Summary
Background
Cervical cancer incidence remains high in several Baltic, central, and eastern European (BCEE) countries, mainly as a result of a historical absence of effective screening programmes. As a catalyst for action, we aimed to estimate the number of women who could be spared from cervical cancer across six countries in the region during the next 25 years, if effective screening interventions were introduced.
Methods
In this population-based study, we applied age–period–cohort models with spline functions within a Bayesian framework to incidence data from six BCEE countries (Estonia, Latvia, Lithuania, Belarus, Bulgaria, and Russia) to develop projections of the future number of new cases of cervical cancer from 2017 to 2040 based on two future scenarios: continued absence of screening (scenario A) versus the introduction of effective screening from 2017 onwards (scenario B). The timespan of available data varied from 16 years in Bulgaria to 40 years in Estonia. Projected rates up to 2040 were obtained in scenario A by extrapolating cohort-specific trends, a marker of changing risk of human papillomavirus (HPV) infection, assuming a continued absence of effective screening in future years. Scenario B added the effect of gradual introduction of screening in each country, under the assumption period effects would be equivalent to the decreasing trend by calendar year seen in Denmark (our comparator country) since the progressive regional introduction of screening from the late 1960s.
Findings
According to scenario A, projected incidence rates will continue to increase substantially in many BCEE countries. Very high age-standardised rates of cervical cancer are predicted in Lithuania, Latvia, Belarus, and Estonia (up to 88 cases per 100 000). According to scenario B, the beneficial effects of effective screening will increase progressively over time, leading to a 50–60% reduction of the projected incidence rates by around 2040, resulting in the prevention of cervical cancer in 1500 women in Estonia and more than 150 000 women in Russia. The immediate launch of effective screening programmes could prevent almost 180 000 new cervical cancer diagnoses in a 25-year period in the six BCEE countries studied.
Interpretation
Based on our findings, there is a clear need to begin cervical screening in these six countries as soon as possible to reduce the high and increasing incidence of cervical cancer over the next decades.
Funding
None.
Introduction
In the next few decades, several million women living in the Baltic, central, and eastern European (BCEE) region will be at high risk of developing cervical cancer. At present, cervical cancer incidence and mortality are higher in BCEE countries than elsewhere in Europe1 and are rising,2, 3, 4 partly due to an absence of screening interventions that are, at best, opportunistic with relatively low coverage and quality.5 Although the introduction of prophylactic vaccination against human papillomavirus (HPV) would substantially reduce the number of future cases of cervical cancer, the full effect, in terms of a reduction in all-ages cervical cancer incidence, will not be detectable for more than 30 years.6 Hence, the implementation of high-quality screening activities can still potentially play a major role in the prevention of cervical cancer and bridge the gap until the longer-term effects of HPV vaccination programmes are seen.
Changes in sexual behaviour and increased exposure to high-risk HPV (eg, HPV-16 or HPV-18 types), the main cause of cervical cancer, led to increasingly higher risks of women developing cervical cancer in the generations of women born around or after the 1930–50s in most European countries,2, 3, 4, 7 especially for those born after World War 2.8 Indirect evidence also suggests that high-risk HPV infection has become progressively more prevalent since the 1960s in several European countries,9, 10, 11 including countries in central and eastern Europe.12, 13 In regions where high-quality, cytology-based cervical cancer screening programmes were implemented several decades ago (eg, in northern Europe), the intervention has countered the increasing generational risks, and incidence has uniformly decreased, making cervical cancer a relatively rare disease. The recorded trends in Nordic countries have provided an important evidence base for the long-term effectiveness of high-quality mass screening programmes since their introduction in the 1960s,14 a period when the incidence of women with cervical cancer was very high and, in some cases (eg, in Denmark), equivalent to that seen in some eastern African populations (Uganda and Zimbabwe) today.1
Research in context.
Evidence before this study
We searched PubMed with the search terms “cervical cancer AND trends AND (Eastern Europe OR baltic)” and “cervical cancer AND (prevention OR screening) AND (Eastern Europe OR baltic)” to assess available evidence for time trends and historical cervical cancer prevention activities in each of the six countries studied in these regions. There was no date or language restriciton. Despite some efforts, screening activities have been absent or, at best, opportunistic with low coverage and quality in the studied countries of Estonia, Lithuania, Latvia, Belarus, Bulgaria, and Russia.
Added value of this study
As a catalyst for action, we analysed cervical cancer incidence from population-based cancer registries in these BCEE countries and projected the number of women that could be spared from cervical cancer diagnoses over the next 25 years in the region upon swift introduction in 2017 of effective screening programmes. Under the assumption that screening-related gains could be as favourable as those shown in the long-term trends in cervical cancer incidence in Denmark, we estimate that 180 000 new cases of cervical cancer could be prevented from 2017 to 2040 in the six countries.
Implications of all the available evidence
The scale of the rapid increase in risk in recent generations of women, most of whom are outside the target age range of the HPV vaccine, and the clear evidence of a prevention effect can and must strengthen the resolve to immediately launch effective screening programmes in Baltic, central, and eastern European countries. A lack of action will result in an increase of the number of women diagnosed with cervical cancer. The use of HPV testing-based screening programmes, as recommended by WHO for countries without established cytology-based programmes, could further accelerate the screening benefits and, in combination with a prompt introduction of HPV vaccination, drastically reduce the burden of cervical cancer.
