Abstract
The expression of miR-143/miR-145 was up-regulated in ischemic stroke (IS), which may be used as biomarkers and/or therapeutic targets for IS. We aimed to investigate the association of rs4705342 and rs4705343 polymorphisms in the promoter of miR-143/145 with risk of IS. The study population comprised 445 patients with IS and 518 controls. The rs4705342 genotype was analyzed by using a TaqMan Assay and the rs4705343 genotype was determined by using a polymerase chain reaction-restriction fragment length polymorphism assay. Relative expression of miR-143/miR-145 was measured by quantitative real-time PCR. We found that the rs4705342 was associated with a decreased risk of IS (TC vs. TT: adjusted OR = 0.74, 95% CI, 0.57–0.97; CC vs. TT: adjusted OR = 0.53, 95% CI, 0.34–0.83). Haplotype analysis showed that the TC haplotype was associated with an increased risk of IS risk (OR = 1.33, 95% CI, 1.01–1.75), whereas the CT haplotype was associated with a decreased risk of IS risk (OR = 0.68, 95% CI, 0.50–0.92). Importantly, patients carrying the rs4705342TC/CC genotypes had a lower level of miR-145 (P = 0.03). We found for the first time that the rs4705342 CC was a protective factor for IS, probably by reducing the level of miR-145.
Atherosclerosis is a chronic inflammatory response of accumulation of white blood cells and proliferation of smooth muscle cell (SMC)1. The pathology of atherosclerosis is complicated, but generally, forms atherosclerotic plaques. The obstruction of heart vessels results in coronary artery disease, and the obstruction of brain vessels results in stroke2,3. In China, stroke is a major cause of death and adult disability, with 2.6 billion new cases and 1.6 billion deaths each year4. Ischemic stroke (IS) is the most common type of stroke, accountable for 43–79% of all strokes4. To date, several non-genetic risk factors have been identified to contribute to the pathogenesis of IS, such as smoking, hypertension, diabetes and heart diseases5,6. Reports involving family and twins studies showed that genetic factors may play key roles in the development of IS7,8,9.
MicroRNAs (miRNAs) are small non-coding single-stranded RNAs, which regulate gene expression and play important roles in various biological functions, such as inflammation, atherosclerosis, and stroke10,11,12,13,14,15,16. miR-143 and miR-145, located on human chromosome 5, are modulators of SMC phenotype. The expression of miR-143 and miR-145 was up-regulated in both atherosclerosis and IS11,17,18,19. Antagomir-mediated prevention of miR-145 level has been found to be atheroprotective13,16. These findings indicate that miR-145 may be used as a biomarker or therapeutic target for IS14.
Previous work has shown that single nucleotide polymorphisms (SNPs) in the promoters of miRNAs may modulate individual’s susceptibility to a variety of human diseases20,21,22,23,24,25,26,27,28,29,30. Recently, several SNPs in the promoter of miR-143/145 cluster have been identified21. Among them, SNPs of rs4705342 T>C and rs4705343 T>C were functional, with the rs4705342T allele having a higher protein-binding affinity and lower promoter activity and the rs4705343C allele displaying a reduced transcriptional activity29,30. Based on this background, we hypothesized that the 2 SNPs may be related to the risk of IS. To test this hypothesis, we performed a case-control study to examine the association of the rs4705342 T>C and rs4705343 T>C and IS risk in a Chinese population. Moreover, expression levels of miR-143/145 in IS patients and their relationship with the 2 SNPs were also explored.
Materials and Methods
Study population
The study protocol was approved by the Ethical Committee of the Affiliated Hospital of Youjiang Medical University for Nationalities. The experiments were performed in accordance with relevant guidelines and regulations. The study population included 518 controls and 445 patients with IS from the department of neurology, Affiliated Hospital of Youjiang Medical University for Nationalities, Guangxi, China between October 2010 and December 2014. Detailed information of inclusion criteria was described in our previous work31. Briefly, an established diagnosis of IS was determined according to clinical manifestations and cranial magnetic resonance imaging and/or computed tomography scans. Patients with hemorrhagic, autoimmune or chronic inflammatory diseases were excluded. There were 308 men and 137 women with a mean age (± standard deviation, SD) of 60.1 (±11.0) years in the IS group. Control subjects were healthy volunteers who were selected from physical examination center of the hospital during the same period. The controls were frequency matched to cases on the basis of age and gender. Clinical information, such as hypertension, diabetes, fasting serum levels of total cholesterol (TCH), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) was abstracted from medical record review. All participants were unrelated Han Chinese who were consecutively selected from the same geographic region. Informed consent was obtained from all subjects.
