Abstract
Background
We evaluated the drug interaction profile of Red Ginseng (RG) with respect to the activities of major cytochrome P450 (CYP) enzymes and the drug transporter P-glycoprotein (P-gp) in healthy Korean volunteers.
Methods
This article describes an open-label, crossover study. CYP probe cocktail drugs, caffeine, losartan, dextromethorphan, omeprazole, midazolam, and fexofenadine were administered before and after RG supplementation for 2 wk. Plasma samples were collected, and tolerability was assessed. Pharmacokinetic parameters were calculated, and 90% confidence intervals (CIs) of the geometric mean ratios of the parameters were determined from logarithmically transformed data using analysis of variance after RG administration versus before RG administration.
Results
Fourteen healthy male participants were evaluated, none of whom were genetically defined as poor CYP2C9, 2C19, and CYP2D6 metabolizers based on genotyping. Before and after RG administration, the geometric least-square mean metabolic ratio (90% CI) was 0.870 (0.805–0.940) for caffeine to paraxanthine (CYP1A2), 0.871 (0.800–0.947) for losartan (CYP2C9) to EXP3174, 1.027 (0.938–1.123) for omeprazole (CYP2C19) to 5-hydroxyomeprazole, 1.373 (0.864–2.180) for dextromethorphan to dextrorphan (CYP2D6), and 0.824 (0.658–1.032) for midazolam (CYP3A4) to 1-hydroxymidazolam. The geometric mean ratio of the area under the curve of the last sampling time (AUClast) for fexofenadine (P-gp) was 0.963 (0.845–1.098). Administration of concentrated RG for 2 wk weakly inhibited CYP2C9 and CYP3A4 and weakly induced CYP2D6. However, no clinically significant drug interactions were observed between RG and CYP and P-gp probe substrates.
Conclusion
RG has no relevant potential to cause CYP enzyme- or P-gp-related interactions.
Clinical trial registration number (ClinicalTrials.gov): NCT02056743.
Keywords: cytochrome P450, drug interaction, P-glycoprotein, Red Ginseng
1. Introduction
Ginseng (Panax ginseng Meyer), one of the most popular herbal medicines, has been used for thousands of years in many eastern countries. Ginsenoside, a saponin that is the main active ingredient of ginseng, is reported to have a wide range of pharmacological activities, including stress reduction and homeostasis maintenance, immunomodulation, antifatigue actions, platelet aggregation inhibition, and anticancer effects [1].
Red Ginseng (RG) is produced when fresh ginseng root is steamed prior to oven drying. The steaming process causes chemical transformations and produces specific ginseng metabolites that have distinctive pharmacological activities [2]. The bioavailability of ginsenosides such as ginsenoside Rg3 and Rh2 in RG is higher relative to those of fresh ginseng [3].
Over the past years, a variety of pharmacokinetic interactions between ginseng and cytochrome P450 (CYP) enzymes have been described. In a review of the in vitro effects of ginsenosides on CYP enzyme activity, it has recently been shown that ginsenoside Rf results in an increase in CYP3A4 activity [4]. In a study using rat hepatocytes, ginseng extracts did not increase the expression of the rat hepatic CYP2B1, CYP3A23, or CYP1A2 genes [5]. However, administration of 500 mg of ginseng (standardized to 5% ginsenosides) in healthy individuals for 4 wk increased CYP3A4 enzyme activity by approximately 34% [6]. In addition, ginsenoside metabolites have been reported to have the potential to inhibit P- glycoprotein (P-gp) [7], [8], [9].
Many of these interaction studies have been performed using fresh ginseng. However, as RG contains a large amount of ginsenoside metabolites (e.g., compound K, Rh2), which are reported to inhibit the enzyme activities of CYP2A6, CYP2C9 and CYP3A4 [10], RG products, when taken as supplements, may affect the activity of CYP enzymes. Additionally, no studies to date have assessed the influence of RG on the activity of P-gp in humans.
The present study was conducted to characterize the influence of RG on the activity of CYP enzymes and P-gp through drug interaction by using probe drugs, caffeine, losartan, dextromethorphan, omeprazole, midazolam, and fexofenadine in healthy human volunteers.
2. Methods
This study was approved by the Ministry of Food and Drug Safety and the Institutional Review Board of Chonbuk National University Hospital (Jeonju, Republic of Korea, IRB No.: CUH 2013-04-002), and was conducted according to the Declaration of Helsinki for biomedical research involving human participants and the Guidelines for Good Clinical Practice. A detailed explanation of the study was provided, and written informed consent was obtained from all participants prior to screening.
2.1. Participants
Healthy volunteers aged 20–55 yr were enrolled. Each individual's health was confirmed by physical examinations, measurements of vital signs, 12-lead electrocardiography (ECG), serology (hepatitis B virus surface antigen, hepatitis B virus surface antibody, hepatitis C virus antibody, and anti-HIV antibody), and routine laboratory assessments (hematology, chemistry, and urinalysis). The individuals were excluded if they had within the previous 30 d consumed drugs known to significantly induce or inhibit drug-metabolizing enzymes, or had taken within the 10 d prior to the first administration of the investigational product any prescription drugs or over-the-counter drugs. Written informed consent was obtained from each of the participants prior to screening, and a detailed explanation of the study was provided.
