Abstract
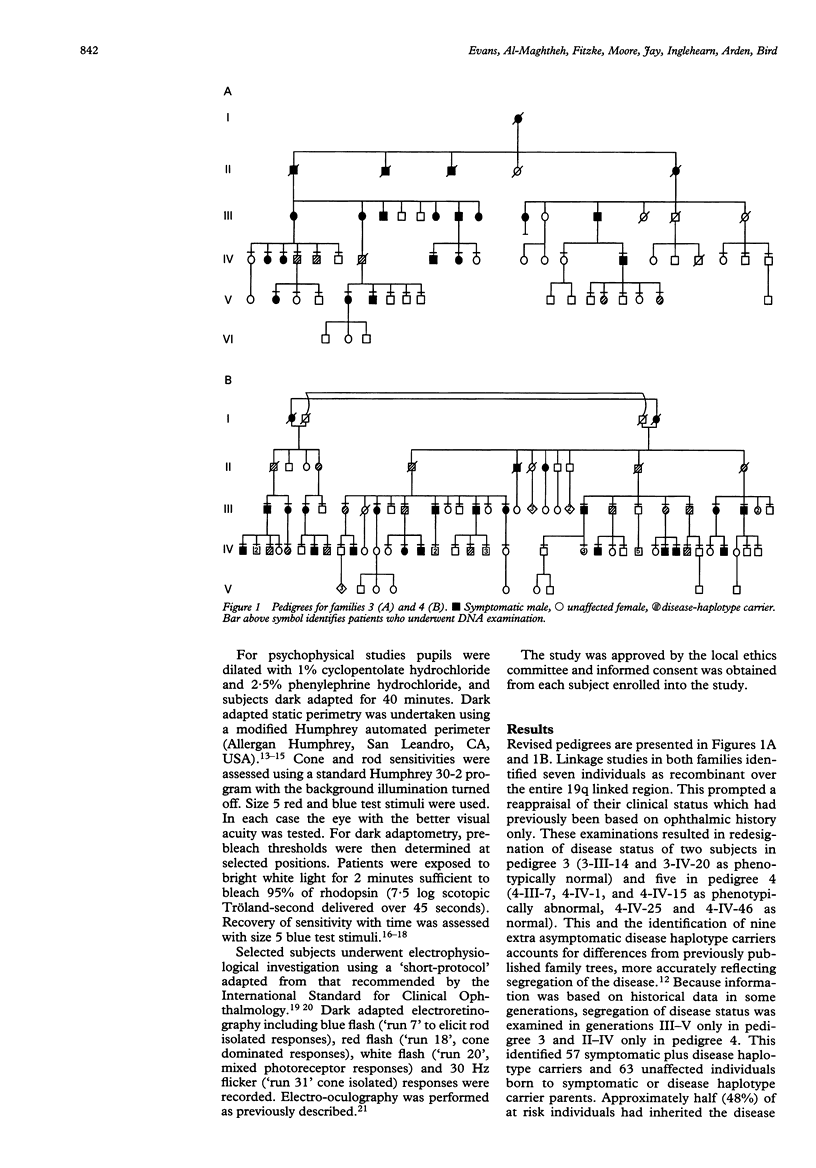
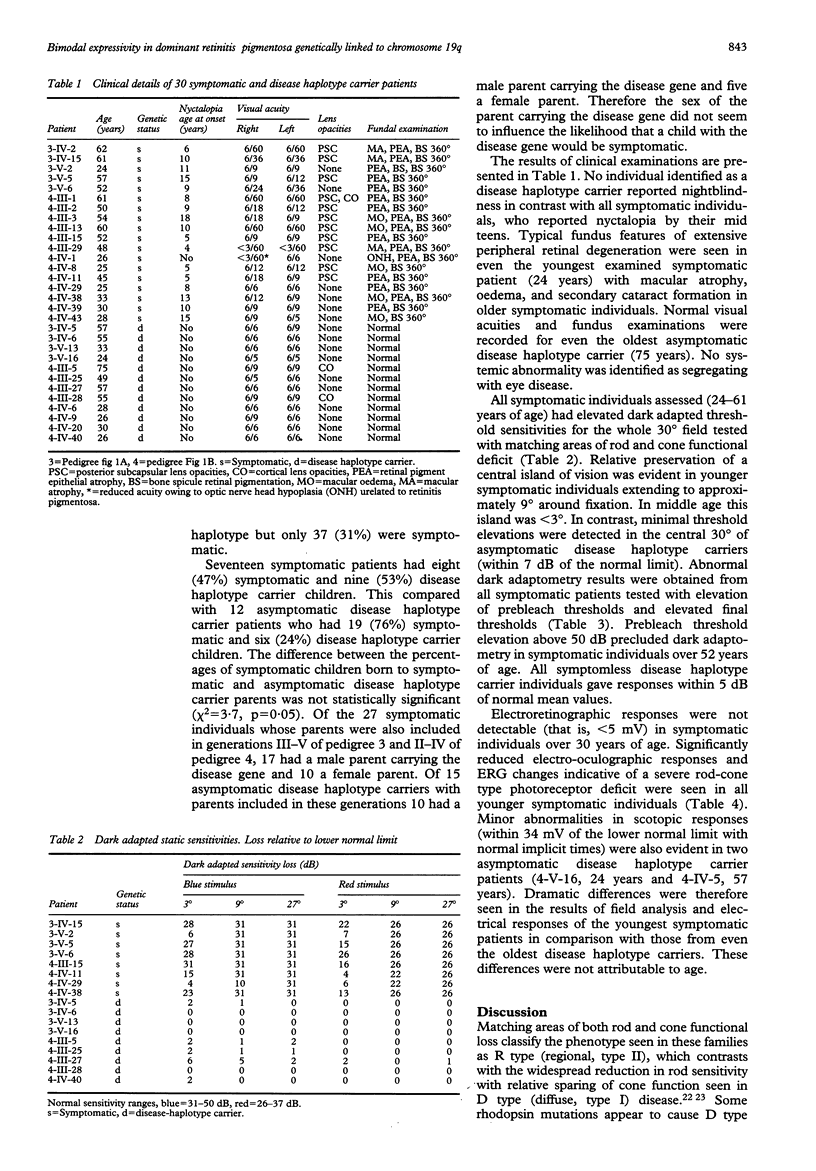
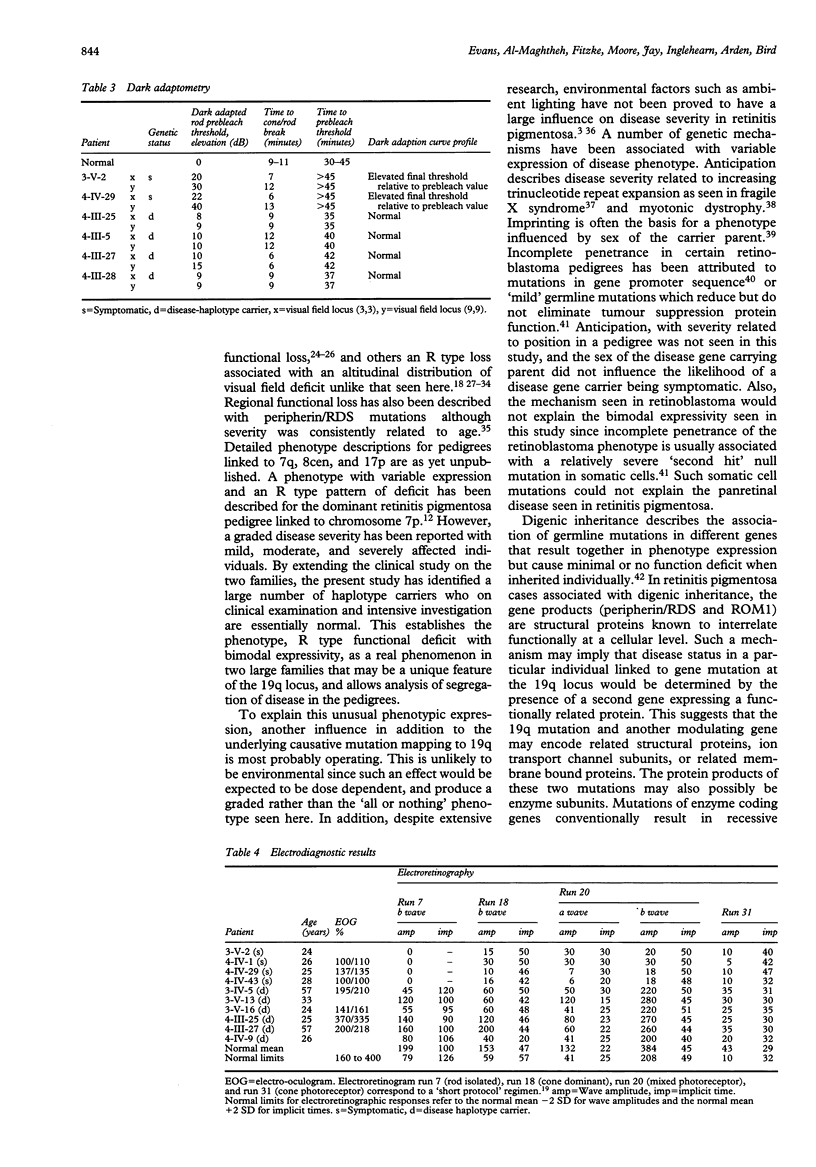
A clinical, psychophysical, and electrophysiologic study was undertaken of two autosomal dominant retinitis pigmentosa pedigrees with a genetic mutation assigned to chromosome 19q by linkage analysis. Members with the abnormal haplotype were either symptomatic with adolescent onset nyctalopia, restricted visual fields, and non-detectable electroretinographic responses by 30 years of age, or asymptomatic with normal fundus appearance and minimal or no psychophysical or electroretinographic abnormalities. There was no correlation in the severity in parents and their offspring. Pedigree analysis suggested that although the offspring of parents with the genetic mutation were at 50% risk of having the genetic defect, the risk of being symptomatic during a working lifetime was only 31%. Such bimodal phenotypic expressivity in these particular pedigrees may be explained by a second, allelic genetic influence and may be a phenomenon unique to this genetic locus. Genetic counselling in families expressing this phenotype can only be based on haplotype analysis since clinical investigations, even in the most elderly, would not preclude the presence of the mutant gene.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arden G. B., Barrada A., Kelsey J. H. New clinical test of retinal function based upon the standing potential of the eye. Br J Ophthalmol. 1962 Aug;46(8):449–467. doi: 10.1136/bjo.46.8.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden G. B., Carter R. M., Hogg C. R., Powell D. J., Ernst W. J., Clover G. M., Lyness A. L., Quinlan M. P. A modified ERG technique and the results obtained in X-linked retinitis pigmentosa. Br J Ophthalmol. 1983 Jul;67(7):419–430. doi: 10.1136/bjo.67.7.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson E. L. Light deprivation and retinitis pigmentosa. Vision Res. 1980;20(12):1179–1184. doi: 10.1016/0042-6989(80)90057-7. [DOI] [PubMed] [Google Scholar]
