Abstract
G protein-coupled receptors (GPCRs) mediate many signal transduction processes in the body. The discovery that these receptors are voltage-sensitive has changed our understanding of their behavior. The M2 muscarinic acetylcholine receptor (M2R) was found to exhibit depolarization-induced charge movement-associated currents, implying that this prototypical GPCR possesses a voltage sensor. However, the typical domain that serves as a voltage sensor in voltage-gated channels is not present in GPCRs, making the search for the voltage sensor in the latter challenging. Here, we examine the M2R and describe a voltage sensor that is comprised of tyrosine residues. This voltage sensor is crucial for the voltage dependence of agonist binding to the receptor. The tyrosine-based voltage sensor discovered here constitutes a noncanonical by which membrane proteins may sense voltage.
Introduction
G protein-coupled receptors (GPCRs) are involved in the vast majority of signal transduction processes. Recently, it was found that the agonist binding and/or activity of many GPCRs, including receptors for acetylcholine (ACh), adrenaline, dopamine, and glutamate, are voltage dependent (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13). For the most extensively studied voltage-sensitive GPCR, the M2 muscarinic acetylcholine receptor (M2R), it was found that its agonist binding affinity is modulated by voltage. Two experimental approaches (3, 4, 6) were used to measure binding affinity: one measured M2R-induced G protein-activated inward-rectifying K+ (GIRK) channel currents, reflecting ACh binding, and the other used direct binding experiments. Both approaches led to the conclusion that the agonist binding affinity of the M2R is voltage dependent; the binding affinity is higher at resting membrane potential than under depolarization.
In a later study, also conducted in the M2R (6), a correlation was demonstrated between the voltage dependence of agonist binding and the voltage dependence of depolarization-induced conformational changes in the orthosteric binding site. This was done with site-directed fluorescence labeling by tetramethylrhodamine maleimide (TMRM) of an endogenous cysteine located near the orthosteric binding site; fluorescence signals acted as reporters of voltage-dependent conformational changes that occur in the orthosteric binding site of the M2R. Finally, we have shown (3) that upon changes in membrane potential, the M2R produces charge-movement associated currents, analogous to the gating currents measured in voltage-gated ion channels (14). Consequently, these currents in M2Rs were likewise called gating currents, although a more appropriate name could be sensing currents. These gating currents were seen in the absence of any ligand and with no permeable ions (except 2 mM Ca2+) in the extracellular solution (3, 6). Unless the very large ions N-methyl-D-glucamine or methylsulfonate were able to reach a binding pocket, the measured gating currents are not likely to have been produced by the movement of ions or charged ligands into and out of the binding pocket. In fact, the charged agonist ACh, as well as an antagonist of the orthosteric site (methoctramine) and an allosteric ligand (gallamine) all reduced the gating currents (15). The voltage dependence of the charge movement measured from these gating currents was seen to correlate with the voltage dependence of the agonist binding affinity and the voltage dependence of the conformational change (3, 6).
The existence of gating currents in the M2R implies that, as in voltage-gated ion channels, the M2R possesses a voltage sensor. In voltage-gated ion channels, the voltage sensor is comprised of a cluster of positively charged amino acids in the fourth transmembrane segment (S4) of the channel which move upon changes in membrane potential through the strong electric field that resides across the cell membrane (16). As such a motif does not exist in the M2R, we aimed to identify residues in the M2R that may behave as its voltage sensor and may underlie the voltage sensitivity of its agonist binding.
Materials and Methods
Xenopus oocytes were prepared, injected, and maintained as previously described (4). Constructs were linearized and transcribed as previously described (3). All mutants were constructed using the QuickChange site-directed mutagenesis kit (Stratagene, San Diego, CA).
Gating current measurements
Gating currents were recorded 3–5 days after RNA injection using the cut-open voltage-clamp technique (17). The external recording solution contained 120 mM N-methyl-D-glucamine, 10 mM HEPES, and 2 mM CaCl2, adjusted to pH 7.4 with methanesulfonic acid. The internal solution was similar but did not contain CaCl2 and contained 2 mM EGTA. Microelectrodes were pulled to a resistance of ∼0.2–0.9 MΩ and filled with 3 M CsCl. Data were sampled at 50 kHz and filtered at 5 kHz. Symmetric capacitive currents were compensated by analog circuitry and subtracted online by using a depolarizing pulse (the P/8 protocol (3)), from a holding potential of +40 mV. Data acquisition was done with pClamp8 (Axon Instruments, Union City, CA). The data were analyzed with an in-house analysis program.
The total charge was calculated from the gating currents, assuming that with the cut-open configuration we record from one-fifth of the membrane area. Charge per receptor was obtained by dividing the total charge measured at each membrane potential by the number of expressed receptors estimated from QNB binding experiments.
Binding experiments
Direct binding of labeled quinuclidinylbenzilate ([3H]QNB; specific activity 47.4 Ci/mmol, PerkinElmer, Waltham, MA) or oxotremorine ([3H]OXO; specific activity 86.4 Ci/mmol, PerkinElmer) to intact individual oocytes was measured as described before (4). Briefly, an oocyte expressing either wild-type (wt) or mutated receptor was incubated in the radioligand solution for 30 s and then washed briefly in ice-cold ND96 solution (96 mM NaCl, 2 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 5 mM HEPES, pH adjusted to 7.4 with NaOH) or 96 mM K+ solution (4 mM NaCl, 96 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 5 mM HEPES, pH adjusted to 7.4 with KOH). The oocyte was then transferred into scintillation solution and radioactivity was counted in a scintillation counter. Membrane potential was modified, as before (3, 4) by varying extracellular K+ concentrations. In these experiments, the GIRK channel was coexpressed to increase the achievable range of membrane potentials. Nonspecific binding was measured by employing the same procedure for oocytes that do not express the receptor. The specific binding of each receptor-expressing oocyte was then calculated by subtracting the nonspecific binding (average of all control oocytes in the batch) from the total binding of the oocyte.
Ionic current measurements
Currents were recorded using the standard two-electrode voltage-clamp technique (Axoclamp 2B amplifier; Axon Instruments). pCLAMP8 software (Axon Instruments) was used for data acquisition and analysis.
GIRK current measurements
GIRK currents were measured as before (4). Briefly, oocytes were injected with 200 pg of the receptor (wt or mutants), 200 pg of each of the two subunits of the GIRK channel (GIRK1 and GIRK2), and 1 ng of the Gαi3 subunit. The oocyte was first clamped at either −80 mV or +40 mV in the ND96 solution (2 mM K+). Basal K+ currents were developed upon replacement of the ND96 solution by a 24 mM K+ solution (72 mM NaCl, 24 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 5 mM HEPES, pH adjusted to 7.4 with KOH). Then, ACh or pilocarpine (Sigma-Aldrich, St. Louis, MO) was applied, and K+ currents, induced by these agonists, appeared. These currents were terminated upon washout of the agonist. After a 10-min wash, the protocol was repeated at the other holding potential. The order of the two holding potentials was selected at random.
