chromatography (HPLC)–electrospray ionization–mass spectrometry. The quantity information of rare ginsenosides was analyzed by HPLC-UV at 203 nm.
Results
The optimal conditions for MAE were using water as solvent with the material ratio of 1:40 (w/v) at a temperature of 145°C, and extracting for 15 min under microwave power of 1,600 W. Seven kinds of rare ginsenosides [20(S)-Rh1, 20(R)-Rh1, Rg6, F4, Rk3, Rk1, and Rg5] had high extraction yields, but those of 20(S)-Rg3 and 20(R)-Rg3 were lower. Compared with the conventional method, the extraction yields of the nine rare ginsenosides were significantly increased.
Conclusion
The results indicate that rare ginsenosides can be extracted effectively by MAE from P. quinquefolius L. in a short time. Microwave radiation plays an important role in MAE. The probable generation process of rare ginsenosides is also discussed in the article. It will be meaningful for further investigation or application of rare ginsenosides.
Keywords: high performance liquid chromatography/mass spectrometry, microwave-assisted extraction, Panax quinquefolius L., rare ginsenosides
1. Introduction
Panax quinquefolius L., which is native to the United States and Canada, is also called American Ginseng in Asia [1]. It has been widely used as a Chinese herbal medicine since the 19th century. In recent years, more attention has been paid to the applications of P. quinquefolius L. in the areas of food and drugs [2], [3], [4]. Modern pharmacological studies have shown that P. quinquefolius L. has immunoenhancing and hypolipidemic activities [5], [6], [7]. It has also been proved to have antifatigue, antiarrhythmia [8], antidiabetic [9], and antioxidative [10], [11] effects.
There are series of chemical components such as saponins, amino acids, saccharides, volatile oils, alkaloids, aliphatic acids, and mineral elements in P. quinquefolius L., among which, ginsenosides are thought to be the main active ingredients [12], [13], [14], [15], [16]. Three types of ginsenosides are found including Dammarane, Oleanane, and Ocotillol, and nearly 40 sorts of ginsenosides in P. quinquefolius L. have been identified [17], [18]. Major ginsenosides, such as Re, Rg1, Rg2, Rb1, Rb2, Rb3, Rc, Rd, Rf, and F1, are present in high concentrations in total saponins and can be easily extracted from P. quinquefolius L. Rare ginsenosides, such as Rh2, Rg3, Rk1, Rg5, Rk3, F4, and Rg6, are considered to be precious ingredients and hard to be extracted. Researchers have paid more attention to rare ginsenosides in recent years. It has been shown that some pharmacological activities, especially the anticancer effect, are related to some of the rare ginsenosides [19], [20]. It is reported that Rh1 has antiallergic, antioxidant, anti-inflammatory, antiamnestic, and antiaging effects and increases hippocampal excitability in rat brains [21], [22], [23], [24]. Ginsenoside Rg5 can induce apoptosis and DNA damage in cancer cells and it is also has a stimulatory effect on osteoblastic cell proliferation [25], [26]. Other rare ginsenosides have potent biological activities such as radical scavenging, vasodilating, neuroprotective, and antitumor activities [27]. Most of the major ginsenosides from P. quinquefolius L. are extracted via accelerated solvent extraction, or extraction assisted by ultrasound or mechanical shaking [28], while some of the rare ginsenosides are extracted via ethanol reflux extraction or degradation. In the study of Qius and Guos, the yields of 20(S)-ginsenoside Rg3 and 20(S)-ginsenoside Rh1 were 0.0155 mg/g and 0.06 mg/g, respectively [29], [30], while there are few reports on yields of other rare ginsenosides like Rk1, Rg5, F4, and Rg6.
Microwave-assisted extraction (MAE) is one kind of sample preparation technology that utilizes microwave energy to heat and extract ingredients in samples into a solvent [31]. Microwave energy is a type of unionized radiation energy that is caused by ionic migration and dipole rotation. It is also a high-frequency wave that can generate energy rapidly and increase the extraction efficiency. Microwave irradiation creates instantaneous polarization of molecules. Dipolar molecules of samples and solvents make polarity movements a billion times per second repeatedly, which leads to vibration of chemical bonds, contact and reaction of the active parts of molecules. Therefore, breakage and recombination of weak chemical bonds are promoted and the chemical structures of constituents waiting for extraction may be inevitably changed [32].
In this study, we used MAE to extract nine kinds of rare ginsenosides, 20(S)-ginsenoside Rh1, 20(R)-ginsenoside Rh1, Rg6, F4, Rk3, 20(S)-ginsenoside Rg3, 20(R)-ginsenoside Rg3, Rk1, and Rg5, from P. quinquefolius L. Contents of ginsenosides were determined by high performance liquid chromatography (HPLC)–electrospray ionization (ESI)–mass spectrometry (MS) and HPLC-UV. Optimum experimental conditions of MAE were confirmed to achieve high target contents. We intended to obtain quantifiable information about the exact yields of rare ginsenosides in P. quinquefolius L by MAE. The investigation of possible rules, which could reveal the process of common ginsenosides converting into rare ginsenosides in MAE method, would help to establish a foundation for developing a quantifiable and novel method for efficiently obtaining rare ginsenosides. It would also be meaningful for further research.