- Blanton S. H., Heckenlively J. R., Cottingham A. W., Friedman J., Sadler L. A., Wagner M., Friedman L. H., Daiger S. P. Linkage mapping of autosomal dominant retinitis pigmentosa (RP1) to the pericentric region of human chromosome 8. Genomics. 1991 Dec;11(4):857–869. doi: 10.1016/0888-7543(91)90008-3. [DOI] [PubMed] [Google Scholar]
- Bundey S., Crews S. J. A study of retinitis pigmentosa in the City of Birmingham. II Clinical and genetic heterogeneity. J Med Genet. 1984 Dec;21(6):421–428. doi: 10.1136/jmg.21.6.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. C., Fitzke F. W., Pauleikhoff D., Bird A. C. Functional loss in age-related Bruch's membrane change with choroidal perfusion defect. Invest Ophthalmol Vis Sci. 1992 Feb;33(2):334–340. [PubMed] [Google Scholar]
- Dryja T. P., Rapaport J., McGee T. L., Nork T. M., Schwartz T. L. Molecular etiology of low-penetrance retinoblastoma in two pedigrees. Am J Hum Genet. 1993 Jun;52(6):1122–1128. [PMC free article] [PubMed] [Google Scholar]
- Ernst W., Faulkner D. J., Hogg C. R., Powell D. J., Arden G. B., Vaegan An automated statis perimeter/adaptometer using light emitting diodes. Br J Ophthalmol. 1983 Jul;67(7):431–442. doi: 10.1136/bjo.67.7.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman G. A., Stone E. M., Gilbert L. D., Sheffield V. C. Ocular findings associated with a rhodopsin gene codon 106 mutation. Glycine-to-arginine change in autosomal dominant retinitis pigmentosa. Arch Ophthalmol. 1992 May;110(5):646–653. doi: 10.1001/archopht.1992.01080170068026. [DOI] [PubMed] [Google Scholar]
- Fishman G. A., Stone E. M., Sheffield V. C., Gilbert L. D., Kimura A. E. Ocular findings associated with rhodopsin gene codon 17 and codon 182 transition mutations in dominant retinitis pigmentosa. Arch Ophthalmol. 1992 Jan;110(1):54–62. doi: 10.1001/archopht.1992.01080130056026. [DOI] [PubMed] [Google Scholar]
- Fishman G. A., Vandenburgh K., Stone E. M., Gilbert L. D., Alexander K. R., Sheffield V. C. Ocular findings associated with rhodopsin gene codon 267 and codon 190 mutations in dominant retinitis pigmentosa. Arch Ophthalmol. 1992 Nov;110(11):1582–1588. doi: 10.1001/archopht.1992.01080230082026. [DOI] [PubMed] [Google Scholar]
