Main Text
The latest advance in employing fluorescent molecular rotors—fluorophores whose fluorescence lifetime and quantum yield are a function of the viscosity of their environment—for measurement and imaging of the viscosity of membranes in Escherichia coli bacteria, is reported in this issue of the Biophysical Journal (1).
Mika et al. (1) use fluorescent microviscosity probes termed “fluorescent molecular rotors” (Fig. 1) based on a BODIPY structure to probe the environment of E. coli bacterial cells. BODIPY fluorescent molecular rotors have previously been shown to be very well suited for fluorescence lifetime imaging (FLIM) microscopy measurement of membrane viscosity (2, 3, 4). The authors show that growth of the bacteria is not inhibited by addition of the BODIPY to the culture, and that the dye stains the inner membrane of bacterial cells, which is of particular interest to biophysicists, because it is considered the main permeability barrier. They find, uniquely to my knowledge, that with 950 cP, the E. coli membrane viscosity is very high compared to that of mammalian cells, at ∼250 cP in SKOV cells at 23°C (5), indicating a very high degree of lipid order. As part of their systematic and detailed study, they also use spheroplasts and lipid vesicles made from E. coli membrane extracts to verify their findings. Spheroplasts retain the high viscosity observed in live E. coli cells, as would be expected because their inner membranes are identical. However, the vesicles produced from lipid membrane extracts do not display unusually high viscosity values, suggesting that cytoskeleton of bacterial cells may play a role in membrane organization.
Figure 1.
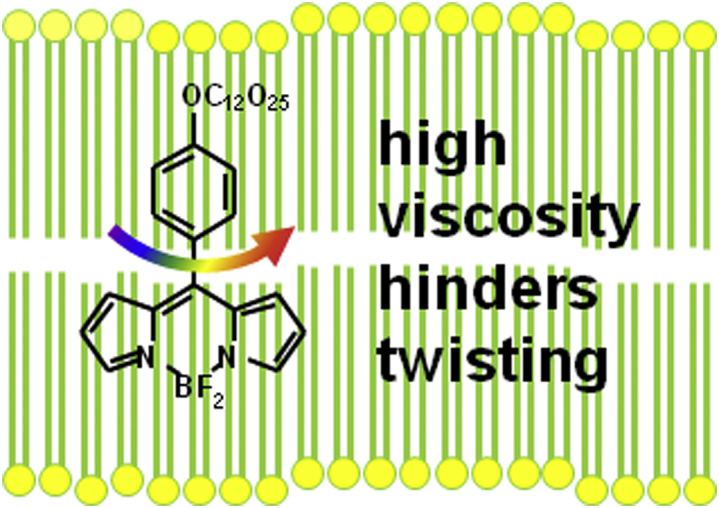
Schematic of fluorescent molecular rotor in cell membrane. A high viscosity slows down internal twisting in the excited state, leading to a long fluorescence lifetime up to several nanoseconds. To see this figure in color, go online.
Fluorescent molecular rotors are not new—in 1896, Schmidt (6) reported the general observation that the same fluorescent dye can have strong and weak fluorescence depending on the dye’s environment. It took many years before this phenomenon was theoretically tackled and understood (7), and we now know that a key characteristic of a fluorescent molecular rotor is that, in the excited state, it can rotate one segment of its structure and thus form a twisted state. It is this intramolecular twisting that depends strongly on the viscosity of the environment and competes with radiative deactivation (8). Loutfy and Arnold (9), Förster and Hoffmann (10) (incidentally the same Förster whose name appears in Förster resonance energy transfer), and others, devised models that show that the quantum yield φ, and thus the fluorescence lifetime τ, is a function of the viscosity η according to a power law, φ = kr τ = c ηx, where 0.1 < x < 1, c is a constant, and kr is the radiative rate constant (7, 8).
