Abstract
Background
We aimed to describe the frequency of vascular inflammatory reactions with second generation drug eluting stents (DES) compared to first generation DES, and analyze the impact on inflammation and neointimal proliferation in a porcine coronary model.
Methods
A total of 26 stents (7 multi-link VISION, 6 CYPHER, 6 TAXUS and 7 XIENCE V) were deployed in the coronary arteries of 10 domestic swine for 28 days, after which each stent was harvested and processed (divided into 8 or 9 segments) for histomorphometric analysis.
Results
A total of 202 histological segments [146 DES and 56 bare metal stents (BMS)] were included in this study. The mean neointimal thickness was significantly reduced in the DES group compared to the BMS group. The DES group had higher injury scores (DES = 0.99 ± 0.79 versus BMS = 0.67 ± 0.44, p < 0.004), inflammatory scores (DES = 2.09 ± 1.54 versus BMS = 0.64 ± 0.98, p < 0.001) and presence of para-strut granulomas (DES = 35% versus BMS = 2%, p < 0.001). In logistic regression analysis, the presence of para-strut granulomas correlated with an area of stenosis > 50% (RR: 6.11, 95% CI: 2.97 to 12.59, p = 0.001). In the DES group, the second generation stents had a lower neointimal area (XIENCE V: 1.64 ± 0.90 mm2) compared to the first generation stents (TAXUS: 2.36 ± 1.56 mm2, p = 0.005; CYPHER 2.78 ± 1.82 mm2, p = 0.001). The XIENCE V stents had lower inflammatory scores and lower frequency of para-strut granulomas compared to the first generation stents.
Conclusions
Second generation DES had a lower incidence of vascular inflammatory reactions compared to first generation DES. This biological phenomenon appears to influence the patterns of neointimal formation.
Keywords: Drug eluting stent, Porcine coronary artery, Vascular inflammation reaction
INTRODUCTION
Coronary artery disease is one of the leading causes of death in developed countries. The introduction of drug-eluting stents (DES) was an important milestone in treating coronary artery disease because of its ability to significantly reduce neointimal proliferation and binary restenosis after coronary angioplasty.1 However, DES have been associated with stent thrombosis and late restenosis, which remain important clinical issues in current treatment and therapies.2 Early experimental and autopsy data have described vascular inflammation reactions (VIRs) with the use of first generation DES.3 Para-strut granulomas and adventitial inflammation, which may be induced by stent polymer hypersensitivity or drug toxicity, have been reported after DES implantation in swine coronary models,4 and inflammatory reactions have been reported to be one of the main reasons for vascular thrombogenesis and delayed healing following DES treatment.5 The biological significance of these findings is unclear due to limited histological data regarding second generation DES, aside from studies reporting that second generation DES provide better safety and efficacy than first generation DES.6,7 Therefore, we performed this randomized controlled study to investigate the frequency of VIRs with the use of second generation DES compared to first generation DES and bare metal stent (BMS), and to analyze the impact on inflammation and neointimal proliferation in a porcine coronary model.
METHODS
Experimental design
Ten purpose-bred Yorkshire swine, 6-7 months of age and weighing 25 to 35 kg were enrolled in the present study. All coronary arteries were randomized for a single stent implantation [multi-link VISION (3.0*12 mm, Abbott Vascular, Santa Clara, CA, USA), XIENCE V (3.0*12 mm, Abbott Vascular), CYPHER (3.0*12 mm, Cordis, Johnson & Johnson) and TAXUS-Liberte (3.0*12 mm, Boston Scientific, Natick, MA, USA)] in each animal. All animals were followed for 28 days post stent implantation. The study protocol was approved by the local institutional animal care and use committee. All animals received humane care in compliance with the Animal Welfare Act and the “Principles of Laboratory Animal Care” formulated by the Institute of Laboratory Animal Resources (National Research Council, NIH Publication No. 85-23, revised 1996).
