Abstract
Background
Inflammation plays an important role in the pathogenesis of cardiovascular disease in patients with advanced chronic kidney disease (CKD). Neutrophil-to-lymphocyte ratio (NLR), an inflammatory biomarker, has not been evaluated in patients who have advanced CKD with peripheral artery disease (PAD) undergoing percutaneous transluminal angioplasty (PTA), especially in Taiwan.
Methods
We retrospectively evaluated 148 advanced CKD (creatinine clearance rate ≤ 30 mL/min/1.73 m2) identified from a prospective registry in our hospital (303 PTA cases in total). Kaplan-Meier analysis with log-rank test was used to study event-free survival, and all univariables (p value < 0.1) were put into multivariate Cox regression analysis.
Results
During the mean follow-up time of 8.6 ± 7.8 months, 35.1% of the cases achieved primary composite endpoint (all-cause mortality or major amputation), 25.5% underwent death from any cause, and 14.9% underwent major or minor amputation. Rutherford grade 6, either NLR or NLR ≥ 3.76, and a history of hypertension had a positively prognostic impact on the occurrence of primary composite endpoint, whereas higher albumin level (≥ 3.0 mg/dL) and technical success had a significantly protective effect. History of hypertension, either NLR or NLR ≥ 3.76, and age were associated with all-cause mortality. In addition, Rutherford 6, higher albumin level (≥ 3.0 mg/dL), technical success, NLR, and age could predict the occurrence of major amputation.
Conclusions
NLR, but not C-reactive protein or platelet-lymphocyte ratio, is an important prognostic predictor of all major clinical outcomes in patients with advanced CKD and PAD receiving PTA. Further studies are warranted to establish a better strategy and healthcare program in this clinical setting.
Keywords: Chronic kidney disease, Inflammation, Peripheral artery disease
INTRODUCTION
Patients with peripheral artery disease (PAD) have a high risk of morbidity and mortality.1 Owing to its clinical feasibility and minimally-invasive nature, percutaneous transluminal angioplasty (PTA) has become a noteworthy approach and usually the first choice of revascularization for the majority of patients with PAD in the current era.2 However, intermediate- or long-term prognosis of PAD undergoing PTA remains poor, with an average of 25% total mortality, 12% cardiovascular death, and 7.5% major amputation.3-5 We3 and other researchers1,6-10 have tried to identify some traditional prognostic determinants, among which advanced chronic kidney disease (CKD)1,10 or end-stage renal disease (ESRD)6,9 could probably independently predict major amputation or death in PAD or PAD receiving PTA. Despite the high prevalence of advanced CKD in patients with PAD undergoing PTA,3,4,6 independent outcome predictors in such clinical setting are not well understood, especially in Taiwan. Apart from traditional cardiovascular risk factors, some specific non-traditional risk factors also play a major role in the modulation of cardiovascular outcomes in patients with advanced CKD.11 These factors should be taken into consideration while evaluating CKD patients with PAD. However, to the best our knowledge, there is no study aimed at the investigation of such outcome predictors, including some non-traditional risk factors, in patients with advanced CKD receiving PTA for PAD.
Inflammation plays an important role in the pathogenesis of cardiovascular disease in patients with advanced CKD and ESRD.11 Some inflammation-relevant risk factors have been reported to be implicated in the prediction of cardiovascular outcomes in CKD/ESRD12-16 or PAD.17,18 However, these factors, especially neutrophil-to-lymphocyte ratio (NLR), are either never or incompletely investigated in PAD patients with advanced CKD/ESRD receiving PTA.
Taiwan, especially the southern part, is an endemic area with a high incidence rate of advanced CKD and ESRD. However, there has been no study to date identifying prognostic factors affecting intermediate-term outcome in patients who have advanced CKD, including ESRD, with PAD undergoing PTA in Taiwan.
The current study aimed to investigate the prognostic factors in patients who have advanced CKD, including ESRD, with PAD undergoing PTA in Taiwan. We hypothesized that NLR, but not high-sensitivity C-reactive protein (hsCRP) level or platelet-to-lymphocyte ratio (PLR), could independently predict intermediate outcomes in this clinical setting.
MATERIALS AND METHODS
Study population
Eligible PAD patients without acute limb ischemia, who were hospitalized for PTA from January, 2011 to June, 2014 were consecutively enrolled in this single-center retrospective study from a prospective registry. There were 148 advanced CKD cases (creatinine clearance rate ≤ 30 mL/min/1.73 m2), including 126 ESRD, identified in a total of 303 PTA cases. The current study protocol, without the necessity of giving an informed consent form, was approved by the institutional review board of the Tainan Municipal Hospital. According to the registry protocol, the clinical data and endpoints were routinely collected and recorded by clinic visits, medical chart review, telephone call or direct contact with the participants or the subjects’ family.
Outcome measurement
The minimal follow-up period in the last enrolled subject would be at least one month, unless death occurred. The primary composite endpoint was the time to first occurrence of total death or major amputation. The secondary endpoints included the individual occurrence of the components of the primary composite endpoint, and all amputation.
Definitions of the underlying disease and endpoints
Definitions of the underlying disease and Rutherford classification have been described in detail elsewhere.4,19 Technical success was defined as all inflow lesions less than 30% and a straight line flow through at least one tibial artery or peroneal artery which have intact flow to the pedal arch.