2. Materials and methods
2.1. Chemicals and reagents
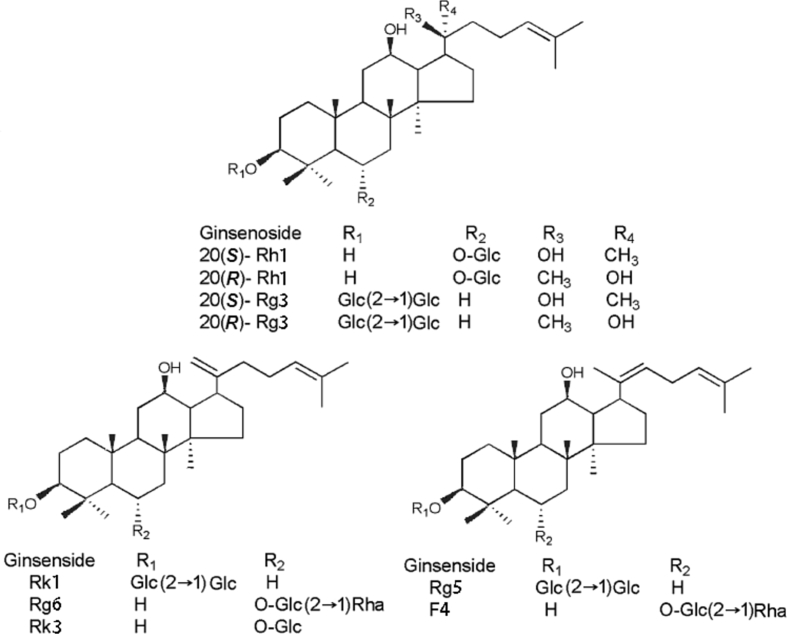
Nine rare ginsenosides, 20(S)-Rh1, 20(R)-Rh1, Rg6, F4, Rk3, 20(S)-Rg3, 20(R)-Rg3, Rk1, and Rg5, were isolated from plants of the genus Panax by our group before [33], with purities > 98%. The structures of the rare ginsenosides were elucidated by nuclear magnetic resonance based on data reported in the literature [27], [34], [35], [36], [37] and shown in Fig. 1. Acetonitrile and methanol were obtained from Fisher Scientific International (chromatographic grade; Pittsburgh, PA, USA) and ultrapure water was obtained from a Milli-Q water-purification system (Millipore, Billerica, MA, USA). Roots of P. quinquefolius L. purchased from Jingyu County, Jilin Province, were smashed and passed through an 80-mesh sieve. Other kinds of solvent used in the experiment, such as ethanol, were analytical grade and purchased from Beijing Chemical Works (Beijing, China).
Fig. 1.
Structures of rare ginsenosides. -Glc, d-glucopyranosyl, -Rha, l-rhamnopyranosyl.
2.2. Instrumental apparatus
The microwave apparatus was composed of a Mars X’press high flux microwave digestion and extraction system (CEM Corporation, San Diego, CA, USA) and polytetrafluoroethylene reactors. A lyophilizer (Martin Christ Corporation, Osterode am Harz, Switzerland) was used for obtaining powder from the products. An Agilent 1200 series HPLC instrument (Santa Clara, CA, USA) coupled with a UV detector (G1316A) was used to analyze the results.
2.3. HPLC-MS
Chromatographic separation of ginsenosides was achieved using gradient elution and the mobile phase consisted of acetonitrile and water. From 0 min to 10 min, the volume ratio of acetonitrile to water was 33:67; from 10 min to 15 min, the ratio linearly changed to 40:60; from 15 min to 40 min, the ratio linearly changed to 60:40; and from 40 min to 70 min, the ratio was kept stable at 60:40. The flow rate was 0.8 mL/min. The chromatography column was an ODS-C18 type (4.6 mm × 250 mm, 5 μm, Acchrom Technologies, Shijiazhuang, China); the temperature of which was kept at 30°C and the detection wavelength was 203 nm. The chromatograms of roots of P. quinquefolius L. obtained by MAE are shown in Fig. 2. An ABI Q-Trap Mass Spectrometer (Applied Biosystems Sciex, Foster City, CA, USA) equipped with an ESI source was used for ESI–MS analysis. Nitrogen (99.999%) was used as the nebulizer and curtain gas. The ion polarity was set to negative mode. The source temperature was set to 480°C. The curtain gas and nebulizer gas were 35 psi and 40 psi, respectively. The ion spray voltage was −4,500 V and mode of collision gas was high. Samples were processed using a declustering potential of −30 V and entrance potential of −10 V to obtain fragment ion information [38], [39]. The Q-trap MS data were collected between 200 m/z and 1,700 m/z with collision energy of −10 V for qualitative analysis.
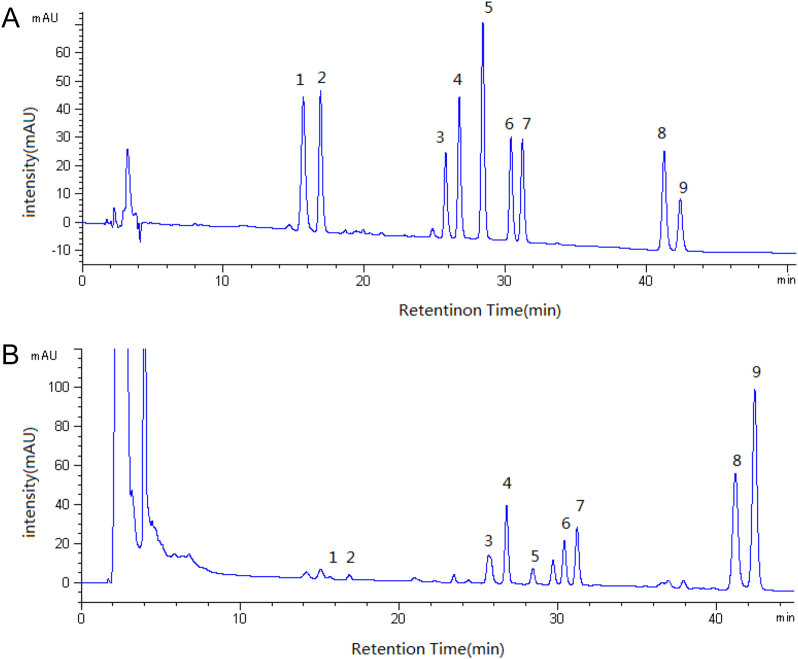
Fig. 2.
High performance liquid chromatography-UV of mixed standard solution (A) and extract of Panax quinquefolius L prepared by microwave-assisted extraction method (B). 1, 20(S)-ginsenoside Rh1; 2, 20(R)-ginsenoside Rh1; 3, Rg6; 4, F4; 5, Rk3; 6, 20(S)-ginsenoside Rg3; 7, 20(R)-ginsenoside Rg3; 8, Rk1; 9, Rg5.
2.4. Sample preparation
In this experiment, nine rare ginsenosides, 20(S)-Rh1 (0.212 mg/mL), 20(R)-Rh1 (0.140 mg/mL), Rg6 (0.040 mg/mL), F4 (0.0760 mg/mL), Rk3 (0.104 mg/mL), 20(S)-Rg3 (0.124 mg/mL), 20(R)-Rg3 (0.152 mg/mL), Rk1 (0.0840 mg/mL), and Rg5 (0.156 mg/mL) were dissolved in 10 mL methanol and used as a standard stock solution.
