Abstract
The role of antigen presenting cells (APCs) in the pathogenesis of autoimmune and other inflammatory diseases is now better understood due to advances in multicolor flow cytometry, gene expression analysis of APC populations, and functional correlation of mouse to human APC populations. A simple but informative nomenclature of conventional and plasmacytoid dendritic cell subsets (cDC1, cDC2, pDC) and monocyte-derived populations incorporates these advances, but accurate subset identification is critical. Ambiguous gating schemes and alterations of cell surface markers in inflammatory condition can make comparing results between studies difficult. Both acute inflammation, such as TLR–ligand stimulation, and chronic inflammation as found in mouse models of autoimmunity can alter DC subset gating. Here, we address these issues using in vivo CpG stimulation as an example of acute inflammation and the non-obese diabetic (NOD) mouse as a model of chronic inflammation. We provide a flow cytometric antibody panel and gating scheme that differentiate 2 monocytic and 3 DC subsets in the spleen both at steady state and after CpG stimulation. Using this method, we observed differences in the composition of NOD DCs that have been previously reported, and newly identified increases in the number of NOD monocyte-derived DCs. Finally, we established a protocol for DC phosphoflow to measure the phosphorylation state of intracellular proteins, and use it to confirm functional differences in the identified subsets. Therefore, we present optimized methods for distinguishing monocytic and DC populations with and without inflammation and/or autoimmunity associated with NOD mice.
Keywords: Dendritic cells, Immune homeostasis, Inflammation, Multicolor flow cytometry, Non-obese diabetic mice, Phosphoflow
1. Introduction
Antigen-presenting cells (APCs) help determine the strength and nature of both the innate and adaptive immune responses by altering the expression of proinflammatory cytokines, chemokines and costimulatory molecules as well as increasing antigen presentation to T cells via major histocompatibility complexes (MHC) in response to environmental cues (Merad et al., 2013). Dendritic cells (DCs) in particular play a vital role in regulating adaptive immune responses by activating pathogen-specific T cells during infection while promoting T cell tolerance to self antigens under homeostatic conditions (Steinman, 2012). Work from many groups has highlighted the heterogeneity within the DC and monocytic compartment (Merad et al., 2013; Guilliams et al., 2014). Dissecting the role each DC subset plays in auto-immunity and other inflammatory diseases is critical for understanding disease pathogenesis and for designing effective immunotherapies.
DCs are broadly characterized by their CD11c and MHCII expression, which can then be further divided using subset-specific markers. A recently proposed framework for classifying the main DC subsets using the nomenclature conventional and plasmacytoid DCs (cDC1, cDC2, pDC) and monocyte-derived populations broadly categorizes both mouse and corresponding human DC subsets based on cell ontogeny and immune function, while permitting variations due to anatomical location and environmental contexts (Guilliams et al., 2014). cDC1 DCs, characterized by their CD8 and/or CD103 expression in mice, cross-present antigen to CD8+ T cells, but can also stimulate CD4+ T cells (Shortman and Heath, 2010; Merad et al., 2013). In contrast, cDC2 DCs primarily drive CD4+ T cell responses due to the prominent expression of MHCII on their cellular surfaces and are typically distinguished by their CD11b expression, but have also been shown to express CD4, CD172a and/or DCIR2 (Dudziak et al., 2007; Lewis and Reizis, 2012; Merad et al., 2013). pDCs, commonly identified by their expression of BST2, B220 and Siglec-H, secrete high levels of type 1 interferon (IFN) upon activation, which in turn activate other downstream immune processes (Merad et al., 2013). Although monocytes do not express MHCII, they do alter immunity via cytokine production (Dominguez and Ardavin, 2010). During inflammation, monocytes can differentiate into monocyte-derived DCs (moDCs) that express MHCII on their surfaces to present antigen to CD4+ T cells; these cells have also been termed activated monocytes, and are usually separated from monocytes by downregulation of Ly6C along with upregulation of MHCII (Tacke et al., 2006; Cheong et al., 2010; Jakubzick et al., 2013). moDCs also produce high amounts of inflammatory cytokines such as TNFα and iNOS during infection (Serbina et al., 2003a,b). In order to understand how these DC populations influence the immune response in mouse studies, separation between these 5 main subsets needs to be well defined.
We are particularly interested in studying DCs from non-obese diabetic (NOD) mice, a strain of mice that is genetically predisposed to autoimmune diabetes. NOD mice begin to spontaneously develop hyperglycemia, a diagnostic marker of diabetes, around 12 weeks of age. This event is caused by beta cell destruction that is preceded by months of insulitis – immune cell infiltration in the pancreatic islets. Although the role of autoreactive T cells is well defined at different stages of disease in NOD mice, the role each specific monocytic and DC subset plays is less clear (Morel, 2013; Price and Tarbell, 2015). Studies have shown that NOD mice have decreased numbers of CD8+ DCs, but increased numbers of pDCs compared to the C57BL/6 strain (Prasad and Goodnow, 2002; Vasquez et al., 2004; Lau-Kilby et al., 2011; Welzen-Coppens et al., 2013), and evidence suggests that both populations contribute to diabetes pathogenesis (Diana et al., 2013; Ferris et al., 2014). At the same time, DCs are also critical for proper maintenance of peripheral self-tolerance via deletion and FoxP3+ regulatory T cells (Tregs) (Lewis and Reizis, 2012; Steinman, 2012). DCIR2+ cDC2s are tolerogenic and induce deletion of autoreactive CD4+ T cells (Price et al., 2015). GM-CSF-derived bone marrow DCs from NOD mice can potently stimulate beta cell-specific Tregs ex vivo that block disease, while NOD cDC subsets may not be able to maintain Treg numbers in vivo (Tarbell et al., 2004, 2007; Price et al., 2014, 2015). Both the ability of DCs to activate pathogenic T cells and maintain peripheral tolerance may be altered in NOD mice due to genetic alterations and/or ongoing inflammation (Price and Tarbell, 2015). Therefore, clear identification of the major DC subsets will facilitate studies to determine the role each subset plays at different stages of autoimmune diabetes pathogenesis.
