Abstract
Background
Although numerous studies of the anticancer activities of Korean Red Ginseng (KRG) have been performed, the therapeutic effect of KRG on leukemia has not been fully elucidated. In this study, we investigated the antileukemia activities of KRG and its cellular and molecular mechanisms.
Methods
An established leukemia tumor model induced by xenografted T cell lymphoma (RMA cells) was used to test the therapeutic activity of KRG water extract (KRG-WE). Direct cytotoxic activity of KRG-WE was confirmed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. The immunomodulatory activities of KRG-WE were verified by immunohistochemistry, nitric oxide production assay. The inhibitory effect of KRG-WE on cell survival signaling was also examined.
Results
Orally administered KRG-WE reduced the sizes of tumor masses. Levels of apoptosis regulatory enzymes and cleaved forms of caspases-3 and -8 were increased by this extract. In addition, expression of matrix metalloproteinase-9, a metastasis regulatory enzyme, was decreased by KRG-WE treatment. The proportion of CD11c+ cells was remarkably increased in the KRG-treated group compared to the control group. However, KRG-WE did not show significant direct cytotoxicity against RMA cells.
Conclusion
Our results strongly suggest that the KRG might have antileukemia activity through CD11c+ cell-mediated antitumor immunity.
Keywords: anticancer activity, cytotoxicity, Korean Red Ginseng, leukemia, Panax ginseng
1. Introduction
Korean Red Ginseng (KRG) has been widely applied as medicine and for tonifying and refection in Korea, China, and Japan. Recently, numerous research studies have found that KRG displays anti-inflammatory, antioxidant, antiobesity, antidiabetic, and anticancer effects [1], [2]. Of these, the anticancer activity of KRG is one of the best-studied areas of ginseng research. To date, anticancer and antitumor activities of KRG water extract (KRG-WE), fine black ginseng, ginseng berry extract, wild ginseng, and their individual saponins have been observed in colon, prostate, liver, pancreas, brain, and stomach cancer cells [3], [4], [5], [6], [7]. Apoptotic activities of ginseng-derived extracts, G-Rg5, G-Rh1, G-Rh2, and G-Rg3, associated with increased levels of caspase 9 and 3 activities have been demonstrated [3], [8]. Antiangiogenesis activity of G-Rp1 was also found in in vitro and in vivo tests [9]. In addition, red ginseng acid polysaccharides were found to activate antitumor immunity mediated by macrophages, natural killer (NK) cells, and cytotoxic T lymphocytes [10], [11].
Although many systemic studies on the anticancer activity of KRG have been performed, the effect of KRG on leukemia has not been fully investigated. Indeed, a few papers have reported the antileukemia effect of orally administered ginseng-derived components from Panax quinquefolius, but not from Panax ginseng [12]. Also, most studies have been performed in in vitro conditions with HL-60, U937, and NB4 cells [13], [14], [15]. Since the number of leukemia patients has been rapidly increasing, we aimed to study the antileukemia activity of KRG and its molecular and cellar mechanisms using a xenograft mouse model bearing RMA (murine T cell lymphoma) cell tumors using water extract of KRG.
2. Materials and methods
2.1. Materials
KRG-WE was provided by Korea Ginseng Cooperation (Daejeon, Korea). The contents of ginsenosides such as G-Rg1, G-Re, G-Rf, G-Rh1, G-Rb1, G-Rc, G-Rb2, and G-Rd in this extract are 1.03, 1.21, 1.04, 0.96, 5.19, 2.02, 1.88, and 0.67 (mg/g dry weight), respectively. Sodium carboxymethylcellulose (Na CMC) and (3-4-5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide were obtained from Sigma Chemical Co. (St Louis, MO, USA). Standard ginsenosides were purchased from Ambo Institute (Daejeon, Korea). Fetal bovine serum and RPMI1640 were purchased from Gibco (Grand Island, NY, USA). The RMA cells used in the present experiments were obtained from ATCC (Rockville, MD, USA). All other chemicals were from Sigma Chemical Co. Total or phospho-specific antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-CD11c and horse radish peroxidase-labeled secondary antibodies were acquired from Abcam (Cambridge, MA, USA) [16], [17], [18].
