Abstract
Background
The induction of cellular defensive genes such as phase II detoxifying and antioxidant enzymes is a highly effective strategy for protection against carcinogenesis as well as slowing cancer development. Transcription factor Nrf2 (nuclear factor E2-related factor 2) is responsible for activation of phase II enzymes induced by natural chemopreventive compounds.
Methods
Red ginseng oil (RGO) was extracted using a supercritical CO2 extraction system and chemical profile of RGO was investigated by GC/MS. Effects of RGO on regulation of the Nrf2/antioxidant response element (ARE) pathway were determined by ARE–luciferase assay, western blotting, and confocal microscopy.
Results
The predominant components of RGO were 9,12-octadecadienoic acid (31.48%), bicyclo[10.1.0]tridec-1-ene (22.54%), and 22,23-dihydrostigmasterol (16.90%). RGO treatment significantly increased nuclear translocation of Nrf2 as well as ARE reporter gene activity, leading to upregulation of heme oxygenase-1 and NAD(P)H:quinone oxidoreductase 1. Phosphorylation of the upstream kinases such as apoptosis signal-regulating kinase (ASK)1, mitogen-activated protein kinase (MAPK) kinase (MKK)4/7, c-Jun N-terminal kinase (JNK), and p38 MAPK were enhanced by treatment with RGO. In addition, RGO-mediated Nrf2 expression and nuclear translocation was attenuated by JNK inhibitor SP600125 and p38 MAPK inhibitor SB202190.
Conclusion
RGO could be used as a potential chemopreventive agent, possibly by induction of Nrf2/ARE-mediated phase II enzymes via ASK1–MKK4/7–JNK and p38 MAPK signaling pathways.
Keywords: antioxidant response element, cytoprotection, nuclear factor E2-related factor 2, phase II enzyme, red ginseng oil
1. Introduction
Carcinogenesis is a multistep process including initiation, promotion, and progression, in which the accumulation of genetic alterations leads to transformation of an initiated cell into a population of preneoplastic cells and eventually a tumor with invasive and metastatic capacities [1]. Recently, many dietary phytochemicals have exhibited beneficial effects on health including prevention, delay, and inhibition of cancer progression [2]. Cancer chemoprevention is defined as the use of natural, synthetic, or biological chemical agents to prevent the early precancerous stage of carcinogenesis [3]. Among the underlying mechanisms of chemopreventive agents is the induction of phase II detoxifying/antioxidant enzymes involved in carcinogen detoxification, and antioxidants, such as heme oxygenase (HO)-1, NAD(P)H:quinone oxidoreductase (NQO)1, aldo–keto reductase (AKR), and glutathione S-transferase (GST). Accumulating evidence shows that transcription factor Nrf2 (nuclear factor-E2-related factor 2) is involved in the regulation of phase II enzymes through the activation of antioxidant response element (ARE), which is located in the promoter region genes encoding these enzymes [4], [5], [6]. Under physiological conditions, Nrf2 is sequestered in the cytoplasm by binding to Keap1 (Kelch-like ECH-associated protein 1, cytosolic repressor). Upon stimulations, however, Nrf2 dissociates from Keap1 and translocates into the nucleus where it binds to ARE to transcriptionally induce defensive genes [7], [8]. Studies of Nrf2-deficient mice have highlighted the importance of phase II detoxifying/antioxidant enzymes in the deactivation of chemical carcinogens, as these mice were more susceptible to carcinogenesis than normal mice were [9], [10].
Many studies have demonstrated that activation of the Nrf2/ARE pathway is related to the upstream kinases, including mitogen-activated protein kinases (MAPKs), protein kinase C, phosphatidylinositol 3-kinase (PI3K), or transmembrane kinase [11]. MAPK families, including c-Jun N-terminal kinase (JNK), extracellular signal-regulating kinases (ERKs) and p38 MAPK are key signaling molecules that respond to mitogenic stimulation or environmental stress, resulting in expression of target proteins [12]. Specific MAPK inhibitors have been shown to block the induction Nrf2 as well as phase II detoxifying/antioxidant enzymes [13], [14]. MAPKs are regulated by their upstream kinases: ERK is activated by MAPK kinase kinase (MKK)1 and MKK2, JNK by MKK4 and MKK7, and p38 MAPK kinase by MKK3, MKK4, and MKK6, which are activated by upstream MAPK kinase kinases (MAPKKKs) [15], [16].
Ginseng (Panax ginseng Meyer) is a representative herbal, which has been widely used in Korea, China, and Japan for about 2,000 years. Red ginseng is made by steaming and drying fresh ginseng, a process that chemically transforms components and acquires special physiological activities such as antioxidant [17], antidiabetic [18], antiobesity [19], and anticarcinogenic [20] effects. Although several pharmacological effects of red ginseng have been studied, they are mostly water-soluble fractions. Moreover, the biological activities and molecular mechanisms of lipid-soluble moieties of red ginseng have been poorly characterized. Lipophilic fractions from red ginseng extracted with hydrophobic organic solvents such as petroleum ether and hexane have been shown to have antithrombotic and antitumor effects [21], [22]. Our group has recently reported anti-inflammatory and hepatoprotective mechanisms of supercritical CO2-extracted red ginseng oil (RGO) in vitro and in vivo [23], [24]. In addition, our previous study has demonstrated the safety of RGO in an acute toxicity study using male and female Sprague–Dawley rats [25].
In the present study, we examined the chemical profile of supercritical CO2-extracted RGO and its underlying mechanisms in the induction of cellular defense system in HepG2 cells.
2. Materials and methods
2.1. Chemicals
Primary antibodies against Nrf2, NQO1, β-actin, and Lamin B were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA); HO-1, phospho (p)-ERK1/2, p-JNK, p-p38, p-Akt, p-MAPK/ERK kinase (MEK)1/2, p-MKK3/MKK6, p-B-Raf, mixed lineage protein kinase (MLK)3, stress-activated protein kinase/ERK kinase-1 (SEK1)/MKK4, p-MKK7, phospho-apoptosis signal-regulating kinase (p-ASK)1, and phospho-transforming growth factor β-activated kinase (p-TAK1) antibodies were purchased from Cell Signaling Technology (Boston, MA, USA). Peroxidase-conjugated secondary anti-rabbit and anti-goat antibodies were purchased from Santa Cruz Biotechnology, and Alexa-Fluor-555-conjugated secondary antibody was from Cell Signaling Technology (Boston, MA, USA). Specific inhibitors including U0126 (MEK1/2 inhibitor), SP600126 (JNK inhibitor), SB202190 (p38 MAPK inhibitor), and LY294002 (PI3 kinase inhibitor) were obtained from Cell Signaling Technology. Triton X-100, polyethylene glycol and other chemicals were from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Preparation of RGO
RGO was prepared as previously described [24]. Dried red ginseng powder was placed into the extraction vessel of a pilot-scale supercritical fluid extraction system (Ilshin Autoclave Co. Ltd., Daejeon, Korea). Extractions with supercritical CO2 were operated at 6,500 psi (relative to 450 bar) in combination with temperature at 65°C. Extracted constituents were collected in a vial that was prefilled with a trapping solvent and maintained at 4°C during the extraction step.
