Abstract
Microfossils, stromatolites, preserved lipids and biologically informative isotopic ratios provide a substantial record of bacterial diversity and biogeochemical cycles in Proterozoic (2500–541 Ma) oceans that can be interpreted, at least broadly, in terms of present-day organisms and metabolic processes. Archean (more than 2500 Ma) sedimentary rocks add at least a billion years to the recorded history of life, with sedimentological and biogeochemical evidence for life at 3500 Ma, and possibly earlier; phylogenetic and functional details, however, are limited. Geochemistry provides a major constraint on early evolution, indicating that the first bacteria were shaped by anoxic environments, with distinct patterns of major and micronutrient availability. Archean rocks appear to record the Earth's first iron age, with reduced Fe as the principal electron donor for photosynthesis, oxidized Fe the most abundant terminal electron acceptor for respiration, and Fe a key cofactor in proteins. With the permanent oxygenation of the atmosphere and surface ocean ca 2400 Ma, photic zone O2 limited the access of photosynthetic bacteria to electron donors other than water, while expanding the inventory of oxidants available for respiration and chemoautotrophy. Thus, halfway through Earth history, the microbial underpinnings of modern marine ecosystems began to take shape.
This article is part of the themed issue ‘The new bacteriology’.
Keywords: Archean, Proterozoic, palaeobiology, carbon cycle, microbial evolution
1. Introduction
The conventional fossil record of bones, shells, tracks and trails traces an evolutionary history of animals nearly 600 Myr in duration. Despite its merits, microbiologists will recognize that this accounting is woefully incomplete with respect to phylogenetic sampling, ecology and time. Universal phylogenies indicate that animals are evolutionary latecomers, occupying distal branches in a tree of life populated principally by microorganisms. And geochronology, in turn, demonstrates that the conventional fossil record documents only the last 15% of recorded Earth history. Thus, phylogeny predicts a long history of microbial evolution prior to the emergence of complex multicellularity, and the geologic record preserves sedimentary rocks in which to search for evidence of early Bacteria and Archaea. Microfossils, stromatolites, organic molecules of biological origin and biologically informative isotope ratios preserved in Proterozoic (2500–541 Ma) rocks document this deeper microbial history of life, quadrupling the conventional record of skeletons. Sedimentary rocks, however, preserve more than a billion years of earlier Earth history, and it was during this interval that the fundamental features of cell biology and metabolism took shape.
Here, we examine Earth's earliest record of microbial evolution (figure 1), from about 3800 to 1800 Ma, focusing in particular on early environmental history, an area of tangible recent progress. There are at least three advantages to this approach: (i) the actual palaeobiological record of life's earliest history is meager and so environmental history may place our strongest constraints on early biological evolution; (ii) an environmental focus circumscribes the oxidants, reductants and nutrients available for early microbial metabolism; and (iii) palaeoenvironmental insights may yield evidence of early constraints on, or opportunities for, evolution that are also recorded in microbial genomes.
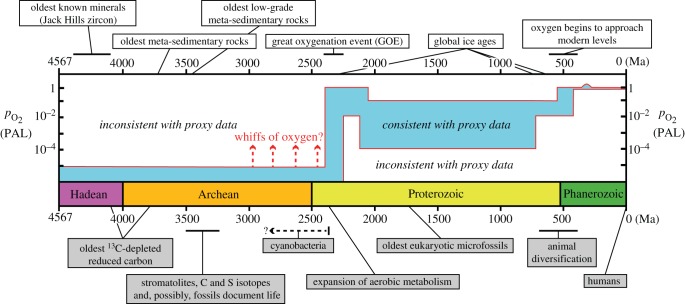
Figure 1.
A time table for Earth's early history, showing the major eons (Hadean, Archean, Proterozoic and Phanerozoic), an estimate of atmospheric oxygen history constructed from geochemical proxy data [1–3] and key environmental (above) and biological (below) events discussed in the text. Oxygen partial pressure in per cent of PAL. (Online version in colour.)
2. A brief outline of Proterozoic palaeobiology
Historically, palaeontologists were sceptical of the idea that bacteria could preserve as fossils, even rejecting reports that later proved to be correct [4]. High-resolution imaging has more recently demonstrated that bacteria can be found in Phanerozoic sedimentary rocks, preserved as films on carbonaceous plant remains, inclusions in precipitated minerals and carbonate rinds templated by cyanobacterial sheaths [5]. Most of these microfossils are simple rods and filaments, whose metabolic or phylogenetic relationships can be inferred, if at all, only by sedimentary association. The Proterozoic Eon (2500–541 Ma; figure 1)—Earth's middle age—is qualitatively distinct. A confluence of ecological circumstance and unique preservational mode provides a micropalaeontological window through which we can view, among other things, aspects of the diversity and environmental distribution of early cyanobacteria and eukaryotes.
A key aspect of Proterozoic microfossil preservation lies in the fact that before the radiations of sponges, radiolarians and, later, diatoms, the marine silica cycle worked differently than it does today. Silica commonly left the oceans as an early stage evaporitic precipitate, redistributing locally within coastal carbonate sediments to form chert concretions that preserve micrometre-scale textures, including microbiological features (figure 2b,c; [6]). Thus, early diagenetic chert nodules preserve a microbiological record of environments in which cyanobacteria were the principal primary producers. Relative to other bacteria, cyanobacteria are commonly large, have distinctive morphologies that reflect phylogenetically distinct patterns of development and life history, and produce extracellular polysaccharide sheaths and envelopes that are resistant to decay and so preserve detailed patterns of cell shape and division [7]. Not all Proterozoic microfossils are phylogenetically tractable, but a number of populations closely resemble morphologically distinct cyanobacteria found today in comparable environments—for example, multicellular Hyella species that live as endoliths within ooids [8].
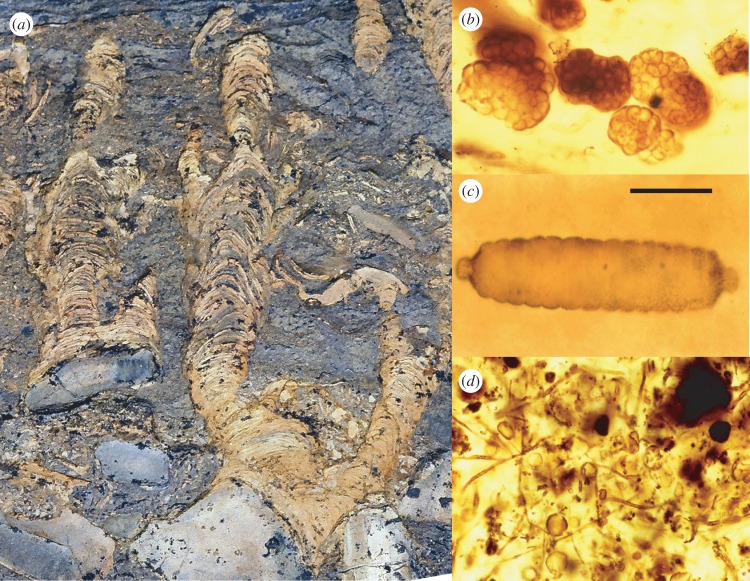
Figure 2.
Evidence for microbial life in Proterozoic rocks. (a) Stromatolites accreting on top of limestone cobbles, ca 800 Ma Limestone-Dolomite Series, central East Greenland; (b) colonies of cyanobacteria-like coccoidal cells, ca 750 Ma Draken Conglomerate, Svalbard; (c) short cyanobacterial trichome, 1400–1500 Ma Billyakh Group, northern Siberia; and (d) filamentous and coccoidal microfossils in iron-rich rocks of the 1860 Ma Gunflint Formation, Ontario. Scale bars: 3 cm in (a), 25 µm in (b,d) and 15 µm in (c). (Online version in colour.)
Thus, while chemoautotrophic and heterotrophic bacteria may occur among preserved populations (for example, simple filaments containing sulfur-rich inclusions that appear to record sulfur-oxidizing bacteria along an oxic–anoxic interface [9]), many Proterozoic bacteria are reasonably, if broadly, interpreted in terms of cyanobacteria, based on a combination of morphology, orientation within sediments and environmental distribution. This does not mean that all Proterozoic cyanobacteria were fully modern in terms of their molecular biology, but rather that the phylogenetic and functional framework built through research on living cyanobacteria can be applied fruitfully to the interpretation of Proterozoic remains [7]. Proterozoic sedimentary rocks also contain evidence of early eukaryotes (e.g. [10–12]) and microfossils preserved in Upper Paleoproterozoic iron formations may preserve a record of iron-loving bacteria (figure 2c; [13]). Informative as these records are, they trickle to a stop in rocks older than about two billion years.
Stromatolites—laminated structures that can be flat-lying, domal, club-shaped or conoidal—are the most conspicuous palaeobiological features of Proterozoic carbonates, extending the record of microbial life in both time and space (figure 2a; [14,15]). Based on modern examples, where process can be linked to preservable sedimentary pattern, Proterozoic stromatolites are convincingly interpreted in terms of accretion by densely interwoven cyanobacterial mat populations whose copious extracellular polymeric exudates trapped fine-grained sediments and provided a framework for penecontemporaneous carbonate precipitation [16]. As most Proterozoic stromatolites accreted in oxic environments, this interpretation is reasonable, but in older rocks, where alternate electron donors would have been available, interpretation grows more tenuous (see below).