Ca2+ dependent Cl− current measurements
M1 muscarinic receptor (M1R)-activated Ca2+ dependent Cl− currents were measured as before (4). Briefly, oocytes were injected with 500 pg of RNA of the receptor and currents were measured 3-4 days after injection. The oocyte was clamped at either −80 mV or +40 mV in the ND96 solution. Then, one concentration of ACh was applied for 10 s and endogenous Ca2+-dependent Cl− currents appeared. After a 20-min wash, a second ACh concentration was applied. Usually, only two to three ACh concentrations were applied to each oocyte, and the response to each concentration was normalized to the response evoked by the maximal concentration of ACh (10 mM in the experiment in Fig. 8 B) measured at the same oocyte.
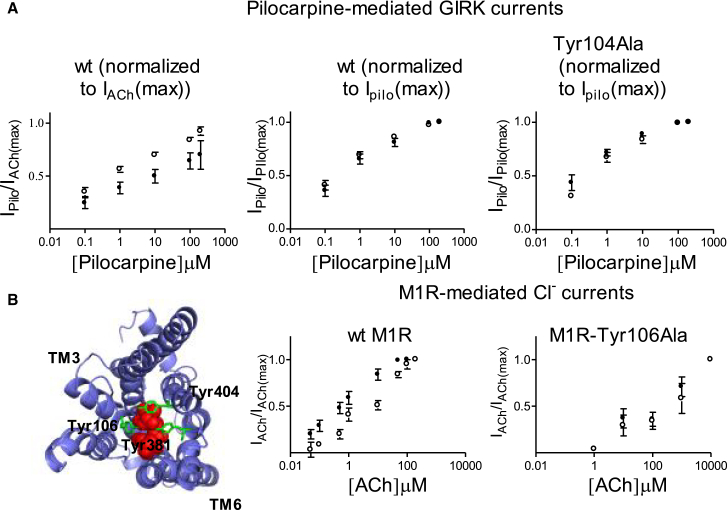
Figure 8.
(A) Voltage dependence of pilocarpine-evoked GIRK currents (Ipilo). Dose-response, obtained at −80 mV (solid circles) and at +40 mV (open circles), from wt (left and middle) and Tyr104Phe (right). The curves in the left plot are normalized to the maximal IACh at each holding potential, whereas the curves in the middle and right plots are normalized to the maximal Ipilo at each holding potential. Each point represents the mean ± SE, n = 6–14. (B) Voltage dependence of M1R- activated Ca2+-dependent Cl− currents. Left: Top view of the M1R, based on (25). Indicated are the orthosteric binding site (red spheres) and the three tyrosines, Tyr106, Tyr381, and Tyr404 (green). Dose-response, obtained at −80 mV (solid circles) and at +40 mV (open circles), from wt M1R (middle, taken from (4)) and M1R-Tyr106Ala (right). The curves are normalized to the maximal IACh at each holding potential. Each point represents the mean ± SE, n = 3–13.
Fluorescence measurements
Oocytes were incubated in ND96 solution with 20 μM TMRM (purchased from Invitrogen, Carlsbad, CA) for 15 min at 4°C. The oocytes were washed and kept in the dark until they were used. The cut-open oocyte voltage-clamp technique with site-directed fluorometry was used to measure voltage-sensitive fluorescence changes from TMRM (6). Fluorescence was obtained through a 40× water-immersion objective (LUMPlan FL N, Olympus, Center Valley, PA). The fluorescence signal was collected by a photodiode and amplified by a patch-clamp amplifier (L/M-EPC-7, LIST Medical Electronics, Darmstadt, West Germany). To improve the signal/noise ratio, the output of the amplifier was integrated over each sampling period by a home-built integrator circuit. A filter cube containing a TMRM filter set and a 530 nm LED (ThorLabs, Newton, NJ) was used for appropriately passing excitation and emission light. Excitation and electrophysiological control was through an SB6711-A4D4 board (Innovative Integration, Simi Valley, CA) and Gpatch, an in-house acquisition program. Recordings were performed at room temperature and averaged between four and nine times to improve the signal/noise ratio. Traces were filtered offline at 4 kHz using a MATLAB routine. The external solution contained 120 mM N-methyl-D-glucamine methanesulfonic acid, 10 mM HEPES, and 2 mM Ca(OH)2 set to pH 7.5; the internal solution was the same, with 2 mM EGTA in place of Ca(OH)2.
Results
Identification of a voltage sensor in the M2R
Based on the crystal structure of the M2R (18), it was inferred that only two charged residues, Asp103 and Asp69, reside within the transmembrane domains of the receptor. In a recent study (13), it was shown that neutralization of Asp103 had no significant effect on the gating currents. Regarding Asp69, it was found (13) that mutating it to alanine abolished the gating currents. As this mutant was shown to express poorly on the cell membrane, these authors did not exclude the possibility that Asp69 is a voltage sensor.
Repeating these experiments with a milder mutation, Asp69Asn, we found that although the expression level of the mutant was similar to that of wt, Asp69Asn did not exhibit gating currents (Fig. S1 A in the Supporting Material). We thus checked whether Asp69Asn also affects the voltage dependence of agonist binding. We found that this is not the case (Fig. S1 C).
The persistence of the voltage sensitivity in Asp69Asn despite the apparent abolishment of the gating currents suggested that this mutation may modify the properties of the gating currents such that gating currents could not be detected using the standard method ((17) and see Materials and Methods) for measuring gating currents.