2.3. GC/MS
Analysis of RGO was performed using an HP-5MS capillary column (30 m × 0.25 mm, 0.25 μm; Agilent Technologies, Santa Clara, CA, USA) in a Gas chromatography–mass spectrometry (GC/MS) (5,975C; Agilent Technologies). Samples were injected into the column and run using split mode (split ratio = 10:1). The helium carrier gas was programmed to maintain a constant flow rate of 1 mL/min. Oven temperature was initially 80°C for 3 min and then finally raised to 300°C at 4°C/min. Compounds were tentatively identified by comparing mass spectra of the peaks with those in the mass spectrum library of NIST 11.
2.4. Cell culture
Human hepatoma HepG2 cell line was purchased from American Type Culture Collection (Manassas, VA, USA). HepG2-C8 cell line was established by the stable transfection of HepG2 cells with p-ARE-T1-luciferase reporter gene as previously described [26]. Both cell lines were maintained in F-12 medium with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 1% essential amino acids, 1% GlutaMAX, and 0.1% insulin. The cells were maintained in a humidified incubator at 37°C in 5% CO2.
2.5. Cell viability assay
HepG2 cells were plated at 105 cells/well in 24-well plates and treated with vehicle (polyethylene glycol: dimethyl sulfoxide, 1:1, v/v) or various concentrations of RGO for 24 h. After incubation, each well was washed twice with phosphate-buffered saline (PBS) followed by incubation with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) for 4 h at 37°C. Dark blue formazan crystals were dissolved with dimethyl sulfoxide and absorbance was measured with a PowerWave XS microplate reader (BioTek Instruments, Inc., Winooski, VT, USA) at 570 nm. The percentage of viable cells was estimated by comparison with vehicle-treated control cells.
2.6. ARE–luciferase activity assay
HepG2-C8 cells were plated at 106 cells/well in 12-well plates and incubated for 24 h. After starvation overnight, cells were treated with RGO for 12 h, washed twice with PBS, and lysed with the reporter lysis buffer supplied for luciferase assay system (Promega, Madison, WI, USA). After centrifugation at 3,000 g for 10 min, a 10-μL aliquot of the supernatant was assayed for luciferase activity with a GloMax luminometer (Promega). The luciferase activity was normalized against protein concentration, determined using a BCA protein assay (Pierce Biotechnology, Rockford, IL, USA), and expressed as fold of induction over luciferase activity of vehicle-treated control cells.
2.7. Preparation of whole-cell, nuclear and cytosolic extracts
Whole-cell extract was prepared using cell lysis buffer containing 20mM Tris (pH 7.5), 135mM sodium chloride, 2mM EDTA, 2mM dithiothreitol, 25mM β-glycerophosphate, 2mM sodium pyrophosphate, 10% glycerol, 1% Triton X-100, 1mM sodium orthovanadate, 10mM sodium fluoride, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 1mM phenylmethanesulfonyl fluoride. Nuclear–cytoplasmic fractionation was conducted using NE-PER Nuclear and Cytoplasmic Extraction Reagents Kit (Pierce Biotechnology), and protein concentrations were determined by BCA protein assay.
2.8. Western blotting
Proteins (whole-cell extracts: 30 μg/lane, nuclear extracts: 10 μg/lane, and cytosolic extracts: 30 μg/lane) were separated on 10% sodium dodecyl sulfate polyacrylamide gels. The separated proteins were electrophoretically transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA) with a semidry transfer system (Bio-Rad, Hercules, CA, USA). The membranes were incubated with blocking solution (5% nonfat milk) for 1 h, and then incubated overnight with proper primary antibodies. After hybridization with primary antibodies, the membranes were washed three times with PBS/Tween 20 (PBST) solution and incubated with horseradish-peroxidase-conjugated secondary antibodies for 1 h. The membranes were again washed three times with PBST, and detected using Western Blotting Luminol reagents (Santa Cruz Biotechnology).
2.9. Confocal microscopy
Cells were cultured in coverglass-bottom dishes. After starvation, the cells were pretreated with specific inhibitors for 1 h and then with RGO for 2 h. The cells were fixed with 4% paraformaldehyde in PBS for 10 min and permeabilized with 0.3% Triton X-100 for 10 min. After incubation with blocking buffer (3% bovine serum albumin in PBS) for 1 h, cells were incubated with Nrf2 antibody (1:100) in 0.3% bovine serum albumin for 2 h. The cells were stained for 2 h with Alexa-Fluor-555-conjugated secondary antibody (1:200) in the dark, and finally incubated with 1 μg/mL of 4′,6′-diamidino-2-phenylindole for 20 min in the dark. Images were obtained using a LSM 510 laser confocal microscope (Zeiss, Jena, Germany).
2.10. Statistical analysis
The data were expressed as mean ± standard deviation values by Microsoft Excel 2010 software (Microsoft Corporation, Redmond, WA, USA). The values were compared with the control using analysis of variance followed by unpaired Student t tests. A value of p < 0.05 was considered significant.
3. Results
3.1. Chemical composition of RGO
Chemical composition of the supercritical CO2-extracted RGO was analyzed for the first time using GC/MS (Table 1). The identified compounds were composed of three major groups including acids (34.37%), alcohols (29.89%), and hydrocarbons (24.07%). RGO contained fatty acids including linoleic acid (C18:2, 31.48%), and palmitic acid (C16:0, 2.89%). Ginseng seed oil has been reported to have oleic acid (C18:1) as the most predominant fatty acid (80%) followed by linoleic acid (16%) and palmitic acid (2%) [27]. Unlike ginseng seed oil, oleic acid was not detected and linoleic acid was the most predominant fatty acid in RGO. A previous study analyzing fatty acid composition of a lipophilic fraction from red ginseng extracted with petroleum ether has shown that linoleic accounts for about 75% of fatty acids in the fraction followed by palmitic acid (10%) [22], which corresponds to our results. For alcohols, β-sitosterol was the most abundant (16.90%) in RGO while ergosta-4,6,22-trien-3α-ol (4.53%), γ-sitosterol (4.31%), stigmasterol (3.14%), stigmasterol, and α-tocopherol (1.01%) were also detected. Stigmasterol and β-sitosterol have been previously found in ginseng seed oil [27]. A hydrocarbon bicyclo(10.1.0)tridec-1-ene was detected with a significant amount (22.54%) in RGO. This compound has been found in the fruit of an Iranian berry (Bereberise integrrima) [28] and the essential oil from buds of Tussilago farfara L [29]. Three compounds including linoleic acid, bicyclo(10.1.0)tridec-1-ene, and β-sitosterol occupied nearly 71% of RGO.