Stromatolites show that benthic photosynthetic populations thrived throughout the photic zone in Proterozoic oceans, inconsistent with speculation that dense bacterial populations made contemporaneous surface waters turbid [17]. While it is tempting to interpret the observed variability of stromatolite macrostructure and microstructure in terms of community heterogeneity, this is complicated by the observation that stromatolite form also reflects both physical and chemical features of the environment [15]. In a few places, silicified microfossils show that mat-building populations and stromatolite microstructure can covary in space [18], strengthening the view that at least some aspects of stromatolite morphology reflect ancient microbial diversity. In general, stromatolites do not accrete in siliciclastic environments, but mat communities can leave distinctive textures on the bedding surfaces of sandstones, extending the fingerprint of benthic mats to a wider range of environments [19,20].
Chemical signatures provide further perspective on Proterozoic microbial diversity. Today, differences in carbon isotopic composition between carbonate sediments and co-occurring organic matter reflect fractionation by photoautotrophs that fix carbon via the Calvin–Benson cycle: photosynthetic organisms preferentially incorporate CO2 containing the lighter stable isotope of carbon, 12C, leaving the ambient seawater from which carbonates precipitate enriched in 13C [21]. Similarly, isotopic differences between sulfate precipitates and sedimentary pyrite document a microbial sulfur cycle in which dissimilatory sulfate reduction plays a key role, enriching product sulfides in 32S, recorded by pyrite precipitation [22]. And the nitrogen isotopic composition of organic molecules indicates a nitrogen cycle in which nitrate is partially reduced by denitrifying bacteria, leaving environmental N incorporated into biomass enriched in 15N [23].
Isotopic measurements of Proterozoic sedimentary rocks suggest that ancient C and S cycles worked broadly as they do today. Nitrogen is a bit trickier, as it can be difficult to know the effects of diagenesis and contamination on the small abundances of N found in ancient organic matter or adsorbed onto clay minerals [24]. A key recent discovery is that, in several Mid-Proterozoic basins, N isotopic values differ between coastal and offshore environments, suggesting that coastal environments contained relatively abundant nitrate, whereas nitrogen fixation provided bioavailable nitrogen in open waters [25]. This observation gains traction both because observed N isotopic compositions vary as a function of environment and because values from basinal rocks do not resemble most present-day values and so are unlikely to be modern contaminants.
Finally, preserved lipids provide a molecular counterpart to conventional microfossils. An evident dearth of steranes in sedimentary rocks older than ca 800 Ma suggests that eukaryotes played a limited role in primary production until late in the Proterozoic Eon [26–28]. Conversely, lipids sourced by purple and green photosynthetic bacteria show that anoxic waters sometimes mixed upward into the Proterozoic photic zone [26], potentially helping to keep oxygen levels low [29].
3. Archean palaeobiology
Documentation of an Archean palaeobiological record is more challenging, in part because relatively few sedimentary rocks survive from this interval, in part because preserved successions are more likely to have been altered by diagenesis or metamorphism and in part because the oldest known rocks record early evolutionary events in alien environments, undermining some of the assumptions that guide interpretation of younger biosignatures.
In general, Archean rocks appear to have been heated to temperatures beyond those at which biomarker lipids can be preserved [30], but isotopes suggest the presence of biological carbon and sulfur cycles, at least back to 3500 Ma and (for carbon) perhaps much earlier (figure 1). In many ways, the most interesting feature of the Archean C isotope record is its similarity to that found in younger rocks: organic carbon with δ13C of −30 to −40‰ (Vienna Pee Dee Belemnite, or VPDB, standard) in association with 0‰ carbonates (figure 1, [31]). Caution is necessary when interpreting the early carbon isotopic record, because abiotic organic syntheses, including those thought to have played a role in the origin of life, also fractionate C isotopes [32,33]. Abiotic fractionation is variable, depending strongly on reactant abundances and generating fractionations comparable to those observed in biological systems only under limited circumstances. Thus, the consistent fractionation observed in Archean organic matter distributed abundantly among a wide range of sedimentary environments is most likely a product of biological carbon fixation [34]. Moreover, as organic matter formed broadly in Archean oceans and not just in local environments characterized by strong chemical gradients, the biological carbon cycle was most probably fuelled by photosynthesis. Observed fractionations are consistent with Rubsico-based fixation, but the Wood–Ljungdahl pathway may also have been important in early ecosystems [35]. Strongly 13C-depleted organic matter in some Late Archean successions suggests an additional, time-bounded influence of methanogenesis and methanotrophy on isotopic composition [36]. The C isotopic composition of graphite in 3700–3800 Ma metasedimentary rocks from Greenland [37] and tiny graphite inclusions within a 4100 Ma zircon [38] potentially push biology still deeper into the past, but a lack of context, especially for the zircon inclusions, makes it difficult to eliminate abiotic alternatives.
The Archean record of sulfur isotopes is challenging to interpret because photochemical processes in the anoxic Archean atmosphere imparted strong isotopic signatures to sulfur species that were later incorporated into Archean sedimentary deposits [39]. Careful measurements of all four stable S isotopes show that Archean photochemical processes fractionated S isotopes in a mass-independent manner, establishing a necessary framework for evaluating potential biological fractionation. Studies of co-occurring barite and organic-associated sulfur support the conclusion that biological processes, including both dissimilatory sulfate reduction [40] and sulfur disproportionation [41], contributed to the S isotopic record observed in Archean sedimentary rocks. Documenting a biological nitrogen cycle may be most challenging of all, but Stüeken et al. [42] interpret distinctive δ15N signatures in terms of biological nitrogen fixation as early as approximately 3200 Ma.
Stromatolites occur in carbonate rocks deposited throughout the Archean Eon, and those younger than about approximately 3000 Ma have textural features reliably associated with microbial trapping and binding in younger rocks (figure 3d, [31,43]). Microbial textures also occur on sandstone bedding surfaces in approximately 2900 Ma successions [44]. The oldest known stromatolites (3400–3500 Ma), however, are more challenging to interpret because they consist almost entirely of precipitated laminae, with textures that can be difficult to distinguish from abiotic precipitates (figure 3a–c). Indeed, Lowe [45] argued in favour of an abiological origin for Early Archean stromatolites. Continuing research has tilted interpretation back toward biogenesis (e.g. [46]), especially in consideration of microscopic textures indicative of continuous organic mats and millimetre-scale tufts [47–50].
Figure 3.
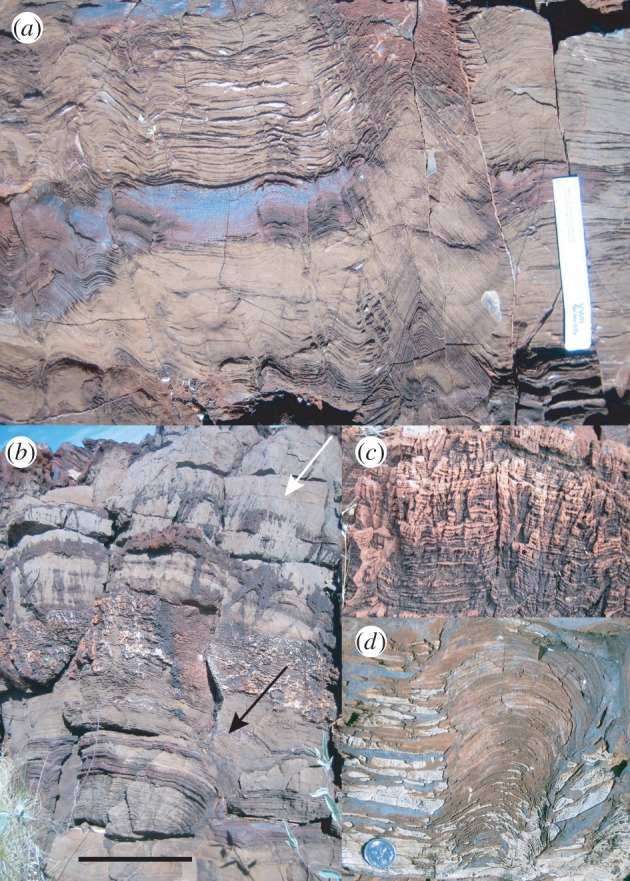
Stromatolites interpreted as evidence for microbial life in Archean rocks. (a) Irregular and conoidal stromatolites in carbonates of the 3450 Ma Strelley Pool Formation, Warrawoona Group, Western Australia; (b) a second view of Strelley Pool carbonate, showing the prevalence of decimeter-scale (originally) aragonite crystals growing upward from the seafloor (white arrow) in close association with precipitated stromatolites (black arrow); (c) laminated seafloor precipitates in the Strelley Pool Formation that blur the lines between biogenic and physically generated laminites; and (d) unambiguously biogenic stromatolite in the approximately 2720 Ma Tumbiana Formation, Fortescue Group, Western Australia. Ruler in (a) is 15 cm; scale bar in (b) is 20 cm for (b), 4 cm for (c) and 15 cm for (d). (Online version in colour.)
The microorganisms that built Earth's oldest known stromatolites remain uncertain [51]. Unlike their Proterozoic counterparts, Archean stromatolites accreted in anoxic waters where electron donors such as Fe2+ and sulfide would have been available for photosynthesis. Experiments and field observations show that anoxygenic autotrophs can accrete stromatolites [52,53], and so early structures may have had little to do with cyanobacteria. Tice et al. [54] argued that a Late Archean increase in the estimated tensile strength and cohesion of microbial mats reflects the global expansion of cyanobacterial mat-builders. Such investigations point the way towards more nuanced textural investigations that may enable cyanobacterial mats to be differentiated from those formed by anoxygenic photosynthetic bacteria.