As a catalyst for the implementation of screening activities, we quantified the maximum potential effect of screening, in terms of the number of cervical cancer cases that could theoretically be prevented in BCEE countries by the year 2040, if screening were introduced by 2017 with a level of effectiveness similar to that seen historically in Denmark. We developed a robust method for the projection that reflect the two underlying factors affecting cervical cancer incidence: period effects that reflect changes in cervical cancer risk through time, mimic the implementation of effective screening programmes, with declining incidence downwards across targeted age groups; and birth cohort effects, which are proxies for changes in sexual behaviour and increased risk of infection with high-risk HPV in successive generations of women.15
Methods
Study design and data sources
In this population-based study, we fitted age–period–cohort (APC) models to incidence data to develop projections of the future number of new cases of cervical cancer in six BCEE countries from 2017 to 2040 under two scenarios. In scenario A, we calculated the number of new cases that would have arisen in the continued absence of screening, and in scenario B, the number of new cases that would have arisen after effective screening from 2017. We selected three Baltic countries (Estonia, Latvia, and Lithuania), and three central or eastern European countries (Belarus, Bulgaria, and Russia), based on the availability of high-quality and representative cancer inicidence data. For all countries except Russia, new cases of invasive cervical cancer were obtained from national population-based cancer registries of the International Agency for Research on Cancer's (IARC) Cancer Incidence in Five Continents series, of which the most recent (volume X) spans to 2007. We also included available data beyond 2007, so the data included in this study are the most recently available in the countries studied at the time of writing. For Russia, data from 2012 were obtained from a national database of regional cancer registries.
Population data were based on the country-specific and age-specific population estimates of 2015–40 from the United Nations Population Division (the 2010 revision).16
Procedures and statistical analysis
Analyses were restricted to include cervical cancer diagnoses at ages 30–74 years because recommended screening ages tend to range from 25–64 years, with beneficial effects starting a few years after screening and extending to 74 years of age. Truncated age-standardised incidence rates (ASR, hereafter referred to as incidence) adjusted for the effect of age using weights of the world standard population were calculated by year of cancer diagnosis.17 We used the APC forecasting model based on our previous work (appendix pp 1–2).3, 7 In brief, Poisson regression models were fitted within a Bayesian generalised additive models framework to summarise trends in terms of age, period, and cohort effects.18 We circumvented the non-identifiability problem that characterises APC models by taking advantage of the consistent association between age and cervical cancer that has been recorded in unscreened populations (ie, a steady rise in prevalence up to roughly 45 years of age, followed by a plateau).19 This pattern was also recorded in populations from Nordic countries in pre-screening periods. In screened populations, rates of cervical cancer plateau earlier—at about 35 years of age—because of the beneficial effect of screening. We could, therefore, constrain incidence rates in both screened and unscreened populations to be equal at ages 45–49 years and 65–69 years, thus enabling the estimation of a unique set of parameters for the age, period, and cohort effects.3, 7, 19, 20
In scenario A, we made forecasts assuming continued absence of effective screening. We obtained expected future incidence rates of cervical cancer up to 2040 by extrapolating period and cohort effects with smoothing functions, under the assumption that age effects remain unchanged in future years. The canonical log link function for APC models for period and cohort effects was replaced by the corresponding first-order approximation of the Taylor series expansion for the exponential, resulting in more conservative projections than those that would be obtained by using the log link function.21 Using a Bayesian framework and Gibbs sampling, we obtained the expected number of cervical cancer cases by running chains of 10 000 iterations after discarding the first 1000 possibly unstable samples. Samples from every tenth iteration were stored and the convergence of the chains was checked. The Rjags library was used as an interface to run the Just Another Gibbs Sampler software (version 4.1.0) within R (version 3.3.0). Prediction intervals for future cancer incidence projections were not reported.
In scenario B, we made forecasts under the assumption that effective screening in all six countries took place from 2017. In this second scenario, we postulated that the gradual effect of implementing effective screening on cervical cancer incidence would be equivalent to the decreasing trend by calendar year recorded in Denmark (our comparator country) upon early implementation of high-quality conventional cytology screening in the late 1960s. The key assumption was that decreases in period-specific effects in Denmark represent an achievable beneficial effect of screening activities on cervical cancer incidence, whereas the absence of such period effects, as seen in BCEE countries, represents an absence of effective screening activities. Although the effect of screening-related decreases was reasonably consistent across Nordic countries since the late 1960s, the largest decreases by calendar period as estimated by the APC analyses occurred in Denmark.3, 7, 20 National projections were based on a scenario in which high-quality screening would be implemented in BCEE countries in 2017, extrapolating the age and cohort effects into the future, while projecting the equivalent period effects only until 2017 and, thereafter, projecting a declining trend with a slope equal to that estimated for period effects in Denmark after the introduction of screening in the period from the late 1960s to 2010 (appendix pp 1–2).3, 7
We estimated the number and proportion of cervical cancer cases that could be prevented by screening during 2017–40 from the difference between projected rates derived in scenario A versus scenario B. Extensive sensitivity analyses were done (appendix).