Table 1.
Chemical composition of red ginseng oil analyzed by GC/MS
| Retention time | Compound | Molecular formula | Peak area (%) |
|---|---|---|---|
| Acids | |||
| 26.98 | Palmitic acid | C16H32O2 | 2.89 |
| 29.23 | Linoleic acid | C18H32O2 | 31.48 |
| Alcohols | |||
| 44.36 | Ergosta-4,6,22-trien-3.alpha.-ol | C28H44O | 4.53 |
| 45.00 | α-tocopherol | C29H50O2 | 1.01 |
| 46.54 | Stigmasterol | C29H48O | 3.14 |
| 47.27 | β-sitosterol | C29H50O | 16.90 |
| 47.64 | γ-sitosterol | C29H50O | 4.31 |
| Hydrocarbons | |||
| 35.79 | Likely bicyclo[10.1.0]tridec-1-ene | C13H22 | 22.54 |
| 43.22 | Stigmastan-3,5-diene | C29H48 | 0.60 |
| 46.87 | Tritetracontane | C43H88 | 0.93 |
| Others | |||
| 49.05 | Stigmast-4-en-3-one | C29H48O | 1.01 |
3.2. Effect of RGO on cell viability
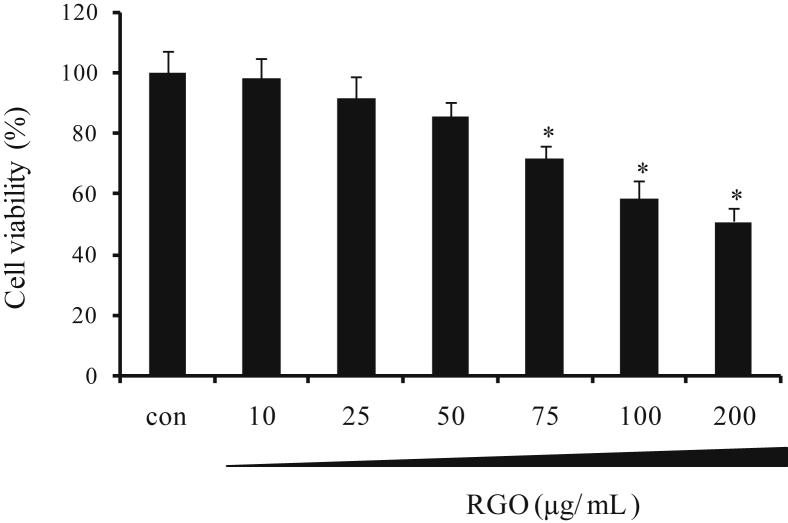
Hepatocytes play a crucial role in the processes of metabolism, detoxification, modification, and excretion of exogenous and endogenous substances, implying that it is a target for the toxic action of xenobiotics or their reactive metabolites [30]. Enhancement of cellular defense system importantly contributes to protection of cells/tissues from damage caused by toxicants or oxidative stress. Consequently, HepG2 human hepatoma cell line is considered as a good model for studying the inductive effect of natural products on phase II detoxifying/antioxidant enzymes, which directly involve in cytoprotective action [31]. In the present study, the viability of HepG2 cells was determined to evaluate the cytotoxic effect of RGO using MTT assay. RGO treatment for 24 h resulted in a decrease of cell viability in a dose-dependent manner (Fig. 1). The treatment of HepG2 cells with 50 μg/mL RGO was unlikely to cause significant cell death, and therefore, RGO concentrations < 50 μg/mL were selected for subsequent experiments of activation of Nrf2/ARE-mediated cytoprotective genes.
Fig. 1.
Effect of RGO on cell viability in HepG2 cells. Cells were treated with vehicle (dimethyl sulfoxide: polyethylene glycol, 1:1, v/v, 0.1%) or RGO at the indicated concentrations for 24 h. Cell viability was assessed with MTT assay. Data represent the average ± SD of three independent experiments with similar results. * p < 0.05 compared to the vehicle-treated control. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; RGO, red ginseng oil.
3.3. Induction of phase II detoxifying/antioxidant enzymes by RGO
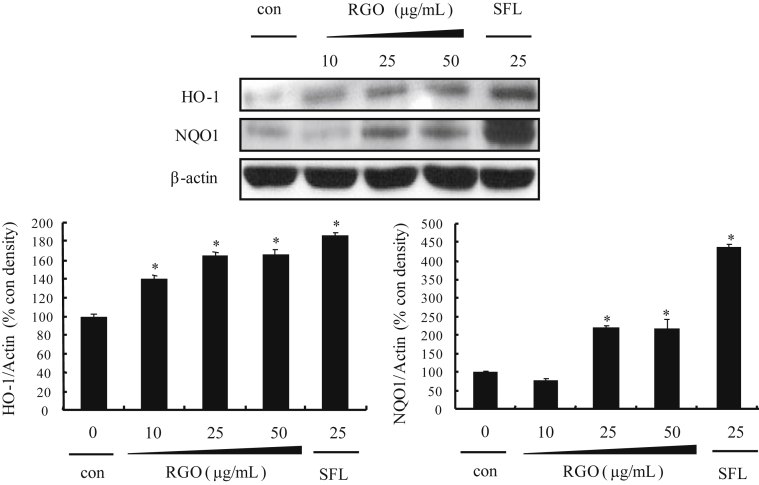
Phase II enzymes play important roles in the protection of cells/tissues from endogenous and/or exogenous carcinogens [32]. Accordingly, we first examined the inductive effect of RGO on the phase II enzymes expression after 24 h treatment. The protein expression levels of HO-1 and NQO1 significantly and dose-dependently increased in RGO-treated HepG2 cells (Fig. 2). RGO treatment at the concentration of 50 μg/mL elevated protein levels of HO-1 and NQO1 by 160% and 200%, respectively, compared to untreated controls. Similarly, expression of HO-1 and NQO1 was strongly induced in the treatment of sulforaphane (SFN), a known phase II genes activator.