Finally, there is the question of Archean microfossils, an area of abiding contention (e.g. [55,56]). Unlike Proterozoic cherts, silica deposits in Archean successions seldom preserve unambiguous microfossils. At least in part, this appears to reflect the silica cycle, which worked differently in Archean and Proterozoic oceans. Sequestration of silica by absorption onto iron oxides deposited in deep basins would have decreased the availability of silica for early digenetic silicification in shallow water settings [57], and in older Archean basins, many cherts reflect potentially fabric-destructive hydrothermal fluids (see below). Nonetheless, a variety of carbonaceous morphologies has been described from Archean cherts and interpreted as microbial fossils. Putative Early Archean include locally abundant 10–30 µm organic spheroids (figure 4d, [58,59]), larger than most younger bacterial fossils and seldom if ever showing evidence of binary fission. If microfossils, they bear more similarity to living L-form bacteria that lack peptidoglycan walls than they do to conventional microbes [60]. Other interpretations are possible, however. Geologic studies show that microstructure-bearing Archean cherts have commonly experienced one or more episodes of hydrothermal fluid flow [61,62]. Thus, it can be instructive to compare organic textures in Archean cherts with those found in Proterozoic rocks altered by hydrothermal fluids.
Figure 4.
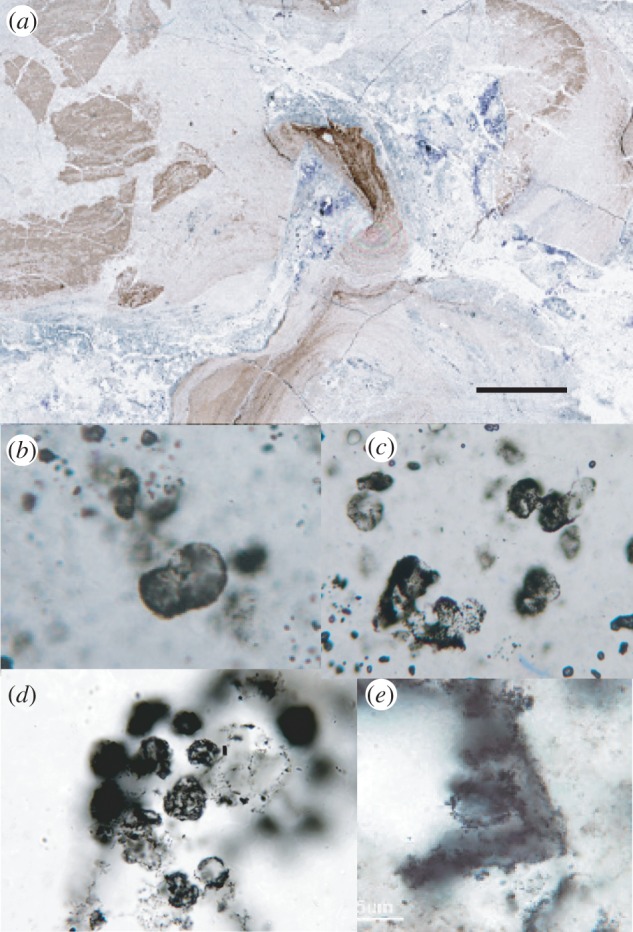
Hydrothermally generated spheroidal microstructures in Neoproterozoic chert and morphologically similar microstructures in Lower Archean chert. (a) Chert thin section, 755–735 Ma Callison Lake Formation, northwestern Canada: darker laminated areas preserve original depositional textures, but light and purple-stained regions show evidence for localized hydrothermal fluid flow that obliterated depositional textures, generating distinctly diagenetic features in their stead; (b,c) carbonaceous spheroids generated diagenetically via organic migration associated with hydrothermal fluid flow in the Callison Lake Formation; (d) morphologically similar carbonaceous microstructures from the 3450 Ma Strelley Pool Formation, Warrawoona Group, Western Australia (thin section courtesy of K. Sugitani); and (e) silicified ghost of a rhombohedral crystal coated by organic matter during hydrothermal fluid flow, Strelley Pool chert. Scale bar: 5 mm in (a), 30 µm in (b) and (c), 25 µm in (d) and 40 µm in (e). (Online version in colour.)
The ca 755–735 Ma Callison Lake Formation, northwestern Canada, comprises a heterogeneous package of siliciclastic and carbonate strata deposited in a series of small rift basins associated with the initial break-up of the supercontinent Rodinia [63]. These locally fossiliferous carbonate and shale deposits record shallow marine sedimentation in a variety of near shore settings, from extensive tidal flats to subtidal shelf environments [64]; however, Callison Lake strata also record a complex post-depositional history, including the isolated development of hydrothermal veins associated with the eruption of ca 717 Ma mafic volcanic rocks [64,65]. Locally, Callison Lake cherts with organically defined textures typical of Neoproterozoic rocks are cut by discrete hydrothermal silica veins (figure 4a). Within the veins, depositional textures have been obliterated, and replacement textures include distinct 10–30 µm carbonaceous spheroids (figure 4b,c). This example shows clearly that hydrothermal fluids can mobilize organic matter and redistribute it into spheroidal microstructures that resemble putative Archean microfossils (figure 4d). Indeed, in Lower Archean cherts, organic matter can be observed to coat mineral surfaces, providing direct evidence for organic mobilization in ancient, hydrothermally influenced sediments (figure 4e).
Other research shows that hydrothermal alterations can also generate filamentous microstructures [61]. Such observations do not necessarily invalidate all reports of Early Archean microfossils, but they underscore the need for caution (and careful petrographic and sedimentological analysis) in interpretation. Among the best candidates for bona fide Early Archean fossils are spheroidal carbonaceous vesicles preserved as compressions in approximately 3200 Ma shales [66]. Like other reported Archean remains, however, these provide few clues to phylogenetic or metabolic interpretation.
The bottom line is that life began before the oldest known sedimentary rocks were deposited, but palaeobiology provides only a sketchy view of early microbial evolution. To enhance our geologic perspective on early life, we need to look for other lines of evidence.
4. Archean environments
Perhaps the most biologically informative statement one can make about early life is that it evolved in anoxic environments [67,68]. More than 50 years ago, low oxygen conditions were inferred for the Archean atmosphere and oceans, based primarily on the temporal distribution of sedimentary iron formation. Sharper quantitative constraints became possible with the documentation of mass-independent fractionation of sulfur isotopes (S-MIF) in Archean sulfates and sulfides [39]; modelling by Pavlov & Kasting [69] suggests that photochemical processes capable of imparting this signature would only occur in an atmosphere with pO2 < 10−5 PAL (present-day level). The oxygen-sensitive minerals siderite, uraninite and pyrite all occur as detrital grains in Archean sandstones [70], but are rare in younger rocks; once again, modelling suggests that such grains would only persist beneath an atmosphere with pO2 < 1.6 × 10−4 PAL [71]. Declines in iron formation, oxygen-sensitive detrital mineral grains and S-MIF all occur at about 2400 Ma, identifying this as a time of major environmental transition when the atmosphere and surface ocean became permanently oxygenated, but not, as we shall see, to present-day levels (figure 1, [1]).
Some Archean successions contain elevated levels of oxygen-mobilized trace elements, including Mo, Cr, Re and U [1], and these enrichments, along with fractionation of chromium isotopes, have been argued to record ‘whiffs’ of oxygen in marine environments 3000 Ma or earlier (figure 1, [72–76]). If correctly interpreted, such geochemical signatures suggest at least transient, cyanobacteria-mediated accumulations of oxygen to low levels, perhaps sufficient to support facultative aerobiosis [77]. More work, however, is required to determine whether these record an early signature of atmospheric oxygen, in part, because these records are at odds with other redox-sensitive records like S-MIF and detrital grains from the same sedimentary successions [78,79]. It should be emphasized that Archean sedimentary rocks are hydrothermally altered as a rule rather than as an exception, and geochemical studies have not adequately demonstrated how post-depositional, more oxygenated hydrothermal fluids may have influenced trace metal records [78,80]. In addition, photochemical generation of reactive oxygen species such as H2O2 has been proposed as an alternative oxidant in early environments [81].
The second most distinctive aspect of the Archean environment is the abundance of dissolved iron, to the point that any reduced sulfur species would be quickly titrated out of solution. Unlike in modern environments, iron would not have been a limiting micronutrient; instead, it would have provided some of the most abundant electron donors and acceptors for both autotrophy and heterotrophy (see below). Thick Archean deposits of deep-water, magnetite-dominated, iron formations stand as evidence of this iron cycling and its side effects (figure 5), including silica scavenging and siderite formation [57,78,82–84]. Unlike the shallower-water, often granular or stromatolitic iron formations in upper Paleoproterozoic successions, Archean iron deposits do not provide a taphonomic window for microfossil preservation.