2.4.1. Extract of P. quinquefolius L. processed by MAE Method
Extract samples of P. quinquefolius L. were obtained by controlling different experimental conditions and parameters of MAE method. Ethanol–water solution with different proportions has an important impact on the yield of rare ginsenosides in MAE, so we investigated the effect of extraction solvent. Ten milliliters of extraction solvent (with progressively increasing volume ratio of ethanol to water from 0% to 100% in 10% increments) and 0.25 g sample were added to polytetrafluoroethylene vessels. When the temperature was increased to 120°C and maintained for 10 min, three products of the samples were processed and detected as a parallel control. We investigated the effects of material weight-to-solvent volume ratio on yields of rare ginsenosides in the process of MAE method. Taking the safe use of extract vessels into consideration, the material weight to solvent volume ratios were 1:16, 1:32, 1:40, 1:48, 1:60, 1:80, 1:100, and 1:120 (w/v). Other conditions were also controlled invariant. The temperature selected was in the range of 90°C to 150°C and extraction time was 5 min to 30 min. Roots of P. quinquefolius L. (0.25 g) were extracted with 10 mL water. Power was set at 400 W, 800 W, and 1,600 W for finding the optimal condition. After extracting, samples were evaporated with a vacuum rotary evaporator and dissolved in 25 mL methanol. The samples were filtered through a 0.22-μm membrane before HPLC detection.
2.4.2. Extract of P. quinquefolius L. processed by conventional extraction methods
Reflux extraction method is the most frequently used conventional method to obtain active pharmaceutical ingredients from traditional Chinese medicine. Roots of P. quinquefolius L. (2.0 g) were extracted with 80 mL water in a 250-mL round bottom flask fitted with a cooling condenser. It was heated to boiling for 1 h, and the residue was rinsed three times with the extraction solvent. After vacuum evaporation, the extract was transferred and diluted with methanol in a 50-mL volumetric flask. Another extraction method taken as contrast was the high temperature and pressure (HTP) method. Powder sample of P. quinquefolius L. root was added into reaction kettle and heated in an air-circulating drying oven. Each sample in high pressure reactor was 0.25 g and was kept at 145℃ with 10 mL water for 15 min reaction. Production of HTP method was lyophilized for futher detection. Finally, different kinds of extract solution were dried by vacuum-rotary evaporation procedure, diluted with methanol and filtered through a 0.22-μm nylon-66 membrane before HPLC.
3. Results and discussion
3.1. HPLC–ESI–MS of rare ginsenosides in extract of P. quinquefolius L. processed by MAE
Because of the complexities and uncertainties of the MAE method, there was an urgent need for another, accurate method to identify compounds in extracts of P. quinquefolius L. obtained by MAE. When peaks were close in chromatography, HPLC–ESI–MS was a valid method to identify what every peak represented. According to the quasi-molecular ions and fragments of every identifiable peak in HPLC-MS spectra, and the retention time of the mixed standard solutions of ginsenosides, the information of what the peaks represented for in HLPC-MS was confirmed respectively. The specific assignments are shown in Table 1. It was clear that there were nearly 30 peaks in HPLC, showing the complexity of the compounds in extracts of P. quinquefolius L. processed by MAE. About 18 types of ginsenosides were identified by the HPLC-MS information under these conditions. This indicated that there were different rare ginsenosides in the extracts processed by MAE.
Table 1.
Electrospray ionization–mass spectrometry ion fragments of Panax quinquefolium L. extract obtained by microwave-assistant extraction under optimized conditions
| Peak identification |
Retention time (min) | Molecular weight |
Main Fragment ions (m/z) |
Other ions (m/z) |
|---|---|---|---|---|
| Rf/Rg1 | 9.332 | 801.1 | 799.6 [M-H]− 835.6 [M-H+Cl]− |
989.4 [M-H+2phenyl+Cl]−, 919.6 [M+C6H12+Cl]−, 897.5 [M+CH3COOH+Cl]−, 589.5, 553.8, 441.4, 283.4 |