Fig. 2.
Effect of RGO on NAD(P)H:quinone oxidoreductase and heme oxygenase-1 protein expression in HepG2 cells. Cells were treated with vehicle (dimethyl sulfoxide: polyethylene glycol, 1:1, v/v, 0.1%) or RGO for 12 h and then an equal amount of proteins from whole cell lysates was analyzed by western blotting. Sulforaphane was used as a positive control. The ratio of immunointensity between the target genes and β-actin was calculated. Data represent means ± standard deviation of three independent experiments with similar results. * p < 0.05 compared to the vehicle-treated control. RG, red ginseng oil.
3.4. Induction of Nrf2/ARE machinery by RGO
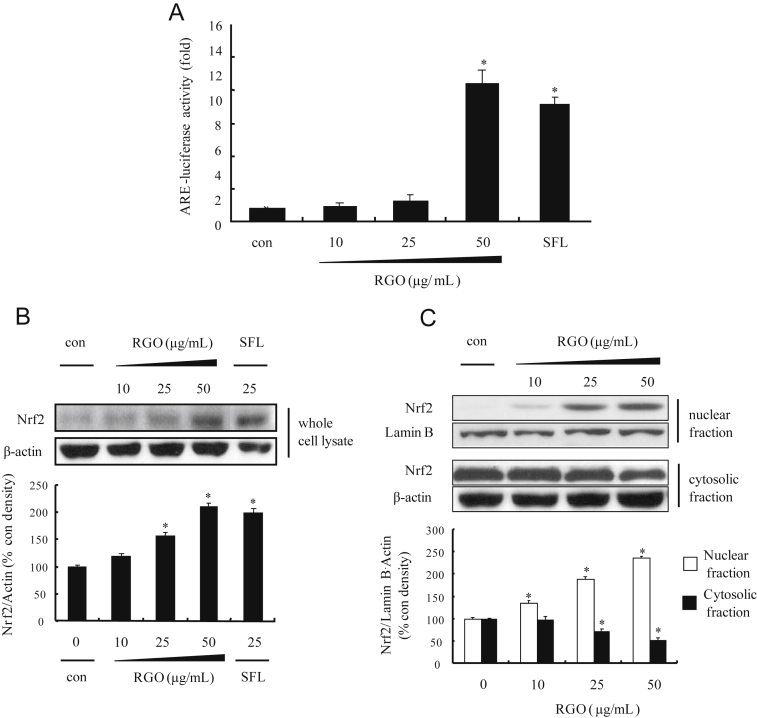
To investigate whether the induction of phase II detoxifying/antioxidant enzymes by RGO was associated with the activation of Nrf2/ARE pathway, we examined the ARE luciferase activity and Nrf2 activation in RGO-treated HepG2-C8 and HepG2 cells, respectively. As shown in Fig. 3A, RGO significantly enhanced ARE luciferase activity in a dose-dependent manner and RGO at 50 μg/mL exerted greater induction than SFN; a potent natural inducer of Nrf2/ARE pathway [33]. ARE is a crucial regulatory element found in the promoter region of many cellular defensive genes encoding phase II detoxifying and/or antioxidant enzymes including NQO1 and HO-1 [5], suggesting that the induction of phase II enzymes by RGO could be mediated by the transcriptional activation of ARE. Based on the potent inductive ability of RGO on the ARE transcriptional activity, the effect of RGO on Nrf2 protein expression and its nuclear translocation was analyzed. Similar to luciferase activity, RGO upregulated protein expression of Nrf2 in a dose-dependent manner (Fig. 3B). In addition, the level of Nrf2 protein in the nucleus was gradually elevated with the dose of RGO, whereas protein level of Nrf2 in the cytoplasm decreased (Fig. 3C). Nuclear accumulation of Nrf2 was enhanced approximately 230% by RGO treatment at 50 μg/mL compared with vehicle-treated controls. These findings indicate that the nuclear translocation of Nrf2 by RGO might facilitate the transcriptional activation of ARE-dependent genes.
Fig. 3.
Effect of RGO on antioxidant response element-reporter gene activity, Nrf2 protein expression, and nuclear translocation in HepG2 cells. (A) HepG2-C8 cells, stably transfected with pARE-T1-luciferase reporter gene, were treated with vehicle (DMSO: PEG, 1:1, v/v, 0.1%) or RGO for 12 h. Luciferase activity was normalized with protein content and expressed as fold induction against vehicle-treated control. (B) Nrf2 expression. Cells were treated with vehicle (DMSO: PEG, 1:1, v/v, 0.1%) or RGO for 2 h and then an equal amount of protein from whole cell lysates was analyzed by western blotting. (C) Levels of Nrf2 in nuclear and cytoplasmic extracts from HepG2 cells treated with RGO for 2 h were evaluated using western blot analysis. Sulforaphane was used as a positive control. The ratio of immunointensity between Nrf2 and β-actin was calculated. Data represent the mean ± standard deviation of three independent experiments with similar results. * p < 0.05 compared to the vehicle-treated control. DMSO, dimethyl sulfoxide; Nrf2, nuclear factor E2-related factor 2; PEG, polyethylene glycol; RGO, red ginseng oil.