Cardiovascular death was defined as death from cardiovascular/cerebrovascular causes. All amputation included major or minor amputation. Major amputation was defined as tissue loss or amputation extending beyond ankle and minor amputation as limited below ankle. Myocardial infarction was defined in accordance with the universal definition proposed in 2012.20
Complete blood counts and differential counts, and biochemical tests
Complete blood counts with automated differential counts, which included total white blood cells, neutrophils, lymphocytes, hemoglobin, and platelets, were obtained at the time of admission. NLR was calculated as the ratio of the neutrophils and lymphocytes, and PLR was calculated as the ratio of the platelets and lymphocytes, all obtained from the same automated blood sample at the time of admission of the study population.
Fasting plasma or serum samples were obtained for routine measurement of all biochemical data and analyzed in our hospital laboratory according to our registry protocol.
Statistical analysis
All variables were presented as mean and standard deviation and skewed data were reported as median (interquartile range). Chi-square or Fisher’s exact test was used for comparison of categorical variables between groups, while Mann-Whitney U test or unpaired Student’s t test was used for continuous variables as appropriate. If more than 1 end point occurred within the follow-up period, only the first event was considered. Kaplan-Meier analysis was used to study patient survival and event-free status, using the log-rank test (Cox-Mantel) to ascertain differences between groups. All univariables with a p value less than 0.1 were put into multi-variate Cox regression analysis by using backward (likelihood ratio) method to select independent covariates stepwise. A p value < 0.05 was regarded as statistically significant. All statistical analyses were performed using SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
High prevalence of ESRD and critical limb ischemia in this cohort
These 148 advanced CKD cases were retrieved from our cohort with 303 PTA cases in total. The percentage of advanced CKD was 48.8%. The baseline clinical characteristics were shown in Table 1. The most prevalent traditional coronary risk factors were diabetes mellitus, hyperlipidemia, and hypertension. Of note, most patients were in stage-5 CKD. Interestingly, a high prevalence of critical limb ischemia (Rutherford class 4 to 6) up to 87% was noticed in this cohort.
Table 1. Baseline clinical, angiographic, and interventional characteristics of 148 advanced chronic kidney disease cases with peripheral artery disease undergoing percutaneous transluminal revascularization.
| PAD cases (n = 148) | |
| Male gender (%) | 83 (56.1) |
| Diabetes mellitus (%) | 129 (87.2) |
| Hypertension (%) | 105 (70.9) |
| Hyperlipidemia (%) | 109 (73.6) |
| Tobacco smoking (%) | 20 (13.5) |
| CAD (%) | 86 (58.1) |
| PCI (%) | 59 (39.9) |
| CABG (%) | 16 (10.8) |
| Old MI (%) | 18 (12.2) |
| Old CVA (%) | 43 (29.1) |
| PUD (%) | 50 (33.8) |
| CKD staging | |
| 4 | 21 (14.2) |
| 5 | 127 (85.8) |
| End-stage renal disease (%) | 126 (85.1) |
| Anemia (%) | 124 (83.8) |
| Bedridden (%) | 28 (18.9) |
| Rutherford classification (%) | |
| 2 (%) | 3 (2.0) |
| 3 (%) | 16 (10.8) |
| 4 (%) | 11 (7.4) |
| 5 (%) | 53 (35.8) |
| 6 (%) | 65 (43.9) |
| Laboratory tests | |
| Albumin, mg/dL | 3.9 ± 2.4 |
| Fasting plasma glucose, mg/dL | 164.5 ± 114.9 |
| hsCRP, mg/L | 4.5 (1.3, 9.5) |
| Total cholesterol, mg/dL | 150.3 ± 45.5 |
| Triglyceride, mg/dL | 129.3 ± 77.0 |
| LDL cholesterol, mg/dL | 92.3 ± 39.4 |
| HDL cholesterol, mg/dL | 38.4 ± 12.8 |
| Hemoglobin, g/dL | 10.3 ± 1.6 |
| Platelet count, 103/μl | 239.8 ± 103.3 |
| NLR | 4.63 (2.8, 7.3) |
| PLR | 169.4 (119.8, 249.0) |
| Angiographic and interventional characteristics | |
| Revascularized site (%) | |
| Iliac (%) | 5 (3.4) |
| F-P (%) | 34 (23.0) |
| IP (%) | 57 (38.5) |
| F-P + IP (%) | 47 (31.8) |
| Iliac + F-P (%) | 4 (2.7) |
| Iliac + F-P+ IP (%) | 1 (0.7) |
| Technical success | 134 (90.5) |
| Technical success in IP PTA (n = 105) | 96 (91.4) |
| Angiosome (%) | 82 (78.1) |
| Boundary (%) | 14 (13.3) |
Values were expressed as mean ± SD, no. (%), or median (interquartile range) whenever appropriate.
CABG, coronary-artery bypass grafting; CAD, coronary artery disease; CKD, chronic kidney disease; CVA, cerebrovascular accident; F-P, femoral-popliteal; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; IP, infrapopliteal; LDL, low-density lipoprotein; MI, myocardial infarction; NLR, neutrophil-to-lymphocyte ratio; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; PLR, platelet-to-lymphocyte ratio; PTA, percutaneous transluminal angioplasty; PUD, peptic ulcer disease.
