Summary
Objective
The purpose of this study was to compare the effectiveness of an outpatient renal dose adjustment alert via a computerized provider order entry (CPOE) clinical decision support system (CDSS) versus a CDSS with alerts made to dispensing pharmacists.
Methods
This was a retrospective analysis of patients with renal impairment and 30 medications that are contraindicated or require dose-adjustment in such patients. The primary outcome was the rate of renal dosing errors for study medications that were dispensed between August and December 2013, when a pharmacist-based CDSS was in place, versus August through December 2014, when a prescriber-based CDSS was in place. A dosing error was defined as a prescription for one of the study medications dispensed to a patient where the medication was contraindicated or improperly dosed based on the patient’s renal function. The denominator was all prescriptions for the study medications dispensed during each respective study period.
Results
During the pharmacist- and prescriber-based CDSS study periods, 49,054 and 50,678 prescriptions, respectively, were dispensed for one of the included medications. Of these, 878 (1.8%) and 758 (1.5%) prescriptions were dispensed to patients with renal impairment in the respective study periods. Patients in each group were similar with respect to age, sex, and renal function stage. Overall, the five-month error rate was 0.38%. Error rates were similar between the two groups: 0.36% and 0.40% in the pharmacist- and prescriber-based CDSS, respectively (p=0.523). The medication with the highest error rate was dofetilide (0.51% overall) while the medications with the lowest error rate were dabigatran, fondaparinux, and spironolactone (0.00% overall).
Conclusions
Prescriber- and pharmacist-based CDSS provided comparable, low rates of potential medication errors. Future studies should be undertaken to examine patient benefits of the prescriber-based CDSS.
Keywords: Renal insufficiency, clinical decision support systems, computerized provider order entry, medication errors, pharmacists
1. Background and Significance
Renal impairment is a well-known risk factor for adverse drug events. Such impairment is often associated with medication dosing errors where a dose is not properly adjusted for renal impairment or dispensed when the medication is contraindicated based on renal function [1–7]. Reports of dose non-adjustment/contraindication in patients requiring renal adjustment range from 19% to 67% and 25% to 69% in the inpatient and ambulatory settings, respectively [2, 3, 6].
Clinical decision support systems (CDSS) have been developed within electronic health records (EHR) to identify patients with renal impairment when such patients are prescribed a medication that requires renal adjustment/contraindication [4, 8–10]. These systems require patient-specific laboratory and demographic data to be available during medication ordering or dispensing. Some systems provide notification and clinical decision support to the prescriber while others target the dispensing pharmacist. While CDSS generally have demonstrated improvements in appropriate drug dosing, most reports are from the inpatient setting [8–11]. Conversely, reports from the outpatient setting are few and restricted to specific medications or populations (e.g., erythropoietin and patients requiring dialysis) [8, 12]. In addition, existing studies do not address which member of the healthcare team is the most appropriate recipient of a notification (alert) from a CDSS [13].
In 2003, Kaiser Permanente Colorado (KPCO) implemented an effective Drug Renal Alert Pharmacy Program (DRAP) where alerts of a potential renal dosing error were made to dispensing pharmacists in the outpatient pharmacy [4]. With the implementation of a new pharmacy dispensing system at KPCO in 2014, this functionality was no longer available. To continue to provide renal dosing alerts, a new system was developed that displays a renal-dose adjustment alert, when indicated, to the prescriber at the time of computerized provider order entry (CPOE) without alerting the dispensing pharmacist. It is unknown if alerting the prescriber is as effective as alerting the dispensing pharmacist.
2. Objectives
The purpose of this study was to compare the effectiveness of a system that alerted prescribers at the time of order entry versus a system that alerted pharmacists at the time of dispensing on the rate of medication dispensing errors for medications requiring dose adjustment or contraindicated in patients with renal impairment.
3. Methods
3.1 Study Design and Setting
This was a retrospective analysis that compared medication error rates due to inappropriately dosed or contraindicated medications in patients with renal impairment between August 1, 2013 and December 31, 2013, when a pharmacist-based CDSS was in place, versus August 1, 2014 and December 31, 2014, when a prescriber-based CDSS program was in place.
This study was conducted at KPCO, a nonprofit, group model, integrated health care delivery system that provides services to over 620,000 members at 29 medical offices in Colorado. Each clinic provides primary care and outpatient pharmacy services, and there is a centralized pharmacy that provides mail order pharmacy services. Kaiser Permanente Colorado utilizes an outpatient electronic health record (EHR) that provides e-prescribing capabilities and interfaces with the internal pharmacy and laboratory systems.
3.2 Study Population
All adult KPCO patients with renal impairment who had a medication dispensed that requires dose adjustment or is contraindicated in patients with renal impairment between either August 1, 2013 and December 31, 2013 or August 1, 2014 and December 31, 2014 were eligible for inclusion. Medications included in the study were amantadine, ciprofloxacin, colchicine, dabigatran, dofetilide, enoxaparin, famciclovir, fondaparinux, gabapentin, glyburide, levofloxacin, metoclopramide, non-steroidal anti-inflammatory drugs (NSAID), sotalol, spironolactone, sulfamethoxazole/trimethoprim, and trimethoprim (► Table 1). The study medications were only those that were included in both systems. Patients with a serum creatinine (SCr) measured within 365 days before the prescription dispense date during the study period whose most proximal value resulted in an SCr below the threshold for the dispensing of a medication requiring renal dose adjustment/contraindication were included. Patients who were receiving dialysis at the time of the prescription were excluded.