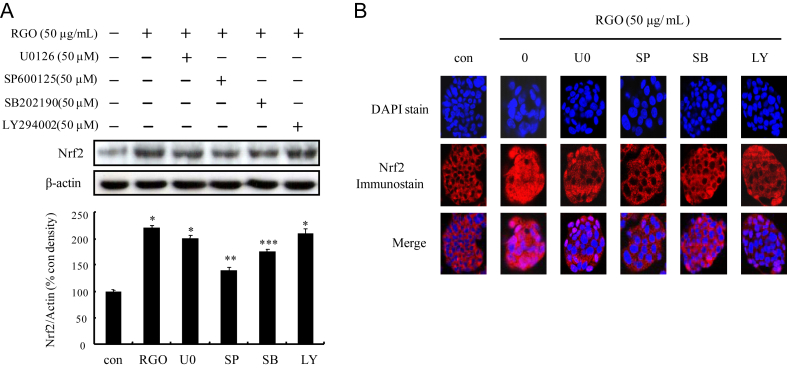
3.5. Regulation of HO-1 and NQO1 expression by RGO through ASK-MKK4/7-JNK and p38 MAPK signaling pathways in HepG2 cells
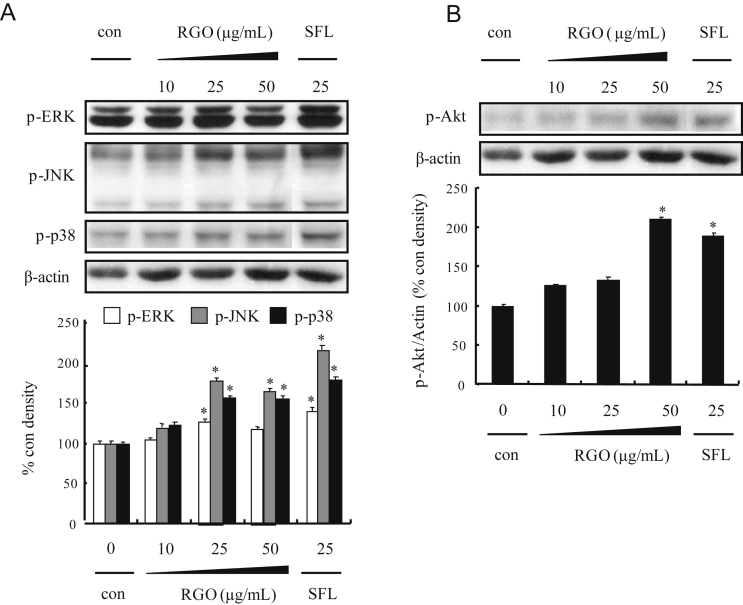
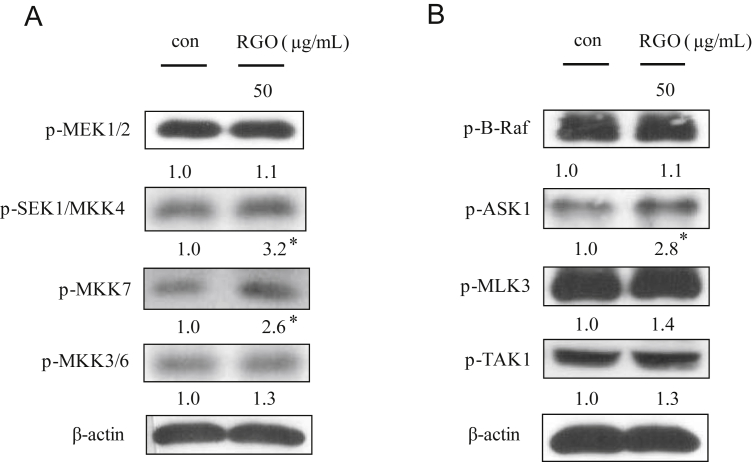
To analyze the underlying mechanisms responsible for RGO-induced phase II enzymes, HepG2 cells were treated with RGO for 1 h and induction of upstream kinase Akt and MAPKs including ERK, JNK and p38 MAPK was determined. As shown in Fig. 4, RGO dose-dependently stimulated phosphorylation of Akt and all three MAPKs. To further address the role of individual Akt and MAPK pathways in RGO-induced Nrf2 expression, specific kinase inhibitors (U0126 for MEK1/2, SB202190 for p38 MAPK, SP600125 for JNK, and LY294002 for Akt) were pretreated for 1 h prior to RGO treatment. The result showed that RGO-induced Nrf2 expression was markedly blocked by JNK inhibitor (SP600125) and significantly inhibited by p38 inhibitor (SB202190), whereas U0126 and LY294002 were unlikely to diminish Nrf2 expression level (Fig. 5A). Furthermore, the RGO-mediated nuclear translocation of Nrf2 was significantly inhibited by SP600125 and SB202190, but not by U0126 or LY294002 (Fig. 5B). These results suggest that RGO-mediated Nrf2 induction is regulated through JNK and p38 MAPK signal transduction pathways. Activation of MAPKs requires tyrosine and threonine phosphorylation by activated MAPKKs (MEK1/2, SEK1/MKK4, MKK7, and MKK3/6). Among the MAPKs, the activation of JNK is catalyzed by two upstream kinases, MKK4 and MKK7 [16], [34]. The activation of MKK4 and MKK7 is mediated by MAPKKKs including MLKs, ASKs, and dual leucine zipper kinases [35], [36]. To further investigate the upstream kinases of JNK and p38 MAPK, we examined the regulatory role of RGO in the phosphorylation of MAPKKs (MKK3/6, MEK1/2, MKK4, and MKK7) and MAPKKKs (TAK1, B-Raf, ASK1, and MLK3). RGO-treated HepG2 cells resulted in the increased phosphorylation levels of MKK4/7 and ASK1, whereas RGO was unlikely to exhibit a significant effect on phosphorylation of MLK3, TAK1, and MKK3/6, implying that RGO directly regulated p38 MAPK phosphorylation without affecting upstream kinases including MKK3/6 and MLK3/TAK1 (Fig. 6). Our findings reveal that the RGO-induced expression of phase II enzymes could be regulated by Nrf2/ARE machinery through activation of ASK1–MKK4/7–JNK and p38 MAPK signaling transduction pathways.
Fig. 4.
Effect of RGO on phosphorylation of mitogen-activated protein kinases and Akt in HepG2 cells. Cells were treated with vehicle (dimethyl sulfoxide: polyethylene glycol, 1:1, v/v, 0.1%) or RGO 1 h. (A) Western blotting for phosphorylation of extracellular signal-regulated kinase, C-Jun N-terminal kinase, and p38. (B) Akt. Sulforaphane was used as a positive control. The ratio of immunointensity between the target genes and β-actin was calculated. Data are expressed as means ± SD of three independent experiments. * p < 0.05 was significantly different from the vehicle-treated control. RGO, red ginseng oil.
Fig. 5.
Effect of specific inhibitor of mitogen-activated protein kinase and Akt on RGO-induced Nrf2 protein expression and nuclear translocation in HepG2 cells. (A) Cells were treated with specific inhibitor for MAPKERK kinase 1/2 (U0126, 50μM), C-Jun N-terminal kinase (SP600125, 50μM), p38 (SB202190, 50μM), and Akt (LY294002, 50μM) for 1 h followed by incubation with RGO (50 μg/mL) for 2 h. Western blotting for protein expression of Nrf2. Data are expressed as means ± SD of three independent experiments. * p < 0.05 compared to the vehicle-treated control. ** p < 0.05 compared to the RGO-treated group. (B) Cells were fixed and immunostained with anti-Nrf2 antibody, whereas the nucleus was stained with 4',6'-diamidino-2-phenylindole. The images of the cellular immunofluorescence were acquired using an LSM 510 laser confocal microscope (Zeiss, Jena, Germany). Nrf2, nuclear factor E2-related factor 2; RGO, red ginseng oil.