Using one common scheme of CD11c, MHCII and CD8 expression to identify the different DC subsets does not allow clear separation of the DCIR2-negative monocyte-derived population from the CD11bint DCIR2+ cDC2 population. In this study, we show a flow cytometric antibody panel that allows distinction of 5 APC subsets, namely the pDCs, CD8+ cDC1, DCIR2+ cDC2, monocytes, and moDCs. Gates established in steady-state mice using this panel could also separate these 5 subsets in inflamed (CpG-treated) splenocytes. This panel and gating facilitated the characterization of differences in NOD APCs, including increased moDCs and the previously described decrease in CD8+ cDC1s. We then applied this flow cytometric antibody panel to measure APC function by phosphoflow, focusing on IFNγ-induced Signal Transducer and Activators of Transcription 1 (STAT1) phosphorylation (pSTAT1). Although all 5 subsets increased pSTAT1 after stimulation, differences in the level and timing of pSTAT1 expression were observed, which highlights the functional differences in these populations and the importance of using a gating strategy that allows clear separation of these 5 subsets.
2. Materials and methods
2.1. Mice
We used 8–10 week old female C57BL/6 J (B6), B6.NOD-(D11Mit167) H2g7/DvsJ (B6.g7) and NOD/ShiLtJ (NOD) mice bred in the NIDDK animal facility. All animals were housed in specific pathogen-free conditions and handled according to the Animal Care and Use Committee of NIDDK at the National Institutes of Health. All experiments were performed with three mice per group.
2.2. Intravenous CpG stimulation
For in vivo TLR stimulation, mice were injected intravenously with 200 μl of CpG [PBS + 5 μg CpG-A (2216, Invivogen, San Diego, CA) + 30 μl DOTAP (Roche, Indianapolis, IN)]. Control mice were injected intravenously with 200 μl of PBS (PBS + 30 μl DOTAP). Spleens were then harvested 12 h post-injection, and prepared as described below.
2.3. Collagenase splenic preparation
Spleens were harvested, placed in ice-cold HBSS supplemented with Ca2+ and Mg2+ (Lonza, Walkersville, MD), and then perfused with 100 U/ml Collagenase III (Worthington Biochemical, Corporation Lakewood, NJ). The perfusion was repeated until the entire spleen lost its deep red color. Spleens were then teased apart into small pieces using two forceps and incubated for 20 min at 37 °C. Collagenase activity was then inhibited by the addition of 10 mM EDTA (Corning, Manassas, VA) and incubated for an additional 5 min at 37 °C. Next, samples were passaged through a 60 μm filter (Millipore, Temecula, CA) and washed with 10 ml of ice-cold 2% FBS (Gemini Bio-Products, Woodland, CA). Red blood cells were lysed with 1 ml of ACK Lysis Buffer (Lonza) per spleen, incubated for 2 min at room temperature (RT) and washed as previously described. Cells were passaged through an additional 60 μm filter, counted and re-suspended to a final concentration of 25 × 106 cells/ml with 2% FBS.
2.4. Surface antibody staining
For staining, 5 × 106 cells were blocked with 50 μl of 20 μg/ml anti-CD16/32 (Biolegend, San Diego, CA) for 15 min on ice. Cells were then incubated with 50 μl of appropriate antibodies for 30 min on ice. Bio-tinylated antibodies were detected by additionally staining with 100 μl of Brilliant Violet 510 Streptavidin (BD Biosciences) for 10 min on ice. 2% FBS was used as the diluent for each stain unless indicated otherwise. The concentration of antibodies and other reagents are listed in Table 1. Because binding of 33D1 (anti-DCIR2) is calcium-dependent (Nussenzweig et al., 1982; Dudziak et al., 2007), it is important to not use EDTA in the staining buffer.
Table 1.
Antibody list and surface stain panel.
| Antibody | Conjugate | Clone | Specificity | Concentration | Company |
|---|---|---|---|---|---|
| Common monocyte/dendritic cell surface markers | |||||
| Dead Cell Stain | Aqua | N/A | Dead cells | 0.2 μl/test | Invitrogen |
| CD3ε | Biotin | 145-2C11 | T cells | 5 μg/ml | Biolegend |
| CD19 | Biotin | 6D5 | B cells | 5 μg/ml | Biolegend |
| Ly6G | Biotin | 1A8 | Neutrophils | 5 μg/ml | Biolegend |
| NKp46 | Biotin | 29A1.4 | NK cells | 10 μg/ml | Biolegend |
| CD11b | APC-Cy7 | M1/70 | CD11b | 1 μg/ml | Biolegend |
| CD11c | PE-Cy7 | N418 | CD11c | 1 μg/ml | Biolegend |
| Ly6C | PB | HK1.4 | Ly6C | 2.5 μg/ml | Biolegend |
| Streptavidin | BV510 | N/A | Biotin | 2 μg/ml | BD Bioscience |
| Live monocytic/dendritic cell panel | |||||
| Siglec-H | APC | 551 | pDCs | 2 μg/ml | Biolegend |
| CD4 | PerCP-Cy5.5 | GK1.5 | CD4 | 1 μg/ml | Biolegend |
| CD8α | BV605 | 53–6.7 | CD8α | 1 μg/ml | Biolegend |
| DCIR2 | PE | 33D1 | CD11b+ DCs | 2 μg/ml | Biolegend |
| RT1B | FiTC | OX-6 | MHCII/IAg7 | 2.5 μg/ml | Biolegend |
| Live pDC panel | |||||
| Siglec-H | APC | 551 | pDC marker | 2 μg/ml | Biolegend |
| CD8α | PerCP Cy5.5 | 53–6.7 | CD8α | 1 μg/ml | Biolegend |
| RT1B | FiTC | OX-6 | MHCII/IAg7 | 2.5 μg/ml | Biolegend |
| BST2 | PE | 927 | BST2/PDCA-1 | 2 μg/ml | Biolegend |
| B220 | BV605 | RA3-6B2 | CD45R/B220 | 1 μg/ml | Biolegend |
| Functional monocytic/dendritic cell panel | |||||
| Siglec-H | PerCP-Cy5.5 | 551 | pDCs | 2 μg/ml | Biolegend |
| CD8α | BV605 | 53–6.7 | CD8α | 1 μg/ml | Biolegend |
| DCIR2 | FITC | 33D1 | CD11b+ DCs | 5 μg/ml | Biolegend |
| RT1B | AF647 | OX-6 | MHCII/IAg7 | 1 μg/ml | BD Bioscience |
| CD206 | PE | C068C2 | Mannose receptor | 2 μg/ml | Biolegend |
| CD209 | PE | 5H10 | DC-SIGN | 2 μg/ml | eBioscience |
2.5. Interferon-gamma stimulation and phosphoflow
Phosphoflow experiments were adapted from previously established protocols (Krutzik and Nolan, 2003). Spleens were prepared with collagenase as described in Section 2.3, except washes were performed with 1% BSA instead of 2% FBS. Prior to sample fixation, BSA low in endotoxin and IgG-free (A2058, Sigma Aldrich, St. Louis, MO) was used to prevent any off-target stimulation of cells prior to sample fixation. After cells were counted, samples were resuspended to a concentration of 25 × 106 cells/ml with Phosphoflow Media [Iscoves Media (Lonza) + 1% BSA (IgG-free, low endotoxin) + 20 mM HEPES (Lonza) + 50 μM beta-mercaptoethanol (Sigma Aldrich)]. Splenocytes were then rested for 10 min in a 37 °C water bath. 10 ng/ml of recombinant mouse IFNγ (R&D, Minneapolis, MN) was added to indicated samples and stimulated for either 5 min or 30 min at 37 °C. Samples were immediately fixed in 4% paraformaldehyde (PFA) (Electron Microscopy Science, Hatfield, PA) at RT for 10 min, washed and then resuspended with 100% ice-cold methanol to a concentration of 50 × 106 cells/ml for at least 30 min. (Cells are stable in methanol for several days.) Cells were then rehydrated with 1% BSA (98% heat shock BSA, A9647, Sigma Aldrich) for 10 min at 4 °C, and resuspended to a concentration of 25 × 106 cells/ml. Cells were then stained for the appropriate intracellular and surface markers as previously described. Table 2 lists a summary of phosphoflow antibodies used in this study.