- Gal A., Orth U., Baehr W., Schwinger E., Rosenberg T. Heterozygous missense mutation in the rod cGMP phosphodiesterase beta-subunit gene in autosomal dominant stationary night blindness. Nat Genet. 1994 May;7(1):64–68. doi: 10.1038/ng0594-64. [DOI] [PubMed] [Google Scholar]
- Gratzer W. Human genetics. Silence speaks in spectrin. Nature. 1994 Dec 15;372(6507):620–621. doi: 10.1038/372620a0. [DOI] [PubMed] [Google Scholar]
- Greenberg J., Goliath R., Beighton P., Ramesar R. A new locus for autosomal dominant retinitis pigmentosa on the short arm of chromosome 17. Hum Mol Genet. 1994 Jun;3(6):915–918. doi: 10.1093/hmg/3.6.915. [DOI] [PubMed] [Google Scholar]
- Hayakawa M., Hotta Y., Imai Y., Fujiki K., Nakamura A., Yanashima K., Kanai A. Clinical features of autosomal dominant retinitis pigmentosa with rhodopsin gene codon 17 mutation and retinal neovascularization in a Japanese patient. Am J Ophthalmol. 1993 Feb 15;115(2):168–173. doi: 10.1016/s0002-9394(14)73920-0. [DOI] [PubMed] [Google Scholar]
- Inglehearn C. F., Carter S. A., Keen T. J., Lindsey J., Stephenson A. M., Bashir R., al-Maghtheh M., Moore A. T., Jay M., Bird A. C. A new locus for autosomal dominant retinitis pigmentosa on chromosome 7p. Nat Genet. 1993 May;4(1):51–53. doi: 10.1038/ng0593-51. [DOI] [PubMed] [Google Scholar]
- Jacobson S. G., Kemp C. M., Sung C. H., Nathans J. Retinal function and rhodopsin levels in autosomal dominant retinitis pigmentosa with rhodopsin mutations. Am J Ophthalmol. 1991 Sep 15;112(3):256–271. doi: 10.1016/s0002-9394(14)76726-1. [DOI] [PubMed] [Google Scholar]
- Jacobson S. G., Voigt W. J., Parel J. M., Apáthy P. P., Nghiem-Phu L., Myers S. W., Patella V. M. Automated light- and dark-adapted perimetry for evaluating retinitis pigmentosa. Ophthalmology. 1986 Dec;93(12):1604–1611. doi: 10.1016/s0161-6420(86)33522-x. [DOI] [PubMed] [Google Scholar]