Procedural description
The animals were pre-anesthetized with an appropriate mixture of pre-anesthetic medication, and dosed per kg of body weight. These drugs included: glycopyrrolate 0.004-0.01 mg/kg, Tiletamine 3-5 mg/kg, and Xylazine 1-2 mg/kg. The injections were given intramuscularly in either the neck or rear muscle quadrant by a qualified animal technologist. When an adequate anesthetic plane was reached, the animals were intubated and isoflurane (1-2%) was administered through a precision vaporizer and a circle absorption breathing system with periodic arterial blood gas monitoring. Monitoring of the animal’s vital signs (heart rate, respiration rate, O2 pulse oximeter, and blood pressure) was performed continuously and recorded at approximately 15-minute intervals. Upon proper depth of anesthesia being reached, the femoral site or neck was prepared with povidone iodine followed by 70% ethanol. A vascular access sheath (7Fr) was placed in the carotid artery by cut-down with a general sterile technique. Before catheterization, heparin (5,000-10,000 U) was injected to maintain an activated clotting time of 250-300 seconds. For each stent deployment, an arterial segment was chosen so that the resulting stent-to-initial artery dimension ratio was ≥ 1.1. The quantitative coronary angiographic (QCA) package was used for these measurements. After allocation of the vessel to an experimental group, the appropriate stent was delivered to the intended site over a guide wire using fluoroscopic guidance. If vascular spasm occurred, 100 μg nitroglycerine was injected. The sheaths were removed when the vital signs became stable, which occurred approximately 30 minutes following stent implantation. Hemostasis was obtained by manual pressure. The artery was ligated with 2-0 silk suture and the incision site closed in 2-3 layers with appropriate suture material. After confirmation of hemostasis, isoflurane was discontinued and the animal was extubated when the gag reflex had returned. Buprenorphine 0.01-0.02 mg/kg IM and flunixin 1-2 mg/kg IV were injected for routine pain management. All animals received aspirin (81 mg) and clopidogrel (75 mg) daily and remained on a normal chow diet during the 28-day follow-up period. At the end of the follow-up period, the animals were euthanized and whole hearts were harvested.
Histological methods
Light Microscopy
An experienced pathologist who was blinded to the groups performed all histomorphometric and histological analyses. For light microscopy, the implanted vessel segments were fixed in 10% formalin, dehydrated in a graded series of ethanol and embedded in methyl methacrylate resin. After polymerization, 2- to 3-mm sections were sawed of each single stent (for a total of 8-9 sections). Sections from the stents were cut on a rotary microtome at 4 to 6 microns, mounted, and stained with hematoxylin and eosin. Segments of the native coronary arteries proximal and distal to the stents were embedded in paraffin. Sections of the vessels were cut on a rotary microtome at 4 to 5 microns, mounted, and stained with hematoxylin-eosin and Movat’s pentachrome. All of the sections were examined by light microscopy for the presence of inflammation, thrombi, neointimal formation, and vessel wall injury (Figure 1).
Figure 1.
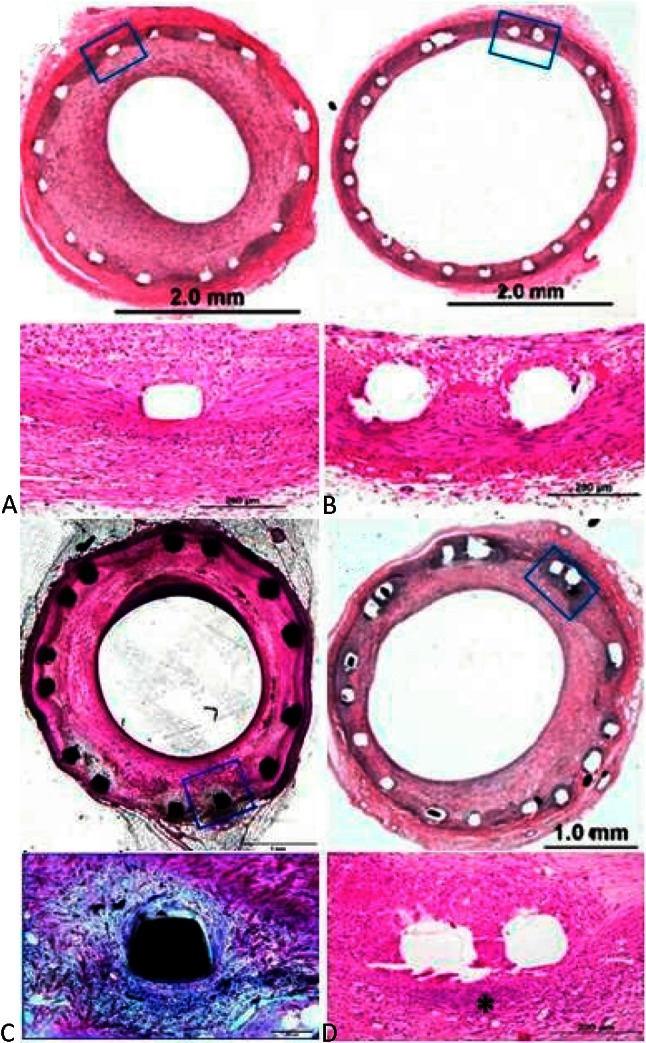
Histological samples of stents. (A) Multi-link VISION stent, (B) XIENCE V stent, (C) CYPHER stent, (D) TAXUS stent. * Granuloma tissue.