DNA isolation and genotyping
Genomic DNA was extracted from eathylene diamine tetraacetic acid-anticoagulated peripheral blood by using a salting-out method32. Genotyping methods were described in detail previously29,30. Briefly, the rs4705342 T>C genotype was analyzed by using a TaqMan Assay on an ABI 7900HT real-time PCR System (Applied Biosystems, CA, USA), and the rs4705343 T>C genotype was identified by using a polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay. Two independent research assistants read the results with a blindness of cases and controls. Distilled water was used as a negative control. Ambiguous genotyping results were verified by sequencing analysis.
Quantitative PCR of miRNA-143 and miR-145
Total RNA was isolated from 1ml of plasma of 46 patients and 46 normal controls using a commercial kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. One microgram of total RNA was reverse transcribed into cDNA utilizing reverse transcription kits from Ribobio Corp., Guangzhou, China (ssD809230154 for miR-143 and ssD809230156 for miR-145). After cDNA conversion, quantitative PCR was done using Power SYBR Master Mix and ABI 7900HT real-time PCR machine (Applied Biosystems, CA, USA). The primers were purchased from Ribobio Corp., China (ssD809230846 for miR-143 and ssD809230848 for miR-145). U6 was used as an internal control. Relative expression levels of miR-143 and miR-145 were computed using comparative Ct method (2−ΔCt).
Statistical analysis
Statistical analyses were done by using the SPSS 19.0 statistical software package (SPSS Inc., Chicago, IL, USA). Continuous variables were displayed as mean ± SD. If the data were normally distributed, the Student’s t-test was used; otherwise, Mann-Whitney U test was used. Categorical variables were expressed as proportions and compared by using chi-squared test. Hardy–Weinberg equilibrium (HWE) was tested by chi-squared test. The association of the rs4705342 T>C and rs4705343 T>C polymorphisms and risk of IS was evaluated by odds ratios (ORs) with 95% confidence interval (CIs). Association analysis and haplotype analysis were performed using SNPstats33. ORs were adjusted based on age, gender, hypertension, diabetes mellitus, TCH, TG, HDL-C, and LDL-C using inverse proportional weights analysis. Both average treatment effect (ATE) and average treatment effect on the treated (ATT) were used to estimate adjusted ORs. The statistical significant criteria was considered as P < 0.05.
Results
The clinical characteristics of cases and controls are shown in Table 1. There was no significant difference between the two groups based on age, gender, and HDL-C (P > 0.05). The frequencies of hypertension and diabetes mellitus in the IS group were significantly higher than those in the control group (P < 0.05). Increased levels of TCH, TG, and LDL-C were observed in the cases compared with the controls (P < 0.001).
Table 1. Clinical characteristics of the study population.