- Jay M. On the heredity of retinitis pigmentosa. Br J Ophthalmol. 1982 Jul;66(7):405–416. doi: 10.1136/bjo.66.7.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan S. A., Farrar G. J., Kenna P., Humphries M. M., Sheils D. M., Kumar-Singh R., Sharp E. M., Soriano N., Ayuso C., Benitez J. Localization of an autosomal dominant retinitis pigmentosa gene to chromosome 7q. Nat Genet. 1993 May;4(1):54–58. doi: 10.1038/ng0593-54. [DOI] [PubMed] [Google Scholar]
- Kajiwara K., Berson E. L., Dryja T. P. Digenic retinitis pigmentosa due to mutations at the unlinked peripherin/RDS and ROM1 loci. Science. 1994 Jun 10;264(5165):1604–1608. doi: 10.1126/science.8202715. [DOI] [PubMed] [Google Scholar]
- Kemp C. M., Jacobson S. G., Roman A. J., Sung C. H., Nathans J. Abnormal rod dark adaptation in autosomal dominant retinitis pigmentosa with proline-23-histidine rhodopsin mutation. Am J Ophthalmol. 1992 Feb 15;113(2):165–174. doi: 10.1016/s0002-9394(14)71529-6. [DOI] [PubMed] [Google Scholar]
- Kim R. Y., al-Maghtheh M., Fitzke F. W., Arden G. B., Jay M., Bhattacharya S. S., Bird A. C. Dominant retinitis pigmentosa associated with two rhodopsin gene mutations. Leu-40-Arg and an insertion disrupting the 5'-splice junction of exon 5. Arch Ophthalmol. 1993 Nov;111(11):1518–1524. doi: 10.1001/archopht.1993.01090110084030. [DOI] [PubMed] [Google Scholar]
- Kranich H., Bartkowski S., Denton M. J., Krey S., Dickinson P., Duvigneau C., Gal A. Autosomal dominant 'sector' retinitis pigmentosa due to a point mutation predicting an Asn-15-Ser substitution of rhodopsin. Hum Mol Genet. 1993 Jun;2(6):813–814. doi: 10.1093/hmg/2.6.813. [DOI] [PubMed] [Google Scholar]
- Lyness A. L., Ernst W., Quinlan M. P., Clover G. M., Arden G. B., Carter R. M., Bird A. C., Parker J. A. A clinical, psychophysical, and electroretinographic survey of patients with autosomal dominant retinitis pigmentosa. Br J Ophthalmol. 1985 May;69(5):326–339. doi: 10.1136/bjo.69.5.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magenis R. E., Toth-Fejel S., Allen L. J., Black M., Brown M. G., Budden S., Cohen R., Friedman J. M., Kalousek D., Zonana J. Comparison of the 15q deletions in Prader-Willi and Angelman syndromes: specific regions, extent of deletions, parental origin, and clinical consequences. Am J Med Genet. 1990 Mar;35(3):333–349. doi: 10.1002/ajmg.1320350307. [DOI] [PubMed] [Google Scholar]