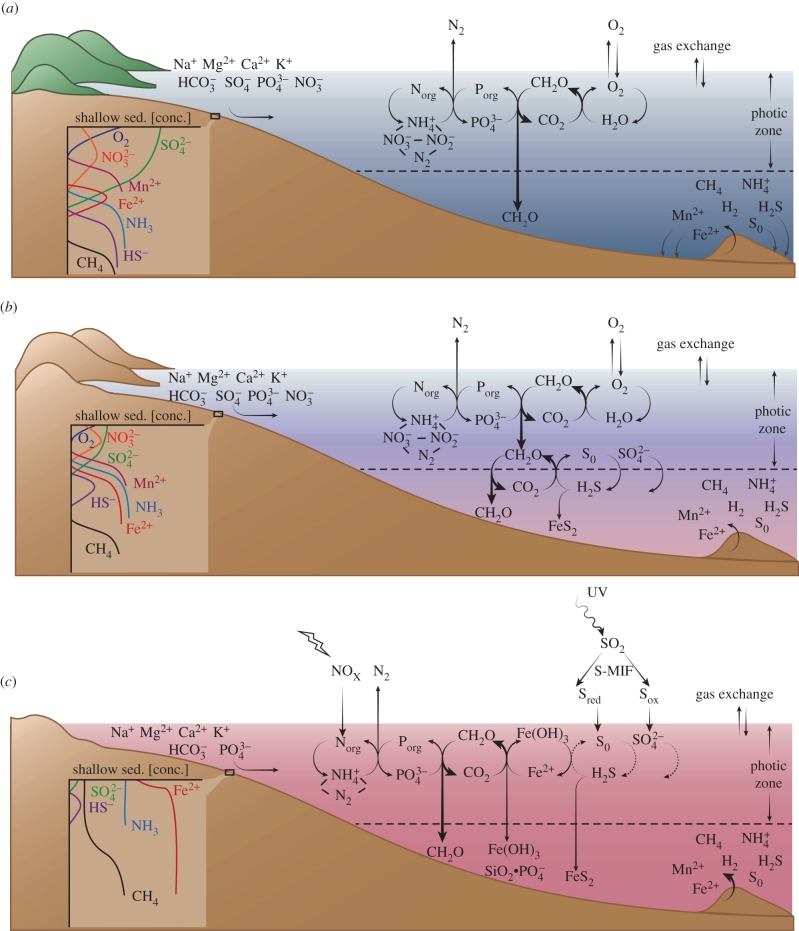
Figure 5.
Diagrams showing how the biological carbon, iron, sulfur, nitrogen and phosphate cycles—and their linkages—have varied through geologic history. From top to bottom, the three panels diagram (a) Phanerozoic (modern), (b) Proterozoic and (c) Archean marine ecosystems. Red tones indicate ferruginous seawater; purple denotes sulfidic water masses; and blue indicates oxic waters. Dotted, thin continuous and thick continuous arrows indicate increasing ecological importance of pathways. Insets show the time-varying distribution of ions and biogenic gases in surficial sediments.
Other critical conditions of the Archean environment, including T, pH and pCO2, are more poorly constrained. To a first order, both liquid water and carbonate precipitation have existed for as long as the available rock record, indicating ocean temperatures and pH not too different from today [85,86]. Given models of stellar evolution that indicate lower luminosity early in our sun's history, this requires higher concentrations of greenhouse gases in the Archean atmosphere, especially CO2 [87]. That said, aspects of preserved limestones and dolostones do suggest that Archean carbonate precipitation differed from that in modern oceans. As noted above, preserved textures in stromatolites and other Lower Archean carbonate lithologies include more abundant abiotically precipitated fabrics [88,89] than at any subsequent time. This temporal characteristic of Archean carbonate rocks has been interpreted in a number of ways: higher carbonate saturation levels (Ω) in surface seawater [86,89], a reduced depth gradient in Ω that reflects anaerobic respiration at depth [90], sediment–water interface effects of anaerobic respiration from increased access to oxides [91], and a change in the dynamics of carbonate inhibition by reduced ions competing for precipitation sites [92]. While the exact temperature range of the Archean climate is still debated, if warmer, temperature would have had a positive effect on precipitation through predictable changes in the rate constant. Carbonate precipitation at the sediment–water interface would also have altered microbial habitats in shallow water environments.
If one thing stands out from the Archean sedimentary and geochemical record, it is the unstable creation and destruction of continental crust. Estimates of when plate tectonic processes began vary, as do hypotheses regarding the protracted and probably episodic growth of Archean continental crust. Several lines of inquiry, however, point to a critical transition towards more silica-rich continental crust generation approximately 3000 Ma, thought to be coincident with the onset of plate tectonics [93]. Only in the Late Archean Eon did large, stable cratons develop, as evidenced by the Earth's earliest thick and laterally extensive platform sedimentary successions [83]. This change in the physical framework of the Earth surface processes would have increased the range and extent of seafloor environments capable of supporting photosynthetically fuelled ecosystems, as well as the timescale on which carbon and other elements would be sequestered in the sedimentary record [94,95].
5. Archean ecosystems
How would the biologic carbon cycle have worked in early anoxic oceans (figure 5)? Canfield et al. [96] argued that before the rise of oxygenic photosynthesis to ecologic and biogeochemical prominence, autotrophy would have been limited by the abundances of electron donors, with ferrous iron permitting anoxygenic photosynthesis at rates about 10% of the present day. When oxygenic photosynthesis began remains uncertain [79], but even if cyanobacteria have at least a Late Archean history, the toxic effects of ferrous iron in solution might have limited rates of oxygenic photosynthesis [97].
In the absence of heterotrophic processes that return carbon dioxide to the environment, the natural experiment of life on the Earth would have been brief. Without O2, aerobic respiration could not have been important. Further, in early anoxic environments, alternative oxidants such as 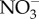 ,
,  and
and 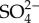 that drive anaerobic respiration in present-day oceans would likewise have been in short supply [98]. This leaves ferric iron as the most abundant oxidant for anaerobic respiration. The idea of an early iron age for the Earth is supported by Fe isotopic ratios, which have been interpreted in terms of repeated redox cycling of iron in Archean oceans [99]; the geochemistry of Archean iron formations also supports the hypothesis that carbon and iron cycling were closely linked in early marine ecosystems (figure 5c, [57,100]). Fermentation and methanogenesis would have contributed to Archean heterotrophy, and carbon isotopes support a quantitatively important role for the latter in Late Archean ecosystems [36]. In the absence of aerobic decay, however, decreased rates of biopolymer breakdown might have limited rates of both metabolisms [101].
that drive anaerobic respiration in present-day oceans would likewise have been in short supply [98]. This leaves ferric iron as the most abundant oxidant for anaerobic respiration. The idea of an early iron age for the Earth is supported by Fe isotopic ratios, which have been interpreted in terms of repeated redox cycling of iron in Archean oceans [99]; the geochemistry of Archean iron formations also supports the hypothesis that carbon and iron cycling were closely linked in early marine ecosystems (figure 5c, [57,100]). Fermentation and methanogenesis would have contributed to Archean heterotrophy, and carbon isotopes support a quantitatively important role for the latter in Late Archean ecosystems [36]. In the absence of aerobic decay, however, decreased rates of biopolymer breakdown might have limited rates of both metabolisms [101].
An additional feature of the Archean carbon cycle is worth noting. In present-day oceans, organic molecules are made available to bacterial heterotrophs by means of secretion, autolysis and viral lysis—what has been termed the ‘microbial loop’. It is estimated that the microbial loop cycles as much as 50% of marine primary production [102]. While this is sometimes viewed as a permutation of the carbon cycle documented for terrestrial communities, in a world without eukaryotes, the microbial loop would have been the carbon cycle (figure 5).
How well can we constrain rates of primary production in early oceans? The mean organic content of Archean shales is not too different from that of their Phanerozoic counterparts [103], but this need not indicate comparable levels of primary production, as organic preservation may have been enhanced in anoxic seas [104]. Macronutrient levels remain uncertain. Bjerrum & Canfield [105] argued that phosphate would have been absorbed onto the surfaces of iron(III) oxide-hydroxides formed in the Archean water column, limiting its biological availability; however, experiments conducted in NaCl solution showed that silica competes strongly with phosphate for absorption sites on ferrihydroxide surfaces [106]. The close relationship of silica and iron in banded iron formations demonstrates that silica adsorption onto oxidized iron was an important process in Archean oceans [57], but further experiments conducted in simulated Archean seawater suggest that despite strong silica adsorption, Ca and Mg ions promote phosphate precipitation on ferrihydroxide surfaces [107]. Consistent with this, other experimental data indicate that the presence of dissolved silica actually increases the retention of phosphorous on ferrihydrate surfaces [108]. Jones et al. [107] estimate that phosphate levels in Early Archean oceans could have been more than an order of magnitude lower than those of present oceans. If correct, this would mean that both the amount and nature of primary production could have been governed by the high Fe : P of early oceans, and only as this ratio declined would cyanobacteria have gained ecological prominence [107]. Other potential participants in the iron and phosphate chemistry of early seawater—for example, green rust and the iron phosphate mineral vivianite—remain to be fully integrated into this picture, providing scope for continued experiments, modelling and both sedimentologically and petrologically constrained analyses of Archean sedimentary rocks.
Uncertainty also attends discussion of nitrogen bioavailability in early oceans (figure 5c). Lightning discharge in the Archean atmosphere would have produced NOx [109], perhaps in abundances sufficient to support the low rates of photosynthesis estimated by Canfield et al. [96] and Jones et al. [107]. Declining pCO2 would have made abiotically fixed nitrogen a diminishing resource through time, however, eventually necessitating biological fixation. Nitrogen fixation is commonly viewed as an early event in evolution (e.g. [24]), but there are dissenters, for example, Boyd & Peters [110], who interpret phylogenetic and genomic data as indicating an Early Proterozoic evolution of nitrogenase, and Sanchez-Baracaldo et al. [111] whose molecular clock estimates indicate a Late Proterozoic radiation of N-fixing cyanobacteria in the phytoplankton.