Table 2.
Phosphoflow antibody table.
| Antibody | Conjugate | Clone | Speci3city | Concentration | Company |
|---|---|---|---|---|---|
| Phosphoflow monocytic/dendritic cell panel | |||||
| CD3ε | Biotin | 145-2C11 | T cells | 5 μg/ml | Biolegend |
| CD19 | Biotin | 6D5 | B cells | 5 μg/ml | Biolegend |
| Ly6G | Biotin | 1A8 | Neutrophils | 5 μg/ml | Biolegend |
| NKp46 | Biotin | 29A1.4 | NK cells | 10 μg/ml | Biolegend |
| CD11b | APC-Cy7 | M1/70 | CD11b | 1 μg/ml | Biolegend |
| CD11c | PE-Cy7 | N418 | CD11c | 1 μg/ml | Biolegend |
| Siglec-H | APC | 551 | pDCs | 2 μg/ml | Biolegend |
| CD8α | BV605 | 53–6.7 | CD8α | 1 μg/ml | Biolegend |
| RT1B | FITC | OX-6 | MHCII/IAg7 | 2.5 μg/ml | Biolegend |
| Ly6C | PB | HK1.4 | Ly6C | 2.5 μg/ml | Biolegend |
| STAT1 (pY701) | PE | 4a | pSTAT1 (Y701) | 2.5 μl/test | BD Bioscience |
| Blank* | PerCP-Cy5.5 | N/A | N/A | N/A | N/A |
| Streptavidin | BV510 | N/A | Biotin | 2 μg/ml | BD Bioscience |
| Monocytic/dendritic cell surface markers that work for phosphoflow | |||||
| CD4 | PE/PerCP-Cy5.5 | GK1.5 | CD4 | 1 μg/ml | Biolegend |
| CD172a | APC | P84 | SIRPα | 2 μg/ml | Biolegend |
| CD205 | APC/PE | NLDC-145 | CD8 DCs | 2 μg/ml | Biolegend |
| Monocytic/dendritic cell surface markers that do not work for phosphoflow | |||||
| CD24 | PE | 30-F1 M1/69 | CD24 | 2 μg/ml | Biolegend |
| CD36 | APC | HM36 | CD36 | 2 μg/ml | Biolegend |
| CD49b | PE | DX5 | NK cells | 4 μg/ml | Biolegend |
| CD103 | APC/PE | 2 E7 | CD103 | 2 μg/ml | Biolegend |
| DCIR2 | FITC and PE | 33D1 | CD11b+ DCs | 2 μg/ml | Biolegend |
The PerCP-Cy5.5 channel was kept free to gate out autofluorescent cells.
2.6. Data acquisition and sample analysis
Samples were collected on a BD LSRII flow cytometer with 4 lasers, and analyzed using FlowJo software 9.8.2 (Treestar, Ashland, OR) on a MAC® workstation. The instrument configuration is provided in Table 3. Instrument calibration was checked daily using rainbow fluorescent particles (BD Biosciences). Voltages were set such that the center of the histograms for the unstained control was around 102 Mean Fluorescence Intensity (MFI) units and the positive peaks for the single stain controls were around 104 MFI units. The compensation matrix was calculated using unstained, single stained and fluorescence minus one (FMO) control samples. FMO controls are samples that include all fluorophores, except the color being analyzed, and have been validated as an optimal negative control (Roederer, 2002). MFI from isotype controls were similar to FMO controls (data not shown). A total of 0.5–1 × 106 events were collected from each sample. Doublets were excluded using side scatter (SSC) height versus SSC width. Dead cells (aqua dead cell stain) and lineage-positive [CD3 (T cells), CD19 (B cells), NKp46 (NK cells) and Ly6G (neutrophils)] were excluded from the data analysis by gating on cells negative for Aqua/Brilliant Violet 510 staining. FMO controls were used to set gates.
Table 3.
LSRII configuration.
| LSRII Dector Configuration | |||
|---|---|---|---|
| Laser | Fluorochrome Detected | Dichroic LP Filter | BP Filter |
| Blue 488 nm | Forward Scatter | ||
| Blue 488 nm | PerCP Cy5.5, PerCP | 685LP | 710/50 |
| Blue 488 nm | FITC, CFSE, GFP, Alexa 488, GrViD, Cy2 | 505LP | 515/20 |
| Blue 488 nm | Side Scatter | 488LP | |
| Green 532 nm | PE Cy7, Alexa 750 | 740LP | 780/40 |
| Green 532 nm | PE, Cy3, Alexa 532 | empty | 575/25 |
| Red 633 nm | APC Cy7, Alexa 750 | 740LP | 780/60 |
| Red 633 nm | APC, Alexa 647 | empty | 660/20 |
| Violet 407 nm | QD605 | 595LP | 605/40 |
| Violet 407 nm | QD545, Pacific Orange, Aqua | 535LP | 560/40 |
| Violet 407 nm | Cblue, ViViD, Pacific Blue | empty | 450/50 |
2.7. Statistical analyses
Homoscedastic 2-tailed t-tests were performed for most analyses. Statistical significance is indicated in the figures where * denotes p<0.05, ** denotes p<0.01, and *** denotes p<0.001.