2.2. Cell culture
RMA cells were cultured in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum and 1% antibiotics (penicillin and streptomycin) at 37°C under 5% CO2 [19], [20].
2.3. Drug treatment
For in vivo experiments, KRG-WE was suspended in 0.5% Na CMC. For the in vitro cytotoxicity test, KRG-WE was suspended in 100% DMSO at a concentration of 100 mg/mL, and the solution was filtered before dilution with culture medium, as reported previously [21].
2.4. Xenograft mouse model experiments
All animal experiments were carried out in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals (IACUC, Seoul, Korea). The experimental protocol was approved by the Animal Experiments Committee of Sungkyunkwan University. C57BL/6 mice (male, age 5 wks; Orient, Sungnam, Korea) were used as our xenograft animal model. Mice were housed individually on a 12-h day/12-h night cycle at 23–27°C and had free access to food and water. Mice were randomly divided into two groups (n = 14/group): (1) a vehicle control group (n = 10): animals received oral administration of 0.5% Na CMC; and (2) a KRG-WE treatment group (n = 14): animals received oral administration of KRG-WE (20 mg/kg) in Na CMC. To produce tumors, each mouse was implanted with RMA cells (1 × 106 cells per animal) subcutaneously in the back next to the right hind leg [19]. Next, KRG-WE (20 mg/kg) or vehicle was administered orally from Day 1 to Day 21. On predetermined days, the tumors were identified and measured with a standard caliper. Tumor volume was calculated as follows:
| Tumor volume (mm3) = [tumor length (mm) × tumor width (mm)2]/2. | (1) |
Mice were sacrificed at Day 25.
2.5. Cell cytotoxicity
Cytotoxicity of KRG-WE against RMA cells was evaluated by a conventional (3-4-5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide assay as previously described [22]. Briefly, the cells were plated in 96-well plates at a density of 1 × 105 cells/well and treated with different concentrations of KRG-WE (0 μg/mL, 200 μg/mL, 400 μg/mL, and 800 μg/mL) for 24 h. The absorbance was measured using a microplate reader at 540 nm.
2.6. Preparation of total lysates from tumor and immunoblotting
Total lysates prepared from cancer tissue were subjected to western blot analysis of cleaved caspase-3, caspase-8, total form of matrix metalloproteinase (MMP)-9, and phospho-forms of p85, a regulatory protein of phosphatidylinositol-4,5-bisphosphate 3-kinase, and protein kinase B (AKT) and visualized as reported previously [21], [23], [24]. Beta-actin was used as a loading control.
2.7. Immunohistochemistry
Paraffin-embedded tissue sections of 4-μm thickness were deparaffinized in xylene and rehydrated in phosphate buffered saline. To block endogenous peroxidase activity, the sections were incubated in 3% H2O2 solution in methanol at room temperature for 10 min. Subsequently, the slides were preincubated in 10% fetal bovine serum to prevent nonspecific binding, and the respective antibody was applied at 4°C overnight. After incubation with the CD11c antibody, the sections were incubated with biotinylated horse radish peroxidase-labeled secondary antibody for 10 min. 3,3′-Diaminobenzidine substrate solution was applied to reveal the antibody staining. Lastly, the tissue sections were counterstained with Gill’s hematoxylin, dehydrated with alcohols, washed in xylene, and mounted [25], [26].
2.8. HPLC analysis
For determination of ginsenosides in KRG-WE, HPLC was conducted as described previously [7].
2.9. Statistical analysis
All data presented in this paper are the mean ± standard deviation of an experiment performed with 14 mice (in vivo studies) or six replicates (in vitro studies). For statistical comparisons, these results were analyzed using Mann–Whitney U tests. A p value < 0.05 was considered statistically significant. All statistical tests were carried out using the computer program SPSS (version 22.0, 2013; IBM Corp., Armonk, NY, USA).