Table 1.
Study Medications and Their eGFR Thresholds and Maximum Daily Dose/Contraindication Status
| Medication | eGFR Threshold (mL/min) | Maximum Daily Dose/Contraindication Status |
|---|---|---|
| Amantadine | <50 | 100 mg |
| <30 | 50 mg | |
| <15 | 29 mg | |
| Ciprofloxacin | <30 | 500 mg |
| Colchicine | <30 | 0.3 mg |
| Dabigatran1 | <30 | contraindicated |
| Dofetilide1 | 40 – <60 | 500 µg |
| 20 – <40 | 250 µg | |
| <20 | contraindicated | |
| Enoxaparin1 | <30 | contraindicated |
| Famciclovir | 40 – <60 | 1000 mg |
| 20 – <40 | 500 mg | |
| <20 | 250 mg | |
| Fondaparinux1 | <30 | contraindicated |
| Gabapentin | 30 – 44 | 1200 mg |
| 15 – 29 | 600 mg | |
| <15 | 300 mg | |
| Glyburide | <51 | contraindicated |
| Levofloxacin | 20 – <50 | 250 mg (most cases) |
| 375 mg (complicated / pneumonia) | ||
| <20 | 125 mg | |
| Metoclopramide | <40 | 20 mg |
| NSAID1,2 | <40 | contraindicated |
| Sotalol1 | 30 – <60 | administer q 24 hours |
| 10 – <30 | administer q 36–48 hours | |
| <10 | individualize dose | |
| Spironolactone | <10 | contraindicated |
| Trimethoprim/ | 15 – <30 | DS |
| Sulfamethoxazole | <15 | contraindicated |
| Trimethoprim | 15 – <30 | 100 |
| <15 | not recommended |
1 High risk medications that are contraindicated at a certain eGFR; medication dose adjustments were made with actual creatinine clearance and body weight
2 Includes ibuprofen, celecoxib, etodolac, flubiprofen, indomethacin, ketoprofen, ketorolac, meloxicam, nabumetone, naproxen, sulindac, and tolmetin
3.3 Intervention
The DRAP was a CDSS in the outpatient pharmacy (pharmacist-based CDSS) implemented at KPCO in 2003 that has been described in detail previously [4]. Briefly, laboratory data (i.e., SCr) and demographic data (i.e., age, sex, height and weight) were downloaded into the pharmacy system nightly to calculate each patient’s estimated creatinine clearance (eCrCl) using the Cockroft-Gault equation so that there was no meaningful time gap in the information being available [14]. When a new or refill prescription for a medication that required renal dose adjustment or was contraindicated at a certain threshold of renal function was put into workflow to be dispensed, an alert was triggered if the patient’s eCrCl was below the threshold or unavailable. The trigger did not allow the medication to be dispensed. A medication decision guide was printed in place of the prescription label, including the result and date of the most recent creatinine and eCrCl if available within the previous year. The guide provided specific dosing guidelines and alternative therapies. The dispensing pharmacist reviewed this information and could contact the prescriber to recommended alternative therapy, if indicated, or bypass the alert based on clinical judgment. Pharmacists had access to the EHR and were able to review a patient’s full medical history before making a recommendation to the prescriber about alternative therapy. Instead of recommending an alternative therapy, the dispensing pharmacist could have also recommended a creatinine test. The pharmacist-based CDSS was activated for both new medication fills and refills, creating multiple opportunities to identify errors throughout the “life” of the prescription. This system demonstrated a 20% reduction in renal dosing errors compared to similar prescriptions before the system was initiated [4].
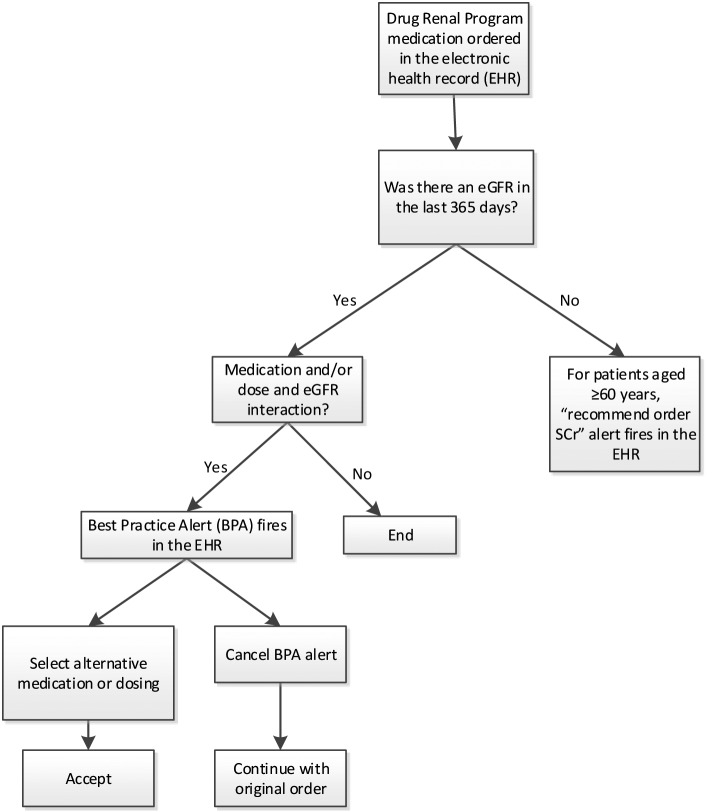
In 2014, KPCO developed a CDSS for the EHR for prescribers (prescriber-based CDSS) in response to procurement of a new pharmacy dispensing system (► Figure 1 for the CDSS process map). The medication list (► Table 1) was updated to include 37 renal medications. If the threshold had been updated from the pharmacist-based CDSS to the prescriber-based CDSS, the more conservative value was used. For example, the NSAID renal dosing threshold for the pharmacist-based system was eCrCl <40 ml/min and for the prescriber-based system, it was changed to eGFR <45 ml/min to better align with chronic kidney disease stage 3b. For the study, eGFR <40 ml/min was used.