| Rc/Rb2/Rb3 | 12.160 | 1,079.27 | 1,077.9 [M-H]− 1,114.8 [M+Cl]− |
1,415.3 [M+2Xylose+Cl]−, 967.7, 945.6, 807.5, 792.9, 283.4 |
| Uncertain | 14.515 | 785.011) | 783.9 [M-H]− 819.7 [M-H+Cl]− |
897.6 [M+phenyl+Cl]−/[M+C7H13O]−2), 846.5 [M+2CH3O]−, 657.5, 483.1, 437.0, 363.2, 283.4 |
| Uncertain | 15.191 | 785.011) | 783.6 [M-H]− 819.8 [M-H+Cl]− |
1,078.9 [M+Glc+Xyl-H]−, 897.4 [M+phenyl+Cl]−/[M+C7H13O]−2), 846.6 [M+2CH3O]−, 676.8, 538.6, 362.9, 281.4 |
| 20(S)-Rh1 | 15.567 | 638.87 | 637.6 [M-H]− 673.4 [M-H+Cl]− |
881.3 [M+Glc+CH3CHO+Cl]−, 802.5 [M-H+rhamnose]−, 783.4 [M+CH3OH+ phenyl+Cl]−, 751.5 [M+phenyl+Cl]−/[M+C7H13O]−2), 584.5, 475.7,362.9 |
| 20(R)-Rh1 | 16.618 | 638.87 | 637.5 [M-H]− 673.4 [M-H+Cl]− |
751.3 [M+phenyl+Cl]−/[M+C7H13O]−2), 683.5 [M+C2H5O]−, 573.4, 475.7, 351.3, 283.4 |
| F2/Rg2 | 23.518 | 785.01 | 783.7 [M-H]− 819.5 [M-H+Cl]− |
1,047.9 [M+2CH3OH+Glc+Cl]−, 897.4 [M+phenyl+Cl]−/[M+C7H13O]−2), 623.0, 458.3, 325.3, 283.4 |
| Rg2/F2 | 24.570 | 785.01 | 783.6 [M-H]− 819.8 [M-H+Cl]− |
1,045.4 [M+2CH3O+Glc+Cl]−, 897.5 [M+phenyl+Cl]−/[M+C7H13O]−2), 675.6, 639.8, 325.4, 293.4, 245.2 |
| Rg6 | 25.726 | 766.49 | 765.5 [M-H]− 801.4 [M+Cl]− |
1,568.6 [2M+Cl]−, 879.5 [M+phenyl+Cl]−/[M+C7H13O]−2), 865.5 [M+2CH3OH +Cl]−, 675.5, 641.5, 283.3 |
| F4 | 26.89 | 766.49 | 765.5 [M-H]− 801.5 [M+Cl]− |
1,568.2 [2M+Cl]−, 1,107.0 [M+Glc+Rha+CH3O]−, 879.5 [M+phenyl+Cl]−/[M+C7H13O]−2), 828.5 [M+C2H3+Cl]−/[M-H +2CH3OH]−, 339.3, 284.4 |
| Rk3 | 28.572 | 620.86 | 619.6 [M-H]− 655.6 [M+Cl]− |
1,239.7 [2M-H]−, 782.7 [M-H+Glc]−, 723.6 [M+C5H8+Cl]−, 665.4 [M+C2H5O]−, 339.4, 269.6 |
| Rh4 | 30.004 | 620.86 | 619.6 [M-H]− 655.6 [M+Cl]− |
1,241.9 [2M]−, 859.6 [M-H+Glc+ phenyl]−, 733.4[M+phenyl+Cl]−/[M+C7H13O]−∗∗, 639.7 [M-O+Cl]−, 518.0, 363.1, 283.4 |
| 20(S)-Rg3 | 30.79 | 785.02 | 783.5 [M-H]− 819.4 [M-H+Cl]− |
1,604.0 [2M-H+Cl]−, 1,568.1 [2M-H]−, 897.4 [M+phenyl+Cl]−, 829.4 [M+C2H5O]−, 281.2 |
| 20(R)-Rg3 | 31.718 | 785.02 | 783.5 [M-H]− 819.4 [M-H+Cl]− |
1,604.9 [2M+Cl]−, 1,568.0 [2M-H]−, 1,225.9 [2(M-Glc)-O]−, 897.5 [M+phenyl+Cl]−, 846.5 [M+2CH3O]−/[M+C2H2+Cl]−, 281.2, 255.3 |
| Rs3 | 37.408 | 826.5 | 825.7 [M-H]− 861.5 [M+Cl]−, |
1,652.1 [2M-H]−, 939.4 [M+phenyl+Cl]−/[M+C7H13O]−2), 783.6, 661.5, 623.6, 513.4 |
| Rk1 | 41.859 | 766.49 | 765.5 [M-H]− 801.5 [M+Cl]− |
1,568.0 [2M+Cl]−, 879.4 [M+phenyl+Cl]−/[M+C7H13O]−2), 828.4 [M+C2H3+Cl]−/[M+2CH3OH-H]−, 465.3, 333.7, 283.3 |
| Rg5 | 43.103 | 766.49 | 765.5 [M-H]− 801.5 [M+Cl]−, |
1,568.1 [2M+Cl]−, 1,532.3 [2M-H]−, 879.5 [M+phenyl+Cl]−/[M+C7H13O]−2), 828.8 [M+C2H3+Cl]−/[M-H +2CH3OH]−, 283.3 |
| Compound K | 47.863 | 622.87 | 657.5 [M-H+Cl]− 621.6 [M-H]− |
1,279.9 [2M-H+Cl]−, 841.3 [2(M-Glc+OH)-C7H12O]−, 741.5 [M+malonyl+Cl]−, 488.4, 465.6, 398.1 |
| 20(S/R)-Rh2 | 49.144 | 622.87 | 657.5 [M-H+Cl]−, 621.6 [M-H]− |
1,281.5[2M+Cl]−, 681.5 [M+CH3COO]−, 641.4 [M-O+Cl]−, 459.7 [M-H-Glc]−, 363.2, 283.3, 255.3 |
Molecular weight was determined from the quasi-molecular ion peaks
Fragment ions were speculated according to compound structures, particular cases, and probable matters of experimental fact. C7H13O−, side chain of ginsenosides
3.2. Validation of quantitative analysis
Calibration curves of nine ginsenosides were obtained by gradually diluting the stock solution into nine concentrations. The linearity of each rare ginsenoside was obtained by plotting the concentrations (x, mg/mL) versus integrated peak areas (Y, mAU·s). The correlation coefficient data (R2) of the calibration curves were all > 0.9995. The limit of detection (LOD) and limit of quantification, which were defined as the lowest concentrations for which signal-to-noise ratios were 3 and 10, respectively, for every peak. The LODs were in the range from 0.18 μg/mL to 0.45 μg/mL and LQDs were in the range from 0.42 μg/mL to 1.62 μg/mL. Intraday and interday precision was in the ranges of 0.56% to 1.22% and 0.42% to 2.06%, respectively. The sample recoveries of nine rare ginsenosides were in the range from 97.97% to 103.24% with the relative standard deviations ranged from 1.534% to 2.939%, which demonstrated favorable accuracy. The details of the parameters are shown in Table 2.
Table 2.