2.2. Study design
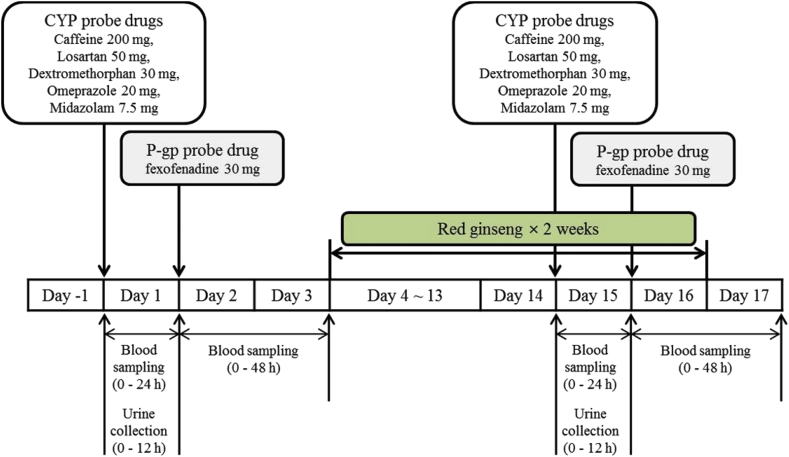
This study was conducted in an open-label, 1-sequence, 2-period crossover design at the Clinical Trial Center of Chonbuk National University Hospital (Jeonju, Republic of Korea; Fig. 1). The following five CYP substrate drugs were used to assess major drug metabolizing enzyme-mediated drug interactions: caffeine (200 mg, Vivarin, Meda Consumer Healthcare Inc., Marietta, GA, USA) as a CYP1A2 substrate; losartan (50 mg, Cozaar, MSD Korea Ltd., Seoul, Korea) as a CYP2C9 substrate; omeprazole (20 mg, Losec capsule, AstraZeneca Korea, Seoul, Korea) as a CYP2C19 substrate; dextromethorphan (15 mg, Robitussin Long-Acting CoughGels, Pfizer Inc., New York, NY, USA) as a CYP2D6 substrate; and midazolam (7.5 mg, Dormicum, Roche Korea, Seoul, Korea) as a CYP3A4 substrate. For assessment of drug transporter protein-mediated drug interactions, fexofenadine (30 mg, Allegra, Handok Pharmaceutical Co., Ltd., Seoul, Korea) was administered as a P-gp substrate.
Fig. 1.
Study design for evaluating the drug interaction of Red Ginseng with respect to the activities of major cytochrome P450 enzymes and P-gp in healthy Korean volunteers. P-gp, P-glycoprotein.
During the pharmacokinetic phase, fasting individuals received a “CYP probe drug cocktail” (caffeine 200 mg + losartan 50 mg + omeprazole 20 mg + dextromethorphan 30 mg + midazolam 7.5 mg along with 240 mL of water) on Days 1 and 15 at 8 am. Blood samples for pharmacokinetic analysis of the five CYP probe drugs were collected before dosing (baseline) and at 0.25 h, 0.5 h, 1 h, 1.5 h, 2 h, 3 h, 4 h, 5 h, 6 h, 8 h, 10 h, 12 h, and 24 h after dosing. On Days 2 and 16, each participant received a “P-gp probe drug” (fexofenadine 30 mg along with 240 mL of water) at 8 am. To determine the pharmacokinetics of fexofenadine, blood was sampled before dosing (baseline) and at 0.5 h, 0.75 h, 1 h, 1.5 h, 2 h, 3 h, 4 h, 6 h, 8 h, 12 h, 24 h, and 48 h after administration. The blood samples were immediately centrifuged at 3,200 rpm (1,800g) for 10 min at 4°C, and the resulting plasma was stored at −70°C until analysis.
Over the subsequent 2 wk (Days 4–17), one pouch (10 mL as the daily recommended dose) of concentrated RG solution (dried ginseng 64%, Hongsamjung Everytime; Korea Ginseng Corp., Daejeon, Korea) was administered once daily. The individuals were hospitalized from the evening on the day before administration of the study drugs (Day −1 and Day 14) to the mornings of Day 3 and Day 17. The individuals were continuously monitored by investigators throughout the study period. Adverse events (AEs) were identified by asking general health-related questions as well as self-reporting by the participants during the study. Physical examinations, routine laboratory assessments and vital sign measurements were performed at predefined regular intervals throughout the study.
2.3. Bioanalytical methods
The plasma concentrations of the parents and metabolites of the five CYP probe drugs (caffeine and paraxanthine, losartan and EXP-3174, omeprazole and 5-hydroxyomeprazole, dextromethorphan and dextrorphan, and midazolam and 1-hydroxymidazolam) and the P-gp probe drug (fexofenadine) were determined using a validated liquid chromatography-tandem mass spectrometry method.
The ginsenoside content [Rb1, Rb2, Rc, Re, F2, Rg1, 20(S)-Rg3, 20(R)-Rg3, 20(S)-Rh1, 20(S)/20(R)-Rh2, 20(R)-Rh1/Rd/F1, compound K, compound O, compound Y, Protopanaxadiol (S), Protopanaxadiol (R), and Protopanaxatriol (S)] of the concentrated RG solution product was analyzed using a GX-281 HPLC System UV/VIS (Gilson, Inc., Middleton, WI, USA; Table 1).
