Abstract
Background
This study aimed to investigate the impact of atorvastatin (Ato) combined with ezetimibe (Eze) for the treatment of carotid atherosclerosis in patients with coronary heart disease (CHD).
Methods
One hundred and forty-eight CHD patients with carotid atherosclerosis were divided into the control (Ato alone) and combination (Ato and Eze) groups. The treatment course was 12 months; patient blood lipids, carotid intima-media thickness (CIMT), and carotid plaque area were measured before and after treatment.
Results
Twelve months after treatment, there was a decrease in the CIMT, and the horizontal and vertical axes of the carotid plaque areas in both groups, compared to pretreatment values. The serum low-density lipoprotein cholesterol (LDL-C) levels were significantly decreased (p < 0.05). There were statistically significant differences (p < 0.05) in the LDL-C (2.12 ± 0.58 mmol/L vs. 2.63 ± 0.56 mmol/L) and CIMT (1.06 ± 0.12 mm vs. 1.13 ± 0.11 mm) levels between the combination and the control groups after treatment. Compared to the control group, the horizontal (0.18 ± 0.06 cm2 vs. 0.19 ± 0.05 cm2) and vertical carotid arterial plaque areas (0.40 ± 0.15 cm2 vs. 0.41 ± 0.17 cm2) of the combination group were reduced after treatment. However, the difference was not statistically significant (p > 0.05).
Conclusions
The combination of Ato and Eze further reduces LDL-C levels and CIMT, and affect the progression of carotid atherosclerosis in CHD patients with hypercholesterolemia.
Keywords: Atorvastatin, Carotid atherosclerosis, Ezetimibe
INTRODUCTION
Dyslipidemia is one of the most important risk factors for cardiovascular disease.1 In patients with high or very high risk of coronary heart disease (CHD) and similar critical conditions, low-density lipoprotein cholesterol (LDL-C) should be reduced by at least 30-50% in order to obtain a clinical benefit.2 However, it is difficult to achieve these target levels with the administration of a statin alone.3 Recent clinical studies showed that the combination of statins with a cholesterol absorption inhibitor (ezetimibe) significantly decreased LDL-C levels in patients with hypercholesterolemia, and resulted in superior lipid-lowering efficacy compared to treatment with a statin alone.4,5
The presence of a plaque is essential for predicting the occurrence of atherosclerotic (AS) events. Only effective interventions can prevent their development.6 Clinical studies show that statins can alter plaque phenotypes and reduce their volume,7 as well as reduce the incidence of adverse cardiovascular events.8 The reported anti-atherosclerotic effects of ezetimibe (Eze) have remained controversial.9,10 Whether the combination of statins and Eze could significantly reduce LDL-C levels, and delay or reverse the atherosclerotic process, remains inconclusive. Accordingly, this study administered a combination of atorvastatin (Ato) and Eze, as well as Ato alone, to CHD patients with hypercholesterolemia, in order to determine the impact of these medications on blood lipids and carotid atherosclerosis-related indicators, as well as to determine whether reducing the LDL-C to lower levels and with wider amplitude could further reduce carotid atherosclerosis.
METHODS
Subjects
One hundred forty-eight patients (88 men and 60 women; mean age of 61.23 ± 12.64 years) with CHD, which was confirmed by coronary angiography that was performed in our department from June 2012 to September 2013, were selected for this study. Among these patients, 64 were diagnosed with type 2 diabetes according to the World Health Organization’s criteria for diagnosis and classification (1999). Patients with blood diseases, hepatonephric dysfunction, severe infectious diseases, and heart failure were excluded from the study. Although patients received lipid-lowering therapy for 3 months before enrollment, they did not achieve the standard goals for LDL-C levels (≤ 2.6 mmol·L-1). These patients were divided into the control and combination groups by the random number table method. The groups were comparable in age, sex, body mass index, blood pressure level, smoking status, and other aspects (Table 1). This study was conducted in accordance with the declaration of Helsinki and with approval from the Ethics Committee of Henan Provincial People’s Hospital. A written informed consent was obtained from each participant.