Infrapopliteal lesions were most commonly intervened (Table 1), consisting of about 71% of those cases receiving infrapopliteal PTA. Nevertheless, the overall technical success rate was high in patients with or without consistent infrapopliteal intervention. In 105 patients with infrapopliteal PTA, the angiosome success rate was still acceptable.
High morbidity and mortality in patients with CKD and ESRD receiving PTA
During the mean follow-up time of 8.6 ± 7.8 months, 35.1% of cases achieved primary composite endpoint. There were 25.5% cases encountering death from any cause, among which near half of cases died due to cardiovascular causes (Table 2). There were 14.9% cases encountering major or minor amputation, among which the majority was major tissue loss or amputation. Clinically-driven target vessel revascularization was performed in 22.3% of the cases.
Table 2. Clinical outcome after percutaneous transluminal revascularization.
| PAD cases (n = 148) | |
| Mean follow-up, M | 8.6 ± 7.8 |
| Recurrent symptoms (%) | 39 (26.4) |
| Intermittent claudication (%) | 6 (4.1) |
| Resting pain (%) | 17 (11.5) |
| Ulcerative wound (%) | 21 (14.2) |
| Gangrene (%) | 14 (9.5)0 |
| Time elapse to develop recurrent symptoms, M | 1.4 ± 3.6 |
| Renal outcome in CKD without end-stage renal disease (n = 22) | |
| Maintained in or improved from the previous stage | 21 (95.5) |
| Progressed into an advanced stage | 1 (4.5) |
| Progressed into end-stage renal disease | 1 (4.5) |
| Total death or major amputation (%) | 52 (35.1) |
| Total death (%) | 38 (25.7) |
| Cardiovascular death in total death | 16 (42.1) |
| All amputation (%) | 22 (14.9) |
| Minor amputation (%) | 5 (3.4) |
| Major amputation (%) | 17 (11.5) |
| Nonfatal MI (%) | 14 (9.5) |
| Nonfatal stroke (%) | 6 (4.1) |
| Target vessel revascularization (%) | 33 (22.3) |
Abbreviations as Table 1. Values were expressed as mean ± SD or no. (%) whenever appropriate.
Independent prognostic determinants of the primary composite endpoint and all secondary endpoints
A comparison of the baseline characteristics of cases with high NLR (≥ 3.76) and low NLR (< 3.76) was shown in Table 3. The cutoff value of NLR was selected according to a previous study focused on the investigation of the prognostic impact of NLR on cardiovascular outcome in patients with CKD.15 The majority of the baseline characteristics was similar except that more cases with higher NLR had poor functional status and higher inflammatory activity.
Table 3. Comparison of baseline characteristics between patients with NLR ≥ 3.76 and those with NLR < 3.76.
| NLR ≥ 3.76 (n = 92) | NLR < 3.76 (n = 56) | p | |
| Age, y | 67.9 ± 9.9 | 69.0 ± 10.5 | 0.52 |
| Male gender (%) | 52 (56.5) | 31 (55.4) | 0.89 |
| Diabetes mellitus (%) | 81 (88.0) | 48 (85.7) | 0.68 |
| Hypertension (%) | 63 (68.5) | 42 (75.0) | 0.4 |
| Hyperlipidemia (%) | 63 (68.5) | 46 (82.1) | 0.07 |
| Tobacco smoking (%) | 15 (16.3) | 5 (8.9) | 0.2 |
| CAD (%) | 54 (58.7) | 32 (57.1) | 0.85 |
| PCI (%) | 38 (41.3) | 21 (37.5) | 0.65 |
| CABG (%) | 12 (13.0) | 4 (7.1) | 0.26 |
| Old MI (%) | 12 (13.0) | 6 (10.7) | 0.67 |
| Old CVA (%) | 27 (29.3) | 16 (28.6) | 0.92 |
| PUD (%) | 28 (30.4) | 22 (39.3) | 0.27 |
| CKD Staging | 0.34 | ||
| 4 | 11 (12.0) | 10 (17.9) | |
| 5 | 81 (88.0) | 46 (82.1) | |
| End-stage renal disease (%) | 80 (87.0) | 45 (80.4) | 0.28 |
| Anemia (%) | 74 (80.4) | 50 (89.3) | 0.16 |
| Bedridden (%) | 22 (23.9) | 6 (10.7) | 0.05 |
| Rutherford classification (%) | 0.55 | ||
| 2 (%) | 1 (1.0) | 2 (3.6) | |
| 3 (%) | 8 (8.7) | 8 (14.3) | |
| 4 (%) | 8 (8.7) | 3 (5.4) | |
| 5 (%) | 32 (34.8) | 21 (37.5) | |
| 6 (%) | 43 (46.7) | 22 (39.3) | |
| Laboratory tests | |||
| Albumin, mg/dL | 4.0 ± 3.1 | 3.8 ± 0.5 | 0.7 |
| Fasting plasma glucose, mg/dL | 172.9 ± 133.1 | 150.7 ± 75.3 | 0.2 |
| hsCRP, mg/L | 6.7 (3.8, 12.7) | 2.1 (0.5, 4.7) | < 0.001 |
| Total cholesterol, mg/dL | 147.8 ± 44.8 | 154.1 ± 46.6 | 0.45 |
| Triglyceride, mg/dL | 128.0 ± 73.0 | 131.3 ± 83.3 | 0.82 |
| LDL cholesterol, mg/dL | 91.1 ± 38.9 | 94.1 ± 40.4 | 0.68 |
| HDL cholesterol, mg/dL | 36.8 ± 12.7 | 40.5 ± 12.9 | 0.12 |
| Hemoglobin, g/dL | 10.3 ± 1.8 | 10.3 ± 1.3 | 0.94 |
| Platelet count, 103/μl | 254.0 ± 115.0 | 216.4 ± 74.8 | 0.02 |
| PLR | 219.9 (166.5, 289.7) | 119.8 (90.9, 154.5) | < 0.001 |
Abbreviations as Table 1. Values were expressed as mean ± SD, no. (%), or median (interquartile range) whenever appropriate.