Table 1.
Ginsenoside contents in Red Ginseng products
| Contents | Ginsenoside concentration |
|
|---|---|---|
| Amount (mg/10 mL) | Composition of ginsenosides (%) | |
| Rb1 | 29.02 ± 0.05 | 28.94 ± 0.25 |
| Rb2 | 4.63 ± 0.27 | 4.62 ± 0.22 |
| Rc | 15.68 ± 0.69 | 15.64 ± 0.52 |
| Re | 8.58 ± 0.01 | 8.55 ± 0.10 |
| F2 | 0.12 ± 0.03 | 0.12 ± 0.03 |
| Rg1 | 4.59 ± 0.02 | 4.58 ± 0.07 |
| 20(S)-Rg3 | 2.95 ± 0.15 | 2.94 ± 0.12 |
| 20(R)-Rg3 | 11.5 ± 0.72 | 11.46 ± 0.06 |
| 20(S)-Rh1 | 15.04 ± 0.56 | 15.00 ± 0.40 |
| 20(S)/20(R)-Rh2 | 0.02 ± 0.01 | 0.02 ± 0.00 |
| 20(R)-Rh1/Rd/F1 | 0.65 ± 0.04 | 0.65 ± 0.03 |
| Compound K | 0.06 ± 0.02 | 0.06 ± 0.02 |
| Compound O | 0.07 ± 0.01 | 0.07 ± 0.01 |
| Compound Y | 5.97 ± 1.32 | 5.96 ± 1.38 |
| Protopanaxadiol(S) | 0.13 ± 0.00 | 0.12 ± 0.00 |
| Protopanaxadiol(R) | 0.19 ± 0.02 | 0.19 ± 0.02 |
| Protopanaxatriol(S) | 1.08 ± 0.08 | 1.08 ± 0.09 |
Values are presented as mean ± standard deviations
2.4. Genotype analysis of CYP2C9, CYP2C19, and CYP2D6
CYP enzyme activity was measured with a PyroMark ID (Biotage AB, Uppsala, Sweden). The CYP2C9*2, CYP2C9*3, CYP2C19*2, and CYP2C19*3 enzymes were measured using a pyrosequencing method, and the CYP2D6*5 enzyme was measured using a polymerase chain reaction (PCR) method.
2.5. Pharmacokinetic and statistical analysis
Individual PK parameters were obtained by noncompartmental methods using Phoenix WinNonlin 6.3 software (Pharsight Corporation, Sunnyvale, CA, USA). The maximum plasma concentration (Cmax) and time to Cmax (tmax) were obtained directly from plasma concentration–time curves. The area under the curve of the last sampling time (AUClast) was calculated using the linear trapezoidal rule. CYP1A2, 2C9, 2C19, 2D6, and 3A4 activities were evaluated by calculating the metabolic ratios of caffeine, losartan, omeprazole, dextromethorphan, and midazolam, respectively; the metabolic ratios were calculated by determining their corresponding metabolite/parent AUC ratios (AUClast, metabolite/AUClast, parent) in plasma.
Statistical analysis was performed using SAS® (Version 9.3, SAS Institute Inc., Cary, NC, USA). Descriptive statistics were used to summarize the pharmacokinetic data, and ANOVA at a 5% significance level was used to compare the pharmacokinetic parameters. The point estimates and 90% confidence intervals (CIs) of the ratios of the geometric means ratios (after RG intake/before RG intake) of the log-transformed Cmax and AUClast were compared. Additionally, because the number of participants was small, we performed the Wilcoxon Signed-Rank test.
As this was a descriptive study to evaluate the drug interaction potential of RG, no formal sample size estimation was performed prospectively for pharmacokinetic evaluations. In retrospect, this study was well powered (> 80% power) to rule out a 20% decrease or 25% increase in AUC when comparing Day 15 and 16 pharmacokinetic parameters to baseline with 90% confidence. Only the AUC of dextromethorphan was not as well powered (59%) at the sample level of confidence.
3. Results
A total of 15 healthy volunteers (with a mean ± standard deviation age of 24.5 ± 1.9 yr, a mean height of 173.9 ± 5.7 cm, and a mean weight of 68.9 ± 9.1 kg) were determined to be eligible for the study and enrolled. After a volunteer chose to withdraw, the data for this individual were excluded from the study; the remaining 14 individuals completed the study. CYP enzyme genotyping was assessed in 14 individuals, and none of the participants were found to be genetically poor metabolizers of CYP2C9*2/*2, *2/*3, or *3/*3, CYP2C19 *2/*2, *2/*3, or *3/*3, or CYP2D6 *5/*5 (Table 2).
Table 2.