| Variables | Controls (n = 518) | IS (n = 445) | P value |
|---|---|---|---|
| Age (years, mean ± SD) | 58.8 (±11.5) | 60.1 (±11.0) | 0.09 |
| Gender (M/F) | 342/176 | 308/137 | 0.29 |
| Hypertension, n (%) | 104 (20.1) | 260 (58.4) | <0.001 |
| Diabetes mellitus, n (%) | 48 (9.3) | 69 (15.5) | 0.003 |
| TCH, mmol/L | 4.69 ± 0.75 | 5.03 ± 0.73 | <0.001 |
| TG, mmol/L | 1.10 ± 0.34 | 1.91 ± 1.12 | <0.001 |
| HDL-C, mmol/L | 1.58 ± 0.35 | 1.56 ± 0.36 | 0.53 |
| LDL-C, mmol/L | 2.28 ± 0.98 | 2.66 ± 0.99 | <0.001 |
IS, ischemic stroke; SD, standard deviation; TCH, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
The association of the rs4705342 T>C and rs4705343 T>C polymorphisms and risk of IS is presented in Table 2. The genotype distributions of the 2 polymorphisms in both cases and controls were in HWE, with P values of 0.56 and 0.95 for the rs4705342 T>C and 0.43 and 0.14 for the rs4705343 T>C. Compared with the rs4705342TT genotype, the rs4705342TC and CC genotypes were associated with decreased risks of IS (TC vs. TT: adjusted OR = 0.74, 95% CI, 0.57–0.97, P = 0.03; CC vs. TT: adjusted OR = 0.53, 95% CI, 0.34–0.83, P = 0.006). However, no significant association of the rs4705343 T>C with IS risk was found (Table 2). After stratification analysis, we also failed to find any association between the two polymorphisms and clinical characteristics of IS (Supplemental Tables I and II).
Table 2. Associaiton between the rs4705342 T>C and rs4705343 T>C polymorphisms and risk of ischemic stroke.
| Polymorphisms | Controls, n = 518 (%) | IS, n = 445 (%) | ATE | P value† | ATT | P value† |
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI)† | Adjusted OR (95% CI)† | |||||
| rs4705342 T>C | ||||||
| TT | 228 (44.0) | 234 (52.6) | 1.00 | 1.00 | ||
| TC | 232 (44.8) | 174 (39.1) | 0.74 (0.57–0.97) | 0.03 | 0.71 (0.49–0.92) | 0.01 |
| CC | 58 (11.2) | 37 (8.3) | 0.53 (0.34–0.83) | 0.006 | 0.51 (0.33–0.81) | 0.004 |
| rs4705343 T>C | ||||||
| TT | 230 (44.4) | 188 (42.2) | 1.00 | 1.00 | ||
| TC | 243 (46.9) | 209 (47.0) | 1.05 (0.81–1.38) | 0.71 | 1.01 (0.74–1.26) | 0.67 |
| CC | 45 (8.7) | 48 (10.8) | 0.93 (0.58–1.49) | 0.75 | 0.71 (0.43–1.16) | 0.17 |
IS, ischemic stroke; ATE, average treatment effect; ATT, average treatment effect on the treated; OR, odds ratio; CI, confidence interval.
†Adjusted by age, gender, hypertension, diabetes mellitus, TCH, TG, HDL-C, and LDL-C.
Haplotype analysis of the rs4705342 T>C and rs4705343 T>C polymorphisms was performed. We found that the 2 polymorphisms were in linkage disequilibrium (LD) (D’ = 0.87, r = 0.34). As shown in Table 3, the TC haplotype was associated with an increased risk of IS risk (OR = 1.33, 95% CI, 1.01–1.75, P = 0.04), whereas the CT haplotype was associated with a decreased risk of IS risk (OR = 0.68, 95% CI, 0.50–0.92, P = 0.012).
Table 3. Haplotype analysis of the rs4705342 T>C and rs4705343 T>C polymorphisms with risk of ischemic stroke.
| Haplotypes | Controls (%) | IS (%) | OR (95% CI) | P value |
|---|---|---|---|---|
| TT | 572 (55.2) | 506 (56.9) | 1.00 | |
| CC | 218 (21.0) | 170 (19.1) | 0.88 (0.70–1.11) | 0.29 |
| TC | 116 (11.2) | 136 (15.3) | 1.33 (1.01–1.75) | 0.04 |
| CT | 130 (12.5) | 78 (8.8) | 0.68 (0.50–0.92) | 0.012 |
IS, ischemic stroke; OR, odds ratio; CI, confidence interval.