While evaluating the independent prognostic role of NLR, we performed multi-variate analyses by considering either the continuous or categorical form of NLR using a different model. Table 4 showed that Rutherford grade 6, either NLR or NLR ≥ 3.76, and a history of hypertension had a positively prognostic impact on the occurrence of all-cause mortality or major amputation, whereas higher albumin level (≥ 3.0 mg/ dL) and technical success had a significantly protective effect in avoiding the occurrence of primary composite endpoint.
Table 4. Uni-variates and multi-variates independently predicting total death or major amputation.
| Uni-variates | Multi-variates | |||||
| Model 1 | Model 2 | |||||
| Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | |
| Age | 1.02 (0.99-1.05) | 0.13 | ||||
| Male gender | 1.06 (0.61-1.84) | 0.85 | ||||
| Diabetes mellitus | 0.59 (0.29-1.22) | 0.15 | ||||
| Hypertension | 2.28 (1.07-4.84) | 0.03 | - | NS | 2.37 (1.04-5.45) | 0.04 |
| Hyperlipidemia | 0.47 (0.26-0.85) | 0.01 | - | NS | - | NS |
| Tobacco smoking | 0.54 (0.22-1.37) | 0.2 | ||||
| CAD | 1.55 (0.87-2.76) | 0.14 | ||||
| PCI | 1.24 (0.72-2.15) | 0.44 | ||||
| CABG | 2.16 (1.05-4.43) | 0.04 | - | NS | - | NS |
| Old MI | 1.29 (0.63-2.66) | 0.49 | ||||
| Old CVA | 1.10 (0.61-1.99) | 0.75 | ||||
| PUD | 0.92 (0.52-1.63) | 0.77 | ||||
| Anemia | 1.64 (0.65-4.14) | 0.3 | ||||
| Bedridden | 2.11 (1.18-3.77) | 0.01 | - | NS | - | NS |
| Rutherford 6 | 2.66 (1.52-4.66) | 0.001 | 3.17 (1.64-6.13) | 0.001 | 3.66 (1.93-6.93) | < 0.001 |
| Albumin ≥ 3.0 | 0.28 (0.15-0.55) | < 0.001 | 0.44 (0.21-0.89) | 0.02 | 0.46 (0.23-0.93) | 0.03 |
| hsCRP | 1.03 (0.99-1.07) | 0.18 | ||||
| Hemoglobin | 0.85 (0.72-1.02) | 0.08 | - | NS | - | NS |
| Platelet | 0.99 (0.96-1.02) | 0.54 | ||||
| NLR | 1.07 (1.04-1.10) | < 0.001 | 1.04 (1.01-1.07) | 0.03 | ||
| NLR ≥ 3.76 | 2.39 (1.25-4.56) | 0.01 | 2.07 (1.00-4.35) | 0.05 | ||
| PLR | 1.00 (1.00-1.00) | 0.73 | ||||
| Technical success | 0.47 (0.24-0.94) | 0.03 | 0.35 (0.16-0.75) | 0.01 | 0.38 (0.17-0.82) | 0.01 |
Abbreviations as Table 1 and 4. CI, confidence interval; NS, non-significant. Multi-variates in model 1 included: hypertension, hyperlipidemia, CABG, bedridden, Rutherford 6, albumin ≥ 3.0, hemoglobin, NLR, and technical success. Multi-variates in model 2 included: hypertension, hyperlipidemia, CABG, bedridden, Rutherford 6, albumin ≥ 3.0, hemoglobin, NLR ≥ 3.76, and technical success.
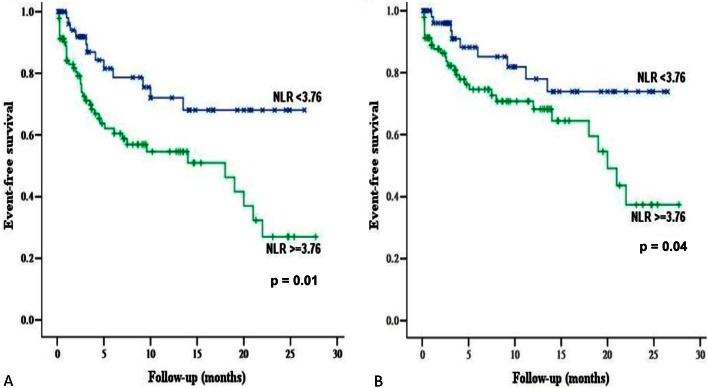
Multivariate analysis showed that history of hypertension, either NLR or NLR ≥ 3.76 (Figure 1), and age could significantly and independently predict all-cause mortality (Table 5). Regarding the occurrence of major tissue loss or amputation, some independent prognostic predictors have been identified shown in Table 6, including Rutherford 6, higher albumin level (≥ 3.0 mg/dL), technical success, NLR, and age. However, Rutherford 6, NLR, and age might independently predict major or minor amputation after PTA (Table 7).