Genotypes and allele frequencies of CYP2C9, CYP2C19, and CYP2D6
| Genotype | No. (frequency, %) | |
|---|---|---|
| CYP2C9 | *1/*1 | 13 (92.9) |
| *1/*3 | 1 (7.1) | |
| CYP2C19 | *1/*1 | 11 (78.6) |
| *1/*3 | 3 (21.4) | |
| CYP2D6 | *1/*1 | 14 (100) |
| Gene deletion | 0 (0) |
3.1. Effects of Red Ginseng on CYP enzymes
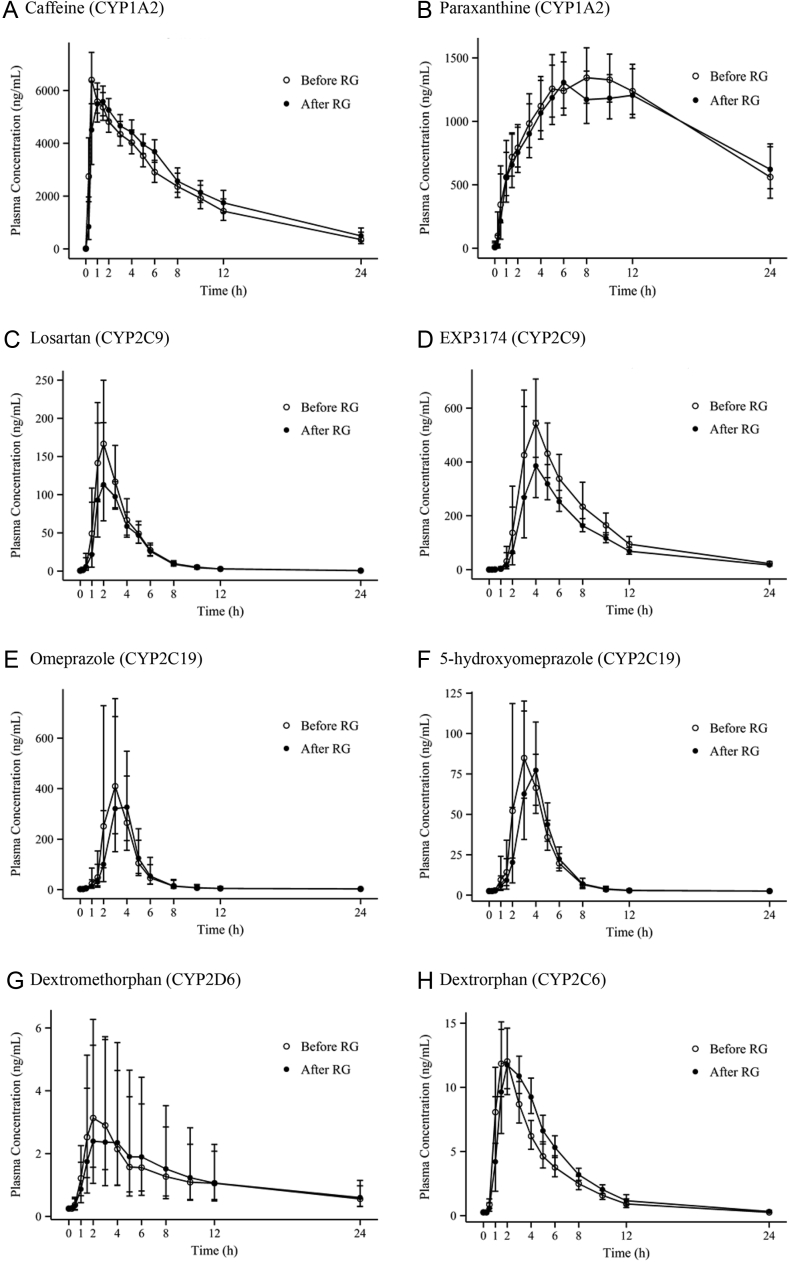
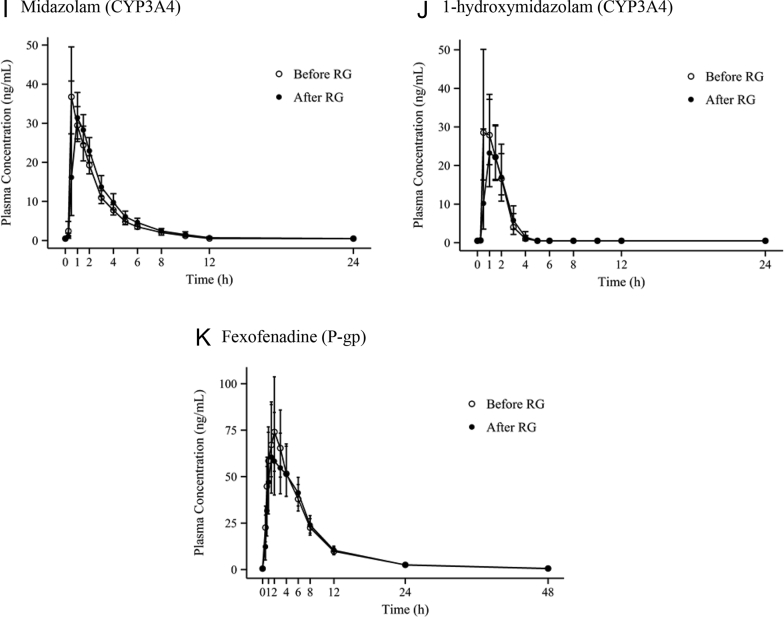
The pharmacokinetic parameters of the 14 participants completing the study according to the protocol were evaluated. The mean plasma concentration–time profiles of the CYP and P-gp probe drugs are shown in Fig. 2. The metabolic ratio between the parents and metabolites of each CYP probe drug are described Table 3.