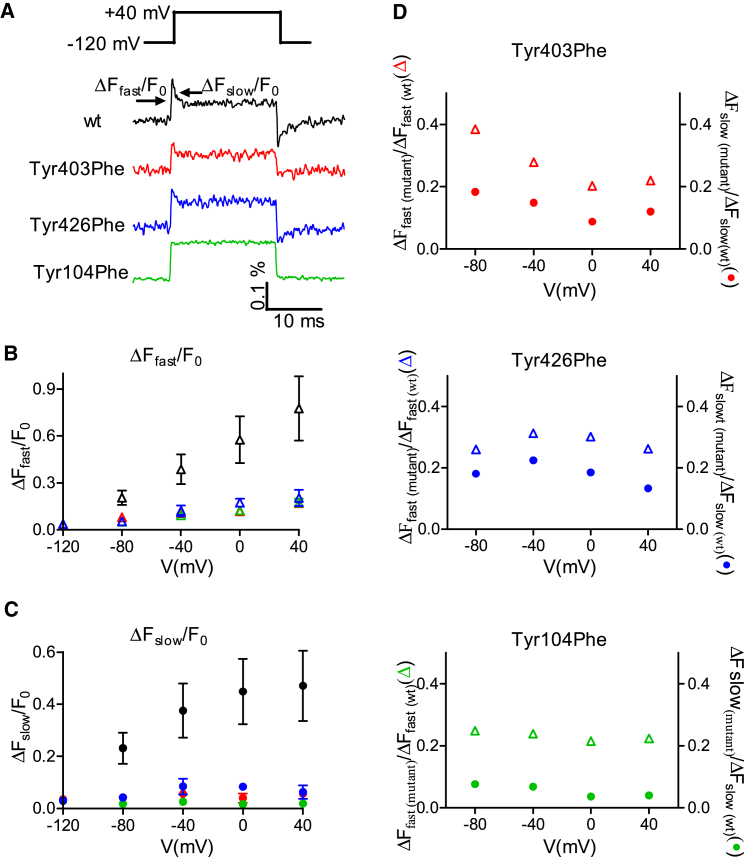
To circumvent this issue, we used another approach to indirectly test whether Asp69Asn produces gating currents. Specifically, we tested whether Asp69Asn produces depolarization-induced conformational change in the orthosteric binding site of the receptor. Such measurements are relevant for detection of gating currents, because in the wt M2R a correlation was found between the voltage dependence of depolarization-induced conformational change in the orthosteric binding site and the voltage dependence of depolarization-induced charge movement (6). We thus examined whether depolarization-induced conformational change occurs in Asp69Asn. The conformational change was measured, as done before in wt M2R (6), from the fluorescence signal evoked by depolarization in M2R labeled with TMRM at its endogenous Cys416, located in the vicinity of the orthosteric binding site. This fluorescence signal (see Fig. 1 D) is comprised of a very fast rise in fluorescence intensity (ΔFfast/F0, where ΔF is the change in fluorescence during the voltage pulse and F0 is the basal fluorescence) followed by a slow decay (ΔFslow/F0). In that study, it was shown that ΔFfast/F0 reflects an electrochromic effect on the TMRM and ΔFslow/F0 reflects a conformational change in or near the orthosteric binding site.
Figure 1.
Gating currents and fluorescence signals recorded from wt M2R. (A) Top view of the M2R, based on (19). Indicated are the orthosteric binding site (red spheres), Cys416 (purple spheres), and the three tyrosines, Tyr104, Tyr403, and Tyr426 (green). (B) Representative recordings of gating currents evoked by depolarizing pulses (see pulse protocol). Similar recordings were obtained from 6–13 oocytes. Here and in Figs. 2, 3, 4, and 5, the currents were normalized to their peak amplitude and were truncated to 50% of their actual peak. (C) The dependence of Q/R on membrane potential (V). Here and in Figs. 2, 4, and 5, Q/R is expressed in electron charge (e) units per receptor molecule. Each point represents the mean ± SE, n = 7–9. (D) A representative fluorescence signal elicited by a depolarizing pulse; the pulse protocol is shown above (taken from (6)). The fluorescence signal is comprised of a very fast rise in fluorescence intensity (ΔFfast/F0) followed by a slow decay (ΔFslow/F0). (E) The dependence of ΔFfast/F0 on V. Each point represents the mean ± SE, n = 4–5.
Fig. S1 D shows that as in the wt (left), a depolarization-induced fluorescence signal comprised of ΔFfast/F0 and ΔFslow/F0 is also seen in Asp69Asn (right). However, a major difference exists between ΔFslow/F0 in the wt and in Asp69Asn. Specifically, ΔFslow/F0 saturates at high depolarizations in the wt, whereas in Asp69Asn it continues to rise linearly with voltage (Fig. S1 E).
The existence of a slow component (ΔFslow/F0) in Asp69Asn indicates that a depolarization-induced conformational change occurs as well, suggesting that this mutant generates gating currents.
Why, then, are the gating currents in Asp69Asn not detected? One possibility is that the gating currents in Asp69Asn became very slow and hence undetectable. We view this possibility as unlikely, because the kinetics of ΔFslow/F0 are not slower in Asp69Asn than in the wt (compare Fig. S1 D, left and right). A second possibility is that the modified voltage dependence of the fluorescence signal reflects modified voltage dependence of the gating charge in the mutant that hampers our ability to detect the gating currents. This may be the case because the gating currents are recorded using a subtraction protocol ((17) and see Materials and Methods). This protocol enables subtraction of the linear capacitive currents from the total current; hence, the remaining currents represent the gating currents. In a subtraction protocol, it is assumed that at extreme voltage ranges there is very little charge movement. Hence, the total current measured at these ranges reflects the linear capacitive currents. Therefore, subtracting the capacitive currents from the total current provides the gating currents.
In Asp69Asn, where ΔFslow/F0 does not saturate (Fig. S1 E), the gating currents presumably increase linearly even at extreme depolarizations. Although it is likely that in Asp69Asn the gating currents will eventually saturate, this clearly occurs at a voltage range more positive than +40 mV. Subtraction in these ranges is not possible with our experimental system, as leak currents that mask the gating currents begin to develop at this voltage range. Therefore, in practice, there will be no voltage range from which the pure capacitive currents could be evaluated. Consequently, using the standard subtraction procedure (17), the gating currents will be subtracted together with the capacitive currents from the total current, and hence, it will not be possible to detect them. Given its voltage-sensitive conformational changes and voltage-dependent agonist binding, we believe that the loss of gating currents described here for the Asp69Asn mutant does not implicate this residue as a key voltage-sensing residue, given its voltage-sensitive conformational changes and voltage-dependent agonist binding.
As the two charged residues in the transmembrane domains of the M2R (Asp103 and Asp69) are probably not voltage-sensing residues, or not responsible for the voltage dependence of agonist binding, we chose to investigate whether the voltage sensor could be comprised of polar residues. In particular, tyrosine has a strong intrinsic dipole moment (19) and so has been posited to sense changes in membrane potential (20). The M2R contains numerous tyrosine residues within its transmembrane domains. Three tyrosine residues in the orthosteric binding site (Fig. 1 A) are of particular interest, as they form a tyrosine lid that surrounds the amine of the orthosteric ligand (21). This lid has been suggested to play a crucial role in controlling both agonist binding and G protein coupling (21, 22). Furthermore, mutating two of these tyrosines to alanine (Tyr403Ala and Tyr426Ala) abolished the voltage dependence of agonist binding (6); the binding affinity was similar at −80 mV and +40 mV. Thus, we hypothesized that the tyrosines in the orthosteric binding site may act as voltage-sensing residues.
To check for this possibility, we mutated these tyrosines to alanine and examined the effect of these mutations on the gating currents. Fig. 1 B shows gating currents elicited from the wt receptor by depolarizing pulses from a holding potential of −120 mV to various membrane potentials. As shown before (23), the gating currents in wt M2R are different from the gating currents in voltage-gated ion channels. These currents are composed of three transitions: a fast one, a slow one, and in between a transition that forms a typical feature, a “bump.” The latter transition is revealed in the ON response only when pulsing to negative potentials. In the OFF response, the bump is seen at all potentials (23). Fig. 1 C shows the voltage dependence of Q/R (in units of electron charge per receptor), where Q is the total charge that moves, obtained by time integration of the ON response of the gating currents, and R is the number of receptors expressed on the cell membrane, evaluated by measurements of binding of saturating concentration of QNB to oocytes from the same batch where the gating currents were measured (for details, see Fig. S2). This procedure for evaluating Q/R was used rather than the alternative one: evaluation of Q/R by fitting the Boltzmann equation to the curve describing the dependence of Q on voltage (Q-V). This is because when the charge moves in multiple steps, as is presumably the case in the M2R, the slope of the Q-V curve fails to reflect the true Q/R (24).