Fig. 1.
Prescriber-based CDSS Process Map
With the prescriber-based CDSS, there were two types of alerts that displayed. One was at the time of CPOE or renewal of a prescription if there was an eGFR in the last 365 days and the medication ordered required renal dose adjustment (► Figure 2 for an example alert) or was contraindicated (► Figure 3 for an example alert) for a patient whose renal function was below the medication’s threshold (► Table 1). The alert provided recommendations for appropriate dosing or alternative therapy. If there was not an eGFR in the last 365 days and the patient’s age was ≥60 years, the alert recommended to order a creatinine to assess renal function (► Figure 1). Prescribers could cancel or ignore the alert based on clinical judgment.
Fig. 2.
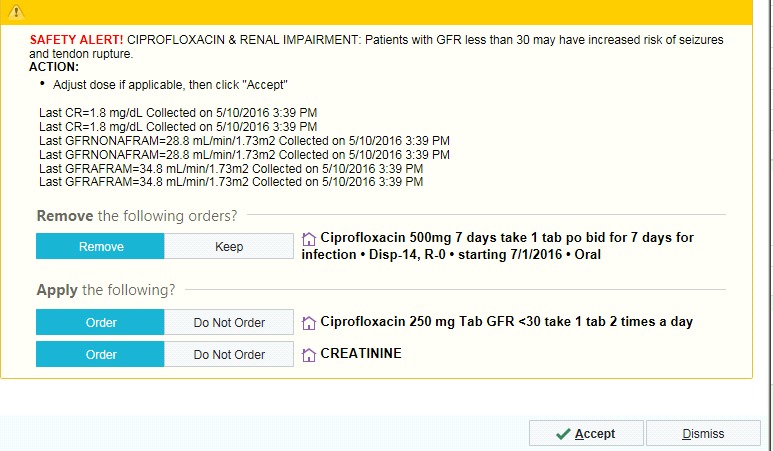
Example of a Dose Adjustment Medication Alert in the Prescriber-based CDSS
Fig. 3.
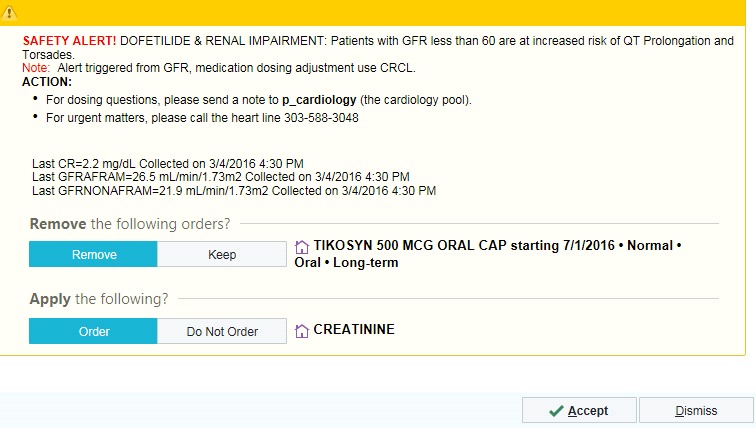
Example of a Contraindicated Medication Alert in the Prescriber-based CDSS
Another type of alert was for surveillance. This alert occurred when the EHR was opened and a patient’s most recent eGFR fell below the threshold but only for specific high-risk maintenance medications that were contraindicated at a certain eGFR (i.e., dabigatran, dofetilide, enoxaparin, fondaparinux, NSAID, or sotalol). This allowed for screening of high risk medications that were being refilled in the pharmacy where renal function may have deteriorated before the prescription was renewed by the prescriber.
The eGFR equation for estimating renal function was based on the laboratory’s ability to automatically report eGFR with all creatinine values and the acceptance of using eGFR for drug dosing in most situations [14–20]. All prescriber-based CDSS medication alerts were based on the patient’s eGFR with the majority dosed on that value. The five anti-arrhythmic and anticoagulant medication (dabigatran, dofetilide, enoxaparin, fondaparinux, or sotalol) alerts were based on the eGFR with recommendations in the alert for the dose to be based on CrCl with actual body weight (ABW) (► Figure 3). This was done because of the narrow range of toxicity of these medications and specific manufacturer dosing recommendations in the package insert.
Before the prescriber-based CDSS was implemented, a 20-minute webinar and continuing medical education videoconference describing the CDSS were presented to prescribers, pharmacists, and nurses. All educational materials were posted for reference on the internal KPCO clinical reference and pharmacy websites. After the prescriber-based CDSS was implemented, the pharmacist-based CDSS was discontinued.