Role of the funding source
The funder of the study had no involvement in data collection, analysis, or interpretation of the results; study design; patient recruitment; writing of the report, or any aspect pertinent to the study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
In total, our analysis included 280 149 recorded cases of cervical cancer in the six BCEE countries and approximately 1060 million women-years of follow-up. The timespan of observation varied between countries, ranging from 16 years in Bulgaria to 40 years in Estonia (table). The observed incidence in 2003–07 varied substantially across countries, ranging from roughly 25–26 cases per 100 000 in Russia, Latvia, and Belarus to about 44–45 per 100 000 in Lithuania and Bulgaria. The APC model effects on fitting age, period, and cohort by country are presented in appendix p 3. No substantial changes in period-specific effects were detected within the observed data in the BCEE countries. Increases in cohort-specific risks in successive generations of women born between the 1940s and 1950s were seen in all studied countries (appendix p 3).
Table.
Observed and projected cases and incidence of cervical cancer under different scenarios for women aged 30–74 years in the six countries
|
Observed |
Projected | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Prediction base (timespan in years) | Mean cases per year, n | Person-years*† | ASR 2003–07 per 100 000 women | ASR 2036–40 with no screening | ASR 2036–40 with screening from 2017 | Cumulative number of incident cases, 2017–40 with no screening | Cumulative number of incident cases, 2017–40 with screening from 2017 | Number of cervical cancer cases potentially preventable by screening (% of total with no screening), 2017–40 with screening from 2017 | |
| Estonia | 1968–2007 (40) | 143 | 0·4 | 35·4 | 64·4 | 31·4 | 4853 | 3392 | 1461 (30%) |
| Lithuania | 1978–2007 (30) | 464 | 1·0 | 45·6 | 87·5 | 43·4 | 16 105 | 11 322 | 4783 (30%) |
| Latvia | 1983–2007 (25) | 192 | 0·7 | 26·8 | 68·4 | 30·1 | 7773 | 5003 | 2770 (36%) |
| Belarus | 1978–2007 (30) | 772 | 2·9 | 26·1 | 67·2 | 30·6 | 34 911 | 22 594 | 12 318 (35%) |
| Bulgaria | 1993–2008 (16) | 1010 | 2·3 | 44·2 | 55·1 | 27·8 | 29 967 | 21 576 | 8392 (28%) |
| Russia | 1993–2012 (20) | 11 043 | 43·1 | 25·2 | 50·2 | 23·3 | 452 173 | 301 999 | 150 175 (33%) |
| Total | .. | .. | .. | .. | .. | .. | .. | .. | 179 899 |
ASR=age-standardised incidence rate.
Average annual figure.
Person-years expressed in millions.
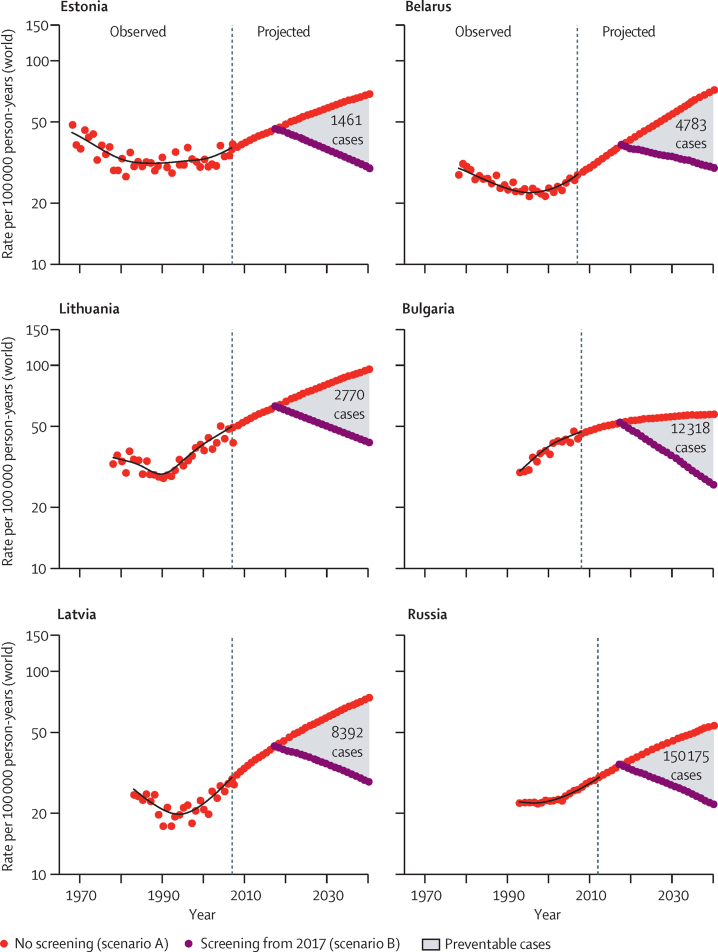
Under the scenario A assumption (no effective screening), the projected cohort effects will continue until 2040 with period effects being quite stable (appendix p 3). Our projections suggest that a failure to introduce screening will result in more than 450 000 new cases of cervical cancer being diagnosed in Russia, 35 000 in Belarus, 30 000 in Bulgaria, and more than 25 000 combined in Estonia, Lithuania, and Latvia during 2017–40. The forecasts to 2040 suggest that the incidence will at least double in the absence of screening in five of the six countries, reaching an incidence of more than 60 per 100 000 for 2036–40 in Estonia, Latvia, and Belarus and more than 85 per 100 000 in Lithuania (figure 1). A smaller increase in the forecasted incidence is predicted in Bulgaria where, despite the high incidence in 2003–07, projections of cohort-specific effects are expected to be more modest (table).