For a voltage sensor to be effective, it must be exposed to the transmembrane electric field. The strength of the electric field can be measured from the fast component of the fluorescence signal (ΔFfast/F0; Fig. 1 D), which reflects an electrochromic effect on the TMRM (6). Electrochromic responses are produced by changes in electric field strength and increase in magnitude linearly as the change in electric field strength increases. The linear electrochromic response of the ΔFfast/F0 signal from TMRM attached to Cys416 (Fig. 1 E) means that the dye is in a position where it is sensitive to changes in the intramembrane electric field. This strongly suggests that the dye is oriented intracellularly and toward the interior of the receptor, near the orthosteric binding site, rather than being oriented extracellularly or toward the lipid membrane.
We began our search for a putative voltage sensor in the agonist binding site with Tyr403. We thus mutated Tyr403 to alanine (Tyr403Ala) and measured the gating currents produced by this mutant. Fig. 2 A shows the voltage dependence of Q/R measured from the ON response. It is seen that the Q/R of Tyr403Ala is significantly smaller (2.2-fold at +40 mV) than that of the wt, whereas the expression levels of the two are similar (Fig. 2 A, inset). Fig. 2 B shows representative currents evoked by pulses to −70 mV and to +20 mV. As seen, the kinetics of the gating currents were also altered in Tyr403Ala. Specifically, the bump, seen in the wt in the ON response at −70 mV, is greatly reduced in Tyr403Ala. Fig. 2 B further shows that, similar to the reduction in the bump, the slow transition was also reduced.
Figure 2.
Effect of various mutants on gating currents and fluorescence signals. (A) The dependence of Q/R on membrane potential (V). Each point represents the mean ± SE, n = 7–9. The average (± SE) expression level of wt and of each mutant is shown in the insets. (B) Representative recordings of gating currents evoked by depolarizing pulses. Similar recordings were obtained from 6–13 oocytes. (C) The dependence of ΔFfast/F0 on V. Each point represents the mean ± SE, n = 4–5.
We then evaluated whether mutating Tyr403 to alanine affects the magnitude of the electric field. Fig. 2 C shows that Tyr403Ala reduced the magnitude of the electric field.
We next repeated the experiments described above for Tyr403Ala with the other two residues of the tyrosine lid, Tyr426 and Tyr104. As seen, Tyr426Ala and Tyr104Ala reduced Q/R, with the strongest effect obtained with Tyr104Ala (2.2-fold in Tyr426Ala, and 4.6-fold in Tyr104Ala) (Fig. 2 A). Like Tyr403Ala, Tyr426Ala and Tyr104Ala also altered the kinetics of the gating currents (Fig. 2 B), with the most dramatic changes seen in Tyr104Ala; the bump had disappeared even in the OFF response, suggesting that the transition that underlies it had been virtually abolished and the slow transition of the gating currents was also abolished. Finally, Tyr426Ala reduced the magnitude of the electric field, whereas Tyr104Ala had no effect on the electric field magnitude (Fig. 2 C).
Tyr104Ala did not affect the electric field, so it is reasonable to conclude that the reduction in Q/R in Tyr104Ala is caused by the mutation changing the actual sensing charge. Regarding Tyr403Ala and Tyr426Ala, where both the electric field strength and Q/R were reduced, deciphering the putative effect of the mutation on the actual sensing charge cannot be achieved by measuring Q alone. This is because Q reflects charge movement in the entire receptor, whereas the electric field was measured only in the vicinity of the fluorophore. We therefore took another approach to test whether the alanine mutations modified the actual sensing charge and therefore at least partially caused the reduction in Q. If Tyr403Ala and Tyr426Ala changed only the electric field, then we expect to see the bump at higher depolarization, where the electric field magnitude is similar to that of the wt in the range where the bump appears. However, this expectation was not met (Fig. 3). As seen in the wt, a bump is not detected at membrane potentials more positive than −30 mV. Although reduced in size, a bump is seen only at the same negative potentials as in the wt. The above results support the conclusion that the reduction in Q/R in both mutants is not solely due to a reduction in the electric field magnitude and is, at least partly, determined by a true effect of the mutations on the actual sensing charge.
Figure 3.
Representative gating currents recorded from wt, Tyr403Ala, and Tyr426Ala induced by depolarizing pulses to the indicated potentials from a holding potential of −120 mV. The arrows indicate the bump. Similar recordings were obtained from 10–13 oocytes.
The results in Figs. 2 and 3 suggest that all three tyrosines of the tyrosine lid are voltage-sensing residues. We next examined whether this voltage sensor accounts for all the gating currents seen in the wt M2R. To do so, we mutated all three tyrosines simultaneously (Tyr403Ala, Tyr426Ala, and Tyr104Ala, denoted the “triple mutant”) and measured the gating currents of the triple mutant. Fig. 4 A shows that Q/R was greatly reduced in the triple mutant (4.3-fold at +40 mV), but a substantial Q/R (∼20%) still remained. Interestingly, the gating currents of the triple mutant are composed only of a fast transition, which seems to be similar to the fast transition in the wt (Fig. 4 B). These results show that the tyrosine-lid voltage sensor cannot account for the fast transition of the gating currents, suggesting that an additional voltage sensor exists in the M2R.
Figure 4.
Effect of the triple mutant on the gating currents. (A) The dependence of Q/R on membrane potential. Each point represents the mean ± SE, n =5. The average (± SE) expression level of wt and the mutant is shown in the insets. (B) Representative recordings of gating currents evoked by depolarizing steps. Similar recordings were obtained from 5 oocytes.
The role of the hydroxyl group of the tyrosines in voltage sensing
Tyrosine is a noncharged residue. The question thus arises how tyrosine could sense voltage. It has been suggested that tyrosine could sense voltage via its hydroxyl group, which produces a dipole that could sense an electric field (16). A straightforward way to test this hypothesis would be to remove the hydroxyl group. This can be done by mutating the tyrosine to a phenylalanine, which is similar in structure to tyrosine but lacks the hydroxyl group. It is expected that this mutant will reduce Q/R.
We thus mutated Tyr403, Tyr426, and Tyr104 to phenylalanine (Tyr403Phe, Tyr426Phe, and Tyr104Phe, respectively) and compared the gating currents of these mutants to those of wt. As seen, all three mutants, like the corresponding alanine mutations (Fig. 2), reduced Q/R (Fig. 5 A; a 2.2-fold reduction in Tyr403Phe, a 2.9-fold reduction in Tyr426Phe, and a 3.2-fold reduction in Tyr104Phe). Also, all mutations, like the corresponding alanine mutations, altered the kinetics of the gating currents (Fig. 5 B); the bump and the slow transition were greatly reduced.
Figure 5.