Table 1. General information of the two groups (x̄ ± s).
| Item | Control group (n = 74) | Combination group (n=74) | p value |
| Gender (M/F, cases) | 44/30 | 40/34 | 0.62 |
| Age (years) | 61.55 ± 9.72 | 60.76 ± 11.56 | 0.65 |
| Body mass index | 24.68 ± 5.42 | 25.23 ± 4.67 | 0.51 |
| Severity of the coronary heart disease | |||
| 1-vessel coronary heart disease | 37 | 35 | 0.74 |
| 2-vessel coronary heart disease | 16 | 17 | 0.84 |
| 3-vessel coronary heart disease | 21 | 22 | 0.86 |
| Coronary revascularisation | |||
| Percutaneous coronary intervention | 18 | 20 | 0.71 |
| Coronary artery bypass grafting | 6 | 7 | 0.77 |
| Disease course | |||
| Stroke (cases) | 12 | 14 | 0.67 |
| Hypertension (cases) | 38 | 36 | 0.74 |
| Diabetics (cases) | 30 | 34 | 0.51 |
| Smoking (cases) | 30 | 26 | 0.5 |
| Systolic pressure (mmHg) | 124.52 ± 12.40 | 126.52 ± 10.22 | 0.29 |
| Diastolic pressure (mmHg) | 72.31 ± 9.52 | 73.45 ± 10.62 | 0.49 |
| Glomerular filtration rate (ml/min) | 64.43 ± 18.22 | 68.43 ± 16.58 | 0.16 |
| Alanine aminotransferase (U/L) | 20.43 ± 14.25 | 22.48 ± 15.07 | 0.4 |
| Aspartate transaminase(U/L) | 31.21 ± 22.82 | 35.12 ± 24.56 | 0.32 |
| Hemoglobin A1C (%) | 6.13 ± 0.82 | 6.15 ± 0.78 | 0.88 |
| Left ventricular ejection fraction (%) | 55.43 ± 10.28 | 56.48 ± 11.09 | 0.55 |
1 mmHg = 0.133 kPa. M/F, man/female.
Drug treatment
As required by the Ethics Committee of the Henan Provincial People’s Hospital, all patients provided their signed informed consent. This study used a randomized, prospective, double-blind, and placebo-controlled design. The control group received oral Ato (Lipitor 20 mg, Pfizer, USA) every night, while the combination group received Eze (Ezetrol 10 mg, Schering-Plough, USA) in the morning and Ato in the evening. Both groups were followed for 12 months. Secondary prevention drugs, such as Bayer aspirin, β-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, and hypoglycemic drugs, to lower blood pressure and sugar levels were routinely administered to the two groups. The glycosylated hemoglobin was controlled at less than 7% in all patients.
Biochemical tests
Blood samples for each patient were collected at two time points (before and after 12 months of treatment). The hematologic, liver function, kidney function, blood lipid, myocardial enzyme, blood glucose, and glycosylated hemoglobin tests were all performed according to protocols for clinical specimen analysis. The blood tests were performed on a Hitachi 7600 automated biochemical analyzer (Hitachi, Tokyo, Japan).
Carotid ultrasonography
Patients underwent carotid ultrasonography before and after 12 months of treatment. The ALOKAα5 color Doppler ultrasound (ALOKA Company, Tokyo, Japan) was operated by an ultrasound specialist.
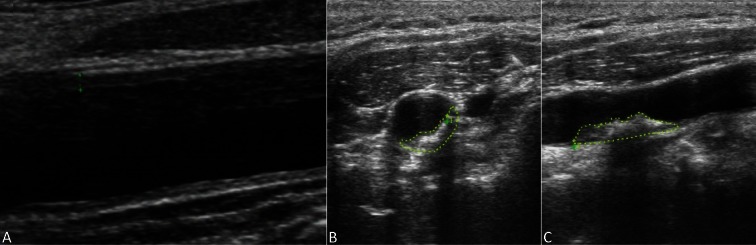
The carotid intima-media thickness (CIMT) at 1 cm below the bilateral carotid arterial bifurcations was measured at the end-diastolic phase three times in order to obtain an average value (Figure 1). The carotid plaque area was measured by detecting the bilateral carotid arteries and carotid arterial bifurcations over the proximal 15-mm-long segment. The atherosclerotic plaque was defined when CIMT ≥ 1.5 mm. All the plaques in these observations were measured using the ALOKAα 10 color Doppler ultrasound. The area integration method to measure the maximal area of horizontal and vertical axes (Figure 1) of the carotid arterial plaques was used for calculation.