3. Results and discussion
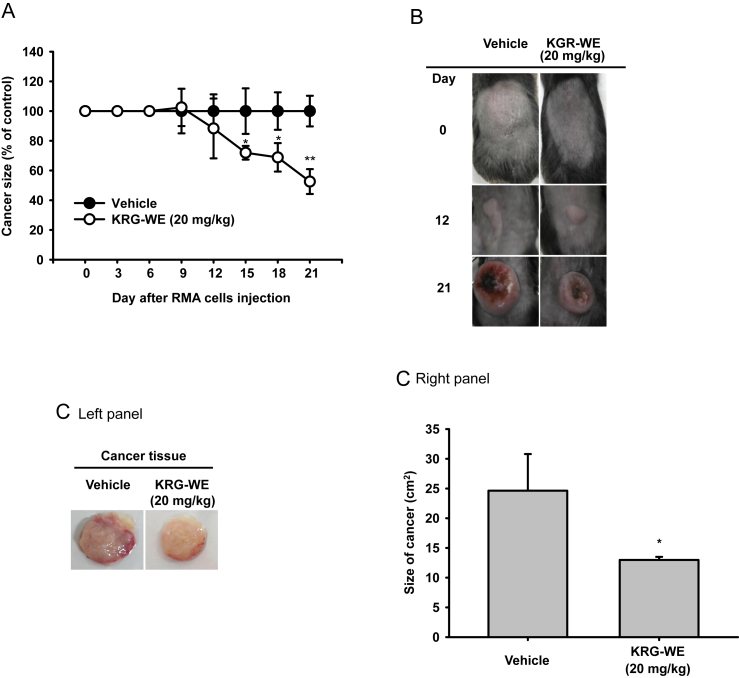
To confirm the anticancer activity of KRG, a xenograft mouse model bearing RMA cell-derived tumors was constructed as reported previously [19]. Injected RMA cells, a subline of the Raucher virus-induced T cell lymphoma RBL-5 (H-2b) [20], efficiently generated tumor tissues in mice (Fig. 1). The tumors in the group with orally administrated KRG-WE (20 mg/kg) were minuscule compared to those of the control group (Fig. 1A), and the excised cancer tissues from the mice were also smaller (Figs. 1B and 1C). These results implied that orally administrated KRG-WE might have antileukemia activity. Various wild, cultivated, and processed forms of ginseng have been reported to have anticancer activity in vivo against hepatocellular carcinoma, Lewis lung carcinoma (LLC-1), and SW480 human colon cancer cells [27], [28], [29], [30], [31]. In addition, we have demonstrated that wild ginseng-derived root powder displayed clear antileukemia activity [7]. Therefore, the present and previous data strongly suggest that ginseng could be a useful herbal medicine for the prevention and treatment of various types of cancers including leukemia, colon cancer, and hepatoma. Indeed, several clinical trials with colon and gastrointestinal cancer patients have demonstrated the effectiveness of ginseng in therapeutic approaches [32], [33].
Fig. 1.
Anticancer activity of Korean Red Ginseng water extract (KGR-WE) in a xenograft mouse bearing RMA cell-derived tumors. (A) RMA cells (1 × 106 cells per mouse) were injected subcutaneously into the back of mice next to the right hind leg, and the mice were orally administered Korean Red Ginseng (20 mg/kg) or vehicle for 3 wks. The induced tumors were identified and the size of the mass was measured on the indicated days. (B) Photograph of xenograft mice bearing RMA cell-derived tumors. (C) The excised tumor tissues were measured (right panel) and photographed (left panel) at Day 21. * p < 0.05 and ** p < 0.01 compared with the control.