Morphometry
A vessel injury score was calculated according to the Schwartz method.8 Quantitative vessel injury severity and neointimal response were derived from the elastic van Gieson-stained sections as follows. Vessel injury at every wire site was determined by the anatomic vessel structures penetrated by each wire. A numeric value from 0 (least injury) to 3 (most injury) was assigned according to injury severity (Table 1). An arterial injury score for each stenotic segment was calculated as the mean injury caused by all coil wires in that segment. The pathologist defined the anatomic and histopathologic basis of this score prospectively, and the scores were calculated as:
Table 1. Definition of vessel injury score and inflammation score.
| Score | Description of Injury |
| 0 | Internal elastic lamina intact; endothelium typically denuded; media compressed but not lacerated |
| 1 | Internal elastic lamina lacerated; media typically compressed but not lacerated |
| 2 | Internal elastic lacerated; media visibly lacerated; external elastic lamina intact but compressed |
| 3 | External elastic lamina lacerated; typically large lacerations of media extending through the external elastic lamina; coil wires sometimes residing in adventitia |
| Score | Description of Inflammation |
| 0 | < 25% struts with fewer than 10 inflammatory cells |
| 1 | Up to 25% struts with more than 10 inflammatory cells |
| 2 | 25-50% struts with more than 10 inflammatory cells |
| 3 | > 50% struts with more than 10 inflammatory cells |
Mean injury score = Sum of the weight for each wire/number of coil wires present
Cross-sectional areas [external elastic lamina (EEL), internal elastic lamina (IEL), and lumen area] of each section were measured with a digital morphometer. Neointimal thickness was measured as the distance from the inner surface of each stent strut to the luminal border. Area measurements were used to calculate vessel layer areas as follows: Media = EEL – IEL; Neointima = IEL – Lumen; % Stenosis = [1 – (Lumen Area / IEL Area) * 100]. Overall neointimal inflammation was scored for each section from 0 to 3 (as defined in Table 1).9 Granulomatous inflammation was defined as localized collections of macrophages, giant cells, lymphocytes, and granulocytes with or without numerous (> 10 cells per ×40 objective field) eosinophils.10 Vessel sections showing two or more struts with granulomatous inflammation were identified as para-strut granulomas. Endothelial coverage was semi-quantified and expressed as the percentage of the lumen circumference covered by the endothelium.
Statistical analysis
Continuous variables were expressed as mean ± SD. The Student’s t test was used to compare continuous variables, and the Mann-Whitney U test was used for skewed distributions. Categorical variables were expressed as both number and percentage, and compared using the chi-square test or Fisher’s exact test. We used binary logistic regression analysis with 95% confidence intervals to determine the predictive role of para-strut granulomas for > 50% area stenosis at 28 days. Data were analyzed with SPSS software version 16.0 for Windows (SPSS, Chicago, Illinois, USA). A p value of less than 0.05 was considered to be statistically significant.