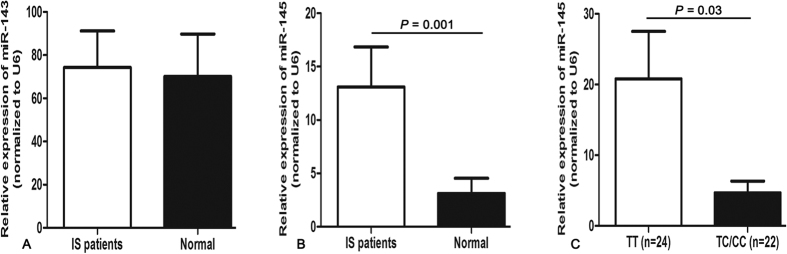
Additionally, plasma levels of miR-143 and miR-145 were examined among cases and controls. Elevated level of miR-145 but not miR-143 was detected in IS patients. Notably, after correlation analysis of the rs4705342 T>C polymorphism with miR-145 expression, we found that patients carrying the rs4705342TC/CC had a lower level of miR-145 compared with those carrying the rs4705342TT (P = 0.03) (Fig. 1). Nevertheless, after comparing the rs4705342 and rs4705343 polymorphisms with relative expression of miR-143 and miR-145 in different gender, we did not find any significant difference (Supplemental Tables III and IV).
Figure 1. Relative expression of miR-143 and miR-145 in ischemic stroke (IS) patients (n = 46) and normal controls (n = 46).
(A) No significant difference of miR-143 between IS patients and normal controls. (B) Increased level of miR-145 in IS patients compared with normal controls (P = 0.001). (C) Decreased level of miR-145 in IS patients carrying the rs4705342TC/CC compared with those carrying the rs4705342TT (P = 0.03). Data are presented as mean ± standard error.
Discussion
This is the first study to evaluate the association between the rs4705342 T>C and rs4705343 T>C polymorphisms in the promoter of miR-143/145 and risk of IS. We demonstrated that the rs4705342 T>C was associated with a reduced risk of IS. The reduced risk was also observed in haplotype analysis. Notably, the rs4705342 T>C was related to abnormal miR-145 expression level in overall analysis but not stratification analysis according to gender. These findings indicate that the rs4705342 T>C may be a protective factor for the etiology of IS.
Atherosclerosis is a major cause of stroke. miR-143 and miR-145 are two SMC-enriched miRNAs, which play important roles in the pathogenesis of atherosclerotic vascular diseases34,35. Increased levels of miR-143 and miR-145 can reduce proliferation of SMCs34,35,36,37. Loss of miR-143 and miR-145 can attenuate the progression of atherosclerosis38. Moreover, circulating miR-145 was significantly increased in IS patients and miR-145 was positively correlated to plasma high-sensitivity C-reactive protein10,11. Taken together, these findings denote that miR-143/145 may be involved in the pathological process of IS. However, not all subjects with altered expression of miR-143/145 develop IS, suggesting that genetic variants may be related to risk of IS.
In 2013, several SNPs in the promoter of miR-143/145 were discovered21. Subsequent reports demonstrated that two of them (i.e., rs4705342 T>C and rs4705343 T>C) were functional with different transcriptional activity29,30. The rs4705343 TC genotype was associated with an increased risk of cervical squamous cell carcinoma30, whereas the rs4705342C allele was associated with a decreased risk of essential hypertension20 and prostate cancer29. In this study, reduced risks of the rs4705342 TC and CC genotypes with IS risk were found. Additionally, the rs4705342C-rs4705343T haplotype had a 0.68-fold decreased risk to develop IS. Previous genome-wide association studies have identified chromosome 5 is a susceptibility loci for IS39,40,41,42. Several coding genes in this region have been reported to link to the risk of IS, such as phosphodiesterase 4D, msh homeobox 2, transforming growth factor beta, and glyceraldehyde-3-phosphate dehydrogenase pseudogene 7140,42. We hypothesized that non-coding genes in this region may be related to the risk of IS. MiR-143/145 located on 5q32 in human. In the current study, we selected the rs4705342 and rs4705343 polymorphisms in the promoter region of miR-143/145 and got the positive results using both ATE and ATT. Base on the above-mentioned observations, the positive results may be biologically reasonable. Further replications are needed to confirm these findings.