High performance liquid chromatography of microwave-assisted extraction
| Ginsenoside | Regression equations | R2 | Linear range (μg/mL) |
LOD (μg/mL) |
LOQ (μg/mL) |
Intraday precision (%) (n=6) |
Interday precision (%) (n=9) |
Recovery (%) |
RSD of recovery (%) |
|---|---|---|---|---|---|---|---|---|---|
| 20(S)-Rh1 | Y=4,749.6x−7.8663 | 0.9996 | 1.06–41.4 | 0.45 | 1.55 | 0.65 | 1.64 | 97.97 | 1.70 |
| 20(R)-Rh1 | Y=6,124.2x−7.6974 | 0.9995 | 0.70–280.0 | 0.42 | 1.40 | 0.68 | 1.53 | 102.01 | 1.30 |
| Rg6 | Y=11,971x−4.592 | 0.9995 | 0.20–80.0 | 0.19 | 0.42 | 0.81 | 1.66 | 100.18 | 1.70 |
| F4 | Y=9,856.9x−4.3474 | 0.9998 | 0.38–152.0 | 0.30 | 0.72 | 0.96 | 1.49 | 102.18 | 0.55 |
| Rk3 | Y=12,051x−12.87 | 0.9995 | 0.52–208.0 | 0.21 | 0.52 | 1.09 | 1.58 | 102.23 | 2.09 |
| 20(S)-Rg3 | Y=4,723.8x−3.5827 | 0.9997 | 0.62–248.0 | 0.37 | 1.34 | 0.98 | 1.93 | 103.24 | 0.93 |
| 20(R)-Rg3 | Y=3,931.6x−3.8801 | 0.9997 | 0.76–304.0 | 0.33 | 1.62 | 0.56 | 1.55 | 103.02 | 0.62 |
| Rk1 | Y=8,670.4x−6.0863 | 0.9996 | 0.42–168.0 | 0.22 | 0.90 | 1.22 | 1.32 | 101.62 | 0.28 |
| Rg5 | Y=2,472.2x−1.9191 | 0.9998 | 0.78–312.0 | 0.18 | 0.75 | 1.03 | 2.06 | 102.28 | 0.40 |
LOD, limit of detection; LOQ, limit of quantification; RSD, relative standard deviation
3.3. Investigation of MAE conditions
3.3.1. Selection of extract solvent
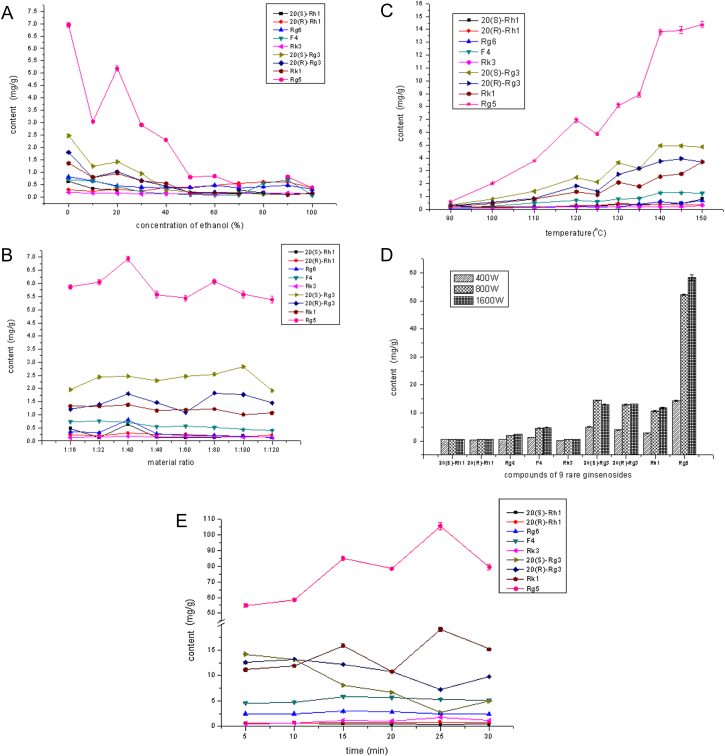
Extraction solvent in MAE method was investigated in the first place. Yields of rare ginsenosides may be various when we use solvent with different ethanol-to-water ratios to extract samples. So we investigated the effect of different ethanol-water rations. Yields of the nine rare ginsenosides extracted from P. quinquefolius L. with various ethanol-water solvent by MAE method were shown in Fig. 3A. The results suggested that increasing the concentration of ethanol decreased the yield of most of the nine rare ginsenosides, and 20(R)-Rh1 had a relatively lower yield than the others. Yield of F4 increased slightly with 90% ethanol–water solution, while other ginsenosides had low extraction yields. So, water was thought to be the better extract solvent. Although it was thought that rare ginsenosides were more easily extracted by solvent with lower polarity, such as ethanol, microwave irradiation played the decisive role in this experiment. Polar solvent and samples could lead to the vibration of molecules under microwave irradiation. High concentrations of rare ginsenosides were probably generated from degradation of major ginsenosides under the effects of microwave radiation. Because polar solvents such as water and samples like major ginsenosides could get more energy and become more active by microwave irradiation, it was possible to obtain more rare ginsenosides when they were extracted by water rather than ethanol via MAE.
Fig. 3.
Results of factors that influenced the extraction yields of nine rare ginsenosides in extract of Panax quinquefolius L. processed by microwave-assisted extraction method. (A) Different concentration of ethanol as extraction solvent. (B) Different material proportion. (C) Different extraction temperature. (D) Different power. (E) Different extraction time.
3.3.2. Selection of the volume to material ratio
Taking the safe use of extract vessels into consideration, the volume to material ratios were 1:16, 1:32, 1:40, 1:48, 1:60, 1:80, 1:100, and 1:120 (w/v). Other conditions were controlled the same as that in the investigation experiment of extraction solvent . When the volume of water was 10 mL (ratio 1:40), most of the ginsenosides had a higher extraction yield, especially Rg5, with the exception of 20(S)-Rg3 (Fig. 3B). Yields of other rare ginsenosides did not increase much as the solvent to material ratio was increased. This indicated that a volume to material ratio of 1:40 (w/v) was suitable.
3.3.3. Selection of proper extraction temperature
For temperature investigation of MAE method, we selected ten temperature points ranging from 90℃ to 150℃. The extraction yields of nine rare ginsenosides were calculated and plotted (Fig. 3C). The extraction yields of Rg5, Rg6, and 20(R)-Rg3 were obviously increased when temperature was maintained at a higher level. However, when the temperature was maintained above 140°C, the increasing in yield of the nine rare ginsenosides was moderate. So, 145°C was thought to be a suitable temperature for MAE.
3.3.4. Selection of MAE power
Power was also a critical factor in MAE, because it may affect the movement of extract solvent and extracted samples. Other optimized conditions of samples were the same as before, except for the power setting at 400 W, 800 W or 1,600 W. Although the yields of 20(S/R)-Rh1 and Rk3 changed little when the power doubled, the extraction yields of 20(S/R)-Rg3, Rk1, and Rg5 were significantly different, especially under the highest power (Fig. 3D). A power setting at 1,600 W was finally determined as the optimized condition.
3.3.5. Selection of extraction time
By controlling the extraction time as the only variable factor, the extraction yields of nine rare ginsenosides were detected (Fig. 3E). The yields of 20(S)-Rh1 and 20(R)-Rh1 had no obvious change, and that of ginsenoside 20(S/R)-Rg3 had moderate decrease trends. Rare ginsenosides F4 and Rg6 had higher extraction yields in samples at 15 min. In addition, with the extraction time longer than 15 min, vapor with partial liquid would overbrim from the extraction vessels to control a favorable pressure in system, so the extraction time should not be too long. Although Rg5 and Rk1 had higher extraction yields at 25 min, the other rare ginsenosides had lower yields at the same time. We confirmed 15 min as the best extraction time.
In conclusion, according to multifactorial consideration, we considered that the optimal conditions for MAE of rare ginesenosides from P. quinquefolius L. were extracting samples with water, maintaining temperature at 145°C for 15 min, and solvent volume to material ratio of 1:40 (w/v). The extraction yields of the nine kinds of rare ginsenosides are shown in Table 3.