RESULTS
The procedures were completed without any complications, and all of the study swine survived until the end of the follow-up period. A total of 202 histological segments (146 DES and 56 BMS) were included in the final analysis. None of the segments had stent strut fractures. The mean neointimal thickness was significantly reduced in the DES group (0.22 ± 0.22 mm) compared to the BMS group (0.32 ± 0.25 mm, p < 0.01). The DES group had higher injury scores (DES = 0.99 ± 0.79 versus BMS = 0.67 ± 0.44, p < 0.004), inflammatory scores (DES = 2.09 ± 1.54 versus BMS = 0.64 ± 0.98, p < 0.001) and the presence of para-strut granulomas (DES = 35% versus BMS = 2%, p < 0.001) compared to the BMS group (Table 2). Table 3 demonstrates the histomorphometric analysis of segments with and without granuloma in the DES group at 28 days. Para-strut granulomas were associated with a higher vascular injury score, more neointimal hyperplasia and poorer endothelialization. In logistic regression, the presence of para-strut granulomas correlated with an area of stenosis > 50% (relative risk 6.11, 95% confidence interval: 2.97 to 12.59, p = 0.001). In the DES group, the XIENCE V stent had a lower inflammatory score (1.65 ± 1.51) compared to the TAXUS (2.57 ± 1.44, p = 0.003) and CYPHER (2.06 ± 1.47, p = 0.02) stents. In addition, the XIENCE V stent had a lower frequency of para-strut granulomas (25%) compared to the TAXUS (47%, p = 0.001) and CYPHER (32%, p = 0.07) stents (Table 4). The XIENCE V stent also had the lowest neointimal area (1.64 ± 0.90 mm2) compared to the TAXUS (2.36 ± 1.56 mm2, p = 0.005) and CYPHER (2.78 ± 1.82 mm2, p = 0.001) stents. Figure 2 illustrates the mean neointimal areas and mean injury scores of different stent segments with para-strut granulomas. We noted that within the same injury score (Figure 2A), XIENCE V stents still had a lower neointimal area (2.79 ± 0.63 mm2) compared with TAXUS (3.27 ± 1.85 mm2, p = 0.344) and CYPHER (4.77 ± 1.29 mm2, p = 0.001) stents (Figure 2B) in the segments with granuloma formation.
Table 2. Analysis of drug-eluting stents (XIENCE V; TAXUS; CYPHER) compared to a bare metal stent (VISION).
| BMS (n = 56) | DES (n = 146) | p value | |
| Angiography | |||
| Baseline reference mean diameter (mm) | 2.75 ± 0.19 | 2.79 ± 0.18 | 0.118 |
| Balloon size (mm) | 3.17 ± 0.07 | 3.22 ± 0.18 | 0.732 |
| Balloon: artery ratio | 1.16 ± 0.09 | 1.15 ± 0.09 | 0.923 |
| Stent: artery ratio | 1.13 ± 0.03 | 1.12 ± 0.04 | 0.065 |
| Histology* | |||
| Endothelialization (%) | 99.11 ± 1.99 | 96.84 ± 6.60 | 0.012 |
| IEL (mm2) | 6.18 ± 0.68 | 6.97 ± 0.95 | 0.001 |
| Lumen area (mm2) | 3.86 ± 1.72 | 4.73 ± 1.58 | 0.001 |
| Neointima area (mm2) | 2.32 ± 1.26 | 1.98 ± 1.30 | 0.117 |
| Mean neointimal thickness (mm) | 0.32 ± 0.25 | 0.22 ± 0.22 | 0.011 |
| Area stenosis (%) | 39.10 ± 23.37 | 29.88 ± 19.32 | 0.009 |
| Injury score | 0.67 ± 0.44 | 0.99 ± 0.79 | 0.004 |
| Inflammation score | 0.64 ± 0.98 | 2.09 ± 1.54 | < 0.001 |
| Number of slides with the presence of para-strut granuloma formation | 3 (2%) | 50 (34%) | < 0.001 |
BMS, bare metal stent; DES, drug-eluting stent; IEL, internal elastic lamina.
* 28 days of follow-up.
Table 3. Histomorphometric analysis of drug-eluting stents with and without granulomas at 28 days of follow-up.
| DES | p value | ||
| (-) Granuloma (n = 96) | (+) Granuloma (n = 50) | ||
| IEL (mm2) | 6.75 ± 0.95 | 7.11 ± 1.01 | 0.188 |
| Lumen area | 5.37 ± 1.23 | 3.52 ± 1.48 | < 0.001 |
| Neointima area (mm2) | 1.53 ± 0.83 | 3.60 ± 1.64 | < 0.001 |
| Mean neointimal thickness (mm) | 0.13 ± 0.12 | 0.44 ± 0.25 | < 0.001 |
| Endothelialization (%) | 98.15 ± 3.58 | 94.32 ± 9.73 | 0.001 |
| Area stenosis (%) | 22.69 ± 12.09 | 50.19 ± 20.21 | < 0.001 |
| Injury score | 0.51 ± 0.37 | 1.93 ± 0.53 | < 0.001 |
Abbreviation in Table 2.