One issue is the bioavailability of molybdenum, a cofactor in the most common and enzymatically efficient form of nitrogenase. Geochemical data suggest that Mo was in short supply before the rise of atmospheric oxygen, and this also might have limited biological nitrogen fixation in early oceans [112]. Such a conclusion, however, depends strongly on the level of Mo necessary to support N-fixation; limited experiments suggest that required levels may, in fact, have been low [113]. Stüeken et al. [42] interpret distinctive δ15N in Mid- to Late Archean samples as a diagnostic signature of Mo-based microbial nitrogen fixation; this conclusion is interesting, not least because it requires Mo solubility in oceans generally interpreted as anoxic. As ever, one must bear in mind the uncertainties associated with diagenesis, hydrothermal alteration and metamorphism. In general, one might imagine that rates of primary production varied spatially in Archean oceans, as they do today, but co-limitation by electron donors, macronutrients and micronutrients very likely limited biological participants in the Archean carbon cycle to fluxes and reservoir sizes well below those observed in modern oceans.
As noted above, isotopes indicate that microorganisms also populated a biological sulfur cycle in Early Archean oceans, but place limits on the extent of this biological participation (figure 5c). NanoSIMS-based analyses of fine-scale isotopic variations in pyrite and organically bound S within approximately 3450 Ma stromatolites reveal a distribution of δ34S and Δ33S best explained by input from sulfur aerosols generated by photochemical processes in the atmosphere and subsequent biological fractionation within the sediments, especially via microbial sulfur disproportionation [41,114]. Other studies, comparing co-occurring barite and pyrite, find evidence for dissimilatory sulfate (or sulfite) reduction [40,115], with generation of the oxidized sulfur required for such metabolism occurring by sulfide-using autotrophy and sulfur disproportionation. Despite evidence for biological sulfur metabolism, however, photochemical signatures persist, indicating that biological cycling of S within seawater and sediments was limited [116]. This reflects, at least in part, low sulfate supply to oceans beneath an anoxic atmosphere [117] and the efficient removal of seawater and pore water sulfur via pyrite precipitation [118]. The early literature spoke of sulfureta in Archean ecosystems, but available evidence suggests that repeated S cycling between microbial autotrophs and heterotrophs must have been limited prior to the rise of atmospheric oxygen.
Finally, how might distinct Archean trace element abundances have influenced early microbial ecosystems? Trace element abundances will ultimately reflect the composition of land masses available for weathering and erosion, fluxes from hydrothermal systems, and redox sensitivity during weathering and transport, and so the inventory of bioavailable trace metals in Archean oceans must have been distinctly different from today's [112,119,120]. For example, Ni abundances in seawater, inferred from Ni : Fe in banded iron formations, began a steady decline between approximately 2700 and 2500 Ma, rendering Ni a declining resource for methanogens that use Ni as a cofactor in several key enzymes [121]. Whether this would have resulted in Ni-starvation among methanogens depends on other factors that would have influenced methanogen abundance and ecology. Declining hydrothermal hydrogen fluxes through time might have also limited autotrophic methanogenesis, while increasing sulfate levels as oxygen gas began to accumulate in the atmosphere and surface oceans would have increased competition among heterotrophs for organic substrates. Thus, as noted for Mo and nitrogen fixation, questions of Ni starvation require consideration of both supply and demand.
Beginning with Frausto de Silva & Williams [122], it has been argued that trace element incorporation into enzymes may reflect the distinct inventory of trace metals in early ecosystems. In anoxic Archean oceans, one would expect elements such as Fe, Mn and Co to be relatively abundant, but Mo, Cu and Zn to be rare [119,120], and phylogenetic analyses of protein structures support the view that environment influenced the early evolution of metallic cofactors (e.g. [123,124]). In particular, the key role of iron in fundamental enzymes is consistent with the inference of an early iron age based on geochemical data. Nonetheless, David & Alm [125] rightly point out that declining Fe availability in increasingly oxygenated Proterozoic and Phanerozoic oceans has not been accompanied by a shift away from Fe use in proteins; indeed the number of Fe-using enzymes has increased through time. This suggests that many enzymes have become tuned for Fe incorporation, making an evolutionary shift to other trace metals difficult; that the evolution of siderophores has helped microorganisms to sustain Fe supply, despite decreasing environmental availability; or both [125].
6. Transition to a more modern biosphere
Starting around 2400 Ma, the sedimentary record displays a clear fingerprint of irreversible Earth surface oxygenation that progressed over a period of ca 200 Myr (figure 1, [1,67]). This ‘Great Oxidation Event’, or GOE, is marked by not only the global appearance in sedimentary basins of oxidized fluvial deposits called red beds [70,126], deep weathering profiles that retain iron [127], sulfate evaporites [128,129] and manganese deposits [130], but also the disappearance of redox-sensitive detrital mineral assemblages [71,131], banded iron formations [82] and perhaps most importantly, S-MIF in sedimentary sulfides [39]. Attempts to constrain the timing of the GOE have focused on the disappearance of S-MIF between 2450 and 2320 Ma [132,133]; however, these geochronological constraints are complicated by two major factors: (i) reports of pre-2450 Ma oxygenesis, or ‘whiffs of oxygen’, as evidenced by trace metal and sulfur isotopic data from various Archean sedimentary basins (see above) and (ii) the possibility that sedimentary sulfides carrying S-MIF signals might have been recycled through multiple sedimentary processes [134]. The youngest occurrence of redox-sensitive minerals, in sandstones dated at 2.415 Ga [130], provides an independent lower constraint on the timing of oxygenation. Between 2.4 and 2.3 Ga, then, Earth's geosphere and biosphere experienced a profound and unidirectional shift in redox environment.
Set against a backdrop of increasingly stable continental crust, H2 escape from the atmosphere and attendant Earth surface oxidation ([135] and references therein), the GOE was ultimately driven by photosynthetic O2 accumulation following a potentially protracted interval when oxygen sinks exceed sources. Many arguments have been put forth for the trigger for this fundamental shift in Earth's redox state, including tectonic evolution models tied to biogeochemical cycling; consensus has not yet been reached, but most discussions now highlight the idea of progressive biospheric oxygenation as opposed to a discrete, short-lived event.
It is also important to note that while oxygen may initially have accumulated to values approaching those of the present day [136], it eventually settled into a stable state in which the atmosphere and surface ocean contained O2 at levels of less than one to a few per cent of current values (figure 1, [1]). In most times and places, the oxygen minimum zone beneath the ocean's surface mixed layer remained anoxic [137]. Ferruginous subsurface waters were more common than sulfidic (euxinic) water masses [138], but sulfidic oxygen minimum zones appear to have been more common early in the Proterozoic Eon than they were later on [2].
How would the GOE have impacted microbial ecosystems? First of all, oxygen gas would have altered the balance of oxidants and reductants available for energy metabolism [139]. O2 in the photic zone would have eliminated alternative electron donors for photosynthesis, cementing the ecological dominance of cyanobacteria as primary producers. That stated, lipid biomarkers preserved in approximately 1600 Ma shales demonstrate that anoxia episodically penetrated the photic zone, supporting anoxygenic photosynthetic bacteria, including cyanobacteria able to downregulate Photosystem II facultatively [26,29]. Indeed, there is reason to believe that as the linkage between carbon and iron cycling weakened into the Proterozoic Eon, the connection between the carbon and sulfur cycles was enhanced (figure 5b).
The GOE would have also expanded the range of oxidants available for respiration and chemosynthesis. Not only did O2 emerge as a persistently available terminal electron acceptor in surficial environments, inventories of sulfate and nitrate would have expanded (e.g. [137]). The result was microbial ecosystems, at least within the mixed layer of the oceans, which more closely resembled those that cycle C, S and N today (figure 5). More generally, metabolic networks expanded with the addition of oxygen-requiring steps in biosynthesis [140]. Iron availability decreased in surface waters, but subsurface anoxia and, especially, hydrogen sulfide would have continued to limit the availability of many biologically important trace elements [119]. Primary production was no longer limited by electron donors, but Mo availability might have placed limits on nitrogen fixation [112,141], and, in the intermediate world of the Proterozoic, P might have been sequestered by adsorption onto iron oxides, limiting rates of primary production [104].
One additional biological change is evident from the fossil record: by at least 1600–1800 Ma, eukaryotes had begun their radiation in the oceans [12,142]. Mitochondria are integral components of crown group eukaryotes, and the GOE enabled the incorporation of proteobacteria able to respire aerobically into nascent eukaryotic cells. (Mitochondria retain the capacity for anaerobic metabolism, suggesting that aerobic respiration was facultative in low oxygen Proterozoic environments [143].) Early eukaryotes were largely phagocytosing heterotrophs [144,145], and this would have added a new ecological dimension to Proterozoic ecosystems. Ecosystem change would continue, with the later development of fully oxic oceans and the evolution of complex multicellular organisms, but in the wake of the GOE, microbial ecosystems gained a modern aspect, interpretable in terms of taxa and metabolisms observable today (figure 5b).