The fluorescent molecular rotor thioflavin T is used to stain amyloid fibrils (11), and protein-protein interactions have also been studied with a fluorescent molecular rotor, CCVJ (12). Here, their quantum yield is very low in solution, but their binding hinders twisting, fluorescence is emitted, and only the on-off feature of the response is of interest. However, a calibration based on measuring the fluorescence lifetime of the rotor in solutions of known viscosity can be used to convert the fluorescence lifetime into a viscosity value. This is theoretically justified by the power law mentioned above, and is a straightforward procedure that allows quantitative measurements to be obtained over a large viscosity range. This is exactly what the authors did in each pixel of their images, thus providing viscosity maps of the inner membrane of E. coli under different experimental conditions (1).
Viscosity is the resistance of a fluid to flow and is a crucial parameter affecting signaling, drug delivery, and diffusion in cells and tissues. Indeed, changes in cell membrane viscosity have been linked to disease and malfunction (7, 8) and rapid diffusion of signaling molecules in the brain extracellular space, for example, determines activation of receptors and ion channels. Knowledge of the viscosity is essential to understand these processes in detail (13). While methods to measure the bulk viscosity are well developed using traditional mechanical rheometers, viscosity maps of microscopic objects, such as single cells, have until recently been hard to obtain. The measurement of viscosity with fluorescence techniques is advantageous because, similarly to other optical techniques, it is minimally invasive and nondestructive and can be applied to living cells and tissues.
A significant advantage of time-resolved measurements of fluorescent molecular rotors is that the fluorescence lifetime is independent of the fluorophore concentration, which is hard to control in cells. FLIM, a mature and routine imaging technique in cell biology, intrinsically separates concentration and viscosity effects (14). BODIPY-based fluorescent molecular rotors have a fluorescence lifetime in the range between hundreds of picoseconds to several nanoseconds, and deliver reasonable quantum yields, making them practically useful. Their fluorescence decays are easily measured with time-correlated single photon counting (TCSPC) combined with confocal scanning microscopy. The innovative use of BODIPY-based fluorescent molecular rotors has been very successful in a number of applications (2, 3, 4), and is a key advance compared to traditional fluorescent molecular rotors with very low quantum yields and short fluorescence lifetimes of the order of a few tens of picoseconds up to a few hundreds of picoseconds (9, 10), which can make their routine use for FLIM impractical.
Unlike in the alternative technique of time-resolved fluorescence anisotropy measurements with rigid fluorophores, no polarized excitation and detection is required and a few thousand photons are sufficient to yield a fluorescence decay, so fluorescence lifetime imaging can be performed easily. This has a number of advantages: fluorescent molecular rotors are not only able to map the viscosity in biological cells, but should also allow monitoring dynamic cellular processes in real-time, as video-rate FLIM is feasible. The fluorescent molecular rotor and FLIM combination may also be a system for superresolution STED FLIM, and single molecule sensitivity of suitable fluorescence microscopes may also be of interest for fluorescent molecular rotor mapping. Moreover, another dye emitting in a different spectral window could be used to probe a different parameter simultaneously with viscosity.
This team, and others, have made excellent progress and valuable contributions in viscosity mapping of various systems, e.g., bacillus spores (3), aerosol droplets (2), eukaryotic cells (5), atmospheric aerosols (15), amyloid aggregating proteins (11), and now the inner membrane of E. coli (1), all based on FLIM of fluorescent molecular rotors. Their interdisciplinary work spanning three disciplines uses the fruits of chemical synthesis, i.e., versatile and advanced fluorescent molecular rotors, and combines them with state-of-the-art TCSPC-based FLIM technology to address the problem of E. coli membrane viscosity. This work shows that fluorescent molecular rotors are very attractive emerging tools in bioimaging. FLIM of fluorescent molecular rotors represents a major advance in terms of straightforward calibration and rapid, real-time, and ultrasensitive detection. Due to their high sensitivity and suitability for applications on the micro- and nanometer scale, fluorescent molecular rotors are ideal probes for monitoring dynamic processes in living cells in real-time with a high spatial and temporal resolution.