Table 4. Comparison of histological characteristics between different drug-eluting stents.
| Everolimus eluting stent | Paclitaxel eluting stent | Sirolimus eluting stent | |
| XIENCE V (n = 52) | TAXUS (n = 47) | CYPHER (n = 47) | |
| Histology† | |||
| IEL (mm2) | 6.25 ± 0.78 | 7.29 ± 0.74* | 7.45 ± 0.85* |
| Lumen area (mm2) | 4.61 ± 1.44 | 4.93 ± 1.70 | 4.67 ± 1.63 |
| Neointima area (mm2) | 1.64 ± 0.90 | 2.36 ± 1.56* | 2.78 ± 1.82* |
| Mean neointimal thickness (mm) | 0.22 ± 0.20 | 0.21 ± 0.24 | 0.28 ± 0.24 |
| Area stenosis (%) | 27.41 ± 16.40 | 32.62 ± 21.96* | 36.79 ± 21.20* |
| Endothelialization (%) | 97.90 ± 3.54 | 92.81 ± 9.76* | 98.3 ± 4.18 |
| Inflammation score | 1.65 ± 1.51 | 2.57 ± 1.44* | 2.06 ± 1.47* |
| Injury score | 0.91 ± 0.79 | 1.10 ± 0.78 | 0.99 ± 1.51 |
| Para-strut granuloma | 13 (25%) | 22 (47%)* | 15 (32%) |
Abbreviation in Table 2.
* p < 0.05 compared with XIENCE V stent. † 28 days of follow-up.
Figure 2.
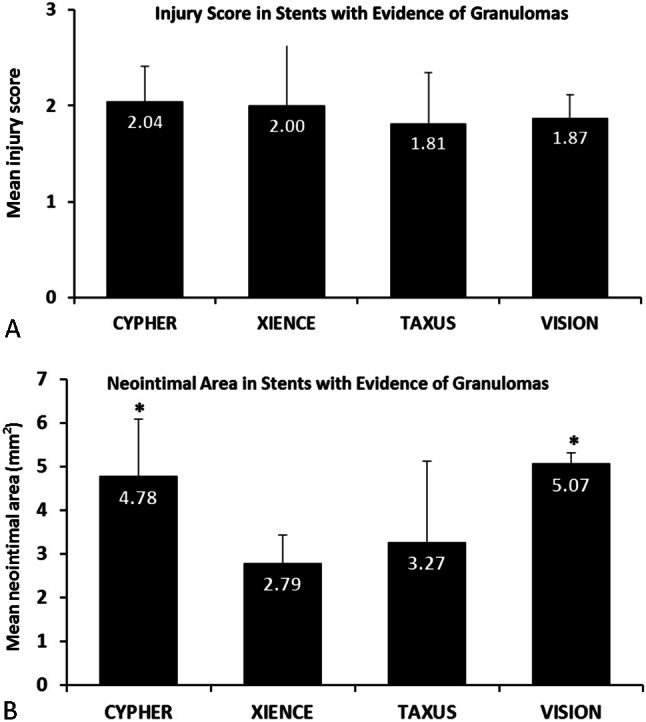
(A) Comparison of mean injury score in stent segments with granulomas. (B) Comparison of neointimal areas in stent segments with granulomas. * p < 0.05 compared with the XIENCE V stent.