Fig. 6.
Effect of RGO on the MAPKK and MAPKKK signaling pathways in HepG2 cells. (A) Effects of RGO on phosphorylation of MAPKK. HepG2 cells were treated with RGO at 50 μg/mL for 30 min. (B) Effects of RGO on phosphorylation of MAPKKK. HepG2 cells were treated with RGO at 50 μg/mL for 15 min. Data are expressed as means ± SD of three independent experiments. * p < 0.05 compared to the vehicle-treated control. MAPKK, mitogen-activated protein kinase kinase; MAPKKK, mitogen-activated protein kinase kinase kinase; RGO, red ginseng oil.
4. Discussion
Consumption of natural products is one of the crucial strategies to prevent the risk of many diseases such as cardiovascular diseases, diabetes, and cancer. This could be achieved through inductive effects of chemopreventive phytochemicals on cellular defense systems, including phase II detoxifying and antioxidant enzymes to eliminate chemical carcinogens and oxidative stress. In the present study, we analyzed the chemical constituents and investigated the chemopreventive properties of RGO. The results demonstrated that RGO induces Nrf2 nuclear translocation and phase II enzymes, including HO-1 and NQO1, through activation of ASK1–MKK4/7–JNK and p38 MAPK signaling pathways in HepG2 cells.
GC/MS analysis revealed that RGO contains many bioactive compounds, in which linoleic acid, β-sitosterol, and bicyclo(10.1.0)tridec-1-ene are major components accounting for about 70% of RGO. Recently, many findings have demonstrated the bioactivities of linoleic acid and β-sitosterol; however, no study has yet reported any biological characteristics of bicyclo(10.1.0)tridec-1-ene. Linoleic acid has been demonstrated to be an inducer of Nrf2 in mouse primary hepatocytes [37]. Our previous study showed that sitosterol might induce cellular protective systems through activation of the Nrf2/ARE pathway in HepG2 cells [38]. In addition, Zhang et al [39] have reported that phytosterins such as β-sitosterol, campesterol, and stigmasterol protected against 4-nitrophenol-induced oxidative stress via enhancement of Nrf2-mediated detoxifying/antioxidant enzymes in rat testes. β-Sitosterol enhances antioxidant enzymes that contribute to the beneficial effects of olive oil consumption [40]. American ginseng oil may be useful as a functional ingredient since it contains various kinds of phytosterols, including squalene, campesterol, stigmasterol, sitosterol, oxidosqualene, and avenasterol, which are considered to promote human health by reducing cholesterol in humans [41].
It has been well documented that phase II enzymes could have significant effects on the prevention of tumor initiation through preventing activation of procarcinogens to reactive intermediates and increasing the neutralization of oxidative stress [32], [42]. HO-1, catalyzing the conversion of heme into biliverdin, free iron, and CO [43], plays an important role in maintaining cellular redox homeostasis against oxidative stress [44]. NQO1, a detoxifying enzyme, catalyzes the two-electron reductive metabolism and detoxification of quinines and their derivatives, resulting in protection of cells from oxidative stress and cancer development [45], [46]. In the field of cancer prevention, phytochemicals have been attracting a lot of interest due to their abilities to induce phase II detoxifying/antioxidant enzymes. A series of phytochemicals such as Epigallocatechin gallate (EGCG), resveratrol, sulforaphane, and quercitrin have been demonstrated as potent inducers of phase II and antioxidant enzymes, including HO-1 and NQO1 [47], [48], [49], [50]. Nrf2 is a key transcription factor in the transcriptional induction of phase II detoxyfying/antioxidant enzymes, via ARE. An in vivo study demonstrated that expression of phase II enzymes such as NQO1 and GSTA1 is markedly abrogated in nrf2-deficient mice as compared with wild-type animals [51]. Exposure of cells to Nrf2 inducers results in enhanced expression of many protective genes [49], [50], [52]. Therefore, activation of Nrf2 contributes to regulation of a powerful cluster of protective genes. One possible mechanism of Nrf2 activation is post-transcriptional modification of Nrf2 by protein kinases, which increase its stability and subsequent transcription activity. Protein kinases play a crucial role in converting various extracellular signals into intracellular responses through serial phosphorylation cascades. Phosphorylation of Nrf2 at serine and threonine residues by protein kinases such as PI3K/Akt, ERK, JNK, and p38 MAPK result in liberation of Nrf2 from Keap1 (Nrf2 inhibitor), enhancing Nrf2 nuclear accumulation, and finally triggering transcriptional activation of phase II enzymes [7], [8]. Phosphorylation is one of the key steps for activation of the Nrf2 pathway; however, the effect of individual protein kinases on Nrf2/ARE signaling systems may depend on cell/tissue types as well as inducers. Phenethyl isothiocyanate activates expression of Nrf2/ARE-mediated phase II enzymes through a JNK1-dependent pathway in HeLa cells [53], whereas p38 MAPK is involved in induction of Nrf2-mediated cellular defense systems by quercitrin in HepG2 cells [54]. Tea catechin EGCG-induced HO-1 expression and Nrf2 nuclear accumulation have been shown through Akt and p38 MAPK signal transduction pathways [55].
In conclusion, the lipid-soluble constituents were determined in RGO with three major compounds including linoleic acid, bicyclo[10.1.0]tridec-1-ene, and β-sitosterol. Moreover, RGO potently induced expression and nuclear translocation of the transcription factor Nrf2, leading to transcriptional activation of ARE-mediated phase II detoxifying/antioxidant enzymes, possibly via ASK1–MKK4/7–JNK and p38 MAPK signaling transduction pathways in HepG2 cells. Our results suggest that RGO might be a new potential source of natural chemopreventive and cellular defensive agents.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
We are grateful to the Korea Ginseng Corporation for the preparation of RGO. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2014R1A2A1A11050006).
Contributor Information
Mira Jun, Email: mjun@dau.ac.kr.
Woo-Sik Jeong, Email: jeongws@inje.ac.kr.
