Abstract
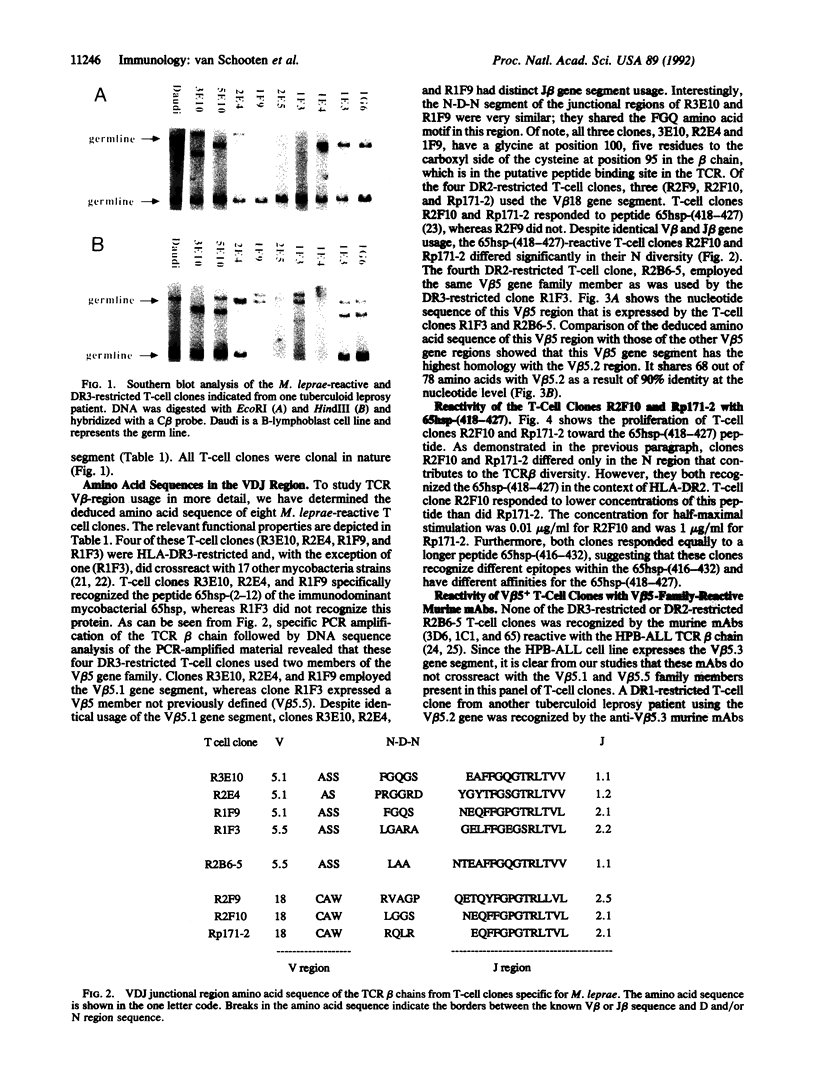
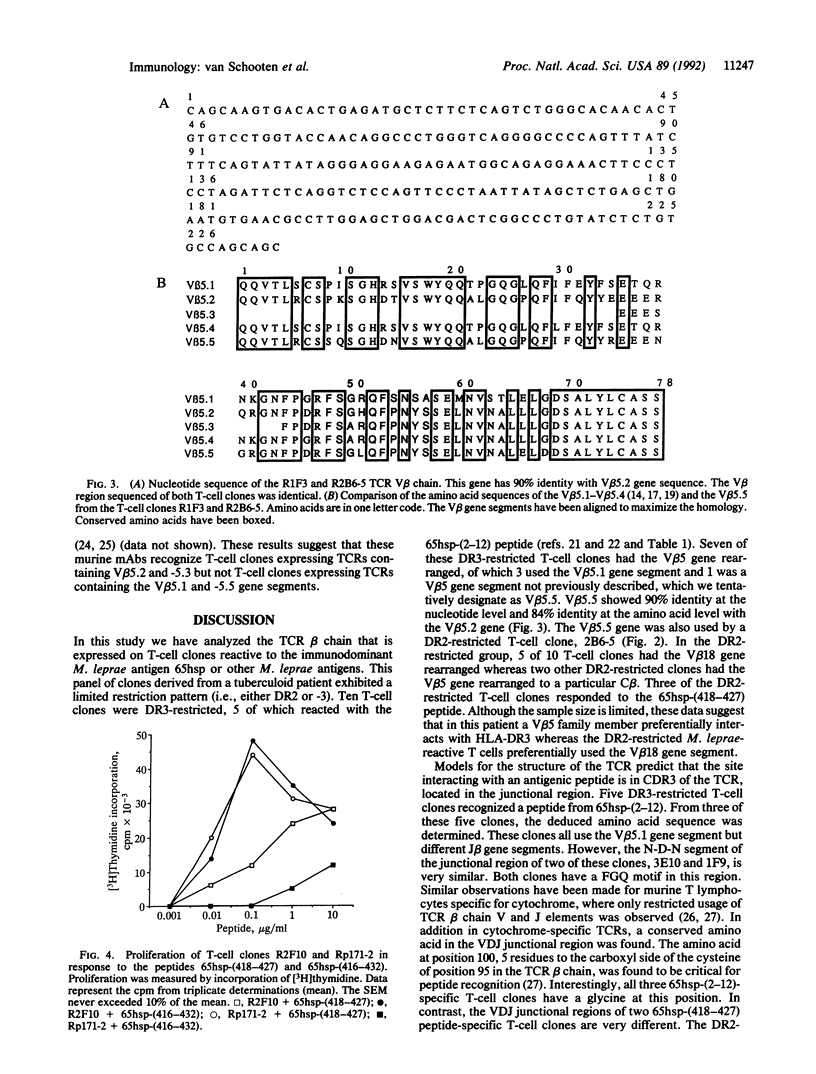
The beta chain of the T-cell antigen receptor present on 20 T-cell clones isolated from a tuberculoid leprosy patient was studied by gene rearrangement and PCR analysis. These T-cell clones all responded to Mycobacterium leprae-encoded protein antigens, and 8 of them specifically recognized peptides of the mycobacterial 65-kDa heat shock polypeptide (65hsp). All T-cell clones studied were HLA-DR-restricted (DR2 or -3). In the DR3-restricted group, 7 of 10 used a beta-chain variable region V beta 5 gene family member, whereas in the DR2-restricted group, 2 of 10 T-cell clones used a V beta 5 gene segment and 5 used the V beta 18 gene segment. The deduced amino acid sequences of the beta chain from 8 T-cell clones have revealed that 3 of 4 DR3-restricted T-cell clones expressed the V beta 5.1 gene segment whereas the fourth DR3-restricted T-cell clone employed a V beta 5 family member not previously described. The V beta 5.1-positive T-cell clones all recognized the same 65hsp peptide from residues 2 to 12. The N-D-N segment (where D is diversity) of the junctional region of these T-cell clones was very similar, despite different beta-chain joining gene segments. Of the 4 DR2-restricted T-cell clones investigated, 3 used the V beta 18 gene segment and recognized the 65hsp peptide from residues 418 to 427. In conclusion, within this panel of M. leprae-reactive T-cell clones, the DR3-restricted T-cell clones mainly used a V beta 5 gene segment, whereas the DR2-restricted clones employed preferentially the V beta 18 gene segment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., van Schooten W. C., Barry M. E., Janson A. A., Buchanan T. M., de Vries R. R. A Mycobacterium leprae-specific human T cell epitope cross-reactive with an HLA-DR2 peptide. Science. 1988 Oct 14;242(4876):259–261. doi: 10.1126/science.2459778. [DOI] [PubMed] [Google Scholar]
- Borst J., Spits H., Voordouw A., de Vries E., Boylston A., de Vries J. E. A family of T-cell receptor molecules expressed on T-cell clones with different specificities for allomajor histocompatibility antigens. Hum Immunol. 1986 Dec;17(4):426–442. doi: 10.1016/0198-8859(86)90302-2. [DOI] [PubMed] [Google Scholar]
- Boylston A. W., Borst J., Yssel H., Blanchard D., Spits H., de Vries J. E. Properties of a panel of monoclonal antibodies which react with the human T cell antigen receptor on the leukemic line HPB-ALL and a subset of normal peripheral blood T lymphocytes. J Immunol. 1986 Jul 15;137(2):741–744. [PubMed] [Google Scholar]