Table 3.
Comparison of rare ginsenosides contents in samples obtained from Panax quinquefolius L. root by different extraction methods
| Method | Extraction yield (total) | Contents of rare ginsenosides [mg/g, mean ± standard deviation (n = 3)] |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 20(S)-Rh1 | 20(R)-Rh1 | Rg6 | F4 | Rk3 | 20(S)-Rg3 | 20(R)-Rg3 | Rk1 | Rg5 | ||
| MAE | 309.78±7.23 | 0.54±0.013 | 0.81±0.0063 | 2.99±0.017 | 5.88±0.0027 | 1.18±0.020 | 8.05±0.18 | 12.12±0.15 | 15.87±0.34 | 84.95±1.22 |
| HR | 185.16±5.18 | 0.041±0.00072 | 0.035±0.0011 | 0.017±0.00078 | 0.098±0.0027 | 0.025±0.0012 | 0.15±0.0015 | 0.098±0.0033 | 0.065±0.0028 | 0.32±0.014 |
| HTP | 120.36±4.45 | 0.0091±0.00035 | 0.0032±0.000090 | 0.00084±0.000043 | – | 0.0017±0.000051 | 0.0037±0.00017 | 0.0017±0.000033 | – | – |
HR, heating reflux; HTP, high temperature and pressure; MAE, microwave-assisted extraction
3.4. Comparison of MAE with other methods
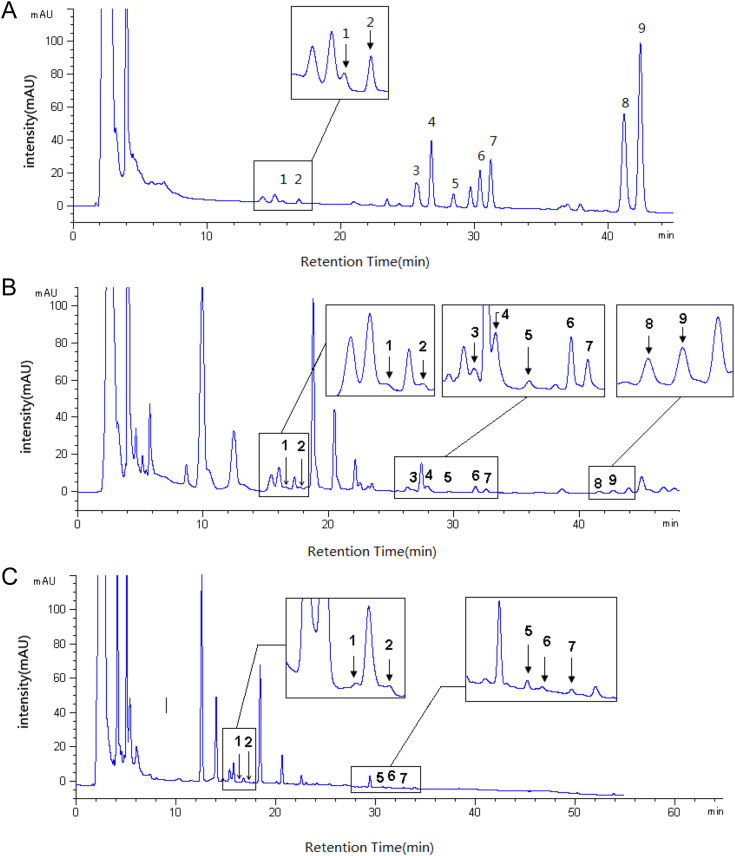
For comparison with MAE, we have investigated conventional heat reflux extraction and HTP extraction [40]. Extraction yields of rare ginsenosides using the two methods were detected by HPLC-UV (Fig. 4). Compared with the chromatogram peaks identified by the two different conventional extraction methods, there were only five kinds of rare ginsenosides at low yields using the HTP method. Although there were nine kinds of ginsenosides detected with the heating reflux method, it required a long time and obtained few target products. MAE was the most efficient among the three methods. The distinct advantages of MAE are shown in Table 3. For MAE, it was obvious that yields of the rare ginsenosides, especially Rk1 and Rg5, were increasing. Extraction yields with MAE were at least 10 times higher than those with heat reflux. The HTP method had the same conditions as MAE, except for microwave radiation. This clearly showed that microwave radiation played an important role in increasing the yields of rare ginsenosides. Although HTP had the same high temperature and pressure as MAE, but without microwave irradiation and with a short extraction time, yields of rare ginsenosides like Rg6, 20(S/R)-Rg3 and Rk3 were still low when extracted in only 15 min by HTP, while other kinds of rare ginsenosides were not detected. Total extraction yields with MAE were the highest among the three methods. This indicates that MAE is an innovative method for increasing the yield of rare ginsenosides, compared with the contrast methods HTP and heat reflux method.
Fig. 4.
High performance liquid chromatography-UV from microwave-assisted extraction (A), heating reflux (B) and high temperature and pressure method (C) of Panax quinquefolius L. 1, 20(S)-ginsenoside Rh1; 2, 20(R)-ginsenoside Rh1; 3, Rg6; 4, F4; 5, Rk3; 6, 20(S)-ginsenoside Rg3; 7, 20(R)-ginsenoside Rg3; 8, Rk1; 9, Rg5.
3.5. Mechanism of rare ginsenoside generation
Some of the major ginsenosides can be degraded to rare ginsenosides, such as degradation of Rd to Rg3. The transformation mechanism of ginsenosides in MAE could be predicted according to the chemical structure of the ginsenosides, especially changes in their sugar moieties. The glycosyl residues of major ginsenosides may be easily reduced when the power and temperature increase. Polar solvents and their interaction with samples in the MAE system were more active under microwave irradiation. This had a particularly significant effect on major ginsenosides. This was why the yield of rare ginsenosides was increased in the present study. Hydrolysis of the rhamnosyl residue at C-6 of ginsenoside Re led to its degradation into ginsenoside Rg1. Ginsenoside Rb1 was hydrolyzed at the glucosyl residue of C-20 and degraded into ginsenoside Rd. Ginsenosides Rg1 and Rd were likely to be the parent compounds of newly formed saponins, including Rk1 and Rg5, respectively. Further hydrolysis of the glucosyl residue at C-20 of Rg1 produced 20(S/R)-Rh1, which then formed Rk3 through dehydration at C-20. Elimination of glycosyl residues occurred at C-20 of common ginsenoside Rb1 made a part amount of Rb1 convert to 20(S/R)-Rg3. After heating by microwave irradiation, 20(S/R)-Rg3 was dehydrated at C-20 and formed Rk1 and Rg5. This explains the increased yields of ginsenosides Rk1 and Rg5 and decreased yield of 20(S/R)-Rg3. During this process, it was evident that microwave power was necessary and high temperature was helpful for these reactions [11], [41].