Figure 1.
The Event-free survival stratified by neutrophil-to-lymphocyte ratio (NLR) by Kaplan-Meier analysis using the log-rank test. (A) Primary composite endpoint (total death or major amputation). (B) Total death. “X” denotes events occurred in patients with NLR < 3.76 and “+” denotes events occurred in patients with NLR ≥ 3.76.
Table 5. Uni-variates and multi-variates independently predicting total death.
| Uni-variates | Multi-variates | |||||
| Model 1 | Model 2 | |||||
| Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | |
| Age | 1.06 (1.03-1.10) | 0.001 | 1.05 (1.02-1.09) | 0.01 | 1.06 (1.03-1.10) | 0.001 |
| Male gender | 0.84 (0.44-1.60) | 0.6 | ||||
| Diabetes mellitus | 0.70 (0.29-1.67) | 0.42 | ||||
| Hypertension | 7.60 (1.83-31.60) | 0.01 | 5.28 (1.23-22.71) | 0.03 | 7.30 (1.74-30.67) | 0.01 |
| Hyperlipidemia | 0.58 (0.309-1.15) | 0.12 | ||||
| Tobacco smoking | 0.27 (0.10-1.14) | 0.07 | - | NS | - | NS |
| CAD | 1.36 (0.69-2.66) | 0.37 | ||||
| PCI | 1.05 (0.55-2.01) | 0.88 | ||||
| CABG | 1.82 (0.76-4.35) | 0.18 | ||||
| Old MI | 1.36 (0.60-3.10) | 0.46 | ||||
| Old CVA | 1.53 (0.79-2.96) | 0.21 | ||||
| PUD | 1.21 (0.63-2.33) | 0.56 | ||||
| Anemia | 1.35 (0.48-3.81) | 0.58 | ||||
| Bedridden | 3.19 (1.67-6.08) | < 0.001 | - | NS | - | NS |
| Rutherford 6 | 1.57 (0.83-2.97) | 0.17 | ||||
| Albumin ≥ 3.0 | 0.34 (0.16-0.72) | 0.01 | - | NS | - | NS |
| hsCRP | 1.00 (0.95-1.05) | 0.96 | ||||
| Hemoglobin | 0.84 (0.68-1.03) | 0.09 | - | NS | - | NS |
| Platelet | 0.98 (0.95-1.02) | 0.3 | ||||
| NLR | 1.05 (1.01-1.09) | 0.01 | 1.04 (1.00-1.08) | 0.04 | ||
| NLR ≥ 3.76 | 2.18 (1.03-4.61) | 0.04 | 2.23 (1.03-4.82) | 0.04 | ||
| PLR | 1.00 (1.00-1.00) | 0.89 | ||||
| Technical success | 0.64 (0.27-1.53) | 0.31 |
Table 6. Uni-variates and multi-variates independently predicting major amputation.
| Uni-variates | Multi-variates | |||||
| Model 1 | Model 2 | |||||
| Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | |
| Age | 0.94 (0.90-0.99) | 0.03 | 0.86 (0.78-0.96) | 0.01 | 0.89 (0.82-0.98) | 0.02 |
| Male gender | 1.06 (0.40-2.78) | 0.91 | ||||
| Diabetes mellitus | 0.57 (0.16-1.98) | 0.37 | ||||
| Hypertension | 0.80 (0.29-2.15) | 0.65 | ||||
| Hyperlipidemia | 0.58 (0.22-1.58) | 0.29 | ||||
| Tobacco smoking | 1.18 (0.34-4.10) | 0.8 | ||||
| CAD | 1.11 (0.42-2.93) | 0.83 | ||||
| PCI | 1.15 (0.44-3.01) | 0.78 | ||||
| CABG | 2.22 (0.64-7.73) | 0.21 | ||||
| Old MI | 0.86 (0.20-3.77) | 0.84 | ||||
| Old CVA | 0.54 (0.16-1.89) | 0.34 | ||||
| PUD | 0.55 (0.18-1.69) | 0.3 | ||||
| Anemia | 3.16 (0.42-23.80) | 0.27 | ||||
| Bedridden | 1.48 (0.48-4.56) | 0.49 | ||||
| Rutherford 6 | 7.90 (2.26-27.58) | 0.001 | 8.55 (1.64-44.53) | 0.01 | 10.00 (1.99-50.21) | 0.01 |
| Albumin ≥ 3.0 | 0.28 (0.07-1.04) | 0.06 | 0.11 (0.01-0.80) | 0.03 | 0.10 (0.02-0.57) | 0.01 |
| hsCRP | 1.05 (1.00-1.11) | 0.08 | - | NS | - | NS |
| Hemoglobin | 0.89 (0.66-1.20) | 0.44 | ||||
| Platelet | 1.01 (0.96-1.05) | 0.79 | ||||
| NLR | 1.08 (1.03-1.13) | 0.001 | 1.09 (1.02-1.17) | 0.01 | ||
| NLR ≥ 3.76 | 2.28 (0.74-7.01) | 0.15 | ||||
| PLR | 1.00 (1.00-1.00) | 0.86 | ||||
| Technical success | 0.34 (0.11-1.04) | 0.06 | 0.07 (0.01-0.39) | 0.002 | 0.09 (0.02-0.43) | 0.003 |
Table 7. Uni-variates and multi-variates independently predicting total amputation.