3. Results
3.1. Steady-state myeloid and dendritic cell gating
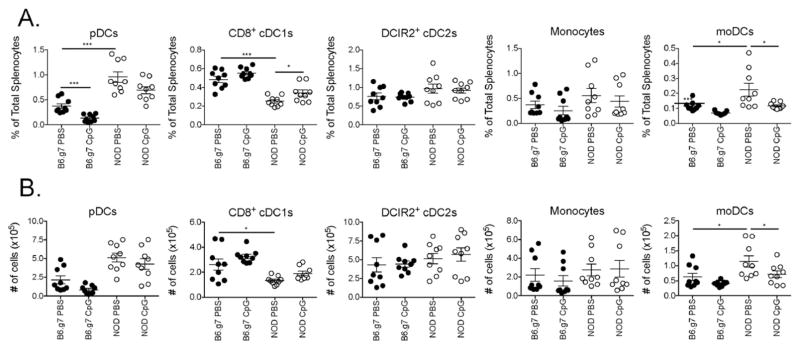
We optimized a flow cytometric antibody panel that is useful for distinguishing the monocytic and DC APC populations in both autoimmune NOD and control B6 mice with and without innate immune stimulation. In order to facilitate direct comparisons between the two strains, B6.g7 mice were used because its MHC region (H2g7) is congenic with the NOD strain, allowing the use of the same MHCII antibody for both strains. With the exception of the percentage of pDCs, a comparison of B6 to B6.g7 mice showed no significant differences in the distribution of APC subsets (Sup. Fig. 1). Consistent with previous reports, NOD mice have more pDCs and fewer CD8+ cDC1s compared to B6 or B6.g7 mice (Prasad and Goodnow, 2002; Vasquez et al., 2004; Lau-Kilby et al., 2011; Welzen-Coppens et al., 2013). We newly identify an increase in monocyte-derived cells in NOD mice.
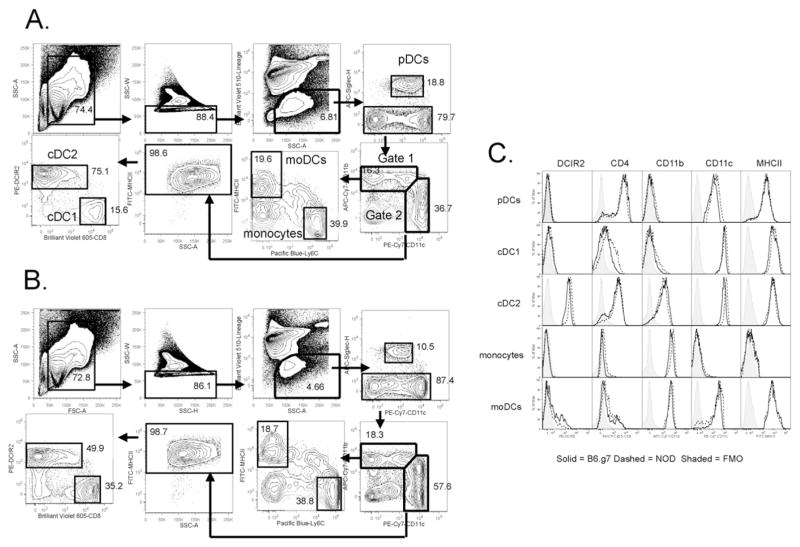
Isolated splenocytes from steady-state mice were stained with the antibodies listed in Table 1 to identify 2 monocytic and 3 DC subsets by flow cytometry. After splenocytes were gated based on forward scatter (FSC-A) versus SSC-A, singlets were identified by SSC-H versus SSC-W and then live lineage (Lin)-negative cells were gated (Fig. 1). The Lin stain included CD3 (T cells), CD19 (B cells), NKp46 (NK cells) and Ly6G (neutrophils). NK cells were identified using NKp46 (CD335) in place of NK1.1 or CD49b because NOD NK cells do not express NK1.1 (Beilke et al., 2012), and CD49b can be expressed on some inflammatory monocytic populations (Gordon and Taylor, 2005). After gating on live Lin− cells, pDCs were identified as being Siglec-H+ CD11cint. NOD pDCs expressed 1.9-fold higher levels of CD11c than B6.g7 pDCs, but still had lower CD11c expression than cDCs (Table 4). Siglec-H negative cells were then plotted for CD11c versus CD11b expression, and two gates were made: 1) CD11bhi cells and 2) CD11chi cells (Fig. 1).
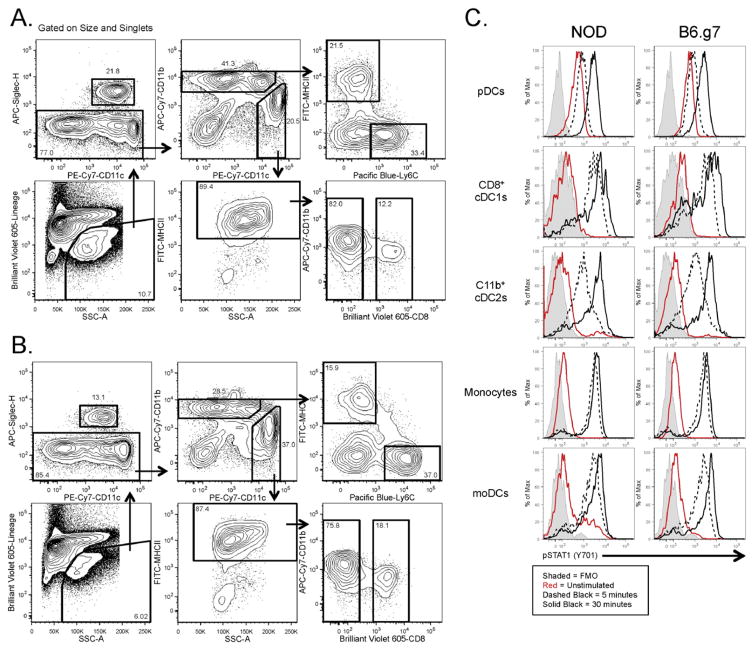
Fig. 1. Flow cytometry analysis of steady–state monocytic and DC spleen populations.