Figure 1.
Measurement of CIMT and carotid arterial plaque areas. (A) Measurements of the CIMT. (B) Measurement of horizontal carotid arterial plaque areas. (C) Measurement of vertical carotid arterial plaque areas. CIMT, carotid intima-media thickness.
Major cardiovascular events
The follow-up period for this study lasted 12 months. Rates of major adverse coronary events, including cardiac death, hospitalization for unstable angina, nonfatal myocardial infarction, coronary revascularization, and stroke, were statistically evaluated. Coronary revascularization included coronary artery bypass surgery and percutaneous coronary intervention therapy.
Statistical analysis
We used the SPSS12.0 statistical software package for analysis. The measurement and counted data were expressed as x̄ ± s and a rate, respectively. We used the paired t-test for intragroup comparisons before and after treatment, while the analysis of variance was used for intergroup comparisons. The chi-square test was used for rate comparison, with a p < 0.05 considered as statistically significant. Means and standard deviations (SDs) for differences between observers were calculated. The inter- and intra-observer error rates (s) were calculated according to the formula s = SD/√2. The coefficient of variation (CV) describes the differences as a percentage of the pooled mean value x̄ and was calculated according to the formula: CV = s × 100%/x̄.
RESULTS
Blood lipid levels
The blood lipid levels of the two groups showed no significant differences before treatment. Twelve months after treatment, the serum total cholesterol (TC), LDL-C, and triglycerides (TG) were significantly reduced (p < 0.01) in both groups. The LDL-C levels in the two groups showed significant differences after treatment (p < 0.01). Twelve months after treatment, statistically significant (p < 0.01) increases in the HDL-C levels were observed in the two groups (Table 2).
Table 2. Changes of blood lipids of the 2 groups before and after the treatment (x̄ ± s).
| Control group (n = 74) | Combination group (n = 74) | |||
| Before | After | Before | After | |
| TC (mmol/l) | 5.98 ± 1.47 | 5.28 ± 1.46* | 5.88 ± 1.45 | 5.06 ± 1.48* |
| LDL-C (mmol/l) | 3.52 ± 0.46 | 2.63 ± 0.56* | 3.57 ± 0.38 | 2.12 ± 0.58*,# |
| HDL-C (mmol/l) | 1.19 ± 0.46 | 1.36 ± 0.44* | 1.17 ± 0.40 | 1.51 ± 0.22* |
| TG (mmol/l) | 2.56 ± 0.64 | 2.26 ± 0.64* | 2.48 ± 0.44 | 2.11 ± 0.48* |
* p < 0.01, compared with that before the treatment; # p < 0.01, compared with the Ato group after the treatment.
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride.
Carotid artery plaque
The intra- and inter-observer variability presented as a coefficient of variation (CV) of 4.72% and 4.49%, respectively. Before treatment, there were no significant differences between the two groups in the CIMT or horizontal and vertical axes of the carotid plaque area. However, the CIMT and horizontal and vertical carotid plaque areas were significantly reduced (p < 0.05; Table 3) 12 months after treatment with more significant differences observed in the CIMT (p < 0.01; Table 3). After treatment, the CIMT improvement in the combination group was much more significant (p < 0.01; Table 3) compared to those in the control group. Although the horizontal and vertical carotid arterial plaque areas were reduced in the combination group after treatment, the difference was not statistically significant (p > 0.05; Table 3).
Table 3. Comparison of carotid atherosclerosis-related indicators before and after the treatment (x̄ ± s).
| Control group (n = 74) | Combination group (n = 74) | |||
| Before | After | Before | After | |
| Plaque area (vertical axis, cm2) | 0.46 ± 0.15 | 0.41 ± 0.17# | 0.45 ± 0.18 | 0.40 ± 0.15# |
| Plaque area (horizontal axis, cm2) | 0.23 ± 0.07 | 0.19 ± 0.05# | 0.22 ± 0.08 | 0.18 ± 0.06# |
| CIMT (mm) | 1.26 ± 0.10 | 1.13 ± 0.11* | 1.27 ± 0.08 | 1.06 ± 0.12*,† |
* p < 0.01, # p < 0.05, compared with that before the treatment; † p < 0.01, compared with the Ato group after the treatment.