Effect of Tyr403Phe, Tyr426Phe, and Tyr104Phe on the gating currents. (A) The dependence of Q/R on membrane potential. Each point represents mean ± SE, n =7–11. The average (± SE) expression level of wt and of each mutant is shown in the insets. (B) Representative recordings of gating currents evoked by depolarizing steps. Similar recordings were obtained from 10–12 oocytes.
Together, the results in Fig. 5 support the conclusion that tyrosine senses voltage through the dipole moment of its hydroxyl group. The similarity between the effect of the phenylalanine mutations (Fig. 5 A) and the alanine mutations (Fig. 2 A) on Q/R suggests that in the alanine mutations, the reduction in Q/R also largely results from the absence of a hydroxyl group in the amino acid side chain.
Is the tyrosine-based voltage sensor responsible for the depolarization-induced conformational change in the orthosteric binding site?
We have shown before that depolarization induces a conformational change in the orthosteric binding site of the M2R (6). We further showed that a correlation exists between the voltage dependence of the conformational change measured in the orthosteric binding site and the voltage dependence of agonist binding (6). The question is then whether the movement of the tyrosines in the tyrosine lid is responsible for the depolarization-induced conformational change. To answer this question, we examined the effect of the phenylalanine mutations of these tyrosines, one at a time, on the conformational change.
The conformational change was evaluated, as before (6), from the slow component of the voltage-sensitive fluorescence signal (ΔFslow/F0) measured from TMRM conjugated to Cys416 of the M2R. Fig. 6 A shows representative fluorescence signals elicited by depolarizing pulses to +40 mV from the wt and the phenylalanine mutations. As seen, the slow component of the fluorescence signal is reduced in Tyr403Phe and Tyr426Phe and was virtually abolished in Tyr104Phe. Results from four similar experiments at different membrane potentials are shown in Fig. 6, B and C. The phenylalanine mutations reduced both the fast component (ΔFfast/F0) and the slow component (ΔFslow/F0) of the signal at all voltages. This loss of the fluorescence signal decay does not appear to be due to the difference in quenching ability between phenylalanine and tyrosine. This is because substitution of Tyr104 by a tryptophan, which is a stronger quencher of many dyes than tyrosine, produces the same effect as phenylalanine (for details, see Fig. S3). This result further suggests that it is the dipole moment of the tyrosine that is critical for voltage sensing.
Figure 6.
Effect of the phenylalanine mutations on the fluorescence signal. (A) Representative fluorescence traces elicited by depolarizing pulses to +40 mV from the various mutants. Similar recordings were obtained from 2–6 oocytes. For all panels, wt is black, Tyr403Phe is red, Tyr426Phe is blue, and Tyr104Phe is green. (B) Dependence of ΔFfast/F0 on V. (C) Dependence of ΔFslow/F0 on V. (D) Comparison between ΔFslow/F0(mutant)/ΔFslow/F0(wt) (solid circles) and ΔFfast/F0(mutant)/ΔFfast/F0(wt) (open triangles). Each point in (B) and (C) represents the mean ± SE, n = 3–7.
It is possible that the reduction in ΔFslow/F0 (i.e., the conformational change) is solely due to the reduction in ΔFfast/F0 (i.e., the electric field magnitude). If this is the case, then we expect that the effect of the mutations on ΔFslow/F0 will be similar to their effect on ΔFfast/F0. Comparing the ratio between ΔFslow/F0(mutant) and ΔFslow/F0(wt) with the ratio between ΔFfast/F0(mutant) and ΔFfast/F0(wt) (Fig. 6 D) reveals that this is not the case. All three mutations reduced ΔFslow/F0 more than they reduced ΔFfast/F0; the most robust reduction is observed in Tyr104Phe. Together, the results in Figs. 5 and 6 suggest that the conformational change in the orthosteric binding site is largely achieved by movement of the tyrosines of the tyrosine lid.
Is the tyrosine-based voltage sensor responsible for the voltage dependence of agonist binding?
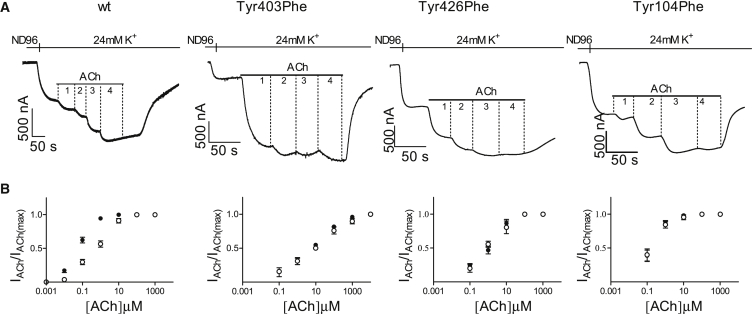
We next asked whether the movement of the tyrosines underlies the depolarization-induced change in agonist binding affinity. To answer this question, we examined the effect of the phenylalanine mutations on the agonist binding affinity. Based on their effect on the conformational change (Fig. 6 C), we expect that the phenylalanine mutations will greatly reduce or even abolish the voltage dependence of agonist binding affinity. The binding affinity of ACh was evaluated, as done previously (4, 6), from dose-response curves measured at two holding potentials, −80 mV and +40 mV, using ACh-induced GIRK currents as a measure for ACh binding (Fig. 7 A). As before, to enable comparison between different holding potentials, the response to each concentration of ACh was normalized to the maximal response evoked by ACh at the same holding potential. As expected, the curves at −80 mV and +40 mV overlapped, indicating that all three phenylalanine mutations abolished the voltage dependence of agonist binding (Fig. 7 B).
Figure 7.
Voltage dependence of agonist binding. (A) Representative recordings of ACh-mediated GIRK currents (IACh) obtained at −80 mV from wt (left column), Tyr403Phe (second column), Tyr426Phe (third column), and Tyr104Phe (right column). The numbers 1–4 represent different ACh concentrations as follows: for wt, 0.01, 0.1, 1, and 100 μM; for Tyr403Phe, 10, 100, 1000, and 10,000 μM; for Tyr426Phe and Tyr104Phe, 1, 10, 100, and 1000 μM. (B) Dose-response curves obtained from several experiments at −80 mV (solid circles) and at +40 mV (open circles) using various concentrations of ACh from wt and various mutants, as indicated. Each point represents the mean ± SE, n = 5–8.
Together, the results in Figs. 6 and 7 suggest that it is the conformational change that is caused by the movement of the tyrosines in the tyrosine lid that underlies the voltage dependence of agonist binding. The results further suggest that the hydroxyl group of the tyrosines is crucial for the voltage dependence of the M2R. These results reinforce our suggestion of a causal relationship between the conformational change and the binding affinity.
Voltage dependence of pilocarpine binding to the M2R
It has been proposed that pilocarpine-mediated M2-receptor activation is oppositely affected by voltage compared to ACh-mediated activation (13). We thus wished to examine whether the voltage sensor described here is also responsible for the voltage sensitivity of pilocarpine binding.