DISCUSSION
In this study, we evaluated VIRs between BMS and two generations of DES, and the impact the VIRs had on vascular healing and neointimal response after stent implantation in porcine coronary arteries. The major findings of this study are as follows: 1) Compared with cobalt chromium-based BMS, inflammatory reactions of the arterial wall were observed in both the first and second generation DES; 2) VIRs which appeared as para-strut granulomas appeared to predict in-stent restenosis in the DES at 28 days of follow-up; 3) Compared with the first generation DES (CYPHER and TAXUS stent), the second generation everolimus-eluting DES (XIENCE V stent) displayed favorable histomorphometric results. Although DES have dramatically reduced the rate of restenosis and target lesion revascularization compared with BMS, experimental and autopsy studies have reported a higher number of VIRs with the first generation DES.10,11 VIRs, characterized by peri-strut inflammation and cell infiltration, can be caused by the stent platform material, anti-proliferative drugs, or coating polymer, and may contribute to stent thrombosis and a delay in healing; however, the biological impact of these reactions remains unclear.12 In the current study, we found a higher inflammation score and frequency of para-strut granulomas in the DES than in cobalt chromium BMS within the same mechanical overstretch, which implies that the stent coating polymer may be an important precursor to a VIR. We also noted that the DES with para-strut granulomas had even higher neointimal proliferation and in-stent restenosis than both the DES without para-strut granulomas and the BMS group, which is comparable with previous studies on BMS.13,14 This may be explained by the presence of granulomas, a hypersensitivity reaction induced by foreign body reactions from metal particles, drugs, or polymers.15 These findings may provide a clearer picture about the role of VIRs in the pathophysiology of stent thrombosis, in-stent restenosis and neoatherosclerosis in both DES and BMS.
Second generation DES have been shown to be safe and efficient compared with first generation DES.16-18 However, only a few studies have reported histomorphometric data of these widely used stents.19,20 Our study provides a comparative analysis of VIRs with polymeric first and second generation DES. A possible explanation for the everolimus-eluting stent outperforming the first generation DES in reducing VIRs is the material used for the stent struts. The first generation DES use a 316L stainless strut containing nickel, chromium, manganese, titanium, and molybdenum, whereas the second-generation everolimus-eluting stents avoid nickel and use a cobalt-chromium strut. The prevalence of hypersensitivity to nickel is about 20%, compared with only 4% and 7% for cobalt and chromium, respectively.21 Another possible reason may be the polymer coating. A layer of everolimus-polymer matrix with a thickness of 5-6 microns is applied to the surface of the stent which is then loaded with 100 μg of everolimus per cm2 of stent surface area with no top coat polymer layer. The permanent durable polymer coating thickness of the XIENCE V (5.3 μm) stent is thinner than the CYPHER (7.2 μm) or TAXUS (15.6 μm) stent,22 and it has previously been reported that the stent polymer is an important potential antigen for chronic inflammation in DES.23 Finally, as observed in preclinical animal models, the low dose of everolimus elution may prompt more rapid and complete stent re-endothelialization.24,25
Second generation DES such as everolimus-eluting stents have a low rate of stent thrombosis, as reported in several large prospective randomized trials.26-29 The current study provides histological evidence of the advantages of second generation everolimus-eluting stents. The combination of thin, facture-resistant struts, low doses of everolimus, and the thromboresistant, non-inflammatory proprieties of a fluorinated polymer may contribute to the lower rates of early stent thrombosis with everolimus-eluting stents.24,30
Limitations
This study had some limitations. First, as with all preclinical studies, there is a lack of any direct correlation of the findings between animal models and human clinical trials.31 There is some controversy with regards to the comparability between human and animal studies on DES.32 One of differences is the temporal response to healing in swine that is substantially prolonged in humans. Therefore, the 28-day results in our study may correspond to a reasonable approximation of 6 months in humans.31 Another difference is that coronary arteries are normal and healthy in a porcine model, in contrast to human subjects whose coronary arteries are tortuous with atherosclerotic changes. However, animal models provide an ideal controlled environment and a reliable platform on which to predict the safety and efficacy of emerging endovascular therapies in humans, as the stages of healing and anatomical features of swine have been found to be remarkably similar to those of humans.33 Second, the sample size was small, and we could only assess vascular injuries, inflammation and para-strut granulomas by semi-quantification methods. Nonetheless, our results still exhibited consistent histological findings that should allow for a meaningful conclusion.
CONCLUSIONS
In the current study, we demonstrated the presence of vascular hypersensitivity and the influence on vascular healing in DES. In addition, second generation DES (everolimus-eluting stent) had a lower incidence of VIRs (para-strut granulomas) compared to first generation DES. This biological phenomenon appears to influence the patterns of neointimal formation and vascular healing. Further studies are warrantied to investigate the impact of VIRs in the pathogenesis of DES thrombosis and restenosis in humans.
Acknowledgments
105swf06 Wan Fang hospital Taipei Medical University.