CIMT, carotid intima-media thickness.
Adverse reactions
Four patients in the control group had elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels over the 12-month follow-up period. However, the elevation was less than 3 times the upper limit of normal. Bicyclol was administered to these patients during therapy, and the transaminases returned to normal levels. Although 3 patients exhibited muscle weakness, there were no changes in their creatine kinase levels. None of the patients had abdominal distension or pain. In the combination group, 3 patients had elevated ALT and AST levels, which were less than 3 times the upper limit of normal. These patients were also given bicyclol, and the transaminases returned to normal levels. One patient had abdominal distention 4 days after administration, but felt no discomfort when the medication was continued. Although 3 patients exhibited muscle weakness, there were no changes in their creatine kinase levels. Neither group exhibited muscle pain, neurologic symptoms, or other clinical adverse reactions.
Comparison of cardiovascular events and safety
Neither group experienced a myocardial infarction or cardiovascular death over the 12-month follow-up period. In the control group, 4 patients were readmitted for angina, 3 of whom subsequently underwent percutaneous coronary stent implantation. In the combination group, 3 patients were readmitted for angina, 2 of whom subsequently underwent percutaneous coronary stent implantation. There were no significant differences (p > 0.05) in the main coronary adverse events and revascularization rates in both groups. Five and 6 patients were admitted for a stroke in the control and combination groups, respectively. There were no significant differences in the rate of stroke between the two groups (p > 0.05).
DISCUSSION
Over the past decade, lipid intervention studies demonstrated that intensive, rational, and effective lipid-lowering therapy could significantly reduce the incidence of adverse cardiovascular events in populations with high cardiovascular risk. Although initial doses of statins could reduce LDL-C by 20-30%, doubling the dose could only reduce it by an extra 5-6%. However, larger doses might compromise safety. Eze represented a novel class of cholesterol-lowering drug, which could selectively inhibit the activity of intestinal Niemann-Pick protein C1L1. This effectively reduced the intestinal absorption of cholesterol, and lowered plasma cholesterol levels and liver cholesterol reserves.11 The combination of statins and Eze blocked the synthesis and absorption of cholesterol, thereby cooperatively reducing LDL-C levels. Recent studies showed that statin therapy in combination with Eze could further reduce LDL-C levels by 6-25.8%, while the incidence of adverse events did not increase.12 Eze not only produced beneficial effects on serum lipids, but also inhibited oxidative stress, increased the expression of endothelial nitric oxide synthase, improved arterial elasticity, and inhibited vascular inflammation.13,14
One study confirmed that the extent of carotid atherosclerosis could predict the risk of CHD.15 This clinical study found that when the tunica media of the carotid arteries was thickened, and the incidence of carotid artery plaque was significantly higher in CHD compared to non-CHD patients.16 The increase in CIMT was the noninvasive indicator for evaluation of early atherosclerosis, and an important risk factor and predictor of cardiovascular disease.17 Although the carotid IMT had been abandoned by the ACC/AHA 2013 guidelines, there are new surrogate markers for initiating statin therapy. Clinical trials have shown that, in patients without ischemic disease, the increased CIMT was related to myocardial infarction (MI) and stroke, and an independent risk factor for atherosclerosis.18 The Cardiovascular Health Study performed a 6.2-year follow-up of 4476 CHD patients with a mean age of 72.5 years. After adjusting for primary and traditional cardiovascular risk factors, the risk for MI or stroke among patients in the highest quintile for CIMT was 3.15 times greater compared to those in the lowest quintile.19 Consequently, many clinical trials still consider CIMT as an important indicator for determining the risk for atherosclerosis, and use it for evaluating the effects of lipid lowering therapy and prognosis of patients with atherosclerosis.