We first attempted to replicate the results of Navarro-Polanco et al. (13) in our experimental system. To this end, we measured pilocarpine-mediated GIRK currents at −80 mV and +40 mV and normalized them, as done in (13), to currents mediated by maximal concentration of ACh. Fig. 8 A, left, shows that with this normalization, the pilocarpine-mediated currents are higher under depolarization than at resting potential, consistent with earlier studies (13). However, to examine the voltage dependence of the binding affinity of pilocarpine, rather than normalizing the GIRK currents to the maximal currents evoked by ACh (13), we normalized the pilocarpine-evoked currents to the maximal current evoked by pilocarpine at the relevant holding potential, as is done routinely in our experiments for other agonists (3, 4, 6). Fig. 8 A, middle, shows that under these conditions, the binding affinity of pilocarpine is similar at −80 mV and +40 mV.
Unraveling the cause for the difference between the voltage dependence of ACh binding and that of pilocarpine is beyond the scope of this work. Nevertheless, because the binding site of pilocarpine is considered to be close to the tyrosine lid (1), we examined the effect of the Tyr104Phe mutation on the binding affinity of pilocarpine. Fig. 8 A, right, shows that this mutation had no significant effect on the binding affinity of pilocarpine or on its voltage sensitivity.
Is the tyrosine-based voltage sensor responsible for the voltage dependence of agonist binding to the M1R?
The M1R, like the M2R, exhibits voltage sensitivity. But in contrast to the M2R, its binding affinity is higher at depolarized potentials than under resting potential (Fig. 8 B and (4)). Like the M2R, the M1R possesses a tyrosine lid in its orthosteric binding site that is composed of three tyrosine residues (Tyr106, Tyr381, and Tyr404) (25), which were shown to be crucial in controlling the binding agonist affinity (26, 27, 28). Thus, we asked whether the tyrosine-based voltage sensor discovered here is also responsible for the voltage dependence of agonist binding to the M1R. To answer this question we mutated one of these tyrosines to alanine (M1R-Tyr106Ala, the homolog of Tyr104 in the M2R) and examined the voltage dependence of the mutant.
The affinity of the receptor was evaluated, as before (4, 29), by measuring ACh-induced Ca2+-dependent Cl− currents (see Materials and Methods) at two holding potentials. The results are shown in Fig. 8 B. It is seen that the affinity of the M1R-Tyr106Ala was similar at −80 mV and +40 mV. This result is compatible with the suggestion that the tyrosine-based voltage sensor described here is also responsible for the voltage dependence of agonist binding to the M1R.
Can the tyrosine-based voltage sensor account for the gating charge?
Theoretical considerations led to the hypothesis that tyrosine could sense voltage via its hydroxyl group, which produces a dipole that can sense an electric field (16). Our results provide the first experimental demonstration, to our knowledge, of a voltage sensor that is comprised of tyrosines rather than charged residues. Furthermore, our results showing that removal of the hydroxyl group from the tyrosine reduced Q/R (Fig. 5) provide experimental support for the essential role of the hydroxyl group in sensing voltage. We will now examine whether this tyrosine-based voltage sensor can account for various aspects of our experimental results. Tyrosines, although noncharged, possess a dipole moment that carries ∼50–90% of the charge of a positively charged amino acid like arginine (19). Thus, if three tyrosines were to take advantage of a focused electric field (30), which the electrochromic response of the TMRM suggests is present near these residues, we could estimate that these three tyrosines could carry 1.5–2.7 e0 if they move across essentially all of the electric field. The measured Q/R of the wt is ∼2.9 e0 (Fig. 1). However, since the Q/R of ∼0.7 e0 remains in the triple mutant, the total charge carried by the tyrosine-based voltage sensor is ∼2.2 e0, which is within the range of the amount of charge that can be carried by three tyrosines.
Based on the above calculation, it is expected that mutation of a single tyrosine will reduce Q/R by 0.5–0.9 e0. On the other hand, our results show that the loss of gating charge produced by a single mutation is larger, ranging from ∼1.5 e0 for Tyr403Ala to 2.4 e0 for Tyr104Ala. However, this is not necessarily surprising, as the analogous neutralization of an individual gating charge in a voltage-gated potassium channel has been shown to result in a reduction in gating charge that is >1 e0/subunit (31, 32, 33). One possible explanation for how removal of a single hydroxyl group could lead to a reduction in gating charge larger than that of a single gating charge is that the hydroxyls interact with other residues within the membrane. For example, the three tyrosines in the tyrosine lid form hydrogen bonds with each other, as well as with other amino acids. In fact, extensive intramolecular networks of hydrogen bonds have been proposed to be crucial for interhelical interactions and normal signaling in GPCRs (34, 35) and may be important for voltage-dependent GPCR signaling (34). Disruption of these networks by the removal of a single hydroxyl group on a tyrosine may result in a reduction in gating charge movement from other residues.
Hydrogen bonding from the hydroxyl group may also explain why the removal of Tyr104 produces a reduction in gating charge that is more extreme than that caused by removal of Tyr403 or Tyr426: Tyr104 hydrogen binds to both of the other tyrosines in the tyrosine lid, whereas both Tyr403 and Tyr426 only hydrogen bond to Tyr104 (21). Thus, removal of Tyr104 disrupts all hydrogen bonds in the tyrosine lid; this disruption may also affect the charge carried by the other two tyrosines and therefore completely abolish the charge movement by this voltage sensor. Indeed, the removal of Tyr104 produces an effect similar to the effect of the triple mutant. Finally, hydrogen bonds themselves may produce dipole moments of their own (36), which may contribute to the total gating charge measured upon changes in membrane potential.
Discussion
We describe here a putative voltage sensor in the M2R. This voltage sensor is situated in the orthosteric binding site of the M2R and is comprised of three tyrosine residues that form a tyrosine lid. We further show that this voltage sensor is crucial for the voltage dependence of agonist binding to the M2R.
The tyrosine-based voltage sensor unraveled here is different from that of the canonical voltage sensor in voltage-gated ion channels. There, the voltage sensor is composed of positively charged amino acids along the S4 helix that move through the electrical field within the membrane (31, 32). Here, the voltage sensor is comprised of tyrosines, which are hydrophobic and polar, but uncharged.
What could be the mechanism by which the tyrosine-based voltage sensor controls the voltage dependence of agonist binding? The tyrosine lid has been suggested to control the agonist binding affinity, producing high affinity when closed and low affinity when open (21, 37). It was further suggested (37) that the conformation of the tyrosine lid is affected by G protein coupling; the lid is closed when the G protein is coupled and open when it is uncoupled. Based on the above and on the cumulative results from this study, we can suggest a qualitative, presumably only partial, mechanism by which the tyrosine-based voltage sensor controls the voltage sensitivity of agonist binding to the M2R. At resting potential, the tyrosine lid is intact, and hence, the receptor exhibits high agonist binding affinity (37). Depolarization induces movement of the tyrosines by means of their hydroxyl group. As a result, the lid is disrupted and consequently, the receptor shifts to a low-affinity state. This mechanism may provide a linkage between the tyrosine-based voltage sensor described here, G protein coupling, and agonist binding affinity.