7. Conclusion
What the geologic record tells us, then, is that for much of our planet's history, both the physical Earth and the biota it supports were far different from those we experience today (figure 5). The first billion years of biological and environmental evolution featured Bacteria and Archaea living in oceans with much iron but little oxygen. Over the next billion years, as oceans came to have less iron but a bit more oxygen, aerobic metabolisms expanded the metabolic diversity of prokaryotic microorganisms, while eukaryotes—hybrid cells with a new and different organization—further increased the diversity and ecological complexity of microbial communities. The ecosystems of life's first two billion years seem alien, but it was within these early oceans that the fundamental biogeochemical circuitries of carbon, sulfur, nitrogen and phosphorus cycling were established—microbial processes that still underpin all functioning ecosystems on the Earth. And within nascent eukaryotic cells, new genetic and cell biological features took root, ultimately making the evolution of complex multicellular organisms possible. Thus, in ways both ecological and evolutionary, we are very much a product of that distant world of the Earth's first iron age.
Acknowledgements
We thank Z. Adam and D. Johnston for reviews of an early version of this manuscript and K. Sugitani for thin sections of Warrawoona chert.
Authors' contributions
All authors contributed to the design, interpretations and writing of this paper.
Competing interests
We have no competing interests.
Funding
A.H.K. acknowledges funding by the Keck Foundation. J.V.S. acknowledges support from the Agouron Institute and Dartmouth College. K.D.B. acknowledges support from MIT and a Junior Fellowship at Harvard.
References
- 1.Lyons TW, Reinhard CT, Planavsky NJ. 2014. The rise of oxygen in Earth's early ocean and atmosphere. Nature 506, 307–315. ( 10.1038/nature13068) [DOI] [PubMed] [Google Scholar]
- 2.Sperling EA, Wolock CJ, Morgan AS, Gill BC, Kunzmann M, Halverson GP, Macdonald FA, Knoll AH, Johnston DT. 2015. Statistical analysis of iron geochemical data suggests limited late Proterozoic oxygenation. Nature 523, 451–454. ( 10.1038/nature14589) [DOI] [PubMed] [Google Scholar]
- 3.Kump LR. 2008. The rise of atmospheric oxygen. Nature 451, 277–278. ( 10.1038/nature06587) [DOI] [PubMed] [Google Scholar]
- 4.Knoll AH, Barghoorn ES, Awramik SM. 1978. New microorganisms from the Aphebian Gunflint Iron Formation, Ontario. J. Paleontol. 52, 976–992. [Google Scholar]
- 5.Ehrlich HL. 2004. Geomicrobiology. New York, NY: Dekker. [Google Scholar]
- 6.Maliva R, Knoll AH, Siever R. 1989. Secular change in chert distribution: a reflection of evolving biological participation in the silica cycle. Palaios 4, 519–532. ( 10.2307/3514743) [DOI] [PubMed] [Google Scholar]
- 7.Knoll AH. 2015. Paleobiological perspectives on early microbial evolution. Cold Spring Harb. Persp. Biol. 7, a018093 ( 10.1101/cshperspect.a018093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green J, Knoll AH, Swett K. 1988. Microfossils in oolites and pisolites from the Upper Proterozoic Eleonore Bay Group, central East Greenland. J. Paleontol. 62, 835–852. ( 10.1017/S0022336000030109) [DOI] [PubMed] [Google Scholar]
- 9.Bailey JV, Corsetti FA, Greene SE, Crosby CH, Liu P, Orphan VJ. 2013. Filamentous sulfur bacteria preserved in modern and ancient phosphatic sediments: implications for the role of oxygen and bacteria in phosphogenesis. Geobiology 11, 397–405. ( 10.1111/gbi.12046) [DOI] [PubMed] [Google Scholar]
- 10.Butterfield NJ, Knoll AH, Swett K. 1994. Paleobiology of the Upper Proterozoic Svanbergfjellet Formation, Spitsbergen. Fossils Strata 34, 1–84. [Google Scholar]
- 11.Butterfield NJ. 2000. Bangiomorpha pubescens n. gen., n. sp.: implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes. Paleobiology 26, 386–404. ( 10.1666/0094-8373(2000)026%3C0386:BPNGNS%3E2.0.CO;2) [DOI] [Google Scholar]
- 12.Knoll AH. 2014. Paleobiological perspectives on early eukaryotic evolution. Cold Spring Harb. Persp. Biol. 6, a016121 ( 10.1101/cshperspect.a016121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cloud PE. 1965. Significance of Gunflint (Precambrian) microflora. Science 148, 27–35. ( 10.1126/science.148.3666.27) [DOI] [PubMed] [Google Scholar]
- 14.Walter MR. 1976. Stromatolites. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 15.Grotzinger JP, Knoll AH. 1999. Proterozoic stromatolites: evolutionary mileposts or environmental dipsticks? Annu. Rev. Earth Planet. Sci. 27, 313–358. ( 10.1146/annurev.earth.27.1.313) [DOI] [PubMed] [Google Scholar]
- 16.Bosak T, Knoll AH, Petroff AP. 2013. The meaning of stromatolites. Annu. Rev. Earth Planet. Sci. 41, 21–44. ( 10.1146/annurev-earth-042711-105327) [DOI] [Google Scholar]
- 17.Butterfield NJ. 2016. Proterozoic photosynthesis – a critical review. Palaeontology 58, 953–972. ( 10.1111/pala.12211) [DOI] [Google Scholar]
- 18.Knoll AH, Wörndle S, Kah L. 2013. Covariance of microfossil assemblages and microbialite textures across a late Mesoproterozoic carbonate platform. Palaios 28, 453–470. ( 10.2110/palo.2013.p13-005r) [DOI] [Google Scholar]
- 19.Noffke N. 2010. Geobiology: microbial mats in sandy deposits from the Archean era to today. Berlin, Germany: Springer. [Google Scholar]
- 20.Mariotti G, Pruss S, Perron JT, Bosak T. 2014. Microbial shaping of sedimentary wrinkle structures. Nat. Geosci. 7, 736–740. ( 10.1038/NGEO2229) [DOI] [Google Scholar]
- 21.Hayes JM. 2001. Fractionation of carbon and hydrogen isotopes in biosynthetic processes. Rev. Mineral. Geochem. 43, 225–278. ( 10.2138/gsrmg.43.1.225) [DOI] [Google Scholar]
- 22.Fike DA, Bradley AS, Rose CV. 2015. Rethinking the ancient sulfur cycle. Annu. Rev. Earth Planet. Sci. 43, 593–622. ( 10.1146/annurev-earth-060313-054802) [DOI] [Google Scholar]
- 23.Ward B. 2012. The global nitrogen cycle. In Fundamentals of geobiology (eds Knoll AH, Canfield DE, Konhauser KO), pp. 36–48. Chichester, UK: Wiley-Blackwell. [Google Scholar]
- 24.Thomazo C, Papineau D. 2013. Biogeochemical cycling of nitrogen on the early Earth. Elements 9, 345–351. ( 10.2113/gselements.9.5.345) [DOI] [Google Scholar]
- 25.Stüeken EE. 2013. A test of the nitrogen-limitation hypothesis for retarded eukaryote radiation: nitrogen isotopes across a Mesoproterozoic basinal profile. Geochim. Cosmochim. Acta 120, 121–139. ( 10.1016/j.gca.2013.06.002) [DOI] [Google Scholar]
- 26.Brocks JJ, Love GD, Summons RE, Knoll AH, Logan GA, Bowden S. 2005. Biomarker evidence for green and purple sulfur bacteria in an intensely stratified Paleoproterozoic ocean. Nature 437, 866–870. ( 10.1038/nature04068) [DOI] [PubMed] [Google Scholar]
- 27.Brocks JJ, Jarrett AJM, Sirantoine E, Kenig F, Moczydłowska M, Porter S, Hope J. 2015. Early sponges and toxic protists: possible sources of cryostane, an age diagnostic biomarker antedating Sturtian Snowball Earth. Geobiology 14, 129–149. ( 10.1111/gbi.12165) [DOI] [PubMed] [Google Scholar]
- 28.Luo GM, Hallmann C, Xie SC, Ruan XY, Summons RE. 2015. Comparative microbial diversity and redox environments of black shale and stromatolite facies in the Mesoproterozoic Xiamaling Formation. Geochim. Cosmochim. Acta 151, 150–167. ( 10.1016/j.gca.2014.12.022) [DOI] [Google Scholar]