Unlike CIMT, carotid plaque might represent the late stage of AS. One study confirmed the closer relationship with CHD, and its ability to predict coronary events much more accurately.20 One meta-analysis reporting 11 items in a total of 54,336 patients showed that carotid artery plaques could predict future MI much more accurately compared with CIMT.21 Carotid ultrasound of 5, 445 non-CHD subjects found that the incidence of coronary artery calcification was higher in those with carotid plaques that were detected by ultrasound.22
Evidence-based medicine revealed that the standards for lipid lowering were often accompanied by regression, delayed development, or stabilization of atherosclerotic plaques. Studies involving western and Japanese populations with acute coronary syndrome (ACS) reported decreased original plaque volumes and CIMT when statins lowered the LDL levels.23,24 Bogiatzi and Spence25 also confirmed that Eze could reduce the carotid atherosclerotic plaque areas, and that measuring the plaque area was more important in evaluating the antiatherosclerotic effects. Luo et al.26 observed that the combination of atorvastatin with ezetimibe could further decrease LDL-C and high sensitivity C-reactive protein levels and affect the progression of carotid atherosclerosis in elderly patients with hypercholesterolemia, which is consistent with the findings in our study. These two studies had similar drug administration and follow-up periods. Nevertheless, there are differences between the two studies. In our study, we observed patients with coronary heart disease and hypercholesterolemia who required lower levels of LDL, and had a higher incidence of adverse cardiovascular events.
However, whether the combination of statins and Eze can reduce LDL-C levels as well as slow the carotid atherosclerotic process, remains controversial. The Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial randomly divided 720 patients with heterozygous hypercholesterolemia into Eze-simvastatin combination and simvastatin alone groups. The baseline of CIMT was 0.68 mm. The mean changes in the CIMT in either group showed no significant differences two years later.10 Most scholars believed that the ENHANCE trial did not deny that the combination of statins with ezetimibe can further reduce the CIMT. Luo P et al.26 argued that the CIMT in ENHANCE was set too low and could not be reduced further. Paraskevas et al.27 discussed the effects of atorvastatin in combination with ezetimibe on CIMT in recent years. They considered the fact that atorvastatin administered at 80 mg/day did not reduce the CIMT when compared with placebo (CASHMERE study) as evidence that the CIMT was set too low (virtually identical to that in ENHANCE).27 Thus, this study could not influence current, intensive, clinical lipid-lowering strategies.28 In our study, the baseline of CIMT was 1.26 mm, which was greater than those in the ENHANCE trial. Our study showed that the CIMT improvement in the combination group was much more significant than in the atorvastatin group after 12 months of treatment. Although the carotid plaque area exhibited a further decreasing trend after treatment, no significant differences were found in the plaque areas of either group. This might be related to the small size of the study population, as well as the relatively short observation period.
In recent years, conclusions about whether Eze could improve the prognosis of CHD patients have not been consistent. The Study of Heart and Renal Protection (SHARP) trial showed that Eze combined with simvastatin could significantly reduce LDL-C levels in patients with chronic renal failure, as well as major atherosclerotic end events such as cardiac death, MI, non-hemorrhagic stroke, and others.29 Although the LDL-C levels decreased in the 499 patients with hypertension, type 2 diabetes, and hyperlipidemia in the Stop Atherosclerosis in Native Diabetics Study (SANDS) after combined lipid adjustment, there was no difference in the incidence of cardiovascular events.30 The recently released IMProved Reduction of Outcomes: Vytorin Efficacy national Trial (IMPROVE-IT) included 18,000 cases of middle- and high-risk ACS patients, who had stable disease, in a multi-center, randomized, double-blind and controlled study. In a 7-year follow-up period, the results showed that the addition of Eze to simvastatin could moderately reduce the incidence of cardiovascular events.31,32 While the 1-year follow-up in our study showed no significant differences in adverse coronary events and stroke between the groups, this might be related to the small size of the study population and the relatively short observation period.
CONCLUSIONS
In conclusion, this study confirmed that combination therapy with Ato and Eze could further reduce plasma LDL-C and CIMT levels. Whether intensive lipid-lowering can delay or reverse the carotid AS process remains controversial, and large-scale clinical trials are needed for confirmation.
ETHICAL APPROVAL
This study was conducted in accordance with the declaration of Helsinki, and proceeded with approval from the Ethics Committee of the Henan Provincial People’s Hospital. Written informed consent was obtained from all participants.