Splenocytes from 8–10 week old (A) NOD or (B) B6.g7 mice were stained, and first gated based on FSC-A and SSC-A, followed by exclusion of doublets. Next, live (Aqua negative) lineage negative (lymphoid and neutrophil lineages) cells were gated. pDCs were identified by Siglec-H expression. Siglec-H negative cells were further analyzed based on CD11b and CD11c expression. CD11bhi cell were further separated into Ly6C+/MHCII− (monocytes) and Ly6C−/MHCII+ (monocyte-derived DCs). CD11chi cells were gated on MHCII+ expression, and subsequently analyzed by CD8 and DCIR2 expression to separate cDC1 CD8+ DCs from cDC2 DCIR2+ DCs. (C) Histograms of key markers are shown for all 5 populations. Representative data shown from more than three independent experiments.
Table 4.
Monocytic and DC phenotype and strain differences.
| Common surface markers between B6.g7 and NOD mice | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pDCs | cDC1s | cDC2s | Monocytes | moDCs | ||||||
| B220 | + | − | − | − | − | |||||
| BST2 | + | − | − | − | − | |||||
| CD4 | + | − | + | − | − | |||||
| CD8α | lo−hi | hi | − | − | − | |||||
| CD24 | − | + | − | − | − | |||||
| CD36 | − | + | − | − | + | |||||
| CD103 | − | + | − | − | − | |||||
| CD205 | − | + | − | − | − | |||||
| CD206 | − | + | − | − | ± | |||||
| CD209 | − | − | − | − | ± | |||||
| Clec9a | − | + | − | − | − | |||||
| DCIR2 | − | − | + | − | − | |||||
| MHCII | int | hi | hi | − | hi | |||||
| Siglec–H | + | − | − | − | − | |||||
| Varying surface markers between B6.g7 and NOD mice
| ||||||||||
| pDCs
|
cDC1s
|
cDC2s
|
Monocytes
|
moDCs
|
||||||
| Strain: | B6 | NOD | B6 | NOD | B6 | NOD | B6 | NOD | B6 | NOD |
|
| ||||||||||
| CD11b | − | − | − | − | 903* | 618 (0.68)** | 4025 | 5347 (1.33) | 3719 | 4218 (1.13) |
| CD11c | 885 | 1687 (1.91) | 7994 | 9563 (1.20) | 8320 | 12928 (1.55) | − | − | 2035 | 1656 |
| CD64 | − | − | 217 | − | − | − | 611 | − | 268 | − |
| CD172a | 268 | 419 (1.56) | − | − | 505 | 797 (1.58) | 424 | 600 (1.42) | 443 | 480 |
| Ly6C | + | −/+ | − | − | − | − | 55042 | 17232 (0.31) | − | − |
Numbers provided are the average MFIs from 3 different mice.
When the difference between B6.g7 and NOD populations are statistically significant, the fold difference can be found in parentheses.
CD11bhi cells (gate 1, Fig. 1) were further subdivided using MHCII and Ly6C expression into MHCII+ Ly6C− monocyte-derived DCs (moDCs) and MHCII− Ly6C+ monocytes. In gate 1, about 20% of the cells were moDCs while approximately 30–35% of the cells were monocytes. Ly6C expression was 3.2-fold lower on NOD monocytes compared to B6.g7 monocytes (Table 4). Some cells in gate 1, especially in B6.g7 mice, express intermediate levels of both Ly6C and MHCII. These cells have been described as a transition phase of Ly6C+ MHCII− monocytes differentiating into the Ly6C− MHCII+ moDCs cells upon activation (Tacke et al., 2006; Jakubzick et al., 2013). The remaining cells stained negatively for both markers (Fig. 1), but expressed intermediate levels of CD11c (data not shown).
CD11chi cells (gate 2, Fig. 1) were confirmed to be cDCs by staining positively for MHCII. The CD11chi MHCII+ cells were then split into two subsets based on DCIR2 and CD8 expression. In NOD mice, DCIR2+ cDC2s constitute about two-thirds of the cDCs while CD8+ cDC1s make up about 15% of this population, a reduction in cDC1s compared to control mice as previously described (Prasad and Goodnow, 2002; Vasquez et al., 2004). Histograms for major DC markers show that most DCIR2+ cDC2s are CD4+ and CD11b+, whereas the CD8+ cDC1s stain negatively for both of these markers (Fig. 1C). Importantly, the DCIR2 marker is largely confined to the cDC2s, and has low or no expression in the monocyte and moDC gates.
The gating scheme shown in Fig. 1 was adopted to optimally separate cDC2s from moDCs; although both are CD11c+ CD11b+ MHCII+, these populations display quite different phenotypes. In some studies, cDCs have been distinguished based on either CD11c expression alone (Sup. Fig. 2A) or by the combination of CD11c and MHCII expression (Sup. Fig. 2B) (Miller et al., 2012; Larsen et al., 2015). These populations were then divided into CD8+ and CD8−cDCs, with the CD8−cDCs corresponding to cDC2 (CD11b+) DCs (Shortman and Heath, 2010; Mildner and Jung, 2014). However, with this gating, the CD8− DC population is a mixed population. Most cells in this gate are DCIR2+, which correspond to cDC2 cells, but some cells are DCIR2 negative with high levels of CD11b, but not CD4 or Ly6C (Sup. Fig. 2A and data not shown). Adding MHCII to the gate (Sup. Fig. 2B) enriched for the DCIR2+ cDC2 subpopulation but the CD11b+ cDC2 gate still contained a minor population that was DCIR2−, CD4−, CD11bhi and Ly6C− that corresponds to the Ly6C− MHCII+ moDCs gated in Fig. 1. Interestingly, these moDCs are slightly higher for CD11b, but slightly lower for CD11c than the DCIR2+ cDC2s, which resulted with the moDCs ending up in gate 1 while the cDC2s ended up in gate 2 (Fig. 1). Because the difference in expression of CD11b and CD11c are small, this separation requires optimal staining and separation for both markers.