Fig. 2.
Mean (standard deviation) plasma concentration–time profiles of a cocktail of five probe drugs and their metabolites, as well as fexofenadine, before and after administration of Red Ginseng. P-gp, P-glycoprotein; RG, Red Ginseng.
Table 3.
Effects of Red Ginseng on metabolic ratios of cytochrome P450 probe drugs
| Enzyme | Analyte ratio | n | After RG | Before RG | Geometric mean ratio, after RG:before RG (90% CI) | CVw (%) | p1) |
|---|---|---|---|---|---|---|---|
| CYP1A2 | Paraxanthine: caffeine | 14 | 0.426 | 0.489 | 0.870 (0.805–0.940) | 11.62 | 0.0012 |
| CYP2C9 | EXP3174: losartan | 14 | 5.427 | 6.233 | 0.871 (0.800–0.947) | 12.61 | 0.0023 |
| CYP2C19 | 5-Hydroxyomeprazole: omeprazole | 14 | 0.220 | 0.214 | 1.027 (0.938–1.123) | 13.50 | 0.5416 |
| CYP2D6 | Dextrorphan: dextromethorphan | 14 | 3.955 | 2.881 | 1.373 (0.864–2.180) | 78.23 | 0.6257 |
| CYP3A4 | 1-Hydroxymidazolam: midazolam | 14 | 0.485 | 0.589 | 0.824 (0.658–1.032) | 34.60 | 0.0785 |
Metabolic ratio = AUClast of metabolite/AUClast of parent
AUClast, area under the plasma concentration–time curve from time 0 to the last measured time; CI, confidence interval; CVw, intra-individual coefficient of variation; RG, Red Ginseng
Wilcoxon Signed-Rank test
The mean Cmax and AUClast of caffeine were 6,692.3 ng/mL and 50,454.8 h*ng/mL, respectively, on Day 1 (before RG intake); these values were 6,222.4 ng/mL and 55,992.3 h*ng/mL, respectively, on Day 15 (after RG intake). The mean Cmax and AUClast of paraxanthine, a metabolite of caffeine, were 1,461.2 ng/mL and 24,682.6 h*ng/mL, respectively, on Day 1 (before RG intake), and these values were 1,444.3 ng/mL and 23,835.0 h*ng/mL, respectively, on Day 15 (after RG intake). The point estimate and 90% CI of the ratios of the geometric means (after RG intake/before RG intake) of Cmax and AUClast of caffeine were 0.930 (0.842–1.026) and 1.110 (1.028–1.198), respectively, and that of the caffeine metabolic ratio (i.e., paraxanthine AUC/caffeine AUC) for the evaluation of CYP1A2 enzyme activity was 0.870 (0.805–0.940).
The mean Cmax and AUClast of losartan were 223.9 ng/mL and 610.3 h*ng/mL, respectively, on Day 1 (before RG intake), but these values decreased to 175.0 ng/mL and 529.4 h*ng/mL, respectively, on Day 15 (after RG intake). The mean Cmax and AUClast of EXP3174, a metabolite of losartan, were 562.5 ng/mL and 3,804.3 h*ng/mL, respectively, on Day 1 (before RG intake) and 457.2 ng/mL and 2,872.8 h*ng/mL, respectively, on Day 15 (after RG intake). The point estimate and 90% CI of the ratios of the geometric means (after RG intake/before RG intake) of Cmax and AUClast of losartan were 0.782 (0.689–0.886) and 0.867 (0.799–0.941), respectively; that of the losartan metabolic ratio, i.e., EXP3174 AUC/losartan AUC, for the evaluation of CYP2C9 enzyme activity was 0.871 (0.800–0.947).
For omeprazole, the mean Cmax and AUClast were 721.784 ng/mL and 1,577.179 h*ng/mL, respectively, on Day 1 (before RG intake), and these values were 626.256 ng/mL and 1,449.431 h*ng/mL, respectively, on Day 15 (after RG intake). The mean Cmax and AUClast of 5-hydroxyomeprazole, a metabolite of omeprazole, were 123.689 ng/mL and 338.141 h*ng/mL, respectively, on Day 1 (before RG intake) and 109.126 ng/mL and 319.016 h*ng/mL, respectively, on Day 15 (after RG intake). The point estimate and 90% CI of the ratios of the geometric means (after RG intake/before RG intake) of Cmax and AUClast of omeprazole were 0.868 (0.697–1.080) and 0.919 (0.814–1.037), respectively, and that of the omeprazole metabolic ratio, i.e., 5-hydroxyomeprazole AUC/omeprazole AUC, for the evaluation of CYP2C19 enzyme activity was 1.027 (0.938–1.123).
The mean Cmax and AUClast of dextromethorphan were 3.623 ng/mL and 18.962 h*ng/mL, respectively, on Day 1 (before RG intake); these values on Day 15 (after RG intake) were 3.334 ng/mL and 17.351 h*ng/mL, respectively. The mean Cmax and AUClast of dextrorphan, a metabolite of dextromethorphan, were 12.679 ng/mL and 54.630 h*ng/mL, respectively, on Day 1 (before RG intake) and 13.663 ng/mL and 68.626 h*ng/mL, respectively, on Day 15 (after RG intake). The point estimate and 90% CI of the ratios of the geometric means (after RG intake/before RG intake) of Cmax and AUClast of dextromethorphan were 0.920 (0.791–1.070) and 0.915 (0.605–1.383), respectively, and that of the dextromethorphan metabolic ratio, i.e., dextrorphan AUC / dextromethorphan AUC, for the evaluation of CYP2D6 enzyme activity was 1.373 (0.864–2.180).