- 29.Johnston DT, Wolfe-Simon F, Pearson A, Knoll AH. 2009. Anoxygenic photosynthesis modulated Proterozoic oxygen and sustained Earth's middle age. Proc. Natl Acad. Sci. USA 106, 16 925–16 929. ( 10.1073/pnas.0909248106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.French KL, et al. 2015. Reappraisal of hydrocarbon biomarkers in Archean rocks. Proc. Natl Acad. Sci. USA 112, 5915–5920. ( 10.1073/pnas.1419563112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schopf JW. 2006. Fossil evidence of Archean life. Phil. Trans. R. Soc. B 361, 869–885. ( 10.1098/rstb.2006.1834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang S, DesMarais D, Mack R, Miller SL, Strathearn GE. 1983. Prebiotic organic syntheses and the origin of life. In Earth’s earliest biosphere (ed. Schopf JW.), pp. 53–92. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 33.McCollom TM, Lollar BS, Lacrampe-Couloume G, Seewald JS. 2010. The influence of carbon source on abiotic organic synthesis and carbon isotope fractionation under hydrothermal conditions. Geochim. Cosmochim. Acta 74, 2717–2740. ( 10.1016/j.gca.2010.02.008) [DOI] [Google Scholar]
- 34.Tice MM, Lowe DR. 2006. The origin of carbonaceous matter in pre-3.0 Ga greenstone terrains: a review and new evidence from the 3.42 Ga Buck Reef Chert. Earth-Sci. Rev. 76, 259–300. ( 10.1016/j.earscirev.2006.03.003) [DOI] [Google Scholar]
- 35.Ragsdale SW, Pierce E. 2008. Acetogenesis and the Wood–Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta Proteins Proteomics 1784, 1873–1898. ( 10.1016/j.bbapap.2008.08.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayes JM. 1994. Global methanotrophy at the Archean-Proterozoic transition. In Early life on Earth (ed. Bengtson S.), pp. 220–236. New York, NY: Columbia University Press. [Google Scholar]
- 37.Rosing MT. 1999. 13C-depleted carbon microparticles in >3700-Ma sea-floor sedimentary rocks from west Greenland. Science 283, 674–676. ( 10.1126/science.283.5402.674) [DOI] [PubMed] [Google Scholar]
- 38.Bell EA, Boehnke P, Harrison TM, Mao WL. 2015. Potentially biogenic carbon preserved in a 4.1 billion-year-old zircon. Proc. Natl Acad. Sci. USA 112, 14 518–14 521. ( 10.1073/pnas.1517557112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farquhar J, Wing BA. 2003. Multiple sulfur isotopes and the evolution of the atmosphere. Earth Planet. Sci. Lett. 213, 1–13. ( 10.1016/S0012-821X(03)00296-6) [DOI] [Google Scholar]
- 40.Roerdink DL, Mason PRD, Farquhar J, Reimer T. 2012. Multiple sulfur isotopes in Paleoarchean barites identify an important role for microbial sulfate reduction in the early marine environment. Earth Planet. Sci. Lett. 331, 177–186. ( 10.1016/j.epsl.2012.03.020) [DOI] [Google Scholar]
- 41.Bontognali TRR, Sessions AL, Allwood AC, Fischer WW, Grotzinger JP, Summons RE, Eiler JM. 2013. Sulfur isotopes of organic matter preserved in 3.45-billion-year-old stromatolites reveal microbial metabolism. Proc. Natl Acad. Sci. USA 109, 15 146–15 151. ( 10.1073/pnas.1207491109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stüeken EE, Buick R, Guy BM, Koehler MC. 2015. Isotopic evidence for biological nitrogen fixation by molybdenum-nitrogenase from 3.2 Gyr. Nature 520, 666–669. ( 10.1038/nature14180) [DOI] [PubMed] [Google Scholar]
- 43.Beukes NJ, Lowe DR. 1989. Environmental control on diverse stromatolite morphologies in the 3000 Myr Pongola Supergroup, South Africa. Sedimentology 36, 383–397. ( 10.1111/j.1365-3091.1989.tb00615.x) [DOI] [Google Scholar]
- 44.Noffke N, Beukes N, Bower D, Hazen RM, Swift DJP. 2008. An actualistic perspective into Archean worlds – (cyano-)bacterially induced sedimentary structures in the siliciclastic Nhlazatse Section, 2.9 Ga Pongola Supergroup, South Africa. Geobiology 6, 5–20. ( 10.1111/j.1472-4669.2007.00118.x) [DOI] [PubMed] [Google Scholar]
- 45.Lowe DR. 1994. Abiological origin of described stromatolites older than 3.2 Ga. Geology 22, 387–390. ( 10.1130/0091-7613(1994)022%3C0387:AOODSO%3E2.3.CO;2) [DOI] [PubMed] [Google Scholar]
- 46.Van Kronendonk MJ, Webb GE, Kamber BS. 2003. Stromatolitic carbonates in the Pilbara Craton: geological and trace element evidence for a marine sedimentary environment of deposition and biogenicity of 3.45 Ga stromatolitic carbonates in the Pilbara Craton, and support for a reducing Archaean ocean. Geobiology 1, 91–108. ( 10.1046/j.1472-4669.2003.00014.x) [DOI] [Google Scholar]
- 47.Allwood AC, Grotzinger JP, Knoll AH, Burch IW, Anderson MS, Coleman ML, Kanik I. 2009. Controls on development and diversity of Early Archean stromatolites. Proc. Natl Acad. Sci. USA 106, 9548–9555. ( 10.1073/pnas.0903323106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tice MM. 2009. Environmental controls on photosynthetic microbial mat distribution and morphogenesis on a 3.42 Ga clastic-starved platform. Astrobiology 9, 989–1000. ( 10.1089/ast.2008.0330) [DOI] [PubMed] [Google Scholar]
- 49.Homann M, Heubeck C, Airo A, Tice MM. 2015. Morphological adaptations of 3.22 Ga-old tufted microbial mats to Archean coastal habitats (Moodies Group, Barberton Greenstone Belt, South Africa). Precambrian Res. 266, 47–64. ( 10.1016/j.precamres.2015.04.018) [DOI] [Google Scholar]
- 50.Westall F, Campbell KA, Gabriel J, Foucher F, Gautret P, Hubert A, Sorieul N, Grassineau N, Guido DM. 2015. Archean (3.33 Ga) microbe-sediment systems were diverse and flourished in a hydrothermal context. Geology 43, 615–618. ( 10.1130/G36646.1) [DOI] [Google Scholar]
- 51.Schirrmeister BE, Sanchez-Baracaldo P, Wacey D. 2016. Cyanobacterial evolution during the Precambrian. Int. J. Astrobiol. 15, 187–204. ( 10.1017/S1473550415000579) [DOI] [Google Scholar]
- 52.Bosak T, Greene SE, Newman K. 2007. A likely role for anoxygenic photosynthetic microbes in the formation of ancient stromatolites. Geobiology 5, 119–126. ( 10.1111/j.1472-4669.2007.00104.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pierson BK, Castenholz RW. 1974. A phototrophic gliding filamentous bacterium of hot springs, Chloroflexus aurantiacus, gen. and sp. nov. Arch. Microbiol. 100, 5–24. ( 10.1007/BF00446302) [DOI] [PubMed] [Google Scholar]
- 54.Tice MM, Thornton DCO, Pope MC, Olszewski TD, Gong J. 2011. Archean microbial mat communities. Annu. Rev. Earth Planet. Sci. 39, 297–319. ( 10.1146/annurev-earth-040809-152356) [DOI] [Google Scholar]
- 55.Schopf JW, Kudryavtsev AB, Czaja AD, Tripathi AB. 2007. Evidence of Archean life: stromatolites and microfossils. Precambrian Res. 158, 141–155. ( 10.1016/j.precamres.2007.04.009) [DOI] [Google Scholar]
- 56.Brasier MD, Green OR, Lindsay JF, McLoughlin N, Steele A, Stoakes C. 2005. Critical testing of Earth's oldest putative fossil assemblage from the 3.5 Ga Apex Chert, Chinaman Creek, Western Australia. Precambrian Res. 140, 55–102. ( 10.1016/j.precamres.2005.06.008) [DOI] [Google Scholar]
- 57.Fischer WW, Knoll AH. 2009. An iron shuttle for deepwater silica in Late Archean and early Paleoproterozoic iron formation. Geol. Soc. Am. Bull. 121, 222–235. ( 10.1130/B26328.1) [DOI] [Google Scholar]
- 58.Sugitani K, Grey K, Nagaoka T, Mimura K, Walter MR. 2009. Taxonomy and biogenicity of Archaean spheroidal microfossils (ca. 3.0 Ga) from the Mount Goldsworthy–Mount Grant area in the northeastern Pilbara Craton, Western Australia. Precambrian Res. 173, 50–59. ( 10.1016/j.precamres.2009.02.004) [DOI] [Google Scholar]
- 59.Sugitani K, Mimura K, Nagaoka T, Lepot K, Takeuchi M. 2013. Microfossil assemblage from the 3400 Ma Strelley Pool Formation in the Pilbara Craton, Western Australia: results form a new locality. Precambrian Res. 226, 59–74. ( 10.1016/j.precamres.2012.11.005) [DOI] [Google Scholar]
- 60.Errington J. 2013. L-form bacteria, cell walls and the origins of life. Open Biol. 3, 120143 ( 10.1098/rsob.120143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pinti DL, Mineau R, Clement V. 2009. Hydrothermal alteration and microfossils artefacts of the 3,465-million-year-old Apex chert. Nat. Geosci. 2, 640–643. ( 10.1038/NGEO601) [DOI] [Google Scholar]
- 62.Marshall AO, Emry JR, Marshall CP. 2012. Multiple generations of carbon in the Apex Chert and implications for preservation of microfossils. Astrobiology 12, 160–166. ( 10.1089/ast.2011.0729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strauss JV, Macdonald FA, Halverson GP, Schrag DP, Knoll AH. 2015. Stratigraphic evolution of the Neoproterozoic Callison Lake Formation. Am. J. Sci. 315, 881–944. ( 10.2475/10.2015.01) [DOI] [Google Scholar]