References
- 1.Surh Y.J. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 2.Dorai T., Aggarwal B.B. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004;215:129–140. doi: 10.1016/j.canlet.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Tsao A.S., Kim E.S., Hong W.K. Chemoprevention of cancer. CA Cancer J Clin. 2004;54:150–180. doi: 10.3322/canjclin.54.3.150. [DOI] [PubMed] [Google Scholar]
- 4.Hayes J.D., McMahon M. Molecular basis for the contribution of the antioxidant responsive element to cancer chemoprevention. Cancer Lett. 2001;174:103–113. doi: 10.1016/s0304-3835(01)00695-4. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen T., Nioi P., Pickett C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao C.R., Gao Z.H., Qu X.J. Nrf2–ARE signaling pathway and natural products for cancer chemoprevention. Cancer Epidemiol. 2010;34:523–533. doi: 10.1016/j.canep.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Lee J.S., Surh Y.J. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005;224:171–184. doi: 10.1016/j.canlet.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 8.Taguchi K., Motohashi H., Yamamoto M. Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16:123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 9.Hu R., Xu C., Shen G., Jain M.R., Khor T.O., Gopalkrishnan A., Lin W., Reddy B., Chan J.Y., Kong A.N.T. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Cancer Lett. 2006;243:170–192. doi: 10.1016/j.canlet.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 10.Khor T.O., Huang M.T., Kwon K.H., Chan J.Y., Reddy B.S., Kong A.N.T. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium–induced colitis. Cancer Res. 2006;66:11580–11584. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- 11.Jeong W.S., Jun M., Kong A.N.T. Nrf2: A potential molecular target for cancer chemoprevention by natural compounds. Antioxid Redox Signal. 2006;8:99–106. doi: 10.1089/ars.2006.8.99. [DOI] [PubMed] [Google Scholar]
- 12.Kong A.N.T., Owuor E., Yu R., Hebbar V., Chen C., Hu R., Mandlekar S. Induction of xenobiotic enzymes by the map kinase pathway and the antioxidant or electrophile response element (ARE/EpRE) Drug Metab Rev. 2001;33:255–271. doi: 10.1081/dmr-120000652. [DOI] [PubMed] [Google Scholar]
- 13.Bak M.J., Ok S., Jun M., Jeong W.S. 6-Shogaol-rich extract from ginger up-regulates the antioxidant defense systems in cells and mice. Molecules. 2012;17:8037–8055. doi: 10.3390/molecules17078037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong G.S., Lee D.S., Li B., Byun E., Kwon D.Y., Park H., Kim Y.C. Protective effect of sauchinone by upregulating heme oxygenase-1 via the p38 MAPK and Nrf2/ARE pathways in HepG2 cells. Planta Med. 2010;76:41–47. doi: 10.1055/s-0029-1185906. [DOI] [PubMed] [Google Scholar]
- 15.Lee J.H., Khor T.O., Shu L., Su Z.-Y., Fuentes F., Kong A.N.T. Dietary phytochemicals and cancer prevention: Nrf2 signaling, epigenetics, and cell death mechanisms in blocking cancer initiation and progression. Pharmacol Ther. 2013;137:153–171. doi: 10.1016/j.pharmthera.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearson G., Robinson F., Gibson T.B., Xu B.E., Karandikar M., Berman K., Cobb M.H. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 17.Ryu J.K., Lee T., Kim D.J., Park I.S., Yoon S.M., Lee H.S., Song S.U., Suh J.K. Free radical-scavenging activity of Korean red ginseng for erectile dysfunction in non-insulin-dependent diabetes mellitus rats. Urology. 2005;65:611–615. doi: 10.1016/j.urology.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 18.Vuksan V., Sung M.K., Sievenpiper J.L., Stavro P.M., Jenkins A.L., Di Buono M., Lee K.-S., Leiter L.A., Nam K.Y., Arnason J.T. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr Metab Cardiovasc Dis. 2008;18:46–56. doi: 10.1016/j.numecd.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Hwang J.T., Lee M.S., Kim H.J., Sung M.J., Kim H.Y., Kim M.S., Kwon D.Y. Antiobesity effect of ginsenoside Rg3 involves the AMPK and PPAR-[gamma] signal pathways. Phytother Res. 2009;23:262–266. doi: 10.1002/ptr.2606. [DOI] [PubMed] [Google Scholar]
- 20.Haniadka R., Popouri S., Palatty P.L., Arora R., Baliga M.S. Medicinal plants as antiemetics in the treatment of cancer: A review. Integr Cancer Ther. 2012;11:18–28. doi: 10.1177/1534735411413266. [DOI] [PubMed] [Google Scholar]
- 21.Lee S.D., Park S.K., Lee E.S., Kim H.M., Lee C.W., Lee K., Lee K.H., Kang M.R., Lee K.S., Lee J. A lipid-soluble red ginseng extract inhibits the growth of human lung tumor xenografts in nude mice. J Med Food. 2010;13:1–5. doi: 10.1089/jmf.2009.1142. [DOI] [PubMed] [Google Scholar]
- 22.Park H.J., Lee J.H., Song Y.B., Park K.H. Effects of dietary supplementation of lipophilic fraction from Panax ginseng on cGMP and cAMP in rat platelets and on blood coagulation. Biol Pharm Bull. 1996;19:1434–1439. doi: 10.1248/bpb.19.1434. [DOI] [PubMed] [Google Scholar]
- 23.Bak M.J., Hong S.G., Lee J.W., Jeong W.S. Red ginseng marc oil inhibits iNOS and COX-2 via NFκB and p38 pathways in LPS-stimulated RAW 264.7 macrophages. Molecules. 2012;17:13769–13786. doi: 10.3390/molecules171213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bak M.J., Jun M., Jeong W.S. Antioxidant and hepatoprotective effects of the red ginseng essential oil in H2O2-treated HepG2 cells and CCl4-treated mice. Int J Mol Sci. 2012;13:2314–2330. doi: 10.3390/ijms13022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bak M.J., Kim K.B., Jun M., Jeong W.S. Safety of red ginseng oil for single oral administration in Sprague–Dawley rats. J Ginseng Res. 2014;38:78–81. doi: 10.1016/j.jgr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeong W.S., Keum Y.S., Chen C., Jain M.R., Shen G., Kim J.H., Li W., Kong A.N.T. Differential expression and stability of endogenous nuclear factor E2-related factor 2 (Nrf2) by natural chemopreventive compounds in HepG2 human hepatoma cells. Int J Biochem Mol Biol. 2005;38:167–176. doi: 10.5483/bmbrep.2005.38.2.167. [DOI] [PubMed] [Google Scholar]