With regard to midazolam, the mean Cmax and AUClast were 41.142 ng/mL and 92.276 h*ng/mL, respectively, on Day 1 (before RG intake); these values were 38.961 ng/mL and 104.053 h*ng/mL, respectively, on Day 15 (after RG intake). The mean Cmax and AUClast of 1-hydroxymidazolam, a metabolite of midazolam, were 38.766 ng/mL and 54.344 h*ng/mL, respectively, on Day 1 (before RG intake) and 30.405 ng/mL and 50.507 h*ng/mL, respectively, on Day 15 (after RG intake). The point estimate and 90% CI of the ratios of the geometric means (after RG intake/before RG intake) of Cmax and AUClast of midazolam were 0.947 (0.756–1.186) and 1.128 (1.000–1.271), respectively, and that of the midazolam metabolic ratio, i.e., 1-hydroxymidazolam AUC/midazolam AUC, for the evaluation of CYP3A4 enzyme activity was 0.824 (0.658–1.032).
3.2. Effects of Red Ginseng on P-glycoprotein
The mean Cmax and AUClast of fexofenadine were 86.283 ng/mL and 528.949 h*ng/mL, respectively, between Days 2 and 3 (before RG intake); between Days 16 and 17 (after RG intake), these values were 76.787 ng/mL and 509.561 h*ng/mL. The point estimate and 90% CI of the ratios of the geometric means (after RG intake/before RG intake) of Cmax and AUClast of fexofenadine were 0.890 (0.681–1.162) and 0.963 (0.845–1.098), respectively.
3.3. Safety assessment
The safety and tolerability of RG or the probe drugs were evaluated in all 14 of the participants. There were 23 adverse events in 10 of the participants, including dizziness (13 events), euphoric mood (6 events), somnolence (2 events), headache (1 event) and nausea (1 event). It is already well known that symptoms such as dizziness, euphoric mood, and somnolence can be generated by midazolam. All adverse events were mild, and all of the test participants who showed adverse sequelae recovered; serious adverse events were not observed.
The severity of all AEs was either mild or moderate, and all were resolved without medical intervention. There were no clinically significant findings upon physical examination, including changes in vital signs or in clinical laboratory evaluations.
4. Discussion
In this study, we used a cocktail technique to examine whether RG affects CYP enzymes and P-gp. This technique employed the concurrent evaluation of many metabolic enzymes and a transport protein to assess induction/suppression effects using probe drugs. Each probe drug was pre-evaluated for specificity for each metabolic enzyme or transport protein and to verify no interactions with the other probes. Therefore, the study utilized a cocktail of drugs that were proven to be sufficient as indicators before proceeding. The probe drugs were selected in accordance with existing studies, such as a Pittsburgh cocktail study [11], a Cooperstown cocktail study [12] and an Inje cocktail study [13]. Many natural materials can affect efflux proteins such as P-gp [14], even though conventional cocktail combinations do not include a drug that probes for this protein. To assess CYP enzyme activity, the present approach incorporated the cocktail combination of the Inje study, a previous drug interaction study that used a cocktail technique to evaluate general drug interactions by calculating metabolic ratios for a probe and its metabolite in plasma at certain time points. In 2012, the Guideline on the Investigation of Drug Interactions published by the European Medicines Agency recommended evaluating the clearance and effect of AUC because metabolite-to-parent drug ratios do not provide a true quantification of effects on enzyme activity [15]. Therefore, our study calculated pharmacokinetic parameters through drug concentration–time curves in plasma to obtain a real quantitative value. In addition, the 2-wk-long administration of the daily recommended dose of ginseng products should be sufficient to induce or inhibit CYP enzymes [16].
The CYP indicator drugs used in this study, including caffeine, losartan, omeprazole, dextromethorphan, and midazolam, are each metabolized in the body by specific CYP enzymes [17], [18], [19], [20], [21].
Based on the results, the activities of the CYP1A2, CYP2C9, and CYP3A4 enzymes were inhibited by RG product intake, whereas CYP2C19 and CYP2D6 were not affected [22], [23]. A previous conventional in vitro study obtained similar results, asserting that ginsenosides Rb1, Rb2, Rc, Re, and Rg1 did not affect CYP enzyme activity but that their metabolites ginsenoside Rh2, compound K, PPD, and PPT did inhibit CYP1A2, CYP2C9, and CYP3A4, with no effect on CYP2C19 and CYP2D6 [4], [5], [10], [24]. However, in this study, administration of the ginseng product at its recommended daily allowance resulted in a small inhibition of CYP1A2, CYP2C9, and CYP3A4 activities, such that even if the drug was administered in combination with CYP substrates, the effect was unlikely to be clinically meaningful.