- Massof R. W., Finkelstein D. Rod sensitivity relative to cone sensitivity in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1979 Mar;18(3):263–272. [PubMed] [Google Scholar]
- Massof R. W., Finkelstein D. Two forms of autosomal dominant primary retinitis pigmentosa. Doc Ophthalmol. 1981 Nov;51(4):289–346. doi: 10.1007/BF00143336. [DOI] [PubMed] [Google Scholar]
- Moore A. T., Fitzke F. W., Kemp C. M., Arden G. B., Keen T. J., Inglehearn C. F., Bhattacharya S. S., Bird A. C. Abnormal dark adaptation kinetics in autosomal dominant sector retinitis pigmentosa due to rod opsin mutation. Br J Ophthalmol. 1992 Aug;76(8):465–469. doi: 10.1136/bjo.76.8.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. T., Fitzke F., Jay M., Arden G. B., Inglehearn C. F., Keen T. J., Bhattacharya S. S., Bird A. C. Autosomal dominant retinitis pigmentosa with apparent incomplete penetrance: a clinical, electrophysiological, psychophysical, and molecular genetic study. Br J Ophthalmol. 1993 Aug;77(8):473–479. doi: 10.1136/bjo.77.8.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restagno G., Maghtheh M., Bhattacharya S., Ferrone M., Garnerone S., Samuelly R., Carbonara A. A large deletion at the 3' end of the rhodopsin gene in an Italian family with a diffuse form of autosomal dominant retinitis pigmentosa. Hum Mol Genet. 1993 Feb;2(2):207–208. doi: 10.1093/hmg/2.2.207. [DOI] [PubMed] [Google Scholar]
- Richards R. I., Holman K., Friend K., Kremer E., Hillen D., Staples A., Brown W. T., Goonewardena P., Tarleton J., Schwartz C. Evidence of founder chromosomes in fragile X syndrome. Nat Genet. 1992 Jul;1(4):257–260. doi: 10.1038/ng0792-257. [DOI] [PubMed] [Google Scholar]
- Sakai T., Ohtani N., McGee T. L., Robbins P. D., Dryja T. P. Oncogenic germ-line mutations in Sp1 and ATF sites in the human retinoblastoma gene. Nature. 1991 Sep 5;353(6339):83–86. doi: 10.1038/353083a0. [DOI] [PubMed] [Google Scholar]