Figure 1.
Observed and projected incidence of cervical cancer in six Baltic, central, and eastern European countries
Forecasts of future cervical cancer rates according to two scenarios: no screening (red circles) and effective screening commencing in 2017 (purple circles). The introduction of effective screening shows a progressive effect similar to that recorded in Denmark after the introduction of screening in the 1960s.
Scenario B projections suggest that the implementation of high-quality cervical cancer screening programmes in BCEE countries from 2017 will have a progressive effect after the introduction of screening, with decreases in cervical cancer incidence projected to 2040 (figure 1). The projected incidence in 2036–40 is approximately half of the projected rates obtained in scenario A of no effective screening, which is about 30 or lower per 100 000 in all selected BCEE countries other than Lithuania, where the incidence increases to roughly 43 per 100 000 (table).
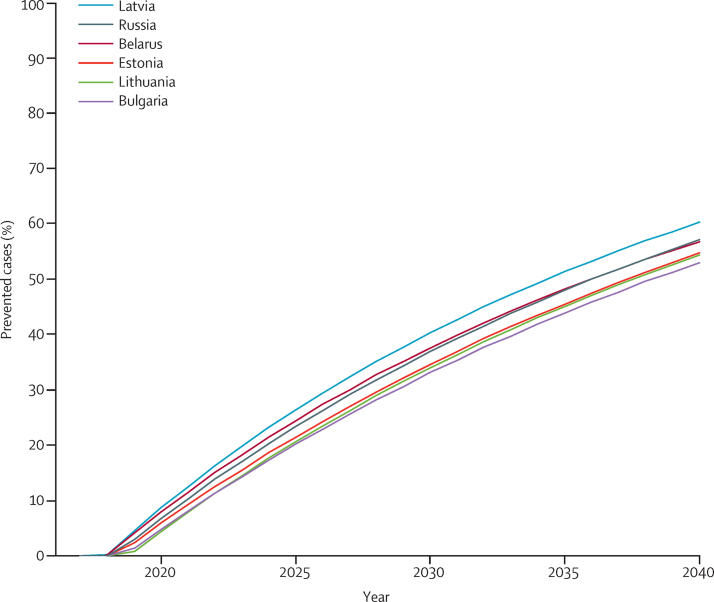
The cumulative number of cervical cancer cases that could be theoretically prevented by screening during 2017–40 varies between countries, from almost 1500 in Estonia to more than 150 000 in Russia (figure 1, table). The percentage of new cases of cervical cancer potentially preventable by screening on average during the period 2017–40 was estimated to range from 28% (Bulgaria) to 36% (Latvia; table). This proportion, however, increases progressively with time, with reductions in incidence being similar across the six countries, reaching 50–60% in 2040, to the no effective screening scenario (figure 2).
Figure 2.
Estimated percentage of cervical cancer cases expected to be potentially preventable by screening in 2040 in six Baltic and eastern European countries
The percentage of cervical cancer cases prevented is calculated as: projected 1–projected 2 × 100/projected 1. Projected cases are derived from a modified age–period–cohort model. Projected 1=projected cases in a scenario that assumes no improvements in present cervical cancer screening activities (scenario A). Projected 2=projected cases in a scenario that assumes that effective screening will start in 2017 (scenario B).
Discussion
Our study supports the notion that effective screening programmes could have a major effect in the next few decades in countries where the incidence of cervical cancer is frequent and implementation of screening activities has been limited. The beneficial effects after implementation of screening in 2017 increase progressively over time, with a projected reduction in cervical cancer incidence of 50–60% by 2040 and with only minor differences across the studied countries that are mainly due to the different projections for the cohort effects.7, 22 Our projections suggest that an increasing number of women in the six BCEE countries will be diagnosed with cervical cancer during the next 30 years in the absence of effective screening. Incidence in 2036–40 is thus predicted to be similar to that observed in women in Denmark (incidence of approximately 68 per 100 000) around 1960,17 before the advent of organised screening. According to our projections, the immediate launch of effective screening programmes would substantially change this adverse future scenario, preventing almost 180 000 new diagnoses of cervical cancer over a 25-year period in the six countries.