CONFLICTS OF INTEREST
All of the authors declare that they have no conflicts of interest regarding this paper.
REFERENCES
- 1.Stehbens WE. Coronary heart disease, hypercholesterolemia, and atherosclerosis. II. Misrepresented data. Exp Mol Pathol. 2001;70:120–139. doi: 10.1006/exmp.2000.2339. [DOI] [PubMed] [Google Scholar]
- 2.Whayne TF., Jr. Assessment of low-density lipoprotein targets. Angiology. 2013;64:411–416. doi: 10.1177/0003319712451115. [DOI] [PubMed] [Google Scholar]
- 3.Weng TC, Yang YH, Lin SJ, Tai SH. A systematic review and meta-analysis on the therapeutic equivalence of statins. J Clin Pharm Ther. 2010;35:139–151. doi: 10.1111/j.1365-2710.2009.01085.x. [DOI] [PubMed] [Google Scholar]
- 4.Toth PP, Foody JM, Tomassini JE, et al. Therapeutic practice patterns related to statin potency and ezetimibe/simvastatin combination therapies in lowering LDL-C in patients with high-risk cardiovascular disease. J Clin Lipidol. 2014;8:107–116. doi: 10.1016/j.jacl.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Foody JM, Toth PP, Tomassini JE, et al. Changes in LDL-C levels and goal attainment associated with addition of ezetimibe to simvastatin, atorvastatin, or rosuvastatin compared with titrating statin monotherapy. Vasc Health Risk Manag. 2013;9:719–727. doi: 10.2147/VHRM.S49840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niccoli G, Liuzzo G, Montone RA, Crea F. Advances in mechanisms, imaging and management of the unstable plaque. Atherosclerosis. 2014;233:467–477. doi: 10.1016/j.atherosclerosis.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Montecucco F, Pende A. Update on the evidence of statin treatment to reduce plaque vulnerability. Drug Metab Lett. 2012;6:151–156. doi: 10.2174/1872312811206030001. [DOI] [PubMed] [Google Scholar]
- 8.Nohara R, Daida H, Hata M, et al. Effect of intensive lipid-lowering therapy with rosuvastatin on progression of carotid intima-media thickness in Japanese patients: Justification for Atherosclerosis Regression Treatment (JART) study. Circ J. 2012;76:221–229. doi: 10.1253/circj.cj-11-0887. [DOI] [PubMed] [Google Scholar]
- 9.Meaney A, Ceballos G, Asbun J, et al. The VYtorin on Carotid intima-media thickness and overall arterial rigidity (VYCTOR) study. J Clin Pharmacol. 2009;49:838–847. doi: 10.1177/0091270009337011. [DOI] [PubMed] [Google Scholar]
- 10.Kastelein JJ, Akdim F, Stroes ES, et al. Simavastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–1443. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- 11.Clearfield MB. A novel therapeutic approach to dyslipidemia. J Am Osteopath Assoc. 2003;103:S16–S20. [PubMed] [Google Scholar]
- 12.Sasaki J, Otonari T, Sawayama Y, et al. Double-dose pravastatin versus add-on ezetimibe with low-dose pravastatin - effects on LDL cholesterol, cholesterol absorption, and cholesterol synthesis in Japanese patients with hypercholesterolemia (PEAS study). J Atheroscler Thromb. 2012;19:485–493. doi: 10.5551/jat.12013. [DOI] [PubMed] [Google Scholar]
- 13.Nochioka K, Tanaka S, Miura M, et al. Ezetimibe improves endothelial function and inhibits Rho-kinase activity associated with inhibition of cholesterol absorption in humans. Circ J. 2012;76:2023–2030. doi: 10.1253/circj.cj-12-0331. [DOI] [PubMed] [Google Scholar]
- 14.Grigore L, Raselli S, Garlaschelli K, et al. Effect of treatment with pravastatin or ezetimibe on endothelial function in patients with moderate hypercholesterolemia. Eur J Clin Pharmacol. 2013;69:341–346. doi: 10.1007/s00228-012-1345-z. [DOI] [PubMed] [Google Scholar]
- 15.Crouse JR., 3rd Carotid and coronary atherosclerosis. What are the connections? Postgrad Med. 1991;90:175–179. doi: 10.1080/00325481.1991.11701043. [DOI] [PubMed] [Google Scholar]