P-gp is not affected by the major ginsenosides of fresh ginseng Rb1, Rb2, and Re, but it is known to be inhibited by compound K, Rh2, PPD, and PPT, which are metabolites of ginsenosides that are produced by intestinal bacteria [7], [8], [9]. In this study, the AUClast of fexofenadine, the P-gp probe drug, did not change after the administration of RG. This result suggests that the contents of compound K, Rh2, PPD and PPT, which inhibit P-gp, increase during the manufacturing process of RG from fresh ginseng. Although fexofenadine is widely used as a P-gp probe drug [25], [26], the use of this compound in this study was limited because fexofenadine is a substrate of OATP1B1, OATP1B3 and MRP2 as well as P-gp [27]. The reasons for several of the differences between the results of existing studies and the present findings are expected to be differences between fresh ginseng and RG products that arise during the processing of ginseng as well as the ethnicities of the study participants.
Because it was performed on healthy volunteers, this study was able to accurately assess drug interaction potentials to minimize confounding factors, such as comorbidities and concomitant medications. However, due to variations in the composition of the RG product as a result of the manufacturing process, it is difficult to expect the same results from all ginseng products. Moreover, unlike medicines, RG products are typically consumed at more than their recommended doses, and there is a need to evaluate the interactions in the body resulting from high doses.
5. Conclusion
In summary, RG does not affect CYP2C19 and CYP2D6, although it does inhibit CYP1A2, CYP2C9, and CYP3A4; however, these results are not clinically relevant because of their small significance in this study. Additionally, it was verified that RG does not affect P-gp. Therefore, this product has no relevant potential to cause metabolic and transport drug interactions.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Acknowledgments
This paper was supported by funding from the Biomedical Research Institute of Chonbuk National University Hospital (CUH 2012-0013).
References
- 1.Choi K.T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 2.Kong B.-M., Park M.-J., Min J.-W., Kim H.-B., Kim S.-H., Kim S.-Y., Yang D.-C. Physico-chemical characteristics of white, fermented and Red Ginseng extracts. J Ginseng Res. 2008;32:238–243. [Google Scholar]
- 3.Qi L.W., Wang C.Z., Du G.J., Zhang Z.Y., Calway T., Yuan C.S. Metabolism of ginseng and its interactions with drugs. Curr Drug Metab. 2011;12:818–822. doi: 10.2174/138920011797470128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henderson G.L., Harkey M.R., Gershwin M.E., Hackman R.M., Stern J.S., Stresser D.M. Effects of ginseng components on c-DNA-expressed cytochrome P450 enzyme catalytic activity. Life Sci. 1999;65:PL209–PL214. doi: 10.1016/s0024-3205(99)00407-5. [DOI] [PubMed] [Google Scholar]
- 5.Yu C.T., Chen J., Teng X.W., Tong V., Chang T.K. Lack of evidence for induction of CYP2B1, CYP3A23, and CYP1A2 gene expression by Panax ginseng and Panax quinquefolius extracts in adult rats and primary cultures of rat hepatocytes. Drug Metab Dispos. 2005;33:19–22. doi: 10.1124/dmd.104.001917. [DOI] [PubMed] [Google Scholar]
- 6.Malati C.Y., Robertson S.M., Hunt J.D., Chairez C., Alfaro R.M., Kovacs J.A., Penzak S.R. Influence of Panax ginseng on cytochrome P450 (CYP)3A and P-glycoprotein (P-gp) activity in healthy participants. J Clin Pharmacol. 2012;52:932–939. doi: 10.1177/0091270011407194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li N., Wang D., Ge G., Wang X., Liu Y., Yang L. Ginsenoside metabolites inhibit P-glycoprotein in vitro and in situ using three absorption models. Planta Med. 2014;80:290–296. doi: 10.1055/s-0033-1360334. [DOI] [PubMed] [Google Scholar]
- 8.Shi J., Cao B., Zha W.B., Wu X.L., Liu L.S., Xiao W.J., Gu R.R., Sun R.B., Yu X.Y., Zheng T. Pharmacokinetic interactions between 20(S)-ginsenoside Rh2 and the HIV protease inhibitor ritonavir in vitro and in vivo. Acta Pharmacol Sin. 2013;34:1349–1358. doi: 10.1038/aps.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J., Zhou F., Wu X., Gu Y., Ai H., Zheng Y., Li Y., Zhang X., Hao G., Sun J. 20(S)-ginsenoside Rh2 noncompetitively inhibits P-glycoprotein in vitro and in vivo: a case for herb–drug interactions. Drug Metab Dispos. 2010;38:2179–2187. doi: 10.1124/dmd.110.034793. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y., Zhang J.W., Li W., Ma H., Sun J., Deng M.C., Yang L. Ginsenoside metabolites, rather than naturally occurring ginsenosides, lead to inhibition of human cytochrome P450 enzymes. Toxicol Sci. 2006;91:356–364. doi: 10.1093/toxsci/kfj164. [DOI] [PubMed] [Google Scholar]