3.4 Study Outcomes
The primary study outcome was the five-month rate of dosing errors among eligible prescriptions. A dosing error was defined as a prescription for one of the 30 study medications dispensed to a patient where the medication was improperly dosed or contraindicated based upon the patient’s renal function. The denominator was all prescriptions for the study medications dispensed during the respective study periods regardless of the patient’s renal function. Non-steroidal anti-inflammatory medications were combined together and reported as one medication class. A single patient could have more than one prescription included if she/he had multiple medications dispensed during the study period that meet the inclusion criteria.
Renal-dosing thresholds were determined using tertiary drug references and manufacturer prescribing information and all thresholds were approved by physicians representing primary care or specialty departments that typically prescribe each medication. Renal function was estimated for both groups by calculated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease (MDRD) equation [15–20]. The five anti-arrhythmic and anticoagulant medication (dabigatran, dofetilide, enoxaparin, fondaparinux, or sotalol) errors were reviewed by calculating CrCl ABW at the time of dispensing to assess whether an error actually occurred.
3.5 Data Analysis
No a priori power/sample size analysis was performed as all eligible patients were included in the study. Prescriber and pharmacist characteristics are reported as percentages. Patient characteristics are reported as means (± standard deviation) and percentages. Error rates are reported as percentages. Characteristics and outcomes were compared between the groups using chi-square tests of association for nominal/ordinal data and t-tests for interval-level data.
4. Results
During the pharmacist- and prescriber-based CDSS study periods, 49,054 and 50,678 prescriptions, respectively, were dispensed for the study medications (► Figure 4). Of these, 878 (1.8%) and 758 (1.5%) prescriptions written for 790 and 704 patients, respectively, were eligible for evaluation (i.e., the patient had a serum creatinine drawn within the past year that indicated an eGFR below the threshold for obligatory dose-adjustment or contraindication). There were 140 pharmacists who utilized the pharmacist-based CDSS during the study period; comprised of doctors of pharmacy (44.9%) and bachelors of science in pharmacy (55.1%). Pharmacists had 0–3 (33.3%), 3–5 (16.1%), 6–11 (37.9%), and 12+ (12.6%) years of experience. A total of 1,345 prescribers utilized the prescriber-based CDSS during the study period; comprised of medical doctors (68.9%), physician assistants (10.4%), nurse practitioners (7.6%), doctors of osteopathic medicine (5.9%), and other prescribers (e.g., optometrists) (7.2%). Prescribers had 0-2 (0.4%), 3-5 (5.9%), 6–11 (20.7%), and 12+ (73.1%) years of experience.
Fig. 4.
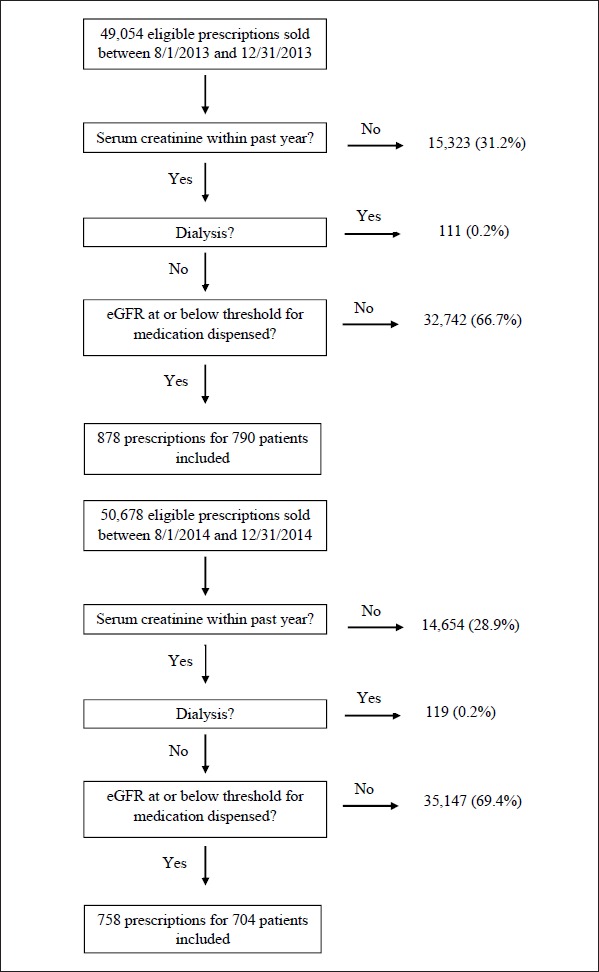
Patient Dispositions
Patients in both groups were similar with respect to age (p=0.128), female sex (p=0.546), eGFR (p=0.665), SCr (p=0.102), and stage of renal function (p=0.464) (► Table 2). The mean lengths of time in days from SCr measurement date to prescription dispense date were 59.1 (± 77) and 60.9 (± 78) for the pharmacist- and prescriber-based CDSS, respectively (p=0.776).
Table 2.