| Uni-variates | Multi-variates | |||||
| Model 1 | Model 2 | |||||
| Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | |
| Age | 0.94 (0.89-0.98) | 0.004 | 0.94 (0.89-0.99) | 0.03 | - | NS |
| Male gender | 1.65 (0.67-4.05) | 0.28 | ||||
| Diabetes mellitus | 0.69 (0.23-2.65) | 0.7 | ||||
| Hypertension | 0.77 (0.32-1.84) | 0.56 | ||||
| Hyperlipidemia | 0.68 (0.28-1.67) | 0.4 | ||||
| Tobacco smoking | 0.87 (0.26-2.95) | 0.83 | ||||
| CAD | 1.39 (0.58-3.31) | 0.46 | ||||
| PCI | 1.11 (0.47-2.59) | 0.82 | ||||
| CABG | 1.58 (0.47-5.35) | 0.46 | ||||
| Old MI | 0.64 (0.15-2.76) | 0.55 | ||||
| Old CVA | 0.56 (0.19-1.67) | 0.3 | ||||
| PUD | 0.39 (0.13-1.17) | 0.09 | - | NS | - | NS |
| Anemia | 1.94 (0.45-8.32) | 0.37 | ||||
| Bedridden | 1.43 (0.53-3.87) | 0.49 | ||||
| Rutherford 6 | 5.43 (2.00-14.77) | 0.001 | - | NS | 3.73 (1.07-12.98) | 0.04 |
| Albumin ≥ 3.0 | 0.35 (0.09-1.26) | 0.11 | ||||
| hsCRP | 1.05 (1.00-1.10) | 0.07 | - | NS | - | NS |
| Hemoglobin | 0.87 (0.66-1.13) | 0.29 | ||||
| Platelet | 1.02 (0.98-1.06) | 0.41 | ||||
| NLR | 1.07 (1.03-1.11) | 0.001 | 1.06 (1.01-1.12) | 0.03 | ||
| NLR ≥ 3.76 | 2.39 (0.88-6.48) | 0.09 | - | NS | ||
| PLR | 1.00 (1.00-1.00) | 0.81 | ||||
| Technical success | 0.47 (0.16-1.39) | 0.17 |
DISCUSSION
Our study reveals, for the first time, that NLR is an important prognostic predictor of total death or major amputation, total death, major amputation, and major or minor amputation after adjusting numerous confounders in patients who have advanced CKD with PAD receiving PTA during an intermediate-term follow-up. In addition, some other predictors have been identified in this clinical situation in Taiwan, which have never been reported in such an endemic area with a high prevalence of advanced CKD and ESRD.
According to the current study, NLR remained significant after adjusting hsCRP and other inflammatory markers in terms of predicted values in mortality and major morbidity, providing further evidence that NLR is an excellent biomarker in this situation. NLR can easily be obtained and calculated. Neutrophil count reflects inflammation, whereas lymphocyte count may reflect general stress and nutrition.15 Although neutrophils have been traditionally considered as one of the components of acute inflammation,15 but during the past decade, neutrophils have received growing attention in the chronic inflammation process;21 recent studies have proposed a linkage of neutrophils to the genesis of atherosclerosis from low grade inflammation in the arterial wall.15,21-23 Neutrophils may secrete a number of cytokines and instruct or activate other immune cells, thus promoting inflammation in the atheroma.21,23 Despite this phenomenon, the role of lymphocytes in atherosclerosis is more complicated, and some studies reveal an association of low peripheral lymphocyte count with the development of atherosclerosis.24,25
Although the prognostic role of NLR has ever been evaluated in CKD and ESRD14-16 or PAD,18 there has been no study investigating the prognostic impact of this factor accompanied by other inflammatory biomarkers in patients with advanced CKD and PAD receiving PTA. Our study showed that NLR could significantly and consistently predict all major outcomes, which was not consistent with the previous study performed in patients with critical limb ischemia.18 In patients with CKD, increased inflammation is associated with the prevalence of PAD.26 Furthermore, specific non-traditional risk factors also play a major role in the modulation of cardiovascular outcomes in patients with advanced CKD,11 probably leading to a different profile of prognostic predictors than patients with non-advanced CKD.
Platelets were found to be evolved in the atherogenesis via secreting proinflammatory cytokines16 and triggering leukocyte transmigration and adhesion.16,27 According to the result of our study, there is no significant role of this marker in patients with advanced CKD and PAD receiving PTA. One previous study16 showed that PLR better predicted inflammation than NLR in ESRD patients. However, the outcome endpoints and patient population of that small study (surrogate markers in ESRD) are totally different from our current study (hard endpoints in advanced CKD and PAD receiving PTA). This discrepancy could be due to different outcome endpoints and study population.