With regard to the potential mechanism, we speculated that the rs4705342 T>C may influence the expression of miR-143 or/and miR-145, and eventually result in the protective effect. We then detected the levels of miR-143 and miR-145 and assessed the expression with the rs4705342 T>C polymorphism. The results confirmed our hypothesis. We found that the rs4705342TC/CC genotypes corresponded to a lower level of miR-145, supporting the idea that genetic polymorphism in the promoter of miRNA may influence the expression of mature miRNA, and finally affect individual’s susceptibility to human diseases. Although it is unknown why only miR-145 was differentially expressed in relation to rs4705342 genotypes, one possible explanation might reside in plasma we used to investigate the expression of miR-143/145. Further studies examining the levels of miR-143/145 in tissue samples may justify these results. Moreover, cell-dependent mechanisms may clarify the reason for this different association.
Although our results are promising, some limitations of the study should be discussed. Firstly, relatively small sample size may result in insufficient power to detect the association of the rs4705342 T>C and rs4705343 T>C with IS risk. Further validations are necessary in independent studies. Secondly, this study is based on hospitalized controls, which may lead to a selection bias. Population-based replication studies are valuable to confirm these findings. Finally, genotype-phenotype analysis cannot be done due to lack of data on smoking and alcohol consumption.
In conclusion, this study provides evidence that the rs4705342 T>C in the promoter of miR-143/145 was associated with a decreased risk of IS, probably by reducing the level of miR-145. These findings, if validated in large cohort studies, may help us to understand the precise effect of miRNA-related SNPs on the etiology of IS.
Additional Information
How to cite this article: Wei, Y.-S. et al. An rs4705342 T>C polymorphism in the promoter of miR-143/145 is associated with a decreased risk of ischemic stroke. Sci. Rep. 6, 34620; doi: 10.1038/srep34620 (2016).
Supplementary Material
Acknowledgments
This study was supported by National Natural Science Foundation (No. 81560552 and 81260234).
Footnotes
The authors declare no competing financial interests.
Author Contributions Y.S.W. designed and wrote the manuscript. Y.X., P.H.L. and J.L.W. performed experiments. Y.X. and Y.F.P. collected samples.
References
- Ross R. Atherosclerosis–an inflammatory disease. The New England journal of medicine 340, 115–126, doi: 10.1056/NEJM199901143400207 (1999). [DOI] [PubMed] [Google Scholar]
- Weber C. & Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nature medicine 17, 1410–1422, doi: 10.1038/nm.2538 (2011). [DOI] [PubMed] [Google Scholar]
- Fromm A., Haaland O. A., Naess H., Thomassen L. & Waje-Andreassen U. Atherosclerosis in Trial of Org 10172 in Acute Stroke Treatment Subtypes among Young and Middle-Aged Stroke Patients: The Norwegian Stroke in the Young Study. Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association, doi: 10.1016/j.jstrokecerebrovasdis.2015.12.019 (2016). [DOI] [PubMed] [Google Scholar]
- Liu L., Wang D., Wong K. S. & Wang Y. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke; a journal of cerebral circulation 42, 3651–3654, doi: 10.1161/STROKEAHA.111.635755 (2011). [DOI] [PubMed] [Google Scholar]
- Shinton R. & Beevers G. Meta-analysis of relation between cigarette smoking and stroke. Bmj 298, 789–794 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P. H. et al. Risk factors for ischemic stroke: a prospective study in Rochester, Minnesota. Annals of neurology 22, 319–327, doi: 10.1002/ana.410220307 (1987). [DOI] [PubMed] [Google Scholar]
- Dichgans M. Genetics of ischaemic stroke. The Lancet. Neurology 6, 149–161, doi: 10.1016/S1474-4422(07)70028-5 (2007). [DOI] [PubMed] [Google Scholar]