Editor: Antoine van Oijen.
References
- 1.Mika J.T., Thompson A.J., Kuimova M.K. Measuring the viscosity of the Escherichia coli plasma membrane using molecular rotors. Biophys. J. 2016;111:1528–1540. doi: 10.1016/j.bpj.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosny N.A., Mohamedi G., Kuimova M.K. Mapping microbubble viscosity using fluorescence lifetime imaging of molecular rotors. Proc. Natl. Acad. Sci. USA. 2013;110:9225–9230. doi: 10.1073/pnas.1301479110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loison P., Hosny N.A., Perrier-Cornet J.M. Direct investigation of viscosity of an atypical inner membrane of Bacillus spores: a molecular rotor/FLIM study. Biochim. Biophys. Acta. 2013;1828:2436–2443. doi: 10.1016/j.bbamem.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y., Stefl M., Kuimova M.K. Molecular rheometry: direct determination of viscosity in Lo and Ld lipid phases via fluorescence lifetime imaging. Phys. Chem. Chem. Phys. 2013;15:14986–14993. doi: 10.1039/c3cp51953h. [DOI] [PubMed] [Google Scholar]
- 5.López-Duarte I., Vu T.T., Kuimova M.K. A molecular rotor for measuring viscosity in plasma membranes of live cells. Chem. Commun. (Camb.) 2014;50:5282–5284. doi: 10.1039/c3cc47530a. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt G.C. Beiträge zur Kenntnis der Fluoreszenz. Ann. Phys. Berlin. 1896;294:103–130. [Google Scholar]
- 7.Haidekker M.A., Theodorakis E.A. Environment-sensitive behavior of fluorescent molecular rotors. J. Biol. Eng. 2010;4:11. doi: 10.1186/1754-1611-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuimova M.K. Mapping viscosity in cells using molecular rotors. Phys. Chem. Chem. Phys. 2012;14:12671–12686. doi: 10.1039/c2cp41674c. [DOI] [PubMed] [Google Scholar]
- 9.Loutfy R.O., Arnold B.A. Effect of viscosity and temperature on torsional relaxation of molecular rotors. J. Phys. Chem. 1982;86:4205–4211. [Google Scholar]
- 10.Förster T., Hoffmann G. Die Viskositätsabhängigkeit der Fluoreszenzquantenausbeuten einiger Farbstoffsysteme. Z. Phys. Chem. 1971;75:63–76. [Google Scholar]
- 11.Thompson A.J., Herling T.W., Kuimova M.K. Molecular rotors provide insights into microscopic structural changes during protein aggregation. J. Phys. Chem. B. 2015;119:10170–10179. doi: 10.1021/acs.jpcb.5b05099. [DOI] [PubMed] [Google Scholar]
- 12.Goh W.L., Lee M.Y., Teo Y.N. Molecular rotors as conditionally fluorescent labels for rapid detection of biomolecular interactions. J. Am. Chem. Soc. 2014;136:6159–6162. doi: 10.1021/ja413031h. [DOI] [PubMed] [Google Scholar]
- 13.Rusakov D.A., Savtchenko L.P., Henley J.M. Shaping the synaptic signal: molecular mobility inside and outside the cleft. Trends Neurosci. 2011;34:359–369. doi: 10.1016/j.tins.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suhling K., Hirvonen L.M., Krstajic N. Fluorescence lifetime imaging (FLIM): basic concepts and some recent developments. Med. Photonics. 2015;27:3–40. [Google Scholar]
- 15.Hosny N.A., Fitzgerald C., Kuimova M.K. Direct imaging of changes in aerosol particle viscosity upon hydration and chemical aging. Chem. Sci. (Camb.) 2016;7:1357–1367. doi: 10.1039/c5sc02959g. [DOI] [PMC free article] [PubMed] [Google Scholar]