- 11.Frye R.F., Matzke G.R., Adedoyin A., Porter J.A., Branch R.A. Validation of the five-drug “Pittsburgh cocktail” approach for assessment of selective regulation of drug-metabolizing enzymes. Clin Pharmacol Ther. 1997;62:365–376. doi: 10.1016/S0009-9236(97)90114-4. [DOI] [PubMed] [Google Scholar]
- 12.Streetman D.S., Bleakley J.F., Kim J.S., Nafziger A.N., Leeder J.S., Gaedigk A., Gotschall R., Kearns G.L., Bertino J.S., Jr. Combined phenotypic assessment of CYP1A2, CYP2C19, CYP2D6, CYP3A, N-acetyltransferase-2, and xanthine oxidase with the “Cooperstown cocktail”. Clinical Pharmacol Ther. 2000;68:375–383. doi: 10.1067/mcp.2000.109519. [DOI] [PubMed] [Google Scholar]
- 13.Ryu J.Y., Song I.S., Sunwoo Y.E., Shon J.H., Liu K.H., Cha I.J., Shin J.G. Development of the “Inje cocktail” for high-throughput evaluation of five human cytochrome P450 isoforms in vivo. Clinical Pharmacol Ther. 2007;82:531–540. doi: 10.1038/sj.clpt.6100187. [DOI] [PubMed] [Google Scholar]
- 14.Izzo A.A., Ernst E. Interactions between herbal medicines and prescribed drugs: an updated systematic review. Drugs. 2009;69:1777–1798. doi: 10.2165/11317010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.European Medicines Agency . European Medicines Agency; 7 Westferry Circus, Canary Wharf, London E14 4HB, United Kingdom: 2012. Guideline on the investigation of drug interactions. [Google Scholar]
- 16.Yang J., Liao M., Shou M., Jamei M., Yeo K.R., Tucker G.T., Rostami-Hodjegan A. Cytochrome p450 turnover: regulation of synthesis and degradation, methods for determining rates, and implications for the prediction of drug interactions. Curr Drug Metab. 2008;9:384–394. doi: 10.2174/138920008784746382. [DOI] [PubMed] [Google Scholar]
- 17.Jacqz-Aigrain E., Funck-Brentano C., Cresteil T. CYP2D6- and CYP3A-dependent metabolism of dextromethorphan in humans. Pharmacogenetics. 1993;3:197–204. doi: 10.1097/00008571-199308000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Karam W.G., Goldstein J.A., Lasker J.M., Ghanayem B.I. Human CYP2C19 is a major omeprazole 5-hydroxylase, as demonstrated with recombinant cytochrome P450 enzymes. Drug Metab Dispos. 1996;24:1081–1087. [PubMed] [Google Scholar]
- 19.Gorski J.C., Hall S.D., Jones D.R., VandenBranden M., Wrighton S.A. Regioselective biotransformation of midazolam by members of the human cytochrome P450 3A (CYP3A) subfamily. Biochem Pharmacol. 1994;47:1643–1653. doi: 10.1016/0006-2952(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 20.Yasar U., Tybring G., Hidestrand M., Oscarson M., Ingelman-Sundberg M., Dahl M.L., Eliasson E. Role of CYP2C9 polymorphism in losartan oxidation. Drug Metab Dispos. 2001;29:1051–1056. [PubMed] [Google Scholar]
- 21.Kalow W., Tang B.K. The use of caffeine for enzyme assays: a critical appraisal. Clinical Pharmacol Ther. 1993;53:503–514. doi: 10.1038/clpt.1993.63. [DOI] [PubMed] [Google Scholar]
- 22.Gurley B.J., Gardner S.F., Hubbard M.A., Williams D.K., Gentry W.B., Cui Y., Ang C.Y. Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in humans. Clinical Pharmacol Ther. 2002;72:276–287. doi: 10.1067/mcp.2002.126913. [DOI] [PubMed] [Google Scholar]
- 23.Gurley B.J., Gardner S.F., Hubbard M.A., Williams D.K., Gentry W.B., Cui Y., Ang C.Y. Clinical assessment of effects of botanical supplementation on cytochrome P450 phenotypes in the elderly: St John's wort, garlic oil, Panax ginseng and Ginkgo biloba. Drugs Aging. 2005;22:525–539. doi: 10.2165/00002512-200522060-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hao M., Zhao Y., Chen P., Huang H., Liu H., Jiang H., Zhang R., Wang H. Structure–activity relationship and substrate-dependent phenomena in effects of ginsenosides on activities of drug-metabolizing P450 enzymes. PLoS One. 2008;3:e2697. doi: 10.1371/journal.pone.0002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson S.M., Davey R.T., Voell J., Formentini E., Alfaro R.M., Penzak S.R. Effect of Ginkgo biloba extract on lopinavir, midazolam and fexofenadine pharmacokinetics in healthy subjects. Current Med Res Opin. 2008;24:591–599. doi: 10.1185/030079908x260871. [DOI] [PubMed] [Google Scholar]
- 26.Penzak S.R., Robertson S.M., Hunt J.D., Chairez C., Malati C.Y., Alfaro R.M., Stevenson J.M., Kovacs J.A. Echinacea purpurea significantly induces cytochrome P450 3A activity but does not alter lopinavir-ritonavir exposure in healthy subjects. Pharmacotherapy. 2010;30:797–805. doi: 10.1592/phco.30.8.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsushima S., Maeda K., Ishiguro N., Igarashi T., Sugiyama Y. Investigation of the inhibitory effects of various drugs on the hepatic uptake of fexofenadine in humans. Drug Metab Dispos. 2008;36:663–669. doi: 10.1124/dmd.107.017814. [DOI] [PubMed] [Google Scholar]