Dynamic models suggest that HPV vaccination is expected to change all-age cervical cancer incidence only after several decades6 and thus will have a limited effect on incidence before 2040. Bulgaria and Latvia (as well as Slovenia, the Czech Republic, Macedonia, Hungary, and Croatia) have integrated HPV vaccines into their national immunisation programme started providing routine vaccination free of charge to adolescent girls.5 Coverage is not, however, reported. In Russia, only small-scale regional vaccination programmes have been implemented, resulting in more than 20 000 girls being vaccinated.23 In most BCEE countries, including Lithuania, Estonia, and Belarus, HPV vaccination has yet to be incorporated into national programmes.5, 23 However, even in the best-case scenario of an accelerated implementation of the vaccine national coverage, the next two to three generations of women beyond the target age for vaccination will be at high risk of cervical cancer.24
So far, none of the studied BCEE countries have established organised, high-quality or high-coverage cervical cancer screening programmes, and roughly the same situation applies to other countries in the region. Until recently, cytology-based screening was mainly opportunistic, with low coverage (at most about 20%)5 and quality control on cytology. Some efforts, however, have been made,25, 26 especially after the release of the European Union guidelines on screening in 2008.27 The Baltic countries of Estonia, Lithuania, and Latvia established organised cytology-based screening programmes around 2005 that partly function, although low coverage, absence of quality assurance, and opportunistic screening outside the main programme are major obstacles.28, 29 In Bulgaria, cervical cancer screening has been opportunistic for the past few decades, and plans for organised screening have not yet started.30 In Russia, and in Belarus and other countries of the former Soviet Union, screening is mainly opportunistic. Since 2002, the Moscow region reorganised screening activities based on a call–recall system (inviting women for screening on a regular basis) and the number of gynaecological examinations increased substantially, although no centralised screening database exists and the coverage of the targeted population is not known.23 Romania and Croatia started organised screening in 2012, but programme indicators have not been reported (Poljak M, University of Ljubjana, personal communication). Indeed, reliable screening indicators are absent in all BCEE countries, and the low participation rates in the screening programme point to a need for central governments to ensure a vastly improved population coverage by organised screening. A particularly positive example comes from Slovenia, where, in a relatively short time and with affordable investment, the country moved from an opportunistic to an organised national screening programme; the result was a dramatic drop in cervical cancer incidence rates, from 15 to six cases per 100 000 during 2003–15.25
None of the BCEE countries seem to have planned for the use of HPV-based screening which, compared with cytology, provides better and more durable negative predictive value against high-grade cervical disease, requires a simpler logistic and health-care infrastructure, is more reproducible, and is likely to be more cost effective.30, 31 The use of the more sensitive HPV testing is recommended by WHO guidelines for countries without an already functioning effective, high-coverage cytology-based programme.32
The comparisons across different areas in Europe sharing similar underlying risk factors allowed us to build projections for BCEE countries in a scenario where the possible future onset of screening activities could theoretically produce a similar effect on cervical cancer risk as that produced historically in Nordic countries. Adopting the principles of preventability, Denmark was chosen as the reference country because, of the Nordic countries, it had the largest screening-related decrease in period effects and therefore represented the largest prevention gain through cytology-based screening.3 Furthermore, rates of cervical cancer in Denmark in the 1960s were even higher compared with BCEE countries, possibly because of differences in sexual behaviour and persistent high-risk HPV infections. Prevalences of cervical cancer in the BCEE countries studied are now approaching those recorded in Denmark in the pre-screening years. Despite varying baseline incidence, the relative effect of screening in driving down incidence has been quite consistent across Nordic countries.7 We therefore believe that the use of different reference countries would not have changed our projected scenarios substantially. Although the estimated risk reduction might be over-optimistic if BCEE countries are unable to implement screening programmes that are as effective as those implemented in Nordic countries in the past, our projection allows an estimate of the upper limit of cervical cancer cases that are preventable by cytology-based programmes. The use of the HPV test as the primary test would most probably enhance screening effectiveness, and lead to an even greater number of prevented cervical cancer cases, provided that high coverage quality control of test results, exhaustive work-up, and, when necessary, treatment of screening-positive women were in place. Additionally, the higher sensitivity of HPV testing enables screening to begin after age 30 years and the adoption of longer screening intervals.
Our approach circumvented the non-identifiability problem of the APC models based on the observation of a uniform age curve for cervical cancer incidence, thus allowing the estimation of a unique set of parameters for period and cohort effects. Although there are no identifiability issues in projecting future incidence rates,33, 34 specific parameterisations of period and cohort trends were essential in our analysis to ensure appropriate interpretation of cervical cancer incidence trends and the development of alternative scenarios of future burden. Screening is expected to deflect the incidence trends downward across all targeted age groups and this should be visible as a period effect, whereas changes in risk factors chiefly manifest themselves as variations in risk across successive birth cohorts of women (ie, a cohort effect). Strong support for such an interpretation of the risk profile of period and cohort effects is gained by the results from previous analyses that compared the effect of long-term screening programmes (in Nordic countries) with their absence (in eastern European and Baltic countries).2, 3 Nordic countries and eastern European countries showed similar increases in cervical cancer prevalence in the birth cohorts born after 1950,3 probably due to a progressive increase in the risk of HPV exposure. However, these regions differ when assessing period effects. In Nordic countries, the strong period-related decreases are visible and the consequences of rising high-risk HPV-related generational risks are largely negated.3, 7, 20 Conversely, in BCEE countries, where screening-related effects are absent, the result is a remarkable and avoidable rise in future cervical cancer incidence, reflecting the increasing risk in successive generations of women.