Higher albumin level is an independently protective factor of total death or major amputation, largely resulting from avoiding major amputation. This factor has been given some attention in patients with PAD undergoing endovascular therapy6,13 in recent years. The interaction of malnutrition with inflammation in CKD patients has been the subject of much interest in the past decade.28 Despite having a different study design and population, our study result is in agreement with the previous reports.6,13
We found that history of hypertension could significantly predict total death or major amputation, mainly mediated through predicting total death. We speculate that it could be due to the underlying characteristics of our study population, being advanced CKD, since hypertension has been reported to be an independent risk factor responsible for cardiovascular outcomes15 and inflammation14 in patients with CKD. Furthermore, despite the fact that this risk factor has garnered less attention regarding cardiovascular outcomes in patients with PAD previously, our study finding is in line with the results of a newly published study1 from a large cohort with 41,882 PAD patients in Germany.
In spite of the potential referral bias and a subject beyond our scope, we3 and others6 have reported a higher prevalence of advanced CKD/ESRD and high Rutherford grade in PAD in Taiwan than in Western countries.7-10 High Rutherford grade is a well-known risk factor for major amputation. It’s recognized to be an important public health regarding how to prevent PAD patients from developing such a critical and terminal situation. Before that troubling point is reached, according to our study result, we should attempt to establish at least one patent flow from thigh to foot to protect the PAD patient from major amputation. Our study showed that the technical success rate of PTA for such a challenging clinical situation could be still high if the procedure is performed by an experienced operator.
There are some limitations to the current study. Although the data is retrieved from a prospective registry, a retrospective analysis might still have some bias. In addition, the current study is limited by a small sample size for purposes of further analysis of the secondary endpoints, with an event rate reduced more than the primary composite endpoint. These limitations decreased the statistical power of our analyses. Furthermore, the prognostic role of calcium-phosphate product is not evaluated in the current study since serum level of both electrolytes is not routinely checked in the registry.
CONCLUSIONS
In conclusion, NLR, but not hsCRP or PLR, might be an important prognostic predictor of all major clinical outcomes in patients with advanced CKD and PAD receiving PTA. Owing to its convenient availability, NLR should be obtained and calculated in patients who have advanced CKD with PAD. Further studies to establish a better strategy and healthcare program to prevent or modify other factors warrant further investigation.
Acknowledgments
This work was supported in part by the Tainan Municipal Hospital Research Grant (RA14005), and a Landmark Project to Promote Innovation and Competitiveness of Clinical Trials by the Excellent Clinical Trial and Research Center in National Cheng Kung University Hospital from the Ministry of Health and Welfare, Taiwan (MOHW103-TDU-B-211-113002 and MOHW104-TDU-B-211-113002).
REFERENCES
- 1.Reinecke H, Unrath M, Freisinger E, et al. Peripheral arterial disease and critical limb ischaemia: still poor outcomes and lack of guideline adherence. Eur Heart J. 2015;36:932–938. doi: 10.1093/eurheartj/ehv006. [DOI] [PubMed] [Google Scholar]
- 2.Kakkar AM, Abbott JD. Percutaneous versus surgical management of lower extremity peripheral artery disease. Curr Atheroscler Rep. 2015;17:479. doi: 10.1007/s11883-014-0479-0. [DOI] [PubMed] [Google Scholar]
- 3.Chen IC, Chao TH, Fang CC, et al. Prognostic determinants of intermediate-term outcome in peripheral artery disease undergoing percutaneous transluminal angioplasty in Taiwan—a single center experience. Atherosclerosis. 2014;235:e269–e270. [Google Scholar]
- 4.Huang HL, Chou HH, Wu TY, et al. Endovascular intervention in Taiwanese patients with critical limb ischemia: patient outcomes in 333 consecutive limb procedures with a 3-year follow-up. J Formos Med Assoc. 2014;113:688–695. doi: 10.1016/j.jfma.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Abularrage CJ, Conrad MF, Hackney LA, et al. Long-term outcomes of diabetic patients undergoing endovascular infrainguinal interventions. J Vasc Surg. 2010;52:314–322. doi: 10.1016/j.jvs.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Chang SH, Tsai YJ, Chou HH, et al. Clinical predictors of long-term outcomes in patients with critical limb ischemia who have undergone endovascular therapy. Angiology. 2014;65:315–322. doi: 10.1177/0003319713515544. [DOI] [PubMed] [Google Scholar]
- 7.Flu HC, Lardenoye JH, Veen EJ, et al. Functional status as a prognostic factor for primary revascularization for critical limb ischemia. J Vasc Surg. 2010;51:360–371. doi: 10.1016/j.jvs.2009.08.051. [DOI] [PubMed] [Google Scholar]