Characteristics of Patients Meeting eGFR Thresholds by CDSS Type
| Characteristic | Pharmacist-Based CDSS (n=790) | Prescriber-Based CDSS (n=704) | P-value |
|---|---|---|---|
| Age (mean, SD) | 72.4 (12.4) | 73.4 (12.4) | 0.128 |
| Male (n, %) | 281(36) | 261(37) | 0.546 |
| Estimated glomerular filtration rate (mean, SD) | 35.9 ml/min (10.4) | 35.8 ml/min (10.2) | 0.665 |
| Serum creatinine (mean, SD) | 1.8 mg/dl (1.2) | 1.9 mg/dl (1.0) | 0.102 |
| Renal function per estimated glomerular filtration rate (n, %) | 0.464 | ||
| Stage 3 (30–59 ml/min) | 551(70) | 503(71) | |
| Stage 4 (15–29 ml/min) | 209(26) | 169(24) | |
| Stage 5 (<15 ml/min) | 30(4) | 32(5) | |
CDSS – clinical decision support system; EHR – electronic health record; SD – standard deviation
Overall, the five-month rate of medication errors due to dispensing of a medication improperly dosed or contraindicated in a patient with renal impairment was 0.38%. Five-month rates of medication errors for all study medications were equivalent between the two groups: 0.36% and 0.40% in the pharmacist- and prescriber-based CDSS, respectively (p=0.523) (► Table 3). The medication with the highest error rate was dofetilide (0.51% overall) while the medications with the lowest rate were dabigatran, fondaparinux, and spironolactone (0.00% overall).
Table 3.
Error Rates by CDSS Type Overall and by Individual Medication
| Medication | Pharmacist-Based CDDS | Prescriber-Based CDDS | ||||
|---|---|---|---|---|---|---|
| Error Count | Total Rx Dispensed | Error Rate | Error Count | Total Rx Dispensed | Error Rate | |
| Amantadine | 4 | 213 | 1.88% | 8 | 221 | 3.62% |
| Ciprofloxacin | 13 | 8091 | 0.16% | 12 | 7862 | 0.15% |
| Colchicine | 7 | 614 | 1.14% | 6 | 632 | 0.95% |
| Dabigatran | 0 | 50 | 0.00% | 0 | 244 | 0.00% |
| Dofetilide | 2 | 43 | 4.65% | 3 | 55 | 5.45% |
| Enoxaparin | 11 | 1147 | 0.96% | 7 | 1353 | 0.52% |
| Famciclovir | 9 | 1287 | 0.70% | 22 | 1311 | 1.68% |
| Fondaparinux | 0 | 22 | 0.00% | 0 | 21 | 0.00% |
| Gabapentin | 37 | 5428 | 0.68% | 40 | 6762 | 0.59% |
| Glyburide | 5 | 211 | 2.37% | 5 | 172 | 2.91% |
| Levofloxacin | 29 | 2103 | 1.38% | 46 | 2717 | 1.69% |
| Metoclopramide | 8 | 696 | 1.15% | 6 | 623 | 0.96% |
| NSAIDS | 28 | 19932 | 0.14% | 32 | 21314 | 0.15% |
| Sotalol | 5 | 170 | 2.94% | 2 | 166 | 1.20% |
| Spironolactone | 0 | 3419 | 0.00% | 0 | 3922 | 0.00% |
| Trimethoprim/ Sulfamethoxazole | 16 | 4915 | 0.33% | 11 | 2745 | 0.40% |
| Trimethoprim | 2 | 713 | 0.28% | 1 | 558 | 0.18% |
| Overall | 176 | 49,054 | 0.36% | 201 | 50,678 | 0.40%* |
* P=0.523
CDSS – clinical decision support system; EHR – electronic health record; Rx – prescriptions
5. Discussion
Patients with renal impairment are at risk for adverse events when they are treated with prescription medications that require renal dose adjustment that aren’t appropriately adjusted/withheld [2, 3]. In our retrospective analysis of patients with renal impairment who were prescribed a medication that required renal dose adjustment or was contraindicated at the patient’s renal function, we found that renal dosing error rates for a prescriber-based CDSS were equivalent to those from a pharmacist-based CDSS. This finding is important as it provides evidence that a CPOE system with a CDSS alert for patients who may require renal dose adjustment can prevent inappropriate dispensing of such medications as effectively as a pharmacy-based system where pharmacists must intervene to prevent inappropriate dispensing. In addition, it is probable that the prescriber-based CPSS is more economical as it likely requires less time and fewer inputs to achieve appropriate renal dosing.
The pharmacist-based CDSS in our analysis was studied previously in an RCT [4]. That evaluation identified a 20% reduction in renal dosing errors but it did not compare the pharmacist-based CPSS to a prescriber-based CDSS [4]. Similar results were found in a recent cluster randomized trial with a 17% reduction in renal dosing errors among physicians who received clinical decision support in both an inpatient and outpatient university health system setting [21]. This investigation utilized similar customized best practice alerts to ours within the Epic EHR. Other investigations of pharmacy- and prescriber-based CDSS in outpatient settings have been performed. Joosten and colleagues described a system where a report was sent to an outpatient pharmacist when a patient’s serum creatinine measurement indicated an eGFR < 40 ml/min [1]. The pharmacist manually reviewed the patient’s medication profile to check for medications requiring dose adjustment due to renal impairment and then, if warranted, sent a recommendation to the prescribing physician. While the system identified a number of patients at risk for an adverse drug event due to renal impairment, no formal assessment of its effectiveness in required renal dose adjustment/withholding was performed [1]. Erler and colleagues evaluated a prescriber-based system in Germany and identified that the use of the system resulted in a >30% trend in the reduction of prescriptions exceeding the standard daily dose for patients with renal impairment compared to prescribers without the system [7]. Twadrous and colleagues conducted a systematic review of CDSS in kidney-related medication prescribing but of the 32 systems studied, only 3 were in an outpatient setting and these targeted very specific medications in dialysis or kidney transplant recipients [8]. We were unable to identify any studies that compared prescriber- vs. pharmacist-based CDSS in patients with renal impairment.