A possible limitation of this study is that the projected generational risks were based on trends recorded in the youngest generations of women, for which data might be sparse, and were assumed to continue to increase across the whole study period. However, we cannot exclude that the direction and magnitude of future generational risks might differ from the assumptions we made in this analysis. Distinct observable changes in cohort effects have been noted in historical trends (eg, a U-shaped pattern in several European countries), with decreases in cervical cancer cases before the introduction of screening reported for generations of women born during 1920–30 followed by risk increases in successive generations. Previous reports have postulated that cohort effects for the risk of some cancers, including cervical cancer, might follow a roughly cyclical pattern.35, 36 However, the patterns found in our study seem irregular and vary by country, mainly related to different changes in sexual behaviour and perhaps parity, which is also a risk factor for cervical cancer. The use of spline functions to parametrise our model enabled a flexible assessment of changing patterns, including possible cyclical patterns, of the cohort effects within the prediction base and a projection of the most recent trend into the future. Mathematical transmission models would help to understand the future generational trends, although they would require a number of additional assumptions about the sexual habits and spread of HPV infection in different populations. Ideally, dynamic models should be integrated with statistical models that enable projections based on recorded incidence data, such as in the present study. Extensive sensitivity analyses using different assumptions were done (appendix). Other sensitivity analyses were also done, including assumptions of a lower effect of the screening-related period effects than that estimated for Denmark. With respect to the quality of incidence data, the completeness of cervical cancer reports is an obvious concern. Notably, the national cancer data from Russia, which comes from population-based registration on a regional basis, might be incomplete. The inclusion of this country in our present study is nevertheless useful because it included the largest population in the BCEE region.
We cannot precisely estimate the future incidence rates and burden of cervical cancer and it is possible that trends will evolve differently from those predicted by our model. Consistent with other studies,9, 37, 38, 39 we did not report uncertainty intervals for cancer incidence projections. Predictions by their nature are fraught with future uncertainty and, until methodological developments allow meaningful uncertainty intervals to be estimated, several authors have explicitly discouraged their reporting.38, 40, 41, 42 Our projections represent the best possible assessment of future scenarios given the data available and it is reassuring that forecasts obtained with a different method (NORDPRED), produced very similar results for the scenario with no future screening improvements. We restricted our analyses to age groups that are usually targeted for cervical cancer screening and extended them to age groups where the protective effect of screening is strong.
At present, cervical cancer screening as well as HPV vaccination are restricted or ineffective in BCEE countries. HPV vaccination is the best strategy for preventing cervical cancer in BCEE countries in the long term, yet strengthening screening activities is a key intervention to prevent a future increase in cervical cancer diagnoses in the next two or three generations of women.5 Protocols combining HPV vaccination of adolescents with several rounds of organised HPV-based screening have been proposed as a viable option in high-risk populations such as the BCEE countries.31, 32 In the absence of action, the cervical cancer risk in women living in these countries might reach levels similar to those seen in some sub-Saharan African countries today43 and in Denmark half a century ago.44
Acknowledgments
Acknowledgments
MPo is supported by the seventh framework programme of Directorate-General Research of the European Commission, through the CoheaHr Network (grant number 603019). We thank the directors and staff of the cancer registries in Europe who collected the data used in this study in Belarus, Bulgaria, Denmark, Estonia, Latvia, Lithuania, and Russia.
Contributors
SV and FB conceived and designed the study, contributed to data collection and analysis and interpretation of the results, and wrote the first draft of the report. SF and MP contributed to data analysis, interpretation of the results, and finalising the manuscript. DZ, MPo, and PV contributed to interpretation of the results. All authors contributed to the interpretation of the data and approved the final version of the report.
Declaration of interests
MPo reports grants and personal fees from Abbott and grants from Merck Sharp & Dohme received during 2007–14; he declares no competing interest since September, 2014. All other authors declare no competing interests.