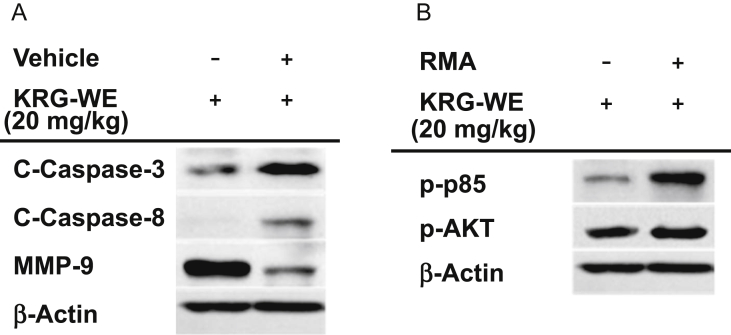
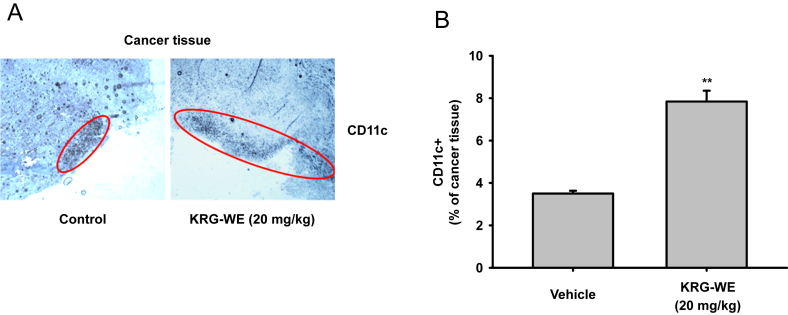
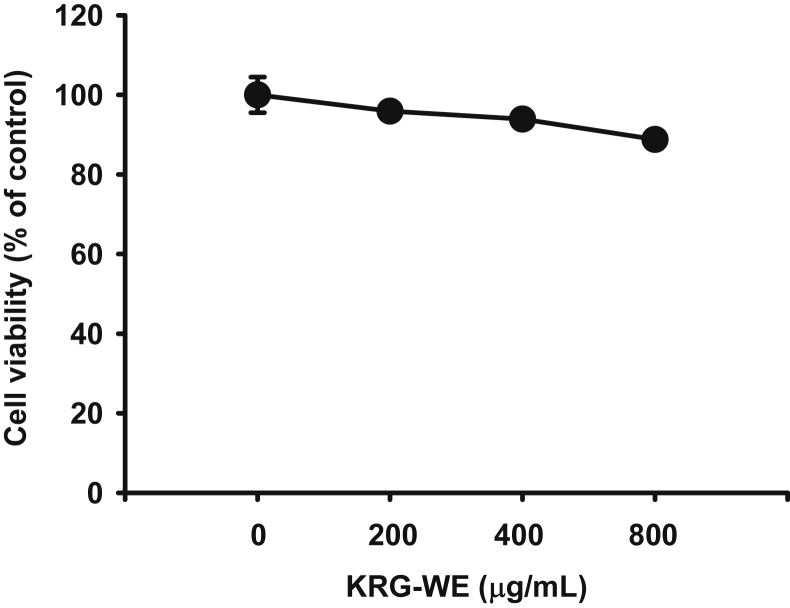
Since a reduced tumor size was observed in the mice treated with KRG-WE, we next examined the mechanism contributing to the inhibition of tumor tissue expansion. The levels of proteins related to cell death and survival, cleaved caspases-3 and -8, MMP-9, phospho-p85, and phospho-AKT, were determined by immunoblot analysis with antibodies specific for total protein and for the cleaved and phospho-proteins that represent the active forms [21], [23], [24]. The levels of cleaved forms of caspases-3 and -8 were significantly increased and the level of total MMP-9 in the KRG-WE group was remarkably decreased in the KRG-WE–treated group compared to the control group (Fig. 2A). Unexpectedly, the levels of phospho-forms of p85 and AKT, which are involved in survival signaling [34], were increased in the KRG-WE-treated group (Fig. 2B). From these results, we assumed that the anticancer activities of KRG-WE are not derived from the direct induction of cytotoxicity through apoptosis but instead are acquired through the stimulation of immune cells such as dendritic cells, neutrophils, and macrophages. To determine whether KRG-WE affects the stimulation of immune cells, we performed immunohistochemical analysis of cancer tissue sections using anti-CD11c antibody. CD11c is a well-known surface molecule expressed in innate immune cells such as macrophages, monocyte, dendritic cells, and neutrophils [35]. As shown in Fig. 3, the proportion of CD11c+ cells was remarkably increased in the KRG-WE treated group compared with the control group. To confirm these data, an in vitro cell proliferation assay was also performed to determine whether KRG-WE directly suppressed the proliferation of RMA cells. As expected, there was no significant cytotoxic effect on RMA cell proliferation under treatment with KRG-WE (even at 400 μg/mL and 800 μg/mL; Fig. 4). Based on the results, we propose that KRG-WE decreases the size of tumor tissue through the recruitment of immune cells and subsequent induction of apoptotic signaling. Exactly how the recruited immune cells arrest the growth of tumor cells is not yet understood. Therefore, we will further explore the suppressive mechanism of KRG-WE on the growth of leukemia cells in vivo in future studies.