The medication we identified with the most errors was gabapentin. Gabapentin is an analgesic with a wide dosing range that is exclusively renally-eliminated by the kidneys; thus, assumed to put patients with chronic kidney disease at risk for central nervous system toxicity [22]. Because gabapentin was prescribed frequently during the study period, the overall error rate was low despite the relatively high number of errors. Conversely, we identified dofetilide with the highest reported error rate. Dofetilide has a narrow therapeutic range and is a class III antiarrhythmic drug that is initiated during hospitalization [23]. Accumulation of dofetilide can produce life-threatening QT prolongation. Dofetilide is recommended to be dose adjusted at eCrCl thresholds between 20–40 ml/min and is contraindicated at a threshold < 20 ml/min [23]. None of the dofetilide errors we identified in either the pharmacist- or prescriber-based CDSS were at the contraindicated level. The error rates we identified with chronic medications may have been higher in the prescriber-based CDSS since refills were not screened and renal function could have changed over the time between prescription orders.
The prescriber-based CDSS we evaluated resulted in low error rates similar to an effective pharmacist-based system [4]. The prescriber-based CDSS has a theoretical disadvantage in that it provides limited surveillance for the period between CPOE in the EHR, potentially a year prior to dispensing for chronic medications, and actual dispensing of the prescription to a patient. When a patient’s EHR is opened, surveillance alerts will display for medications that require dose adjustment/are contraindicated at a certain level of renal function. This inadequacy did not occur in the pharmacist-based CDSS because every time a potentially offending medication was dispensed, an alert would be triggered. To overcome this theoretical disadvantage, patients’ medication and laboratory data could be electronically monitored over time and an alert automatically displayed to the prescriber or dispensing pharmacist whenever a patient’s clinical profile changes.
There is another potential issue with prescriber-based CDSS. It has been reported that EHR alerts displayed during CPOE are commonly ignored [9, 25–27]. While prescribers may be more likely to act on the severity of the alert [25], it is unknown how prescribers view the severity of renal dose adjustment alerts. The implications of ignored prescriber-based alerts without the potential for pharmacist renal dosing review is substantial as prescribers may develop a prejudice towards alerts and disregard potentially valuable prescribing advice.
Conversely, a prescriber-based CDSS has potential advantages over pharmacist-based CDSS. A prescriber-based CDSS does not depend upon prescriptions being dispensed at a health system’s internal pharmacy. With point of prescribing alerting, the medication is already dose adjusted appropriately and the patient can take the prescription to any pharmacy for dispensing. In addition, prescriber-based CDSS is likely more efficient. It identifies a potential error at the point of prescribing before the order is transmitted to the pharmacy; thus, eliminating any need for a pharmacist to contact the prescriber to recommend renal dose adjustment/withholding of the medication. Furthermore, prescriber-based CDSS is recommended by the Office of the National Coordinator for Health Information Technology [28]. Among other evidence-based recommendations, the guidelines recommend “CDS(S) alerts are displayed in the relevant clinical context” and “Critical patient information is visible during the order entry process”. These are important components of the studied prescriber-based CDSS.
The strengths of our study were its large sample size, ability to incorporate laboratory, medical, and pharmacy data, and comparison of two distinct CDSS. However, our study had limitations that need to be taken into account. While pharmacists and prescribers were the recipients of the interventions in this study, results were analyzed at the prescription-level due to data collection limitations. The analysis of prescription-level data could potentially lead to the effects of pharmacist/prescriber factors (e.g., experience with respective CDSS, ability to override renal dose warning) on the results not being accounted for. In addition, analysis at this level results in low numerical error rates. Other investigators have used other metrics to describe error rates (e.g., use of patients with renal dysfunction). We were able to ascertain only limited information on the characteristics of the prescribers and pharmacists during the respective study periods. We did not utilize such information to adjust our results for potential confounding as the literature does not provide information on which prescriber/pharmacist characteristics are likely to impact renal dosing medication errors.
We were unable to examine if the study medications were refills or new prescriptions. We were unable to determine the individual patient’s benefit from appropriate/non-appropriate dose adjustment/withholding. While few CDSS trials have reported clinical outcomes [29], we identified no study that has reported on a major outcome benefit (e.g., reduced hospitalization, decreased mortality, increased health-related quality of life). We were unable to perform a cost comparison of the studied CDSS. Costs associated with developing and implementing a CDSS have been reported with the most time intensive piece being development of the contents of the CDSS [30]. We are aware of no study that compared the cost of use of two distinct types of CDSS. Additionally, we did not measure prescriber satisfaction with the prescriber-based CDSS. If prescribers were unsatisfied, they may be more likely to ignore renal dosing alerts.