- Chothia C., Boswell D. R., Lesk A. M. The outline structure of the T-cell alpha beta receptor. EMBO J. 1988 Dec 1;7(12):3745–3755. doi: 10.1002/j.1460-2075.1988.tb03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Levitt M., Amit A. G., Mariuzza R. A., Phillips S. E., Poljak R. J. The predicted structure of immunoglobulin D1.3 and its comparison with the crystal structure. Science. 1986 Aug 15;233(4765):755–758. doi: 10.1126/science.3090684. [DOI] [PubMed] [Google Scholar]
- Claverie J. M., Prochnicka-Chalufour A., Bougueleret L. Implications of a Fab-like structure for the T-cell receptor. Immunol Today. 1989 Jan;10(1):10–14. doi: 10.1016/0167-5699(89)90058-3. [DOI] [PubMed] [Google Scholar]
- Concannon P., Pickering L. A., Kung P., Hood L. Diversity and structure of human T-cell receptor beta-chain variable region genes. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6598–6602. doi: 10.1073/pnas.83.17.6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danska J. S., Livingstone A. M., Paragas V., Ishihara T., Fathman C. G. The presumptive CDR3 regions of both T cell receptor alpha and beta chains determine T cell specificity for myoglobin peptides. J Exp Med. 1990 Jul 1;172(1):27–33. doi: 10.1084/jem.172.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Dembić Z., Haas W., Weiss S., McCubrey J., Kiefer H., von Boehmer H., Steinmetz M. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature. 1986 Mar 20;320(6059):232–238. doi: 10.1038/320232a0. [DOI] [PubMed] [Google Scholar]
- Engel I., Hedrick S. M. Site-directed mutations in the VDJ junctional region of a T cell receptor beta chain cause changes in antigenic peptide recognition. Cell. 1988 Aug 12;54(4):473–484. doi: 10.1016/0092-8674(88)90068-2. [DOI] [PubMed] [Google Scholar]
- Fink P. J., Matis L. A., McElligott D. L., Bookman M., Hedrick S. M. Correlations between T-cell specificity and the structure of the antigen receptor. Nature. 1986 May 15;321(6067):219–226. doi: 10.1038/321219a0. [DOI] [PubMed] [Google Scholar]
- Haanen J. B., Ottenhoff T. H., Voordouw A., Elferink B. G., Klatser P. R., Spits H., De Vries R. R. HLA class-II-restricted Mycobacterium leprae-reactive T-cell clones from leprosy patients established with a minimal requirement for autologous mononuclear cells. Scand J Immunol. 1986 Jan;23(1):101–108. doi: 10.1111/j.1365-3083.1986.tb01947.x. [DOI] [PubMed] [Google Scholar]
- Hochgeschwender U., Simon H. G., Weltzien H. U., Bartels F., Becker A., Epplen J. T. Dominance of one T-cell receptor in the H-2Kb/TNP response. Nature. 1987 Mar 19;326(6110):307–309. doi: 10.1038/326307a0. [DOI] [PubMed] [Google Scholar]