- MacClellan L. R. et al. Familial aggregation of ischemic stroke in young women: the Stroke Prevention in Young Women Study. Genetic epidemiology 30, 602–608, doi: 10.1002/gepi.20171 (2006). [DOI] [PubMed] [Google Scholar]
- Brass L. M., Isaacsohn J. L., Merikangas K. R. & Robinette C. D. A study of twins and stroke. Stroke; a journal of cerebral circulation 23, 221–223 (1992). [DOI] [PubMed] [Google Scholar]
- Jia L., Hao F., Wang W. & Qu Y. Circulating miR-145 is associated with plasma high-sensitivity C-reactive protein in acute ischemic stroke patients. Cell biochemistry and function 33, 314–319, doi: 10.1002/cbf.3116 (2015). [DOI] [PubMed] [Google Scholar]
- Gan C. S., Wang C. W. & Tan K. S. Circulatory microRNA-145 expression is increased in cerebral ischemia. Genet Mol Res 11, 147–152, doi: 10.4238/2012.January.27.1 (2012). [DOI] [PubMed] [Google Scholar]
- Volny O., Kasickova L., Coufalova D., Cimflova P. & Novak J. microRNAs in Cerebrovascular Disease. Advances in experimental medicine and biology 888, 155–195, doi: 10.1007/978-3-319-22671-2_9 (2015). [DOI] [PubMed] [Google Scholar]
- Koutsis G., Siasos G. & Spengos K. The emerging role of microRNA in stroke. Curr Top Med Chem 13, 1573–1588 (2013). [DOI] [PubMed] [Google Scholar]
- Maitrias P. et al. MicroRNA deregulation in symptomatic carotid plaque. Journal of vascular surgery 62, 1245–1250 e1241, doi: 10.1016/j.jvs.2015.06.136 (2015). [DOI] [PubMed] [Google Scholar]
- Rink C. & Khanna S. MicroRNA in ischemic stroke etiology and pathology. Physiological genomics 43, 521–528, doi: 10.1152/physiolgenomics.00158.2010 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharap A., Bowen K., Place R., Li L. C. & Vemuganti R. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism 29, 675–687, doi: 10.1038/jcbfm.2008.157 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santulli G. microRNAs Distinctively Regulate Vascular Smooth Muscle and Endothelial Cells: Functional Implications in Angiogenesis, Atherosclerosis, and In-Stent Restenosis. Advances in experimental medicine and biology 887, 53–77, doi: 10.1007/978-3-319-22380-3_4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Nazari-Jahantigh M., Neth P., Weber C. & Schober A. MicroRNA-126, -145, and -155: a therapeutic triad in atherosclerosis? Arteriosclerosis, thrombosis, and vascular biology 33, 449–454, doi: 10.1161/ATVBAHA.112.300279 (2013). [DOI] [PubMed] [Google Scholar]
- Santovito D. et al. Overexpression of microRNA-145 in atherosclerotic plaques from hypertensive patients. Expert opinion on therapeutic targets 17, 217–223, doi: 10.1517/14728222.2013.745512 (2013). [DOI] [PubMed] [Google Scholar]
- Fu X., Guo L., Jiang Z. M., Zhao L. S. & Xu A. G. An miR-143 promoter variant associated with essential hypertension. Int J Clin Exp Med 7, 1813–1817 (2014). [PMC free article] [PubMed] [Google Scholar]
- Li L. et al. Association between polymorphisms in the promoter region of miR-143/145 and risk of colorectal cancer. Human immunology 74, 993–997, doi: 10.1016/j.humimm.2013.04.019 (2013). [DOI] [PubMed] [Google Scholar]
- Li L. et al. Interactions of miR-34b/c and TP-53 polymorphisms on the risk of nasopharyngeal carcinoma. Tumour Biol 34, 1919–1923, doi: 10.1007/s13277-013-0736-9 (2013). [DOI] [PubMed] [Google Scholar]
- Gao L. B. et al. A genetic variant in the promoter region of miR-34b/c is associated with a reduced risk of colorectal cancer. Biol Chem 394, 415–420, doi: 10.1515/hsz-2012-0297/j/bchm.just-accepted/hsz-2012-0297/hsz-2012-0297.xml[pii] (2013). [DOI] [PubMed] [Google Scholar]
- Li L. et al. Interactions of miR-34b/c and TP53 Polymorphisms on the Risk of Intracranial Aneurysm. Clin Dev Immunol 2012, 567586, doi: 10.1155/2012/567586 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. et al. Association Between Genetic Polymorphisms in the Promoter Regions of Let-7 and Risk of Papillary Thyroid Carcinoma: A Case-Control Study. Medicine 94, e1879, doi: 10.1097/MD.0000000000001879 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y. et al. Genetic variants in the promoters of let-7 family are associated with an increased risk of major depressive disorder. Journal of affective disorders 183, 295–299, doi: 10.1016/j.jad.2015.04.035 (2015). [DOI] [PubMed] [Google Scholar]