REFERENCES
- 1.Stone GW, Ellis SG, Cannon L, et al. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA. 2005;294:1215–1223. doi: 10.1001/jama.294.10.1215. [DOI] [PubMed] [Google Scholar]
- 2.Moreno R, Fernández C, Hernández R, et al. Drug-eluting stent thrombosis: results from a pooled analysis including 10 randomized studies. J Am Coll Cardiol. 2005;45:954–959. doi: 10.1016/j.jacc.2004.11.065. [DOI] [PubMed] [Google Scholar]
- 3.Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation. 2007;115:1440–1455. doi: 10.1161/CIRCULATIONAHA.106.666800. [DOI] [PubMed] [Google Scholar]
- 4.Wilson GJ, Nakazawa G, Schwartz RS, et al. Comparison of inflammatory response after implantation of sirolimus- and paclitaxel-eluting stents in porcine coronary arteries. Circulation. 2009;120:141–149. doi: 10.1161/CIRCULATIONAHA.107.730010. [DOI] [PubMed] [Google Scholar]
- 5.Inoue T, Croce K, Morooka T, et al. Vascular inflammation and repair: implications for re-endothelialization, restenosis, and stent thrombosis. JACC Cardiovasc Interv. 2011;4:1057–1066. doi: 10.1016/j.jcin.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson GJ, Huibregtse BA, Pennington DE, Dawkins KD. Comparison of the SYNERGY with the PROMUS (XIENCE V) and bare metal and polymer-only Element control stents in porcine coronary arteries. EuroIntervention. 2012;8:250–257. doi: 10.4244/EIJV8I2A39. [DOI] [PubMed] [Google Scholar]
- 7.Lowe HC, Schwartz RS, Mac Neill BD, et al. The porcine coronary model of in-stent restenosis: current status in the era of drug-eluting stents. Catheter Cardiovasc Interv. 2003;60:515–523. doi: 10.1002/ccd.10705. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol. 1992;19:267–274. doi: 10.1016/0735-1097(92)90476-4. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz RS, Edelman ER, Carter A, et al. Preclinical evaluation of drug-eluting stents for peripheral applications: recommendations from an expert consensus group. Circulation. 2004;110:2498–2505. doi: 10.1161/01.CIR.0000145164.85178.2E. [DOI] [PubMed] [Google Scholar]
- 10.Wilson GJ, Nakazawa G, Schwartz RS, et al. Comparison of inflammatory response after implantation of sirolimus- and paclitaxel-eluting stents in porcine coronary arteries. Circulation. 2009;120:141–149. doi: 10.1161/CIRCULATIONAHA.107.730010. [DOI] [PubMed] [Google Scholar]
- 11.Virmani R, Guagliumi G, Farb A, et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation. 2004;109:701–705. doi: 10.1161/01.CIR.0000116202.41966.D4. [DOI] [PubMed] [Google Scholar]
- 12.Nebeker JR, Virmani R, Bennett CL, et al. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J Am Coll Cardiol. 2006;47:175–181. doi: 10.1016/j.jacc.2005.07.071. [DOI] [PubMed] [Google Scholar]
- 13.Kornowski R, Hong MK, Virmani R, et al. Granulomatous ‘foreign body reactions’ contribute to exaggerated in-stent “restenosis.”. Coron Artery Dis. 1999;10:9–14. doi: 10.1097/00019501-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Yutani C, Ishibashi-Ueda H, Suzuki T, Kojima A. Histologic evidence of foreign body granulation tissue and de novo lesions in patients with coronary stent restenosis. Cardiology. 1999;92:171–177. doi: 10.1159/000006967. [DOI] [PubMed] [Google Scholar]
- 15.Kornowski R, Hong MK, Tio FO, et al. In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol. 1998;31:224–230. doi: 10.1016/s0735-1097(97)00450-6. [DOI] [PubMed] [Google Scholar]
- 16.Sarno G, Lagerqvist B, Fröbert O, et al. Lower risk of stent thrombosis and restenosis with unrestricted use of ‘new-generation’ drug-eluting stents: a report from the nationwide Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Eur Heart J. 2012;33:606–613. doi: 10.1093/eurheartj/ehr479. [DOI] [PubMed] [Google Scholar]