Another limitation is that we did not examine benefit of using both systems concurrently or in a randomized fashion. Randomization or assignment of patients to the pharmacist-based CDSS was not feasible since with the implementation of a new pharmacy dispensing system at KPCO in 2014, the pharmacist-based CDSS functionality was no longer available. Randomization of patients to the prescriber-based CDSS or placebo could place the control group at increased risk of potentially severe adverse reactions. In the ambulatory care setting, a lack of interoperability between outpatient EHR and outpatient pharmacy information systems does not support renal dosing CDSS alerts at all of the stages of the medication use process, particularly at the dispensing stage. Providing renal dosing CDSS at only one stage is not ideal, but most outpatient pharmacies are unable to create renal dosing CDSS at the dispensing stage because renal function test results are often unavailable in the outpatient pharmacy to support the system.
Both the pharmacist-based and prescriber-based CDSS systems required a serum creatinine result to be available over the past year. In the ambulatory care setting for a general health system population, patients may not have a creatinine value available over the past year. Using a shorter time gap between the creatinine result and the prescription could contribute to unnecessary laboratory monitoring in stable ambulatory patients. National nephrology guidelines, KDIGO and KDOQI, support assessing renal function at least annually in patients with CKD [31].
6. Conclusions
This study of approximately 100,000 prescriptions for medications that require renal dose adjustment in patients with renal impairment identified that a prescriber- and pharmacist-based CDSS provided comparable, low rates of potential medication errors. Enhancements to the prescriber-based CDSS should focus on improvement of continuous surveillance of patients with renal impairment and to identify those who develop renal impairment while receiving a medication that requires renal dose adjustment. In addition, future studies should be undertaken to examine cost-effectiveness and patient benefits of a prescriber-based CDSS and examine the effect of layering a prescriber-based and pharmacist-based CDSS in combination.
Acknowledgements
Financial support for this project was provided by the KPCO Pharmacy Department. We wish to thank Thomas J Koehler, RPh for his contribution of programming the CDSS renal dose adjustment alerts in the EHR and Assistant KPCO Nephrology Regional Department Chief Brent Arnold, MD for his leadership in developing and sponsoring the prescriber-based CDSS.
Footnotes
Clinical Relevance Statement
The presented data compared prescriber- and pharmacist-based CDSS on their ability to prevent potential medication errors for medications that require renal dose adjustment in patients with renal impairment. That the prescriber-based CDSS provided comparable, low rates of potential medication errors is important as the Office of the National Coordinator for Health Information Technology recommends that prescriber-based CDSS in an EHR be the national standard.
Conflicts of Interest
The authors declare that they have no conflicts of interest in the research.
Human Subjects Protection
The study was performed in compliance with the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects and was deemed exempt from review by the KPCO Institutional Review Board since it was performed during routine work by the KPCO Pharmacy Department.
References
- 1.Joosten H, Drion L, Boogerd K, Van der Pijl E V, Slingerland RJ, Slaets J P, Jansen TJ, Schwantje Gans RO, Bilo HJ. Optimizing drug prescribing and dispensing in subjects at risk for drug errors due to renal impairment: improving drug safety in primary healthcare by low eGFR alerts. BMJ Open 2013; 3: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yap C, Dunhan D, Thompson J, Baker D. Medication dosing errors for patients with renal insufficiency in ambulatory care. Jt Comm J Qual Patient Saf 2005; 31: 514-521. [DOI] [PubMed] [Google Scholar]
- 3.Farag A, Garg A, Li L, Jain AK. Dosing errors in prescribed antibiotics for older persons with CKD: a retrospective time series analysis. Am J Kidney Dis 2014; 63: 422–428. [DOI] [PubMed] [Google Scholar]
- 4.Bhardwaja B, Carroll N, Raebel M, Chester EA, Korner EJ, Rocho BE, Brand DW, Magid DJ. Improving prescribing safety in patients with renal insufficiency in the ambulatory setting: the Drug Renal Alert Pharmacy (DRAP) program. Pharmacotherapy 2011; 31: 346–356. [DOI] [PubMed] [Google Scholar]
- 5.Patel HR, Prunchnicki MC, Hall LE. Assessment for chronic kidney disease service in high-risk patient at community health clinics. Ann Pharmacother 2005; 39: 22-27. [DOI] [PubMed] [Google Scholar]
- 6.Long CL, Rabel MA, Price DW, Magid DJ. Compliance with dosing guidelines in patients with chronic kidney disease. Ann Pharmacother 2004; 38: 853-858. [DOI] [PubMed] [Google Scholar]
- 7.Erler A, Beyer M, Petersen J, Saal K, Rath T, Rochon J, Haefeli WE, Gerlach FM. How to improve drug dosing for patients with renal impairment in primary care – a cluster-randomized controlled trial. BMC Fam Pract 2012; 13: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tawadrous D, Shariff SZ, Haynes RB, Iansavichus AV, Jain AK, Garg AX. Use of clinical decision support systems for kidney-related prescribing: a systematic review. Am J Kidney Dis 2011; 58: 903-914. [DOI] [PubMed] [Google Scholar]