Fig. 2.
Effect of Korean Red Ginseng water extract (KGR-WE) on the expression of proteins involved in apoptotic cell death and survival pathways. Levels of cleaved (C)-caspases-3 and 8, total form of matrix metalloproteinase (MMP)-9, phospho-forms of protein kinase B (p-AKT), p85, and β-actin in RMA tumor tissues were determined by immunoblotting analysis.
Fig. 3.
Effect of Korean Red Ginseng water extract (KGR-WE) on the infiltration level of CD11c+ cells. (A) The level of infiltrated CD11c+ cells was analyzed by immunohistochemistry after tissue sectioning. Photographs were taken under microscopy. (B) The cells were counted. * p < 0.05 and ** p < 0.01 compared with the control.
Fig. 4.
Effect of Korean Red Ginseng water extract (KGR-WE) on the viability of RMA cells. (3-4-5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide assay was conducted to determine the dose-dependent viability of RMA cells treated with KGR-WE for 24 h. **p < 0.01 compared with the control group.
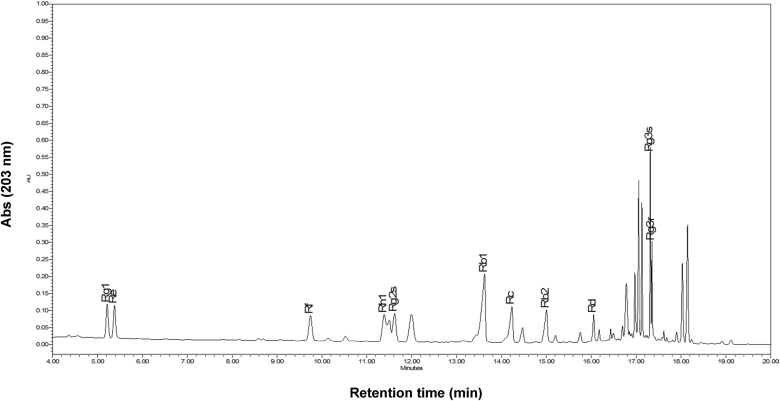
The anticancer activity of ginseng is generally considered to be due to the regulation of immune cells including macrophages and NK cells [5], [14]. In addition, various components of ginseng, such as compound K, G-Rh1, G-F2, G-Rg3, and G-Rp1, or ginseng-derived preparations were identified as anti-cancer saponins [36], [37], [38], [39], [40]. Even though this extract includes these components according to HPLC analysis (Fig. 5), their anti-cancer activity seems to be marginal. In fact, the extract had no direct cytotoxicity in an in vitro test (Fig. 4), and, more importantly, the levels of CD11c+ cells were increased in tumor tissues (Fig. 3). It was reported that acid polysaccharide fractions are able to stimulate cellular responses of innate immune cells such as macrophages, dendritic cells, and NK cells [41]. The acid polysaccharides were also shown to activate these cells to secrete cytotoxic molecules such as nitric oxide and tumor necrosis factor-α in vitro [10], [41]. Indeed, acid polysaccharides are reported to account for 74% of total KRG-WE [42]. Considering these findings, it is possible that the antileukemia activity of KRG-WE might be generated by acid polysaccharides. We will test this possibility through further study.
Fig. 5.
HPLC profile of Korean Red Ginseng water extract (KGR-WE). Ginsenosides from KGR-WE were identified.
In summary, KRG-WE has been demonstrated to exhibit strong anticancer activity in a xenograft mouse model bearing RMA cell-derived tumors without showing direct cytotoxicity. Immunoblot analysis indicated that apoptotic signaling components such as cleaved caspases-3 and -8 were increased and the total form of MMP-9 was decreased by KRG-WE administration. In addition, the number of CD11c+ cells was remarkably increased in the KRG-WE–treated group compared with the control group. These results strongly suggest that KRG-WE might have the ability to reduce the incidence of leukemia through the recruitment of immune cells and subsequent induction of apoptotic signaling in leukemia cells.
Conflicts of interest
All authors have no conflicts of interest to declare.
Contributor Information
Jong-Hoon Kim, Email: jhkim1@chonbuk.ac.kr.
Jae Youl Cho, Email: jaecho@skku.edu.