- Sima X., Sun H., Zhou P. & You C. A Potential Polymorphism in the Promoter of Let-7 is Associated With an Increased Risk of Intracranial Aneurysm: A Case-Control Study. Medicine 94, e2267, doi: 10.1097/MD.0000000000002267 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. et al. Pri-Mir-34b/C and Tp-53 Polymorphisms are Associated With The Susceptibility of Papillary Thyroid Carcinoma: A Case-Control Study. Medicine 94, e1536, doi: 10.1097/MD.0000000000001536 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H. et al. A functional variant in miR-143 promoter contributes to prostate cancer risk. Arch Toxicol 90, 403–414, doi: 10.1007/s00204-014-1396-2 (2016). [DOI] [PubMed] [Google Scholar]
- Liang Y. et al. A Functional Polymorphism in the Promoter of MiR-143/145 Is Associated With the Risk of Cervical Squamous Cell Carcinoma in Chinese Women: A Case-Control Study. Medicine 94, e1289, doi: 10.1097/MD.0000000000001289 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y. S., Lan Y., Meng L. Q. & Nong L. G. The association of L-selectin polymorphisms with L-selectin serum levels and risk of ischemic stroke. Journal of thrombosis and thrombolysis 32, 110–115, doi: 10.1007/s11239-011-0587-4 (2011). [DOI] [PubMed] [Google Scholar]
- John S. W., Weitzner G., Rozen R. & Scriver C. R. A rapid procedure for extracting genomic DNA from leukocytes. Nucleic acids research 19, 408 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sole X., Guino E., Valls J., Iniesta R. & Moreno V. SNPStats: a web tool for the analysis of association studies. Bioinformatics 22, 1928–1929, doi: 10.1093/bioinformatics/btl268 (2006). [DOI] [PubMed] [Google Scholar]
- Vengrenyuk Y. et al. Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arteriosclerosis, thrombosis, and vascular biology 35, 535–546, doi: 10.1161/ATVBAHA.114.304029 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M. et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes & development 23, 2166–2178, doi: 10.1101/gad.1842409 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettger T. et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. The Journal of clinical investigation 119, 2634–2647, doi: 10.1172/JCI38864 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riches K. et al. Elevated expression levels of miR-143/5 in saphenous vein smooth muscle cells from patients with Type 2 diabetes drive persistent changes in phenotype and function. Journal of molecular and cellular cardiology 74, 240–250, doi: 10.1016/j.yjmcc.2014.05.018 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala F. et al. MiR-143/145 deficiency attenuates the progression of atherosclerosis in Ldlr-/-mice. Thrombosis and haemostasis 112, 796–802, doi: 10.1160/TH13-11-0905 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gretarsdottir S. et al. Localization of a susceptibility gene for common forms of stroke to 5q12. American journal of human genetics 70, 593–603, doi: 10.1086/339252 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson-Ardnor S. et al. Linkage of ischemic stroke to the PDE4D region on 5q in a Swedish population. Stroke; a journal of cerebral circulation 36, 1666–1671, doi: 10.1161/01.STR.0000174188.04716.8d (2005). [DOI] [PubMed] [Google Scholar]
- Nilsson-Ardnor S. et al. Genome-wide linkage scan of common stroke in families from northern Sweden. Stroke; a journal of cerebral circulation 38, 34–40, doi: 10.1161/01.STR.0000251643.37454.16 (2007). [DOI] [PubMed] [Google Scholar]
- Carty C. L. et al. Meta-Analysis of Genome-Wide Association Studies Identifies Genetic Risk Factors for Stroke in African Americans. Stroke; a journal of cerebral circulation 46, 2063–2068, doi: 10.1161/STROKEAHA.115.009044 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.