- 8.Desormais I, Aboyans V, Bura A, et al. Anemia, an independent predictive factor for amputation and mortality in patients hospitalized for peripheral artery disease. Eur J Vasc Endovasc Surg. 2014;48:202–207. doi: 10.1016/j.ejvs.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Patel VI, Mukhopadhyay S, Guest JM, et al. Impact of severe chronic kidney disease on outcomes of infrainguinal peripheral arterial intervention. J Vasc Surg. 2014;59:368–375. doi: 10.1016/j.jvs.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Lacroix P, Aboyans V, Desormais I, et al. Chronic kidney disease and the short-term risk of mortality and amputation in patients hospitalized for peripheral artery disease. J Vasc Surg. 2013;58:966–971. doi: 10.1016/j.jvs.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Kahn MR, Robbins MJ, Kim MC, Fuster V. Management of cardiovascular disease in patients with kidney disease. Nat Rev Cardiol. 2013;10:261–273. doi: 10.1038/nrcardio.2013.15. [DOI] [PubMed] [Google Scholar]
- 12.do Sameiro-Faria M, Ribeiro S, Costa E, et al. Risk factors for mortality in hemodialysis patients: two-year follow-up study. Dis Markers. 2013;35:791–798. doi: 10.1155/2013/518945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishii H, Aoyama T, Takahashi H, et al. Serum albumin and C-reactive protein levels predict clinical outcome in hemodialysis patients undergoing endovascular therapy for peripheral artery disease. Atherosclerosis. 2013;227:130–134. doi: 10.1016/j.atherosclerosis.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 14.Okyay GU, Inal S, Oneç K, et al. Neutrophil to lymphocyte ratio in evaluation of inflammation in patients with chronic kidney disease. Ren Fail. 2013;35:29–36. doi: 10.3109/0886022X.2012.734429. [DOI] [PubMed] [Google Scholar]
- 15.Solak Y, Yilmaz MI, Sonmez A, et al. Neutrophil to lymphocyte ratio independently predicts cardiovascular events in patients with chronic kidney disease. Clin Exp Nephrol. 2013;17:532–540. doi: 10.1007/s10157-012-0728-x. [DOI] [PubMed] [Google Scholar]
- 16.Turkmen K, Erdur FM, Ozcicek F, et al. Platelet-to-lymphocyte ratio better predicts inflammation than neutrophil-to-lymphocyte ratio in end-stage renal disease patients. Hemodial Int. 2013;17:391–396. doi: 10.1111/hdi.12040. [DOI] [PubMed] [Google Scholar]
- 17.Mueller T, Hinterreiter F, Luft C, et al. Mortality rates and mortality predictors in patients with symptomatic peripheral artery disease stratified according to age and diabetes. J Vasc Surg. 2014;59:1291–1299. doi: 10.1016/j.jvs.2013.11.063. [DOI] [PubMed] [Google Scholar]
- 18.Chan C, Puckridge P, Ullah S, et al. Neutrophil-lymphocyte ratio as a prognostic marker of outcome in infrapopliteal percutaneous interventions for critical limb ischemia. J Vasc Surg. 2014;60:661–668. doi: 10.1016/j.jvs.2014.03.277. [DOI] [PubMed] [Google Scholar]
- 19.Chao TH, Tseng SY, Chen IC, et al. Cilostazol enhances mobilization and proliferation of endothelial progenitor cells and collateral formation by modifying vasculo-angiogenic biomarkers in peripheral arterial disease. Int J Cardiol. 2014;172:e371–e374. doi: 10.1016/j.ijcard.2013.12.295. [DOI] [PubMed] [Google Scholar]
- 20.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1598. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Döring Y, Drechsler M, Soehnlein O, Weber C. Neutrophils in atherosclerosis: from mice to man. Arterioscler Thromb Vasc Biol. 2015;35:288–295. doi: 10.1161/ATVBAHA.114.303564. [DOI] [PubMed] [Google Scholar]
- 22.Drechsler M, Megens RT, van Zandvoort M, et al. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122:1837–1845. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- 23.Drechsler M, Doring Y, Megens RT, Soehnlein O. Neutrophilic granulocytes-promiscuous accelerators of atherosclerosis. Thromb Haemost. 2011;106:839–848. doi: 10.1160/TH11-07-0501. [DOI] [PubMed] [Google Scholar]
- 24.Tsiantoulas D, Sage AP, Mallat Z, Binder CJ. Targeting B cells in atherosclerosis: closing the gap from bench to bedside. Arterioscler Thromb Vasc Biol. 2015;35:296–302. doi: 10.1161/ATVBAHA.114.303569. [DOI] [PubMed] [Google Scholar]
- 25.Nunez J, Minana G, Bodi V, et al. Low lymphocyte count and cardiovascular diseases. Curr Med Chem. 2011;18:3226–3233. doi: 10.2174/092986711796391633. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Mohler ER, 3rd, Xie D, et al. Risk factors for peripheral arterial disease among patients with chronic kidney disease. Am J Cardiol. 2012;110:136–141. doi: 10.1016/j.amjcard.2012.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borissoff JI, Spronk HM, ten Cate H. The hemostatic system as a modulator of atherosclerosis. N Engl J Med. 2011;364:1746–1760. doi: 10.1056/NEJMra1011670. [DOI] [PubMed] [Google Scholar]
- 28.Peev V, Nayer A, Contreras G. Dyslipidemia, malnutrition, inflammation, cardiovascular disease and mortality in chronic kidney disease. Curr Opin Lipidol. 2014;25:54–60. doi: 10.1097/MOL.0000000000000045. [DOI] [PubMed] [Google Scholar]