References
- 1.Xiaoguang C., Hongyan L., Xiaohong L., Zhaodi F., Yan L., Lihua T., Rui H. Cancer chemopreventive and therapeutic activities of red ginseng. J Ethnopharmacol. 1998;60:71–78. doi: 10.1016/s0378-8741(97)00133-5. [DOI] [PubMed] [Google Scholar]
- 2.Kim J.H., Hahm D.H., Yang D.C., Kim J.H., Lee H.J., Shim I. Effect of crude saponin of Korean Red Ginseng on high-fat diet-induced obesity in the rat. J Pharmacol Sci. 2005;97:124–131. doi: 10.1254/jphs.fp0040184. [DOI] [PubMed] [Google Scholar]
- 3.Kim S.J., Kim A.K. Anti-breast cancer activity of fine black ginseng (Panax ginseng Meyer) and ginsenoside Rg5. J Ginseng Res. 2015;39:125–134. doi: 10.1016/j.jgr.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nabavi S.F., Sureda A., Habtemariam S., Nabavi S.M. Ginsenoside Rd and ischemic stroke; a short review of literatures. J Ginseng Res. 2015;39:299–303. doi: 10.1016/j.jgr.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang S., Min H. Ginseng, the ‘immunity boost’: the effects of Panax ginseng on immune system. J Ginseng Res. 2012;36:354–368. doi: 10.5142/jgr.2012.36.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C.Z., Cai Y., Anderson S., Yuan C.S. Ginseng metabolites on cancer chemoprevention: an angiogenesis link? Diseases. 2015;3:193–204. doi: 10.3390/diseases3030193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park J.G., Kang W.S., Park K.T., Park D.J., Aravinthan A., Kim J.H., Cho J.Y. Anticancer effect of joboksansam, Korean wild ginseng germinated from bird feces. J Ginseng Res. 2016;40:304–308. doi: 10.1016/j.jgr.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung H., Bae J., Ko S.K., Sohn U.D. Ultrasonication processed Panax ginseng berry extract induces apoptosis through an intrinsic apoptosis pathway in HepG2 cells. Arch Pharm Res. 2016;39:855–862. doi: 10.1007/s12272-016-0760-6. [DOI] [PubMed] [Google Scholar]
- 9.Park T.Y., Park M.H., Shin W.C., Rhee M.H., Seo D.W., Cho J.Y., Kim H.M. Anti-metastatic potential of ginsenoside Rp1, a novel ginsenoside derivative. Biol Pharm Bull. 2008;31:1802–1805. doi: 10.1248/bpb.31.1802. [DOI] [PubMed] [Google Scholar]
- 10.Byeon S.E., Lee J., Kim J.H., Yang W.S., Kwak Y.S., Kim S.Y., Choung E.S., Rhee M.H., Cho J.Y. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean Red Ginseng. Mediators Inflamm. 2012;2012:732860. doi: 10.1155/2012/732860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du X.F., Jiang C.Z., Wu C.F., Won E.K., Choung S.Y. Synergistic immunostimulating activity of pidotimod and red ginseng acidic polysaccharide against cyclophosphamide-induced immunosuppression. Arch Pharm Res. 2008;31:1153–1159. doi: 10.1007/s12272-001-1282-6. [DOI] [PubMed] [Google Scholar]
- 12.Durairaj P., Miller S.C. Neoplasm prevention and immuno-enhancement mediated by daily consumption of a proprietary extract from North American ginseng by elderly mice of a cancer-prone strain. Phytother Res. 2013;27:1339–1344. doi: 10.1002/ptr.4880. [DOI] [PubMed] [Google Scholar]
- 13.Kim S.H., Cho S.S., Simkhada J.R., Lee H.J., Kim S.W., Kim T.S., Yoo J.C. Enhancement of 1,25-dihydroxyvitamin D3- and all-trans retinoic acid-induced HL-60 leukemia cell differentiation by Panax ginseng. Biosci Biotechnol Biochem. 2009;73:1048–1053. doi: 10.1271/bbb.80823. [DOI] [PubMed] [Google Scholar]
- 14.Jo S., Lee H., Kim S., Lee C.H., Chung H. Korean Red Ginseng extract induces proliferation to differentiation transition of human acute promyelocytic leukemia cells via MYC-SKP2-CDKN1B axis. J Ethnopharmacol. 2013;150:700–707. doi: 10.1016/j.jep.2013.09.036. [DOI] [PubMed] [Google Scholar]