Because of the widespread prophylactic use of rare ginsenosides and the increasingly significant roles they play therapeutically, scientists have tried to find efficient to obtain rare ginsenosides. The MAE method obtained higher extraction yields of the nine ginsenosides than conventional heat reflux or HTP extraction. We demonstrated optimized conditions for MAE of rare ginsenosides extracted from P. quinquefolius L. HPLC–ESI–MS was a valid method for identifying the components in extracts of P. quinquefolius L. and their quantitative detection. We acquired particular information about every target peak using this method. The extraction yields of rare ginsenosides using the MAE method were the highest among the different test methods. MAE was a novel and effective way for obtaining substantial amounts of rare ginsenosides within a short time. The investiagtion of rare ginsenosides in P. quinquefolius L. processed by MAE method showed promising application value in producing high content of active pharmaceutical ingredients like rare ginsenosides. It also had significant meaning for discovering the conversion rules of other constituents in traditional Chinese medicines processed by MAE method.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgments
China has launched a new US$2.5 billion program to fast-track research and development into technologies considered key to China’s economic and social development. This article was supported and completed, under the financial aid of the National Science-technology Support Plan Projects (Grant No. 2007BAI38B05).
References
- 1.China Pharmacopoeia Committee . 2010 ed. Traditional Chinese Medicine Science and Technology Press of China; Beijing: 2010. Chinese pharmacopoeia; pp. 122–123. [Google Scholar]
- 2.Yang W.Z., Hu Y., Wu W.Y., Ye M., Guo D.A. Saponins in the genus Panax L. (Araliaceae): a systematic review of their chemical diversity. Phytochemistry. 2014;106:7–24. doi: 10.1016/j.phytochem.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Szeto Y.T., Sin Y.S., Pak S.C., Kalle W. American ginseng tea protects cellular DNA within 2 h from consumption: results of a pilot study in healthy human volunteers. Int J Food Sci Nutr. 2015;66:815–818. doi: 10.3109/09637486.2015.1088937. [DOI] [PubMed] [Google Scholar]
- 4.Xie J.T., Wang C.Z., Ni M., Wu J.A., Mehendale S.R., Aung H.H., Foo A., Yuan C.S. American ginseng berry juice intake reduces blood glucose and body weight in Ob/Ob mice. Food Sci. 2007;72:S590–S594. doi: 10.1111/j.1750-3841.2007.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L., Yao Y., Sang W., Yang X., Ren G. Structural features and immunostimulating effects of three acidic polysaccharides isolated from Panax quinquefolius. Int J Biol Macromol. 2015;80:77–86. doi: 10.1016/j.ijbiomac.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Yin J., Zhang H., Ye J. Traditional Chinese medicine in treatment of metabolic syndrome. Endocr Metab Immune Disord Drug Targets. 2008;8:99–111. doi: 10.2174/187153008784534330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao J.Y., Zhou D.X., Qu P.G. Experimental study on enhancing immune function of American ginseng. J Zhejiang Chin Med Univ. 2011;35:755–757. [Google Scholar]
- 8.Bao W.F., Li B.H., Yang B.Y. Pharmacological advances of research on Panax quinquefolius L. Nat Prod Res Dev. 1998;10:103–108. [Google Scholar]
- 9.Chen B., Qian H., Zhao B.T., Chen S.L. The research progress of ginseng and American ginseng effect on diabetes. Ginseng Res. 2011;1:33–36. [Google Scholar]
- 10.Zheng C.H., Chen J.Q. Study on extraction of total flavonoids from Panax quinquefolius and its antioxidation effects. J Anhui Agri Sci. 2012;40:15903–15907. [Google Scholar]
- 11.Kan K.S., Yamabe N., Kim H.Y., Yokozawa T. The changes in the constituents of American ginseng caused by heat-processing and its antioxidant activity. J Trad Med. 2010;27:97–108. [Google Scholar]
- 12.Bao W.F., Li H.B., Yang B.Y. Advances in the study of chemical constituents of Panax quinquefolius L. J Shenyang Pharm Univ. 1998;15:149–153. [Google Scholar]
- 13.Lemmon H.R., Sham J., Chau L.A., Madrenas J. High molecular weight polysaccharides are key immunomodulators in North American ginseng extracts: characterization of the ginseng genetic signature in primary human immune cells. J Ethnopharmacol. 2012;142:1–13. doi: 10.1016/j.jep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Punja Z.K. American ginseng: research developments, opportunities, and challenges. J Ginseng Res. 2011;35:368–374. doi: 10.5142/jgr.2011.35.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y., Song F.R., Liu S.Y., Li X.G. Study of the saponins of Panax quinquefolius L. China J Chinese Materia Medica. 1998;23:551–552. [PubMed] [Google Scholar]
- 16.Bao J.C., Liu G., Zheng Y.L., Zhang C.X. Research of the saponins of Panax quinquefolius L. Ginseng Res. 2004;1:7–9. [Google Scholar]
- 17.Kim D.H. Chemical diversity of Panax ginseng, Panax quinquefolius, and Panax notoginseng. J Ginseng Res. 2012;36:1–15. doi: 10.5142/jgr.2012.36.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keum Y.S., Han S.S., Chun K.S., Park K.K., Park J.H., Lee S.K., Surh Y.J. Inhibitory effects of the ginsenoside Rg3 on phorbol ester-induced cyclooxygenase-2 expression, NF-kappaB activation and tumor promotion. Mutat Res. 2003;523–524:75–85. doi: 10.1016/s0027-5107(02)00323-8. [DOI] [PubMed] [Google Scholar]