Supplementary Material
References
- 1.Ferlay J, Soerjomataram I, Dikshit R. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Arbyn M, Antoine J, Magi M. Trends in cervical cancer incidence and mortality in the Baltic countries, Bulgaria and Romania. Int J Cancer. 2011;128:1899–1907. doi: 10.1002/ijc.25525. [DOI] [PubMed] [Google Scholar]
- 3.Vaccarella S, Lortet-Tieulent J, Plummer M, Franceschi S, Bray F. Worldwide trends in cervical cancer incidence: Impact of screening against changes in disease risk factors. Eur J Cancer. 2013;49:3262–3273. doi: 10.1016/j.ejca.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 4.Bray F, Lortet-Tieulent J, Znaor A, Brotons M, Poljak M, Arbyn M. Patterns and trends in human papillomavirus-related diseases in Central and Eastern Europe and Central Asia. Vaccine. 2013;31(suppl 7):H32–H45. doi: 10.1016/j.vaccine.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 5.Poljak M, Seme K, Maver PJ. Human papillomavirus prevalence and type-distribution, cervical cancer screening practices and current status of vaccination implementation in Central and Eastern Europe. Vaccine. 2013;31(suppl 7):H59–H70. doi: 10.1016/j.vaccine.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 6.Van de Velde N, Boily MC, Drolet M. Population-level impact of the bivalent, quadrivalent, and nonavalent human papillomavirus vaccines: a model-based analysis. J Natl Cancer Inst. 2012;104:1712–1723. doi: 10.1093/jnci/djs395. [DOI] [PubMed] [Google Scholar]
- 7.Vaccarella S, Franceschi S, Engholm G, Lonnberg S, Khan S, Bray F. 50 years of screening in the Nordic countries: quantifying the effects on cervical cancer incidence. Br J Cancer. 2014;111:965–969. doi: 10.1038/bjc.2014.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foley G, Alston R, Geraci M, Brabin L, Kitchener H, Birch J. Increasing rates of cervical cancer in young women in England: an analysis of national data 1982–2006. Br J Cancer. 2011;105:177–184. doi: 10.1038/bjc.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet. 2004;364:249–256. doi: 10.1016/S0140-6736(04)16674-9. [DOI] [PubMed] [Google Scholar]
- 10.Simms I, Fairley CK. Epidemiology of genital warts in England and Wales: 1971 to 1994. Genitourin Med. 1997;73:365–367. doi: 10.1136/sti.73.5.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laukkanen P, Koskela P, Pukkala E. Time trends in incidence and prevalence of human papillomavirus type 6, 11 and 16 infections in Finland. J Gen Virol. 2003;84:2105–2109. doi: 10.1099/vir.0.18995-0. [DOI] [PubMed] [Google Scholar]
- 12.Uuskula A, Puur A, Toompere K, DeHovitz J. Trends in the epidemiology of bacterial sexually transmitted infections in eastern Europe, 1995–2005. Sex Transm Infect. 2010;86:6–14. doi: 10.1136/sti.2009.037044. [DOI] [PubMed] [Google Scholar]
- 13.Tichonova L, Borisenko K, Ward H, Meheus A, Gromyko A, Renton A. Epidemics of syphilis in the Russian Federation: trends, origins, and priorities for control. Lancet. 1997;350:210–213. doi: 10.1016/S0140-6736(97)01382-2. [DOI] [PubMed] [Google Scholar]
- 14.Laara E, Day NE, Hakama M. Trends in mortality from cervical cancer in the Nordic countries: association with organised screening programmes. Lancet. 1987;1:1247–1249. doi: 10.1016/s0140-6736(87)92695-x. [DOI] [PubMed] [Google Scholar]
- 15.IARC . IARC Handbooks of Cancer Prevention Volume 10: Cervix Cancer Screening. IARC Press; Lyon: 2005. http://www.iarc.fr/en/publications/pdfs-online/prev/handbook10/HANDBOOK10.pdf (accessed June 21, 2016). [Google Scholar]
- 16.United Nations. Department of Economic and Social Affairs. Population Division World Population Prospects: The 2012 revision, highlights and advance tables. Working paper no. ESA/P/WP.228. https://esa.un.org/unpd/wpp/publications/Files/WPP2012_HIGHLIGHTS.pdf (accessed June 21, 2016).
- 17.Doll R, Payne P, Waterhouse J. vol. 1. Union Internationale Contre le Cancer; Geneva: 1966. (Cancer Incidence in Five Continents). [Google Scholar]
- 18.Clayton D, Schifflers E. Models for temporal variation in cancer rates. I: age-period and age-cohort models. Stat Med. 1987;6:449–467. doi: 10.1002/sim.4780060405. [DOI] [PubMed] [Google Scholar]
- 19.Plummer M, Peto J, Franceschi S. Time since first sexual intercourse and the risk of cervical cancer. Int J Cancer. 2012;130:2638–2644. doi: 10.1002/ijc.26250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bray F, Loos AH, McCarron P. Trends in cervical squamous cell carcinoma incidence in 13 European countries: changing risk and the effects of screening. Cancer Epidemiol Biomarkers Prev. 2005;14:677–686. doi: 10.1158/1055-9965.EPI-04-0569. [DOI] [PubMed] [Google Scholar]
- 21.Moller B, Fekjaer H, Hakulinen T. Prediction of cancer incidence in the Nordic countries: empirical comparison of different approaches. Stat Med. 2003;22:2751–2766. doi: 10.1002/sim.1481. [DOI] [PubMed] [Google Scholar]
- 22.Vaccarella S, Franceschi S, Bray F. The incremental benefits of implementing effective cervical cancer screening. Int J Cancer. 2015;138:254–255. doi: 10.1002/ijc.29700. [DOI] [PubMed] [Google Scholar]