- 15.Park S.E., Park C., Kim S.H., Hossain M.A., Kim M.Y., Chung H.Y., Son W.S., Kim G.Y., Choi Y.H., Kim N.D. Korean Red Ginseng extract induces apoptosis and decreases telomerase activity in human leukemia cells. J Ethnopharmacol. 2009;121:304–312. doi: 10.1016/j.jep.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 16.Endale M., Park S.C., Kim S., Kim S.H., Yang Y., Cho J.Y., Rhee M.H. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology. 2013;218:1452–1467. doi: 10.1016/j.imbio.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Piao J., Lee J.Y., Weon J.B., Ma C.J., Ko H.J., Kim D.D., Kang W.S., Cho H.J. Angelica gigas Nakai and Soluplus-based solid formulations prepared by hot-melting extrusion: Oral absorption enhancing and memory ameliorating effects. PLoS One. 2015;10:e0124447. doi: 10.1371/journal.pone.0124447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho J.Y., Kim A.R., Yoo E.S., Baik K.U., Park M.H. Ginsenosides from Panax ginseng differentially regulate lymphocyte proliferation. Planta Med. 2002;68:497–500. doi: 10.1055/s-2002-32556. [DOI] [PubMed] [Google Scholar]
- 19.Park J.G., Kim S.C., Kim Y.H., Yang W.S., Kim Y., Hong S., Kim K.H., Yoo B.C., Kim S.H., Kim J.H. Anti-inflammatory and antinociceptive activities of anthraquinone-2-carboxylic acid. Mediators Inflamm. 2016;2016:1903849. doi: 10.1155/2016/1903849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Bruijn M.L., Schumacher T.N., Nieland J.D., Ploegh H.L., Kast W.M., Melief C.J. Peptide loading of empty major histocompatibility complex molecules on RMA-S cells allows the induction of primary cytotoxic T lymphocyte responses. Eur J Immunol. 1991;21:2963–2970. doi: 10.1002/eji.1830211210. [DOI] [PubMed] [Google Scholar]
- 21.Vivanco I., Sawyers C.L. The phosphatidylinositol 3-kinase–AKT pathway in human cancer. Nature Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 22.Yayeh T., Jung K.H., Jeong H.Y., Park J.H., Song Y.B., Kwak Y.S., Kang H.S., Cho J.Y., Oh J.W., Kim S.K. Korean Red Ginseng saponin fraction downregulates proinflammatory mediators in LPS stimulated RAW264. 7 cells and protects mice against endotoxic shock. J Ginseng Res. 2012;36:263–269. doi: 10.5142/jgr.2012.36.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devarajan E., Sahin A.A., Chen J.S., Krishnamurthy R.R., Aggarwal N., Brun A.M., Sapino A., Zhang F., Sharma D., Yang X.H. Down-regulation of caspase 3 in breast cancer: a possible mechanism for chemoresistance. Oncogene. 2002;21:8843–8851. doi: 10.1038/sj.onc.1206044. [DOI] [PubMed] [Google Scholar]
- 24.Merdad A., Karim S., Schulten H.J., Dallol A., Buhmeida A., Al-Thubaity F., Gari M.A., Chaudhary A.G., Abuzenadah A.M., Al-Qahtani M.H. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer: MMP-9 as a potential biomarker for cancer invasion and metastasis. Anticancer Res. 2014;34:1355–1366. [PubMed] [Google Scholar]
- 25.Hume D.A., Perry V.H., Gordon S. Immunohistochemical localization of a macrophage-specific antigen in developing mouse retina: phagocytosis of dying neurons and differentiation of microglial cells to form a regular array in the plexiform layers. J Cell Biol. 1983;97:253–257. doi: 10.1083/jcb.97.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hans C.P., Weisenburger D.D., Greiner T.C., Gascoyne R.D., Delabie J., Ott G., Müller-Hermelink H.K., Campo E., Braziel R.M., Jaffe E.S. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 27.Lee J.J., Kwon H.K., Jung I.H., Cho Y.B., Kim K.J., Kim J.L. Anticancer activities of ginseng extract fermented with Phellinus linteus. Mycobiology. 