3.2. Inflammatory-state myeloid and dendritic cell gating and composition
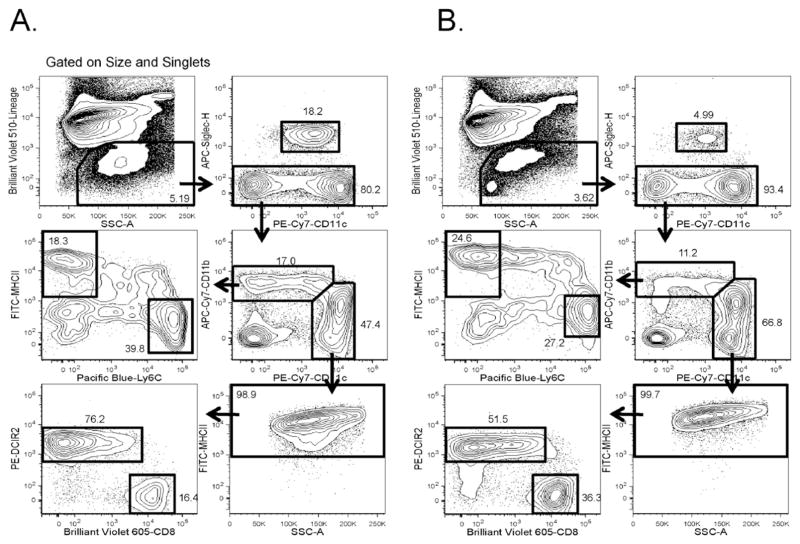
The gating approach described in Fig. 1 can also be used in an inflammatory context such as TLR stimulation. We used a 12 h in vivo stimulation with CpG as one example of an innate inflammatory signal. Splenocytes isolated from CpG-treated NOD and B6.g7 mice were stained, and the gates established with unstimulated splenic samples were used to analyze inflamed splenocytes (Fig. 2). The gates remained qualitatively similar between homeostatic and inflamed conditions, but CpG treatment did alter the composition of splenocytes, resulting in a 25% loss in both NOD and B6.g7 live, Lin− myeloid cells. This correlated with a 59% decrease in B6.g7 pDCs, but a 7% decrease in NOD pDCs. Differences in subset distribution between NOD and B6.g7 mice and with or without CpG stimulation are shown in Fig. 3. Within gate 1 (Fig. 1), CpG treatment significantly reduced the number of moDCs in NOD mice, and the same trend was observed in B6.g7 mice. The reduction of these cells in the spleen could be due to either maturation-induced cell death or migration, or a phenotypic alteration of the cells (Asselin-Paturel et al., 2005; Swirski et al., 2009). Although not quantified as a separate population here, the percentage of transitional Ly6C+ MHCII+ cells increased as expected because immune activation triggers the differentiation of Ly6C+ MHCII− cells to Ly6C− MHCII+ cells (Jakubzick et al., 2013). Cells within gate 2 displayed fewer changes with CpG but, a significant increase in the percentage of CD8+ cDC1s was detected (Fig 3). Although these observed changes are specific to the stimulus and time points measured (and other stimuli will have different changes in these markers), these data suggest that some markers are more robust in an inflammatory setting.
Fig. 2. Flow cytometry analysis of monocytic and DC spleen populations after CpG stimulation.
Splenocytes from 8–10 week old (A) NOD or (B) B6.g7 mice treated with CpG for 12 h. Splenocytes were then gated as described in Fig. 1 to identify monocytic and DC populations. Representative data shown from more than three independent experiments.
Fig. 3. Cellular composition of monocytic and DC spleen populations in NOD and B6.g7 mice.
The percentage (A) and the number (B) of total splenocytes for each population described in Fig. 1 is shown for 8–10 week old NOD and B6.g7 mice treated with and without CpG as indicated. The data shown was pooled from three separate experiments. Each dot represents 1 mouse.
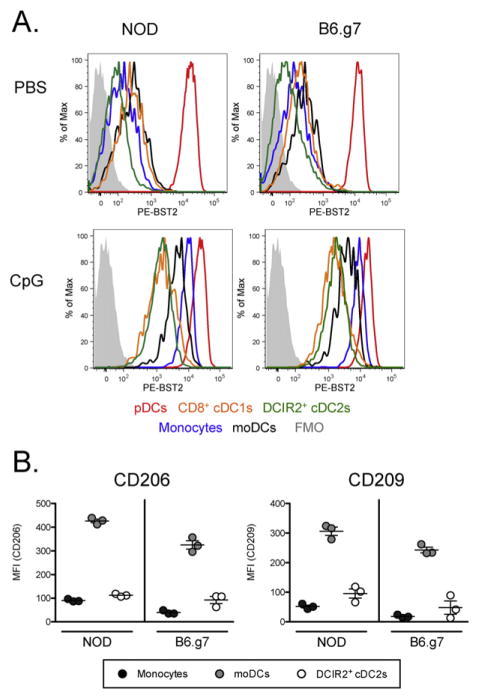
3.3. Differences between moDCs and cDC2: expression of CD206 and CD209, and upregulation of BST2 with inflammation
Under homeostatic conditions, most cells with high levels of BST2/PDCA-1 are pDCs, but after 12 h of CpG stimulation, other APCs, especially monocytes and moDCs upregulate BST2 (Fig. 4 and Sup. Fig. 4). Thus, BST2 is not a good marker for identifying pDCs in this inflammatory context, and even without stimulation, BST2 is not as specific a marker as Siglec-H (Fig. 4A). The differential expression of BST2 after CpG stimulation shows another phenotypic difference between moDCs (MFI = 3504) and cDC2 cells (MFI = 1308).
Fig. 4. Differential expression of BST2, CD206 and CD209 on monocytic and DC spleen populations.
(A) 8–10 week old NOD and B6.g7 mice were treated with either PBS or CpG. After 12 h, splenocytes were harvested and stained for BST2 in addition to the stain described in Fig. 1. BST2 expression for the populations gated in Figs. 1 and 2 are represented by overlayed histograms in the indicated colors. The FMO control is shown (filled grey). (B) The expression of CD206 and CD209 was measured on Ly6C+ monocytes (black dot), MHCII+ moDCs (gray dot) and cDC2 DCIR2+ DCs (white dot) from 8–10 week old NOD and B6.g7 mice. The data shown is representative of two separate experiments.
To confirm that the moDCs we have gated are positive for other described markers for this population, splenocytes were stained for CD206 (Mannose Receptor) and CD209 (DC-Sign) expression. Although both monocytes and DCIR2+ cDC2 cells express low amounts of CD206 and CD209, Ly6C−MHCII+ CD11bhi moDCs exhibited the highest levels of CD206 and CD209 expression (Fig. 4B). These results are consistent with other published reports (Cheong et al., 2010), and support the conclusion that Ly6C− MHCII+ CD11bhi cells are moDCs and distinct from cDC2s.