- 19.Shin Y.W., Bae E.A., Kim D.H. Inhibitory effect of ginsenoside Rg5 and its metabolite ginsenoside Rh3 in an oxazolone-induced mouse chronic dermatitis model. Arch Pharm Res. 2006;29:685–690. doi: 10.1007/BF02968253. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Y., Shen L.H., Zhang J.T. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin. 2005;26:143–149. doi: 10.1111/j.1745-7254.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- 21.Park E.K., Choo M.K., Han M.J., Kim D.H. Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int Arch Allergy Immunol. 2004;133:113–120. doi: 10.1159/000076383. [DOI] [PubMed] [Google Scholar]
- 22.Zhu D., Wu L., Li C.R., Wang X.W., Ma Y.J., Zhong Z.Y., Zhao H.B., Cui J., Xun S.F., Huang X.L., Zhou Z., Wang S.Q. Ginsenoside Rg1 protects rat cardiomyocyte from hypoxia/reoxygenation oxidative injury via antioxidant and intracellular calcium homeostasis. J Cell Bio Chem. 2009;108:117–124. doi: 10.1002/jcb.22233. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y.Z., Chen J., Chu S.F., Wang Y.S., Wang X.Y., Chen N.H., Zhang J.T. Improvement of memory in mice and increase of hippocampal excitability in rats by ginsenoside Rg1’s metabolites ginsenoside Rh1 and protopanaxatriol. J Pharmacol Sci. 2009;109:504–510. doi: 10.1254/jphs.08060fp. [DOI] [PubMed] [Google Scholar]
- 24.Jung J.S., Ahn J.H., Le T.K., Kim D.H., Kim H.S. Protopanaxatriol ginsenoside Rh1 inhibits the expression of matrix metalloproteinases and the in vitro invasion/migration of human astroglioma cells. Neurochem Int. 2013;63:80–86. doi: 10.1016/j.neuint.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Liang L.D., He T., Du T.W., Fan Y.G., Chen D.S., Wang Y. Ginsenoside-Rg5 induces apoptosis and DNA damage in human cervical cancer cells. Mol Med Rep. 2015;11:940–946. doi: 10.3892/mmr.2014.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siddiqi M.H., Siddiqi M.Z., Ahn S., Kang S., Kim Y.J., Veerappan K., Yang D.U., Yang D.C. Stimulative effect of ginsenosides Rg5:Rk1 on murine osteoblastic MC3T3-E1 cells. Phytother Res. 2014;28:1447–1455. doi: 10.1002/ptr.5146. [DOI] [PubMed] [Google Scholar]
- 27.Ha Y.W., Lim S.S., Ha I.J., Na Y.C., Seo J.J., Shin H., Son S.H., Kim Y.S. Preparative isolation of four ginsenosides from Korean red ginseng (steam-treated Panax ginseng C. A. Meyer), by high-speed counter-current chromatography coupled with evaporative light scattering detection. J Chromatogr A. 2007;1151:37–44. doi: 10.1016/j.chroma.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 28.Ligor T., Ludwiczuk A., Wolski T., Buszewski B. Isolation and determination of ginsenosides in American ginseng leaves and root extracts by LC-MS. Anal Bioanal Chem. 2005;383:1098–1105. doi: 10.1007/s00216-005-0120-8. [DOI] [PubMed] [Google Scholar]
- 29.Qiu N.N., Liu J.P., Ai M., Li P.Y. Chemical constituents and bioavailability of saponins from the leaves and stems of Panax quinquefolius L. Nat Prod Res Dev. 2012;24:1393–1423. [Google Scholar]
- 30.Guo N., Fu R., Dou D.Q. Chemical constituents of Panax quinquefolius. Chin J Med Chem. 2006;16:172–174. [Google Scholar]
- 31.Li H.B., Wang Y., Li J.F., Li X.M. Application of microwave assisted extraction technology to extraction of natural products. Mod Food Sci Technol. 2005;21:148–150. [Google Scholar]
- 32.Mandal V., Mohan Y., Hemalatha S. Microwave assisted extraction – an innovative and promising extraction tool for medicinal plant research. Pharmacognosy Rev. 2007;1:7–18. [Google Scholar]
- 33.Li L.J., Jin Y.R., Wang X.Z., Liu Y., Wu Q., Shi X.L., Li X.W. Ionic liquid and aqueous two-phase extraction based on salting-out coupled with high-performance liquid chromatography for the determination of seven rare ginsenosides in Xue-Sai-Tong injection. J Sep Sci. 2015;38:3055–3062. doi: 10.1002/jssc.201500363. [DOI] [PubMed] [Google Scholar]
- 34.Park I.H., Kim N.Y., Han S.B., Kim J.M., Kwon S.W., Kim H.J., Park M.K., Park J.H. Three new dammarane glycosides from heat processed ginseng. Arch Pharm Res. 2002;25:428–432. doi: 10.1007/BF02976595. [DOI] [PubMed] [Google Scholar]
- 35.Ryu J.H., Park J.H., Eun J.H., Jung J.H., Sohn D.H. A dammarane glycoside from Korean red ginseng. Phytochemistry. 1997;44:931–933. [Google Scholar]
- 36.Xiang W.J., Guo C.Y., Ma L., Hu L.H. Dammarane-type glycosides and long chain sesquiterpene glycosides from Gynostemma yixingense. Fitoterapia. 2010;81:248–252. doi: 10.1016/j.fitote.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Kang D.I., Lee J.Y., Yang J.Y., Jeong S.M., Lee J.H., Nah S.Y., Kim Y. Evidence that the tertiary structure of 20(S)-ginsenoside Rg(3) with tight hydrophobic packing near the chiral center is important for Na(+) channel regulation. Biochem Biophys Res Commun. 2005;333:1194–1201. doi: 10.1016/j.bbrc.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 38.Yang R.J., Li X.W., Yao H., Zhang M.C., Jin Y.R. Determination of ten rare ginsenosides in three kinds of injection by SPE and HPLC. Chromatographia. 2012;75:281–287. [Google Scholar]
- 39.Bai Y.P., Zhao L.S., Qu C.L., Meng X.Z., Zhang H.Q. Microwave degradation of floatation-enriched ginsenoside extract from Panax quinquefolius L. Leaf J Agric Food Chem. 2009;57:10252–10260. doi: 10.1021/jf902153a. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y.T., You J.Y., Yu Y., Qu C.L., Zhang H.R., Ding L., Zhang H.Q., Li X.W. Analysis of ginsenosides in Panax ginseng in high pressure microwave-assisted extraction. Food Chem. 2008;110:161–167. doi: 10.1016/j.foodchem.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 41.Wang D., Liao P.Y., Zhu H.T., Chen K.K., Xu M., Zhang Y.J., Yang C.R. The processing of Panax notoginseng and the transformation of its saponin components. Food Chem. 2012;132:1808–1813. [Google Scholar]