2009;37:21–27. doi: 10.4489/MYCO.2009.37.1.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C.Z., Yuan C.S. Potential role of ginseng in the treatment of colorectal cancer. Am J Chin Med. 2008;36:1019–1028. doi: 10.1142/S0192415X08006545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lv Q., Rong N., Liu L.J., Xu X.L., Liu J.T., Jin F.X., Wang C.M. Antitumoral activity of (20R)- and (20S)-ginsenoside Rh2 on transplanted hepatocellular carcinoma in mice. Planta Med. 2016;82:705–711. doi: 10.1055/s-0042-101764. [DOI] [PubMed] [Google Scholar]
- 30.Seo E.Y., Kim W.K. Red ginseng extract reduced metastasis of colon cancer cells in vitro and in vivo. J Ginseng Res. 2011;35:315–324. doi: 10.5142/jgr.2011.35.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong V.K., Cheung S.S., Li T., Jiang Z.H., Wang J.R., Dong H., Yi X.Q., Zhou H., Liu L. Asian ginseng extract inhibits in vitro and in vivo growth of mouse lewis lung carcinoma via modulation of ERK-p53 and NF-kappaB signaling. J Cell Biochem. 2010;111:899–910. doi: 10.1002/jcb.22778. [DOI] [PubMed] [Google Scholar]
- 32.Wang C.Z., Anderson S., Du W., He T.C., Yuan C.S. Red ginseng and cancer treatment. Chin J Nat Med. 2016;14:7–16. doi: 10.3724/SP.J.1009.2016.00007. [DOI] [PubMed] [Google Scholar]
- 33.Park J.M., Lee H.J., Yoo J.H., Ko W.J., Cho J.Y., Hahm K.B. Overview of gastrointestinal cancer prevention in Asia. Best Pract Res Clin Gastroenterol. 2015;29:855–867. doi: 10.1016/j.bpg.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Gao M., Kong Y., Yang G., Gao L., Shi J. Multiple myeloma cancer stem cells. Oncotarget. 2016 doi: 10.18632/oncotarget.8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beyth S., Borovsky Z., Mevorach D., Liebergall M., Gazit Z., Aslan H., Galun E., Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 36.Choi K., Choi C. Proapoptotic ginsenosides Compound K and Rh 2 enhance Fas-induced cell death of human astrocytoma cells through distinct apoptotic signaling pathways. Cancer Res Treat. 2009;41:36–44. doi: 10.4143/crt.2009.41.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H.S., Lim J.M., Kim J.Y., Kim Y., Park S., Sohn J. Panaxydol, a component of Panax ginseng, induces apoptosis in cancer cells through EGFR activation and ER stress and inhibits tumor growth in mouse models. Int J Cancer. 2016;138:1432–1441. doi: 10.1002/ijc.29879. [DOI] [PubMed] [Google Scholar]
- 38.Kim M.Y., Yoo B.C., Cho J.Y. Ginsenoside-Rp1-induced apolipoprotein A-1 expression in the LoVo human colon cancer cell line. J Ginseng Res. 2014;38:251–255. doi: 10.1016/j.jgr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J.H., Nao J.F., Zhang M., He P. 20 (s)-ginsenoside Rg3 promotes apoptosis in human ovarian cancer HO-8910 cells through PI3K/Akt and XIAP pathways. Tumor Biol. 2014;35:11985–11994. doi: 10.1007/s13277-014-2497-5. [DOI] [PubMed] [Google Scholar]
- 40.Mao Q., Zhang P.H., Wang Q., Li S.L. Ginsenoside F 2 induces apoptosis in humor gastric carcinoma cells through reactive oxygen species-mitochondria pathway and modulation of ASK-1/JNK signaling cascade in vitro and in vivo. Phytomedicine. 2014;21:515–522. doi: 10.1016/j.phymed.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 41.Wang L., Yao Y., Sang W., Yang X., Ren G. Structural features and immunostimulating effects of three acidic polysaccharides isolated from Panax quinquefolius. Int J Biol Macromol. 2015;80:77–86. doi: 10.1016/j.ijbiomac.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Choi K.T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]