- 27.Lee M.H., Kim S.S., Cho C.W., Choi S.Y., In G., Kim K.T. Quality and characteristics of ginseng seed oil treated using different extraction methods. J Ginseng Res. 2013;37:468–474. doi: 10.5142/jgr.2013.37.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halimi M., Vahedi H., Lari J., Nasrabadi M. Chemical composition of n-hexane extratcs of the fruit from Bereberinse integrrima of Iran. Der Pharmacia Sin. 2011;2:27–30. [Google Scholar]
- 29.Liu Y.F., Yang X.W., Wu B. GC-MS analysis of essential oil constituents from buds of Tussilago farfara L. J Chinese Pharm Sci. 2006;15:10–14. [Google Scholar]
- 30.Grant D.M. Detoxification pathways in the liver. J Inherit Metab Dis. 1991;14:421–430. doi: 10.1007/BF01797915. [DOI] [PubMed] [Google Scholar]
- 31.Wilkening S., Stahl F., Bader A. Comparison of primary human hepatocytes and hepatoma cell line HepG2 with regard to their biotransformation properties. Drug Metab Dispos. 2003;31:1035–1042. doi: 10.1124/dmd.31.8.1035. [DOI] [PubMed] [Google Scholar]
- 32.Talalay P. Chemoprotection against cancer by induction of phase 2 enzymes. Biofactors. 2000;12:5–11. doi: 10.1002/biof.5520120102. [DOI] [PubMed] [Google Scholar]
- 33.Clarke J.D., Dashwood R.H., Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269:291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall C.J. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev. 1994;4:82–89. doi: 10.1016/0959-437x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 35.Cuevas B.D., Abell A.N., Johnson G.L. Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene. 2007;26:3159–3171. doi: 10.1038/sj.onc.1210409. [DOI] [PubMed] [Google Scholar]
- 36.Wang X., Destrument A., Tournier C. Physiological roles of MKK4 and MKK7: insights from animal models. Biochim Biophys Acta. 2007;1773:1349–1357. doi: 10.1016/j.bbamcr.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Cui Y., Wang Q., Yi X., Zhang X. Effects of fatty acids on CYP2A5 and Nrf2 expression in mouse primary hepatocytes. Biochem Genet. 2016;54:29–40. doi: 10.1007/s10528-015-9697-6. [DOI] [PubMed] [Google Scholar]
- 38.Kang H.S., Park M.J., Jin K.S., Kim Y.H., Jun M., Lim H.J., Jo W.K., Kim J.S., Jeong W.S. Regulatory roles of Chrysanthemum zawadskii roots in nuclear factor E2-related factor 2/antioxidant response element pathway. Food Sci Biotechnol. 2008;17:367–372. [Google Scholar]
- 39.Zhang Y., Song M., Rui X., Pu S., Li Y., Li C. Supplemental dietary phytosterin protects against 4-nitrophenol-induced oxidative stress and apoptosis in rat testes. Toxicol Rep. 2015;2:664–676. doi: 10.1016/j.toxrep.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vivancos M., Moreno J.J. β-Sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radical Biol Med. 2005;39:91–97. doi: 10.1016/j.freeradbiomed.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 41.Beveridge T.H.J., Li T.S.C., Drover J.C.G. Phytosterol content in American ginseng seed oil. J Agric Food Chem. 2002;50:744–750. doi: 10.1021/jf010701v. [DOI] [PubMed] [Google Scholar]
- 42.Nair S., Li W., Kong A.N.T. Natural dietary anti-cancer chemopreventive compounds: redox-mediated differential signaling mechanisms in cytoprotection of normal cells versus cytotoxicity in tumor cells. Acta Pharmacol Sin. 2007;28:459–472. doi: 10.1111/j.1745-7254.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- 43.Abraham N.G., Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 44.Gozzelino R., Jeney V., Soares M.P. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 45.Lim J.H., Kim K.M., Kim S.W., Hwang O., Choi H.J. Bromocriptine activates NQO1 via Nrf2-PI3K/Akt signaling: novel cytoprotective mechanism against oxidative damage. Pharm Res. 2008;57:325–331. doi: 10.1016/j.phrs.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Siegel D., Gustafson D.L., Dehn D.L., Han J.Y., Boonchoong P., Berliner L.J., Ross D. NAD(P)H:quinone oxidoreductase 1: role as a superoxide scavenger. Mol Pharmacol. 2004;65:1238–1247. doi: 10.1124/mol.65.5.1238. [DOI] [PubMed] [Google Scholar]
- 47.Chen C.Y., Jang J.H., Li M.H., Surh Y.J. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res Commun. 2005;331:993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- 48.Keum Y.S., Yu S., Chang P.P.J., Yuan X., Kim J.H., Xu C., Han J., Agarwal A., Kong A.N.T. Mechanism of action of sulforaphane: inhibition of p38 mitogen-activated protein kinase isoforms contributing to the induction of antioxidant response element–mediated heme oxygenase-1 in human hepatoma HepG2 Cells. Cancer Res. 2006;66:8804–8813. doi: 10.1158/0008-5472.CAN-05-3513. [DOI] [PubMed] [Google Scholar]
- 49.Tanigawa S., Fujii M., Hou D.X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radical Biol Med. 2007;42:1690–1703. doi: 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 50.Wu C.C., Hsu M.C., Hsieh C.W., Lin J.B., Lai P.H., Wung B.S. Upregulation of heme oxygenase-1 by epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways. Life Sci. 2006;78:2889–2897. doi: 10.1016/j.lfs.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 51.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 52.Morimitsu Y., Nakagawa Y., Hayashi K., Fujii H., Kumagai T., Nakamura Y., Osawa T., Horio F., Itoh K., Iida K. A sulforaphane analogue that potently activates the Nrf2-dependent detoxification pathway. J Biol Chem. 2002;277:3456–3463. doi: 10.1074/jbc.M110244200. [DOI] [PubMed] [Google Scholar]
- 53.Keum Y.S., Owuor E.D., Kim B.R., Hu R., Kong A.N.T. Involvement of Nrf2 and JNK1 in the activation of antioxidant responsive element (ARE) by chemopreventive agent phenethyl isothiocyanate (PEITC) Pharm Res. 2003;20:1351–1356. doi: 10.1023/a:1025737622815. [DOI] [PubMed] [Google Scholar]
- 54.Granado-Serrano A.B., Martín M.A., Bravo L., Goya L., Ramos S. Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: involvement of p38. Chem Biol Interact. 2012;195:154–164. doi: 10.1016/j.cbi.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 55.Andreadi C.K., Howells L.M., Atherfold P.A., Manson M.M. Involvement of Nrf2, p38, B-Raf, and nuclear factor-κB, but not phosphatidylinositol 3-kinase, in induction of Hemeoxygenase-1 by dietary polyphenols. Mol Pharmacol. 2006;69:1033–1040. doi: 10.1124/mol.105.018374. [DOI] [PubMed] [Google Scholar]