- 23.Rogovskaya SI, Shabalova IP, Mikheeva IV. Human papillomavirus prevalence and type-distribution, cervical cancer screening practices and current status of vaccination implementation in Russian Federation, the Western countries of the former Soviet Union, Caucasus region and Central Asia. Vaccine. 2013;31(suppl 7):H46–H58. doi: 10.1016/j.vaccine.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 24.Campos NG, Castle PE, Wright TC, Jr, Kim JJ. Cervical cancer screening in low-resource settings: a cost-effectiveness framework for valuing tradeoffs between test performance and program coverage. Int J Cancer. 2015;137:2208–2219. doi: 10.1002/ijc.29594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maver PJ, Seme K, Korac T. Cervical cancer screening practices in central and eastern Europe in 2012. Acta Dermatovenerol Alp Pannonica Adriat. 2013;22:7–19. [PubMed] [Google Scholar]
- 26.Altobelli E, Lattanzi A. Cervical carcinoma in the European Union: an update on disease burden, screening program state of activation, and coverage as of March 2014. Int J Gynecol Cancer. 2015;25:474–483. doi: 10.1097/IGC.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 27.Arbyn M, Anttila A, Jordan J, editors. European guidelines for quality assurance in cervical cancer screening. Second edn. Office for Official Publications of the European Communities; Luxembourg: 2008. p. 291.http://bookshop.europa.eu/en/european-guidelines-for-quality-assurance-in-cervical-cancer-screening-pbND7007117/ (accessed June 21, 2016). [Google Scholar]
- 28.Elfstrom KM, Arnheim-Dahlstrom L, von KL, Dillner J. Cervical cancer screening in Europe: Quality assurance and organisation of programmes. Eur J Cancer. 2015;51:950–968. doi: 10.1016/j.ejca.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Viberga I, Poljak M. Cervical cancer screening in Latvia: a brief history and recent improvements (2009–2011) Acta Dermatovenerol Alp Pannonica Adriat. 2013;22:27–30. [PubMed] [Google Scholar]
- 30.Poljak M, Rogovskaya SI, Kesic V. Recommendations for cervical cancer prevention in Central and Eastern Europe and Central Asia. Vaccine. 2013;31(suppl 7):H80–H82. doi: 10.1016/j.vaccine.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 31.Bosch FX, Robles C, Diaz M. HPV-FASTER: broadening the scope for prevention of HPV-related cancer. Nat Rev Clin Oncol. 2015;13:119–132. doi: 10.1038/nrclinonc.2015.146. [DOI] [PubMed] [Google Scholar]
- 32.WHO . WHO guidelines for screening and treatment of precancerous lesions for cervical cancer prevention. World Health Organization; Geneva: 2013. http://apps.who.int/iris/bitstream/10665/94830/1/9789241548694_eng.pdf (accessed June 21, 2016). [PubMed] [Google Scholar]
- 33.Carstensen B. Age-period-cohort models for the Lexis diagram. Stat Med. 2007;26:3018–3045. doi: 10.1002/sim.2764. [DOI] [PubMed] [Google Scholar]
- 34.Holford TR. Understanding the effects of age, period, and cohort on incidence and mortality rates. Annu Rev Public Health. 1991;12:425–457. doi: 10.1146/annurev.pu.12.050191.002233. [DOI] [PubMed] [Google Scholar]
- 35.Dimitrov BD. Cyclic patterns of cancer incidence in males by body site: data from the USA for the period 1973–1989. J BUON. 2000;5:81–84. [Google Scholar]
- 36.Xu M, Powers DA. Bayesian ridge estimation of age-period-cohort models. In: Schoen R, editor. Dynamic Demographic Analysis. Springer International Publishing; Switzerland: 2016. pp. 337–359. [Google Scholar]
- 37.Mistry M, Parkin DM, Ahmad AS, Sasieni P. Cancer incidence in the United Kingdom: projections to the year 2030. Br J Cancer. 2011;105:1795–1803. doi: 10.1038/bjc.2011.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moller H, Fairley L, Coupland V. The future burden of cancer in England: incidence and numbers of new patients in 2020. Br J Cancer. 2007;96:1484–1488. doi: 10.1038/sj.bjc.6603746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sasieni P, Adams J. Changing rates of adenocarcinoma and adenosquamous carcinoma of the cervix in England. Lancet. 2001;357:1490–1493. doi: 10.1016/S0140-6736(00)04646-8. [DOI] [PubMed] [Google Scholar]
- 40.Bray F, Moller B. Predicting the future burden of cancer. Nat Rev Cancer. 2006;6:63–74. doi: 10.1038/nrc1781. [DOI] [PubMed] [Google Scholar]
- 41.Moller B, Weedon-Fekjaer H, Haldorsen T. Empirical evaluation of prediction intervals for cancer incidence. BMC Med Res Methodol. 2005;5:21. doi: 10.1186/1471-2288-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutherford MJ, Thompson JR, Lambert PC. Projecting cancer incidence using age-period-cohort models incorporating restricted cubic splines. Int J Biostat. 2012;8:33. doi: 10.1515/1557-4679.1411. [DOI] [PubMed] [Google Scholar]
- 43.Parkin DM, Bray F, Ferlay J, Jemal A. Cancer in Africa 2012. Cancer Epidemiol Biomarkers Prev. 2014;23:953–966. doi: 10.1158/1055-9965.EPI-14-0281. [DOI] [PubMed] [Google Scholar]
- 44.Engholm G, Ferlay J, Christensen N. NORDCAN—a Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010;49:725–736. doi: 10.3109/02841861003782017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.