- 16.Craven TE, Ryu JE, Espeland MA, et al. Evaluation of the associations between carotid artery atherosclerosis and coronary artery stenosis. A case-control study. Circulation. 1990;82:1230–1242. doi: 10.1161/01.cir.82.4.1230. [DOI] [PubMed] [Google Scholar]
- 17.Peters SA, Bots ML. Carotid intima-media thickness studies: study design and data analysis. J Stroke. 2013;15:38–48. doi: 10.5853/jos.2013.15.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keo HH, Baumgartner I, Hirsch AT, et al. Carotid plaque and intima-media thickness and the incidence of ischemic events in patients with atherosclerotic vascular disease. Vasc Med. 2011;16:323–330. doi: 10.1177/1358863X11419997. [DOI] [PubMed] [Google Scholar]
- 19.O’Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 20.Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012;220:128–133. doi: 10.1016/j.atherosclerosis.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 21.Brook RD, Bard RL, Patel S, et al. A negative carotid plaque area test is superior to other noninvasive atherosclerosis studies for reducing the likelihood of having underlying significant coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:656–662. doi: 10.1161/01.ATV.0000200079.18690.60. [DOI] [PubMed] [Google Scholar]
- 22.Polak JF, Tracy R, Harrington A, et al. Carotid artery plaque and progression of coronary artery calcium: the multi-ethnic study of atherosclerosis. J Am Soc Echocardiogr. 2013;26:548–555. doi: 10.1016/j.echo.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365:2078–2087. doi: 10.1056/NEJMoa1110874. [DOI] [PubMed] [Google Scholar]
- 24.Hiro T, Kimura T, Morimoto T, et al. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol. 2009;54:293–302. doi: 10.1016/j.jacc.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 25.Bogiatzi C, Spence JD. Ezetimibe and regression of carotid atherosclerosis: importance of measuring plaque burden. Stroke. 2012;43:1153–1155. doi: 10.1161/STROKEAHA.111.640789. [DOI] [PubMed] [Google Scholar]
- 26.Luo P, Li L, Wang LX, et al. Effects of atorvastatin in combination with ezetimibe on carotid atherosclerosis in elderly patients with hypercholesterolemia. Genet Mol Res. 2014;13:2377–2384. doi: 10.4238/2014.April.3.10. [DOI] [PubMed] [Google Scholar]
- 27.Paraskevas KI, Veith FJ, Mikhailidis DP. Carotid intima-media thickness and ezetimibe: the end of a misunderstanding? Curr Vasc Pharmacol. 2011;9:381–384. doi: 10.2174/157016111796197198. [DOI] [PubMed] [Google Scholar]
- 28.Gandhi MJ. Does ‘ENHANCE’ diminish confidence in ezetimibe? J Assoc Physicians India. 2008;56:665–666. [PubMed] [Google Scholar]
- 29.Sharp Collaborative Group. Study of Heart and Renal Protection (SHARP): randomized trial to assess the effects of lowering low-density lipopmtein cholesterol among 9,438 patients with chronic kidney disease. Am Heart J. 2010;160:785–794. doi: 10.1016/j.ahj.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Fleg JL, Mete M, Howard BV, et al. Effect of statins alone versus statins plus ezetimibe on carotid atherosclerosis in type 2 diabetes: the SANDS (Stop Atherosclerosis in Native Diabetics Study) trial. J Am Coll Cardiol. 2008;52:2198–2205. doi: 10.1016/j.jacc.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cannon CP, Giugliano RP, Blazing MA, et al. Rationale and design of IMPROVE-IT (IM-Proved Reduction of Outcomes: Vytorin Efficacy Intemationala1): comparison of ezetimibe/simvastatin versus simvastatin mono-therapy on cardiovascular outcomes in patients with acute coronary syndromes. Am Heart J. 2008;156:826–832. doi: 10.1016/j.ahj.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 32.Kohno T. Report of the American Heart Association (AHA) Scientific Sessions 2014, Chicago. Circ J. 2015;79:34–40. doi: 10.1253/circj.CJ-14-1329. [DOI] [PubMed] [Google Scholar]