- Steinmetz R. L., Polkinghorne P. C., Fitzke F. W., Kemp C. M., Bird A. C. Abnormal dark adaptation and rhodopsin kinetics in Sorsby's fundus dystrophy. Invest Ophthalmol Vis Sci. 1992 Apr;33(5):1633–1636. [PubMed] [Google Scholar]
- Sullivan L. J., Makris G. S., Dickinson P., Mulhall L. E., Forrest S., Cotton R. G., Loughnan M. S. A new codon 15 rhodopsin gene mutation in autosomal dominant retinitis pigmentosa is associated with sectorial disease. Arch Ophthalmol. 1993 Nov;111(11):1512–1517. doi: 10.1001/archopht.1993.01090110078029. [DOI] [PubMed] [Google Scholar]
- Tsilfidis C., MacKenzie A. E., Mettler G., Barceló J., Korneluk R. G. Correlation between CTG trinucleotide repeat length and frequency of severe congenital myotonic dystrophy. Nat Genet. 1992 Jun;1(3):192–195. doi: 10.1038/ng0692-192. [DOI] [PubMed] [Google Scholar]
- Weleber R. G., Carr R. E., Murphey W. H., Sheffield V. C., Stone E. M. Phenotypic variation including retinitis pigmentosa, pattern dystrophy, and fundus flavimaculatus in a single family with a deletion of codon 153 or 154 of the peripherin/RDS gene. Arch Ophthalmol. 1993 Nov;111(11):1531–1542. doi: 10.1001/archopht.1993.01090110097033. [DOI] [PubMed] [Google Scholar]
- Wells J., Wroblewski J., Keen J., Inglehearn C., Jubb C., Eckstein A., Jay M., Arden G., Bhattacharya S., Fitzke F. Mutations in the human retinal degeneration slow (RDS) gene can cause either retinitis pigmentosa or macular dystrophy. Nat Genet. 1993 Mar;3(3):213–218. doi: 10.1038/ng0393-213. [DOI] [PubMed] [Google Scholar]
- al-Maghtheh M., Gregory C., Inglehearn C., Hardcastle A., Bhattacharya S. Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Hum Mutat. 1993;2(4):249–255. doi: 10.1002/humu.1380020403. [DOI] [PubMed] [Google Scholar]
- al-Maghtheh M., Inglehearn C. F., Keen T. J., Evans K., Moore A. T., Jay M., Bird A. C., Bhattacharya S. S. Identification of a sixth locus for autosomal dominant retinitis pigmentosa on chromosome 19. Hum Mol Genet. 1994 Feb;3(2):351–354. doi: 10.1093/hmg/3.2.351. [DOI] [PubMed] [Google Scholar]