3.4. Functional analysis of subsets: phosphoflow after in vitro stimulation
One practical application of this gating strategy is the study of the signaling pathways present in these 5 subsets using intracellular phosphoflow staining (Krutzik and Nolan, 2003). We optimized isolation conditions and antibodies for use with the necessary PFA fixation and methanol permeabilization. Although the 33D1 epitope was no longer available following PFA/methanol treatment, the cDC2s were distinguished by gating on CD11chi MHCII+ CD8− CD11bint cells, and the moDCs by CD11cint MHCII+ CD8− CD11bhi (Fig. 5). cDC2, but not moDCs, expressed CD4, confirming this gating separation (data not shown). Other markers had slightly altered staining properties with this protocol, but still could be used with similar gates as with fresh cells, whereas other related markers were not useful with PFA/methanol-treated cells (Fig. 5A and Table 2). This protocol was then used to measure phosphorylated STAT1 after stimulating splenocytes in vitro with IFNγ for either 5 or 30 min and identify differential responses in the 5 subsets. pDCs had higher basal pSTAT1 but took longer to upregulate it after IFNγ stimulation. After 30 min of stimulation, both cDC populations continued to phosphorylate STAT1 whereas the moDCs and monocytes exhibited relatively little increase in pSTAT1 levels beyond the 5 min time point (Fig 5B). Therefore, this confirms functional differences between carefully gated cDC2 and moDC populations.
Fig. 5. Phosphoflow for monocytic and DC populations.
Splenocytes were harvested and pooled from 8–10 week old NOD and B6.g7 mice for signaling experiments. The gating scheme for the fixed and permeabilized cells isolated from (A) NOD mice and (B) B6.g7 mice. (C) Splenocytes were stimulated in vitro with 10 ng/ml IFN-gamma for 5 min (dashed black) and 30 min (solid black), and then stained intracellularly for pSTAT1. Unstimulated cells are represented by solid red line. Fluorescence minus one is represented by solid gray histogram. The data shown is representative of three separate experiments.
4. Discussion
The data presented here characterizes a gating approach that can be used to distinguish 5 key monocytic and DC populations in both NOD and B6 mice with and without acute inflammation. Many studies have used gating schemes that lump all CD11c+ CD11b+ cells together, yet evidence shows clear functional and phenotypic differences between the two populations (Merad et al., 2013; Guilliams et al., 2014; Mildner and Jung, 2014). Although both moDCs and cDC2s can present antigen, cDCs are important for initiation of new T cell responses in lymphoid tissues whereas moDC are more important in the context of ongoing inflammation (Serbina et al., 2003a,b; Rydstrom and Wick, 2007; Lewis and Reizis, 2012; Merad et al., 2013). We focus our analysis here on spleen DCs, and DCIR2 is less apparent in DCs from other sites, but as we show in Fig. 5, careful gating with the levels of CD11b and CD11c also help separate these cells, along with other distinguishing markers such as CD4 on cDC2 and CD206 and CD209 in moDCs (Cheong et al., 2010; Lewis and Reizis, 2012; Merad et al., 2013). In mouse strains other than NOD, CD64 is also a useful marker for separating moDCs from cDC2 (Tan et al., 2003). In addition, the use of Siglec-H allowed proper identification of pDCs in both steady-state and inflammatory conditions, and was more specific than either B220 or BST2 even without stumulation. This approach yielded a single homogenous population that expressed the B220, BST2 and CD11c surface antigens.
From this analysis of 5 DC and monocyte populations in both the steady-state and with acute inflammation, we can summarize differences that occur in autoimmune-prone NOD mice (Fig. 3). As described previously, NOD mice have more pDCs, and retain more of them after CpG treatment compared to B6.g7, and NOD mice have fewer CD8+ cDC1s (Prasad and Goodnow, 2002; Vasquez et al., 2004; Lau-Kilby et al., 2011; Welzen-Coppens et al., 2013). We newly describe higher numbers of moDCs in NOD mice compared to B6.g7 that decrease with CpG stimulation. Increases in this monocyte-derived population could be a result of or cause of the increases autoimmunity in NOD mice. We also observe lower or no expression of Ly6C and CD64 respectively (Table 4) (Philbrick et al., 1990; Tan et al., 2003; Nikolic et al., 2005). NOD neutrophils are Ly6G positive as expected but do not express Ly6C (data not shown). NOD pDCs are comprised of two populations—a Ly6C+ and a Ly6C−population, whereas all B6.g7 pDCs express Ly6C. Other markers also showed differential expression in NOD DCs. For example, CD11c and CD172a (Sirpα) are generally higher in NOD DCs. This gating scheme allows clean separation of some key populations for further functional analysis of differences between populations and between strains.
In summary, we describe protocols that are capable of separating distinct DC subsets in both homeostatic and inflammatory contexts, and use this scheme to identify alterations in these subsets in NOD mice and with CpG treatment. We have also adapted an already-established protocol to study signaling mechanisms within these myeloid subsets, which will allow us to better understand how these subsets regulate the strength and nature of the adaptive immune response.
Supplementary Material
Acknowledgments
We would like to acknowledge Alice Franks (Diabetes, Endocrinology, and Obesity Branch, NIDDK) for help with mouse husbandry and the NIDDK/NHLBI flow core and Dr. Phil McCoy for flow cytometry support. We thank the member of the Tarbell Lab: Dr. Chie Iwamura, Dr. William Coley and Dr. Yongge Zhao for helpful discussions. This work was supported by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases.
MBD designed and performed experiments, analyzed data and wrote the manuscript; MJR designed and performed experiments and analyzed data; KVT designed experiments, analyzed data and wrote the manuscript. The authors have no conflicts of interest to disclose.
Abbreviations
- APCs
antigen presenting cells
- B6.g7
B6.NOD–D11Mit167) H2g7/DvsJ
- B6
C57BL/6
- cDCs
conventional dendritic cells
- DCs
dendritic cells
- FMO
fluorescence minus one
- FSC-A
forward scatter
- IFN
interferon
- Lin
lineage
- MHC
major histocompatibility complexes
- MFI
mean fluorescence intensity
- moDCs
monocyte-derived dendritic cells
- NOD
non-obese diabetic
- PFA
paraformaldehyde
- pDCs
plasmacytoid dendritic cells
- Tregs
regulatory T cells
- RT
room temperature
- SSC
side scatter
- pSTAT1
Signal Transducer and Activators of Transcription 1 phosphorylation
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jim.2015.08.015.