- Leiden J. M., Strominger J. L. Generation of diversity of the beta chain of the human T-lymphocyte receptor for antigen. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4456–4460. doi: 10.1073/pnas.83.12.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipoldova M., Boylston A. W., Yssel H., Owen M. J. T-cell receptor V beta 5 usage defines reactivity to a human T-cell receptor monoclonal antibody. Immunogenetics. 1989;30(3):162–168. doi: 10.1007/BF02421201. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Schneider R., Lees R. K., Howe R. C., Acha-Orbea H., Festenstein H., Zinkernagel R. M., Hengartner H. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988 Mar 3;332(6159):40–45. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- Oksenberg J. R., Stuart S., Begovich A. B., Bell R. B., Erlich H. A., Steinman L., Bernard C. C. Limited heterogeneity of rearranged T-cell receptor V alpha transcripts in brains of multiple sclerosis patients. Nature. 1990 May 24;345(6273):344–346. doi: 10.1038/345344a0. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Klatser P. R., Ivanyi J., Elferink D. G., de Wit M. Y., de Vries R. R. Mycobacterium leprae-specific protein antigens defined by cloned human helper T cells. Nature. 1986 Jan 2;319(6048):66–68. doi: 10.1038/319066a0. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. H., Haberman A. M., Gerhard W., Caton A. J. Structure-function relationships among highly diverse T cells that recognize a determinant from influenza virus hemagglutinin. J Exp Med. 1990 Dec 1;172(6):1643–1651. doi: 10.1084/jem.172.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schooten W. C., Elferink D. G., Van Embden J., Anderson D. C., De Vries R. R. DR3-restricted T cells from different HLA-DR3-positive individuals recognize the same peptide (amino acids 2-12) of the mycobacterial 65-kDa heat-shock protein. Eur J Immunol. 1989 Nov;19(11):2075–2079. doi: 10.1002/eji.1830191116. [DOI] [PubMed] [Google Scholar]
- Van Schooten W. C., Ottenhoff T. H., Klatser P. R., Thole J., De Vries R. R., Kolk A. H. T cell epitopes on the 36K and 65K Mycobacterium leprae antigens defined by human T cell clones. Eur J Immunol. 1988 Jun;18(6):849–854. doi: 10.1002/eji.1830180604. [DOI] [PubMed] [Google Scholar]
- Wilkinson D., de Vries R. R., Madrigal J. A., Lock C. B., Morgenstern J. P., Trowsdale J., Altmann D. M. Analysis of HLA-DR glycoproteins by DNA-mediated gene transfer. Definition of DR2 beta gene products and antigen presentation to T cell clones from leprosy patients. J Exp Med. 1988 Apr 1;167(4):1442–1458. doi: 10.1084/jem.167.4.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig K. W., Ota K., Endo N., Seidman J. G., Rosenzweig A., Weiner H. L., Hafler D. A. Shared human T cell receptor V beta usage to immunodominant regions of myelin basic protein. Science. 1990 May 25;248(4958):1016–1019. doi: 10.1126/science.1693015. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Anatoniou D., Clark S. P., Yanagi Y., Sangster R., Van den Elsen P., Terhorst C., Mak T. W. Sequence and expression of transcripts of the human T-cell receptor beta-chain genes. Nature. 1984 Dec 6;312(5994):521–524. doi: 10.1038/312521a0. [DOI] [PubMed] [Google Scholar]