One puzzling result from this study is that although the voltage dependence of binding affinity has been abolished by the tyrosine mutations (Fig. 7), these mutations did not completely abolish Q/R (Figs. 2 and 5). This may seem to be in conflict with our suggestion that a causal relationship exists between the charge movements and the voltage sensitivity of the M2R (3). A possible way to resolve this seeming conflict is as follows. We showed that a single mutation is sufficient to abolish the voltage dependence of agonist binding (Fig. 7). This finding suggests that even a local disruption of the lid, caused by a movement of a single tyrosine, is sufficient to shift the receptor to a low-affinity state and hence to abolish the voltage sensitivity. When this happens, Q/R remains substantial as it reflects charge carried by the remaining tyrosines and by the putative additional voltage sensor that is responsible for the fast transition.
The tyrosine voltage sensor unraveled here is probably not the only voltage sensor in the M2R. This conclusion is based on the observation that the triple mutant exhibits gating currents that are composed only of the fast transition. This putative second voltage sensor is not likely to be involved in controlling the voltage sensitivity of agonist binding in the M2R. This conclusion is based on the finding that the tyrosine mutants abolished the voltage sensitivity without affecting the fast transition.
Further research is needed to identify this putative voltage sensor. Interestingly, a voltage-sensitive transition of a sodium ion has recently been described (38) that may account for this fast transition of the gating current. However, for this mechanism to be applied here, it would be necessary to assume that N-methyl-D-glucamine or low concentrations of Ca2+ could replace the sodium ion, because Na+ was not present in our experiments.
We have described here a tyrosine-based voltage sensor and proposed a possible mechanism for its action. An interesting question is whether this type of voltage sensor and this proposed mechanism also apply to other voltage-sensitive GPCRs. Several GPCRs that were found to be voltage sensitive (e.g., the α2 adrenergic receptor, the metabotropic glutamate 1 receptor (mGluR1), and the dopamine D2 receptor (1, 9, 12)) also contain tyrosines in their transmembrane domains that could potentially serve as voltage sensors. For example, the voltage-sensitive mGluR1 possesses an extracellular binding site, and thus, it is unlikely that its voltage sensitivity is due to a direct effect of voltage on the orthosteric binding site. However, mGluR1 has a membranal allosteric binding site (39) that controls the binding affinity of the orthosteric site (40) and contains a tyrosine (Tyr805, situated in transmembrane domain 6 (41)) that may sense the electric field and serve as a voltage sensor. Thus, although the tyrosine lid described here for the M2R may not have a correlate in other GPCRs, it is possible that in other receptors, other tyrosine-based voltage sensors employ different mechanisms to control the voltage dependence of agonist binding. Further research is needed to examine the mechanism of voltage sensitivity in other GPCRs.
Author Contributions
O.B.-A., M.F.P., and Y.B.-C. designed research, performed research, and wrote the manuscript. N.D. designed research and performed research. H.P. and F.B. designed research and wrote the manuscript.
Acknowledgments
This work was supported by a grant from the National Institute for Psychobiology in Israel, founded by the Charles E. Smith Family, to Y.B.-C., by an internal research grant from the Open University in Israel to Y.B.-C., and by National Institutes of Health grant R01-GM030376 to F.B., with extra support to M.F.P. from F31-NS081954. O.B.-A. was supported by a fellowship from the Open University in Israel.
Editor: Henry Colecraft.
Footnotes
Ofra Barchad-Avitzur and Michael F. Priest contributed equally to this work.
Supporting Results and three figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)30757-3.
Supporting Material
References
- 1.Rinne A., Mobarec J.C., Bünemann M. The mode of agonist binding to a G protein-coupled receptor switches the effect that voltage changes have on signaling. Sci. Signal. 2015;8:ra110. doi: 10.1126/scisignal.aac7419. [DOI] [PubMed] [Google Scholar]
- 2.Ben Chaim Y., Bochnik S., Parnas H. Voltage affects the dissociation rate constant of the m2 muscarinic receptor. PLoS One. 2013;8:e74354. doi: 10.1371/journal.pone.0074354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Chaim Y., Chanda B., Parnas H. Movement of “gating charge” is coupled to ligand binding in a G-protein-coupled receptor. Nature. 2006;444:106–109. doi: 10.1038/nature05259. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Chaim Y., Tour O., Parnas H. The M2 muscarinic G-protein-coupled receptor is voltage-sensitive. J. Biol. Chem. 2003;278:22482–22491. doi: 10.1074/jbc.M301146200. [DOI] [PubMed] [Google Scholar]
- 5.Bolton T.B., Zholos A.V. Potential synergy: voltage-driven steps in receptor-G protein coupling and beyond. Sci. STKE. 2003;2003:pe52. doi: 10.1126/stke.2102003pe52. [DOI] [PubMed] [Google Scholar]
- 6.Dekel N., Priest M.F., Bezanilla F. Depolarization induces a conformational change in the binding site region of the M2 muscarinic receptor. Proc. Natl. Acad. Sci. USA. 2012;109:285–290. doi: 10.1073/pnas.1119424109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahaut-Smith M.P., Martinez-Pinna J., Gurung I.S. A role for membrane potential in regulating GPCRs? Trends Pharmacol. Sci. 2008;29:421–429. doi: 10.1016/j.tips.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Pinna J., Gurung I.S., Mahaut-Smith M.P. Direct voltage control of signaling via P2Y1 and other Galphaq-coupled receptors. J. Biol. Chem. 2005;280:1490–1498. doi: 10.1074/jbc.M407783200. [DOI] [PubMed] [Google Scholar]
- 9.Ohana L., Barchad O., Parnas H. The metabotropic glutamate G-protein-coupled receptors mGluR3 and mGluR1a are voltage-sensitive. J. Biol. Chem. 2006;281:24204–24215. doi: 10.1074/jbc.M513447200. [DOI] [PubMed] [Google Scholar]
- 10.Ong B.H., Ohsaga A., Maruyama Y. G protein modulation of voltage-sensitive muscarinic receptor signalling in mouse pancreatic acinar cells. Pflugers Arch. 2001;441:604–610. doi: 10.1007/s004240000459. [DOI] [PubMed] [Google Scholar]
- 11.Sahlholm K., Barchad-Avitzur O., Århem P. Agonist-specific voltage sensitivity at the dopamine D2S receptor--molecular determinants and relevance to therapeutic ligands. Neuropharmacology. 2011;61:937–949. doi: 10.1016/j.neuropharm.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Sahlholm K., Marcellino D., Århem P. Voltage-sensitivity at the human dopamine D2S receptor is agonist-specific. Biochem. Biophys. Res. Commun. 2008;377:1216–1221. doi: 10.1016/j.bbrc.2008.10.117. [DOI] [PubMed] [Google Scholar]