References
- Asselin-Paturel C, Brizard G, Chemin K, Boonstra A, O’Garra A, Vicari A, Trinchieri G. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J Exp Med. 2005;201:1157. doi: 10.1084/jem.20041930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilke JN, Meagher CT, Hosiawa K, Champsaur M, Bluestone JA, Lanier LL. NK cells are not required for spontaneous autoimmune diabetes in NOD mice. PLoS One. 2012;7:e36011. doi: 10.1371/journal.pone.0036011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, Koh H, Rodriguez A, Idoyaga J, Pack M, Velinzon K, Park CG, Steinman RM. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143:416. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana J, Simoni Y, Furio L, Beaudoin L, Agerberth B, Barrat F, Lehuen A. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med. 2013;19:65. doi: 10.1038/nm.3042. [DOI] [PubMed] [Google Scholar]
- Dominguez PM, Ardavin C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol Rev. 2010;234:90. doi: 10.1111/j.0105-2896.2009.00876.x. [DOI] [PubMed] [Google Scholar]
- Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- Ferris ST, Carrero JA, Mohan JF, Calderon B, Murphy KM, Unanue ER. A minor subset of Batf3-dependent antigen-presenting cells in islets of Langerhans is essential for the development of autoimmune diabetes. Immunity. 2014;41:657. doi: 10.1016/j.immuni.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, Yona S. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14:571. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van Rooijen N, Grainger JR, Belkaid Y, Ma’ayan A, Riches DW, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutzik PO, Nolan GP. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A. 2003;55:61. doi: 10.1002/cyto.a.10072. [DOI] [PubMed] [Google Scholar]
- Larsen J, Weile C, Antvorskov JC, Engkilde K, Nielsen SM, Josefsen K, Buschard K. Effect of dietary gluten on dendritic cells and innate immune subsets in BALB/c and NOD mice. PLoS One. 2015;10:e0118618. doi: 10.1371/journal.pone.0118618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau-Kilby AW, Kretz CC, Pechhold S, Price JD, Dorta S, Ramos H, Trinchieri G, Tarbell KV. Interleukin-2 inhibits FMS-like tyrosine kinase 3 receptor ligand (fit3L)-dependent development and function of conventional and plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2011;108:2408. doi: 10.1073/pnas.1009738108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KL, Reizis B. Dendritic cells: arbiters of immunity and immunological tolerance. Cold Spring Harb Perspect Biol. 2012;4:a007401. doi: 10.1101/cshperspect.a007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity. 2014;40:642. doi: 10.1016/j.immuni.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, Pandey G, Leboeuf M, Elpek KG, Helft J, Hashimoto D, Chow A, Price J, Greter M, Bogunovic M, Bellemare-Pelletier A, Frenette PS, Randolph GJ, Turley SJ, Merad M. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol. 2012;13:888. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel PA. Dendritic cell subsets in type 1 diabetes: friend or foe? Front Immunol. 2013;4:415. doi: 10.3389/fimmu.2013.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic T, Bouma G, Drexhage HA, Leenen PJ. Diabetes-prone NOD mice show an expanded subpopulation of mature circulating monocytes, which preferentially develop into macrophage-like cells in vitro. J Leukoc Biol. 2005;78:70. doi: 10.1189/jlb.1104662. [DOI] [PubMed] [Google Scholar]
- Nussenzweig MC, Steinman RM, Witmer MD, Gutchinov B. A monoclonal antibody specific for mouse dendritic cells. Proc Natl Acad Sci U S A. 1982;79:161. doi: 10.1073/pnas.79.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philbrick WM, Maher SE, Bridgett MM, Bothwell AL. A recombination event in the 5′ flanking region of the Ly-6C gene correlates with impaired expression in the NOD, NZB and ST strains of mice. Embo J. 1990;9:2485. doi: 10.1002/j.1460-2075.1990.tb07427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad SJ, Goodnow CC. Cell-intrinsic effects of non-MHC NOD genes on dendritic cell generation in vivo. Int Immunol. 2002;14:677. doi: 10.1093/intimm/dxf034. [DOI] [PubMed] [Google Scholar]
- Price JD, Tarbell KV. The role of dendritic cell subsets and innate immunity in the pathogenesis of type 1 diabetes and other autoimmune diseases. Front Immunol. 2015;6:288. doi: 10.3389/fimmu.2015.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JD, Beauchamp NM, Rahir G, Zhao Y, Rieger CC, Lau-Kilby AW, Tarbell KV. CD8+ dendritic cell-mediated tolerance of autoreactive CD4+ T cells is deficient in NOD mice and can be corrected by blocking CD40L. J Leukoc Biol. 2014;95:325. doi: 10.1189/jlb.0113013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JD, Hotta-Iwamura C, Zhao Y, Beauchamp NM, Tarbell KV. DCIR2+ cDC2 DCs and Zbtb32 restore CD4+ T cell tolerance and inhibit diabetes. Diabetes. 2015 doi: 10.2337/db14-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roederer M. Compensation in flow cytometry. Curr Protoc Cytom. 2002;Chapter 1(Unit 1):14. doi: 10.1002/0471142956.cy0114s22. [DOI] [PubMed] [Google Scholar]
- Rydstrom A, Wick MJ. Monocyte recruitment, activation, and function in the gut-associated lymphoid tissue during oral Salmonella infection. J Immunol. 2007;178:5789. doi: 10.4049/jimmunol.178.9.5789. [DOI] [PubMed] [Google Scholar]
- Serbina NV, Kuziel W, Flavell R, Akira S, Rollins B, Pamer EG. Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity. 2003a;19:891. doi: 10.1016/s1074-7613(03)00330-3. [DOI] [PubMed] [Google Scholar]
- Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003b;19:59. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke F, Ginhoux F, Jakubzick C, van Rooijen N, Merad M, Randolph GJ. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J Exp Med. 2006;203:583. doi: 10.1084/jem.20052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan PS, Gavin AL, Barnes N, Sears DW, Vremec D, Shortman K, Amigorena S, Mottram PL, Hogarth PM. Unique monoclonal antibodies define expression of Fc gamma RI on macrophages and mast cell lines and demonstrate heterogeneity among subcutaneous and other dendritic cells. J Immunol. 2003;170:2549. doi: 10.4049/jimmunol.170.5.2549. [DOI] [PubMed] [Google Scholar]
- Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199:1467. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbell KV, Petit L, Zuo X, Toy P, Luo X, Mqadmi A, Yang H, Suthanthiran M, Mojsov S, Steinman RM. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J Exp Med. 2007;204:191. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez AC, Feili-Hariri M, Tan RJ, Morel PA. Qualitative and quantitative abnormalities in splenic dendritic cell populations in NOD mice. Clin Exp Immunol. 2004;135:209. doi: 10.1111/j.1365-2249.2003.02359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welzen-Coppens JM, van Helden-Meeuwsen CG, Leenen PJ, Drexhage HA, Versnel MA. The kinetics of plasmacytoid dendritic cell accumulation in the pancreas of the NOD mouse during the early phases of insulitis. PLoS One. 2013;8:e55071. doi: 10.1371/journal.pone.0055071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.