- 17.Garg S, Serruys P, Onuma Y, et al. 3-year clinical follow-up of the XIENCE V everolimus-eluting coronary stent system in the treatment of patients with de novo coronary artery lesions: the SPIRIT II trial (Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients with de novo Native Coronary Artery Lesions). JACC Cardiovasc Interv. 2009;2:1190–1198. doi: 10.1016/j.jcin.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Kedhi E, Joesoef KS, McFadden E, et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet. 2010;375:201–209. doi: 10.1016/S0140-6736(09)62127-9. [DOI] [PubMed] [Google Scholar]
- 19.Joner M, Nakazawa G, Finn AV, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52:333–342. doi: 10.1016/j.jacc.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 20.Carter AJ, Brodeur A, Collingwood R, et al. Experimental efficacy of an everolimus eluting cobalt chromium stent. Catheter Cardiovasc Interv. 2006;68:97–103. doi: 10.1002/ccd.20769. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen SH, Dang TP, Macpherson C, et al. Prevalence of patch test results from 1970 to 2002 in a multi-centre population in North America (NACDG). Contact Dermatitis. 2008;58:101–106. doi: 10.1111/j.1600-0536.2007.01281.x. [DOI] [PubMed] [Google Scholar]
- 22.Sheiban I, Villata G, Bollati M, et al. Next-generation drug-eluting stents in coronary artery disease: focus on everolimus-eluting stent (Xience V). Vasc Health Risk Manag. 2008;4:31–38. doi: 10.2147/vhrm.2008.04.01.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen JP, Hou D, Pendyala L, et al. Drug-eluting stent thrombosis: the Kounis hypersensitivity-associated acute coronary syndrome revisited. JACC Cardiovasc Interv. 2009;2:583–593. doi: 10.1016/j.jcin.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Joner M, Nakazawa G, Finn AV, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52:333–342. doi: 10.1016/j.jacc.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 25.Nakazawa G, Shinke T, Ijichi T, et al. Optical coherence tomography and histopathology assessment after implantation of first- and second-generation drug-eluting stents in a porcine coronary model. Circ J. 2014;78:2665–2673. doi: 10.1253/circj.cj-14-0315. [DOI] [PubMed] [Google Scholar]
- 26.Valgimigli M, Tebaldi M, Borghesi M, et al. Two-year outcomes after first- or second-generation drug-eluting or bare-metal stent implantation in all-comer patients undergoing percutaneous coronary intervention: a pre-specified analysis from the PRODIGY study. JACC Cardiovasc Interv. 2014;7:20–28. doi: 10.1016/j.jcin.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Smits PC, Vlachojannis GJ, McFadden EP, et al. Final 5-year follow-up of a randomized controlled trial of everolimus- and paclitaxel-eluting stents for coronary revascularization in daily practice: the COMPARE trial. JACC Cardiovasc Interv. 2015;doi: 10.1016/j.jcin.2015.03.028 doi: 10.1016/j.jcin.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 28.Otsuka F, Vorpahl M, Nakano M, et al. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation. 2014;129:211–223. doi: 10.1161/CIRCULATIONAHA.113.001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kedhi E, Gomes ME, Lagerqvist B, et al. Clinical impact of second-generation everolimus-eluting stent compared with first-generation drug-eluting stents in diabetes mellitus patients: insights from a nationwide coronary intervention register. JACC Cardiovasc Interv. 2012;5:1141–1149. doi: 10.1016/j.jcin.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 30.Kiaei D, Hoffman AS, Horbett TA. Tight binding of albumin to glow discharge treated polymers. J Biomater Sci Polym Ed. 1992;4:35–44. [PubMed] [Google Scholar]
- 31.Virmani R, Kolodgie FD, Farb A, Lafont A. Drug eluting stents: are human and animal studies comparable? Heart. 2003;89:133–138. doi: 10.1136/heart.89.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teirstein PS. Living the dream of no restenosis. Circulation. 2001;104:1996–1998. [PubMed] [Google Scholar]
- 33.Schwartz RS, Edelman ER, Carter A, et al. Drug-eluting stents in preclinical studies: recommended evaluation from a consensus group. Circulation. 2002;106:1867–1873. doi: 10.1161/01.cir.0000033485.20594.6f. [DOI] [PubMed] [Google Scholar]