- 13.Navarro-Polanco R.A., Moreno Galindo E.G., Tristani-Firouzi M. Conformational changes in the M2 muscarinic receptor induced by membrane voltage and agonist binding. J. Physiol. 2011;589:1741–1753. doi: 10.1113/jphysiol.2010.204107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstrong C.M., Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature. 1973;242:459–461. doi: 10.1038/242459a0. [DOI] [PubMed] [Google Scholar]
- 15.Kupchik Y.M., Barchad-Avitzur O., Parnas H. A novel fast mechanism for GPCR-mediated signal transduction—control of neurotransmitter release. J. Cell Biol. 2011;192:137–151. doi: 10.1083/jcb.201007053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bezanilla F. The voltage-sensor structure in a voltage-gated channel. Trends Biochem. Sci. 2005;30:166–168. doi: 10.1016/j.tibs.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Stefani E., Bezanilla F. Cut-open oocyte voltage-clamp technique. Methods Enzymol. 1998;293:300–318. doi: 10.1016/s0076-6879(98)93020-8. [DOI] [PubMed] [Google Scholar]
- 18.Haga K., Kruse A.C., Kobayashi T. Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature. 2012;482:547–551. doi: 10.1038/nature10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Momany F.A., McGuire R.F., Scheraga H.A. Energy parameters in polypeptides. VII. Geometric parameters, partial atomic charges, nonbonded interactions, hydrogen bond interactions, and intrinsic torsional potentials for the naturally occurring amino acids. J. Phys. Chem. 1975;79:2361–2381. [Google Scholar]
- 20.Bezanilla F. The voltage sensor in voltage-dependent ion channels. Physiol. Rev. 2000;80:555–592. doi: 10.1152/physrev.2000.80.2.555. [DOI] [PubMed] [Google Scholar]
- 21.Kruse A.C., Ring A.M., Kobilka B.K. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature. 2013;504:101–106. doi: 10.1038/nature12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregory K.J., Hall N.E., Christopoulos A. Identification of orthosteric and allosteric site mutations in M2 muscarinic acetylcholine receptors that contribute to ligand-selective signaling bias. J. Biol. Chem. 2010;285:7459–7474. doi: 10.1074/jbc.M109.094011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zohar A., Dekel N., Parnas H. New mechanism for voltage induced charge movement revealed in GPCRs—theory and experiments. PLoS One. 2010;5:e8752. doi: 10.1371/journal.pone.0008752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bezanilla F., Villalba-Galea C.A. The gating charge should not be estimated by fitting a two-state model to a Q-V curve. J. Gen. Physiol. 2013;142:575–578. doi: 10.1085/jgp.201311056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thal D.M., Sun B., Christopoulos A. Crystal structures of the M1 and M4 muscarinic acetylcholine receptors. Nature. 2016;531:335–340. doi: 10.1038/nature17188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Z.-L., Saldanha J.W., Hulme E.C. Transmembrane domains 4 and 7 of the M(1) muscarinic acetylcholine receptor are critical for ligand binding and the receptor activation switch. J. Biol. Chem. 2001;276:34098–34104. doi: 10.1074/jbc.M104217200. [DOI] [PubMed] [Google Scholar]
- 27.Ward S.D.C., Curtis C.A.M., Hulme E.C. Alanine-scanning mutagenesis of transmembrane domain 6 of the M(1) muscarinic acetylcholine receptor suggests that Tyr381 plays key roles in receptor function. Mol. Pharmacol. 1999;56:1031–1041. doi: 10.1124/mol.56.5.1031. [DOI] [PubMed] [Google Scholar]
- 28.Spalding T.A., Ma J.-N., Burstein E.S. Structural requirements of transmembrane domain 3 for activation by the M1 muscarinic receptor agonists AC-42, AC-260584, clozapine, and N-desmethylclozapine: evidence for three distinct modes of receptor activation. Mol. Pharmacol. 2006;70:1974–1983. doi: 10.1124/mol.106.024901. [DOI] [PubMed] [Google Scholar]
- 29.Minami K., Vanderah T.W., Harris R.A. Inhibitory effects of anesthetics and ethanol on muscarinic receptors expressed in Xenopus oocytes. Eur. J. Pharmacol. 1997;339:237–244. doi: 10.1016/s0014-2999(97)01354-x. [DOI] [PubMed] [Google Scholar]
- 30.Asamoah O.K., Wuskell J.P., Bezanilla F. A fluorometric approach to local electric field measurements in a voltage-gated ion channel. Neuron. 2003;37:85–97. doi: 10.1016/s0896-6273(02)01126-1. [DOI] [PubMed] [Google Scholar]
- 31.Aggarwal S.K., MacKinnon R. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron. 1996;16:1169–1177. doi: 10.1016/s0896-6273(00)80143-9. [DOI] [PubMed] [Google Scholar]
- 32.Seoh S.-A., Sigg D., Bezanilla F. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+ channel. Neuron. 1996;16:1159–1167. doi: 10.1016/s0896-6273(00)80142-7. [DOI] [PubMed] [Google Scholar]
- 33.Ishida I.G., Rangel-Yescas G.E., Islas L.D. Voltage-dependent gating and gating charge measurements in the Kv1.2 potassium channel. J. Gen. Physiol. 2015;145:345–358. doi: 10.1085/jgp.201411300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X.C., Sun K., Cao C. GPCR activation: protonation and membrane potential. Protein Cell. 2013;4:747–760. doi: 10.1007/s13238-013-3073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venkatakrishnan A.J., Deupi X., Babu M.M. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494:185–194. doi: 10.1038/nature11896. [DOI] [PubMed] [Google Scholar]
- 36.Kristof W., Zundel G. Proton transfer in and polarizability of hydrogen bonds in proteins. Tyrosine-lysine and glutamic acid-lysine hydrogen bonds—IR investigations. Biophys. Struct. Mech. 1980;6:209–225. [Google Scholar]
- 37.DeVree B.T., Mahoney J.P., Sunahara R.K. Allosteric coupling from G protein to the agonist-binding pocket in GPCRs. Nature. 2016;535:182–186. doi: 10.1038/nature18324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vickery Owen N., Zachariae U. Structural mechanisms of voltage sensing in G protein-coupled receptors. Structure. 2016;24:997–1007. doi: 10.1016/j.str.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu H., Wang C., Stevens R.C. Structure of a class C GPCR metabotropic glutamate receptor 1 bound to an allosteric modulator. Science. 2014;344:58–64. doi: 10.1126/science.1249489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson M.P., Nisenbaum E.S., Kingston A.E. Allosteric modulators of metabotropic glutamate receptors: lessons learnt from mGlu1, mGlu2 and mGlu5 potentiators and antagonists. Biochem. Soc. Trans. 2004;32:881–887. doi: 10.1042/BST0320881. [DOI] [PubMed] [Google Scholar]
- 41.Malherbe P., Kratochwil N., Mutel V. Mutational analysis and molecular modeling of the allosteric binding site of a novel, selective, noncompetitive antagonist of the metabotropic glutamate 1 receptor. J. Biol. Chem. 2003;278:8340–8347. doi: 10.1074/jbc.M211759200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.