- 9.Boussaid A, Caruba T, Karras A. Validity of a clinical decision rule-based alert system for drug dose adjustments in patients with renal failure intended to improve pharmacist’s’ analysis of medication orders in hospitals. IJMI 2013; 82: 964–972. [DOI] [PubMed] [Google Scholar]
- 10.Terrell KM, Perkins AJ, Hui SL, Callahan CM, Dexter PR, Miller DK. Computerized decision support for medication dosing in renal insufficiency: a randomized, controlled trial. Ann Emerg Med 2010; 56: 623-629. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen AL, Henriksen DP, Marinakis C, Hellebek A, Birn H, Nybo M, Sondergaard J, Nymark A, Pedersen C. Drug dosing in patients with renal insufficiency in a hospital setting using electronic prescribing and automated reporting of estimated glomerular filtration rate. Basic Clin Pharmacol Toxicol 2014; 114: 407-413. [DOI] [PubMed] [Google Scholar]
- 12.Wang HY, Lu CL, Wu MP, Huang M, Huang Y. Effectiveness of an integrated CPOE decision-supporting system with clinical pharmacist monitoring practice in preventing antibiotic dosing errors. Int J Clin Pharmacol Ther 2012; 50: 375-382. [DOI] [PubMed] [Google Scholar]
- 13.Kuperman GJ, Bobb A, Payne T, Avery A, Gandhi TK, Burns G, Classen DC, Bates DW. Medication – related clinical decision support in computerized provider order entry systems: a review. J Am Med Inform Assoc 2007; 14: 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cockcroft DW, Gault HM. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31-41. [DOI] [PubMed] [Google Scholar]
- 15.National Kidney Disease Education Program. CKD and drug dosing: information for providers. 2015. [cited December 2015]; Available from: http://www.niddk.nih.gov/health-information/health-communication-programs/nkdep/a-z/ckd-drug-dosing/Documents/ckd-drug-dosing-508.pdf. [Google Scholar]
- 16.Hudson JQ, Nyman HA. Use of estimated glomerular filtration rate for drug dosing in the chronic kidney disease patient. Curr Opin Nephrol Hypertens 2011; 20: 482–491. [DOI] [PubMed] [Google Scholar]
- 17.Levey A, Bosch JP, Lewis JB, Greene T, Nancy R, Roth D. A more accurate method to estimate glomerular filtration rate. Ann Intern Med 1999; 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 18.Nyman HA, Dowling TC, Hudson JQ, Peter WL, Joy MS, Nolin TD. Comparative evaluation of the Cock-croft-Gault equation and the modification of the diet in renal disease (MDRD) study equation for drug dosing: An opinion of the Nephrology Practice and Research Network of the American College of Clinical Pharmacy. Pharmacotherapy 2011; 31: 1130–1144. [DOI] [PubMed] [Google Scholar]
- 19.Jones G. Estimating renal function for drug dosing decisions. Clin Biochem Rev 2011; 32: 81–88. [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens LA, Nolin TD, Richardson MM, Feldman HI, Lewis JB, Rodby R, Townsend R, Okparavero A, Zhang YL, Schmid CH, Levey AS. Comparison of drug dosing recommendations based on measured GFR and kidney function estimating equations. Am J Kidney Dis 2009: 54: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Awdishu L, Coates CR, Lyddane A, Tran K, Daniels CE, Lee J, El-Kareh R. The impact of real-time alerting on appropriate prescribing in kidney disease: a cluster randomized controlled trial. J Am Med Inform Assoc 2016; 23: 609–616. [DOI] [PubMed] [Google Scholar]
- 22.Zand L, McKian KP, Qian Q. Gabapentin toxicity in patients with chronic kidney disease: a preventable cause of mobidity. Am J Med 2010; 123: 367–373. [DOI] [PubMed] [Google Scholar]
- 23.Mounsey J P, DiMarco J P. Dofetilide. Circulation. 2000; 102: 2665–2670. [DOI] [PubMed] [Google Scholar]
- 24.Cho I, Slight SP, Nanji KC, Seger DL, Maniam N, Dykes PC, Bates DW. Understanding physicians‘ behavior toward alerts about nephrotoxic medications in outpatients: a cross-sectional analysis. BMC Nephrol 2014;15: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isaac T, Weissman JS, Davis RB, Massagli M, Cyrulik A, Sands DZ, Weingart SN. Overrides of medication alerts in ambulatory care. Arch Intern Med 2009; 169: 305-311. [DOI] [PubMed] [Google Scholar]
- 26.Weingart SN, Toth M, Sands DZ, Aronson MD, Davis RB, Phillips RS. Physicians’ decisions to override computerized drug alerts in primary care. Arch Intern Med 2003; 163: 2625-2631. [DOI] [PubMed] [Google Scholar]
- 27.Galanter WL, Moja J, Lambert BL. Using computerized provider order entry and clinical decision support to improve prescribing in patients with decreased GFR. Am J Kidney Dis 2010; 56: 809-812. [DOI] [PubMed] [Google Scholar]
- 28.The Office of the National Coordinator for Health Information Technology. Self assessment. Computerized provider order entry with decision support. 2014. [cited December 2015]; Available from: https://www.healthit.gov/sites/safer/files/guides/SAFER_CPOE_sg007_form.pdf. [Google Scholar]
- 29.Garg A, Adhikari N, McDonald H, Rosas-Arellano M, Devereaux PJ, Beyene J, Sam J, Haynes RB. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes. JAMA 2005; 293: 1223–1238. [DOI] [PubMed] [Google Scholar]
- 30.Field TS, Rochon P, Lee M, Gavendo L, Subramanian S, Hoover S, Baril J, Gurwitz J. Costs associated with developing and implementing a computerized clinical decision support system for medication dosing for patients with renal insufficiency in the long-term care setting. J Am Med Inform Assoc 2008; 15: 466-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150. [Google Scholar]