- 64.Macdonald FA, et al. 2010. Calibrating the Cryogenian. Science 327, 1241–1243. ( 10.1126/science.1183325) [DOI] [PubMed] [Google Scholar]
- 65.Mustard PS, Roots CF. 1997. Rift-related volcanism, sedimentation, and tectonic setting of the Mount Harper Group, Ogilvie Mountains, Yukon Territory. Geol. Surv. Can. Bull. 492, 1–92. ( 10.4095/208670) [DOI] [Google Scholar]
- 66.Javaux EJ, Marshall CP, Bekker A. 2010. Organic-walled microfossils in 3.2 billion-year-old shallow-marine siliciclastic deposits. Nature 463, 934–938. ( 10.1038/nature08793) [DOI] [PubMed] [Google Scholar]
- 67.Holland HD. 2006. The oxygenation of the atmosphere and oceans. Phil. Trans. R. Soc. B 361, 903–915. ( 10.1098/rstb.2006.1838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin WF, Sousa FL. 2016. Early microbial evolution: the age of anaerobes. Cold Spring Harb. Perspect. Biol. 8, a018127 ( 10.1101/cshperspect.a018127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pavlov AA, Kasting JF. 2002. Mass-independent fractionation of sulfur isotopes in Archean sediments: strong evidence for an anoxic Archean atmosphere. Astrobiology 2, 27–41. ( 10.1089/153110702753621321) [DOI] [PubMed] [Google Scholar]
- 70.Roscoe SM. 1969. Huronian rocks and uraniferous conglomerates in the Canadian Shield. Geol. Surv. Pap. Can. 68, 1–205. [Google Scholar]
- 71.Johnson JE, Gerpheide A, Lamb MP, Fischer WW. 2014. O2 constraints from Paleoproterozoic detrital pyrite and uraninite. Bull. Geol. Soc. Am. 126, 813–830. ( 10.1130/B30949.1) [DOI] [Google Scholar]
- 72.Planavsky NJ, Asael D, Hofmann A, Reinhard CT. 2014. Evidence for oxygenic photosynthesis half a billion years before the Great Oxidation Event. Nature 7, 283–286. ( 10.1038/ngeo2122) [DOI] [Google Scholar]
- 73.Crowe SA, Døssing LN, Beukes NJ, Bau M, Kruger SJ, Frei R, Canfield DE. 2013. Atmospheric oxygenation three billion years ago. Nature 501, 535–538. ( 10.1038/nature12426) [DOI] [PubMed] [Google Scholar]
- 74.Frei R, Gaucher C, Poulton SW, Canfield DE. 2009. Fluctuations in Precambrian atmospheric oxygenation recorded by chromium isotopes. Nature 461, 250–253. ( 10.1038/nature08266) [DOI] [PubMed] [Google Scholar]
- 75.Anbar AD, et al. 2007. A whiff of oxygen before the great oxidation event? Science 317, 1903–1906. ( 10.1126/science.1140325) [DOI] [PubMed] [Google Scholar]
- 76.Kendall B, Reinhard CT, Lyons TW, Kaufman AJ, Poulton SW, Anbar AD. 2010. Pervasive oxygenation along late Archaean ocean margins. Nat. Geosci. 3, 647–652. ( 10.1038/NGEO942) [DOI] [Google Scholar]
- 77.Stolper DA, Revsbech NP, Canfield DE. 2010. Aerobic growth at nanomolar oxygen concentrations. Proc. Natl Acad. Sci. USA 107, 18 755–18 760. ( 10.1073/pnas.1013435107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pufahl PK, Hiatt EE. 2012. Oxygenation of the Earth's atmosphere–ocean system: a review of physical and chemical sedimentologic responses. Mar. Pet. Geol. 32, 1–20. ( 10.1016/j.marpetgeo.2011.12.002) [DOI] [Google Scholar]
- 79.Farquhar J, Zerkle AL, Bekker A. 2011. Geological constraints on the origin of oxygenic photosynthesis. Photosynth. Res. 107, 11–36. ( 10.1007/s11120-010-9594-0) [DOI] [PubMed] [Google Scholar]
- 80.Fischer WW, Hemp J, Johnson JE. 2015. Manganese and the evolution of photosynthesis. Orig. Life Evol. Biosph. 45, 351–357. ( 10.1007/s11084-015-9442-5) [DOI] [PubMed] [Google Scholar]
- 81.Frei R, Crowe SA, Bau M, Polat A, Fowle DA, Døssing LA. 2016. Oxidative elemental cycling under the low O2 Eoarchean atmosphere. Sci. Rep. 6, 21058 ( 10.1038/srep21058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bekker A, Slack JF, Planavsky N, Krapez B, Hofmann A, Konhauser KO, Rouxel OJ. 2010. Iron formation: the sedimentary product of a complex interplay among mantle, tectonic, oceanic, and biospheric processes. Econ. Geol. 105, 467–508. ( 10.2113/gsecongeo.105.3.467) [DOI] [Google Scholar]
- 83.Beukes NJ. 1987. Facies relations, depositional environments and diagenesis in a major early Proterozoic stromatolitic carbonate platform to basinal sequence, Campbellrand Subgroup, Transvaal Supergroup, Southern Africa. Sed. Geol. 54, 1–46. ( 10.1016/0037-0738(87)90002-9) [DOI] [Google Scholar]
- 84.Stefurak EJT, Lowe DR, Zentner D, Fischer WW. 2014. Primary silica granules -- a new mode of Paleoarchean sedimentation. Geology 42, 283–286. ( 10.1130/G35187.1) [DOI] [Google Scholar]
- 85.Arndt NT, Nisbet EG. 2012. Processes on the young Earth and the habitats of early life. Annu. Rev. Earth Planet. Sci. 40, 521–549. ( 10.1146/annurev-earth-042711-105316) [DOI] [Google Scholar]
- 86.Grotzinger JP, Kasting JF. 1993. New constraints on Precambrian ocean composition. J. Geol. 101, 235–243. ( 10.1086/648218) [DOI] [PubMed] [Google Scholar]
- 87.Kasting JF. 1987. Theoretical constraints on oxygen and carbon dioxide concentrations in the Precambrian atmosphere. Precambrian Res. 34, 205–229. ( 10.1016/0301-9268(87)90001-5) [DOI] [PubMed] [Google Scholar]
- 88.Grotzinger JP. 1989. Facies and evolution of Precambrian carbonate depositional systems: emergence of the modern platform archetype. SEPM Spec. Publ. 44, 79–106. [Google Scholar]
- 89.Sumner DY, Grotzinger JP. 2004. Implications for Neoarchaean ocean chemistry from primary carbonate mineralogy of the Campbellrand-Malmani Platform, South Africa. Sedimentology 51, 1273–1299. ( 10.1111/j.1365-3091.2004.00670.x) [DOI] [Google Scholar]
- 90.Higgins JA, Fischer WW, Schrag DP. 2009. Oxygenation of the ocean and sediments: consequences for the seafloor carbonate factory. Earth Planet. Sci. Lett. 284, 25–33. ( 10.1016/j.epsl.2009.03.039) [DOI] [Google Scholar]
- 91.Bergmann KD, Grotzinger JP, Fischer WW. 2013. Biological influences on seafloor carbonate precipitation. Palaios 28, 99–115. ( 10.2110/palo.2012.p12-088r) [DOI] [Google Scholar]
- 92.Sumner DY, Grotzinger JP. 1996. Were kinetics of Archean calcium carbonate precipitation related to oxygen concentration? Geology 24, 119–122. ( 10.1130/0091-7613(1996)024%3C0119:WKOACC%3E2.3.CO;2) [DOI] [PubMed] [Google Scholar]
- 93.Dhuime B, Wuestefled A, Hawkesworth CJ. 2015. Emergence of modern continental crust about 3 billion years ago. Nat. Geosci. 8, 552–555. ( 10.1038/ngeo2466) [DOI] [Google Scholar]
- 94.Knoll AH. 1979. Archean photoautotrophy: some alternatives and limits. Orig. Life Evol. Biospheres 9, 313–327. ( 10.1007/BF00926824) [DOI] [PubMed] [Google Scholar]
- 95.Lalonde SV, Konhauser KO. 2015. Benthic perspective on Earth's oldest evidence for oxygenic photosynthesis. Proc. Natl Acad. Sci. USA 112, 995–1000. ( 10.1073/pnas.1415718112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Canfield DE, Rosing MT, Bjerrum C. 2006. Early anaerobic metabolisms. Phil. Trans. R. Soc. B 361, 1819–1836. ( 10.1098/rstb.2006.1906) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Swanner ED, Mloszewska AM, Cirpka OA, Schoenberg R, Konhauser KO, Kappler A. 2015. Modulation of oxygen production in Archaean oceans by episodes of Fe(II) toxicity. Nat. Geosci. 8, 126–130. ( 10.1038/NGEO2327) [DOI] [Google Scholar]
- 98.Anbar AD. 2008. Elements and evolution. Science 322, 1481–1483. ( 10.1126/science.1163100) [DOI] [PubMed] [Google Scholar]
- 99.Busigny V, Planavsky NJ, Jézéquel D, Crowe S, Louvat P, Moureau J, Viollier E, Lyons TW. 2014. Iron isotopes in an Archean ocean analogue. Geochim. Cosmochim. Acta 133, 443–462. ( 10.1016/j.gca.2014.03.004) [DOI] [Google Scholar]
- 100.Kappler A, Pasquero C, Konhauser KO, Newman DK. 2005. Deposition of banded iron formations by anoxygenic phototrophic Fe(II)-oxidizing bacteria. Geology 33, 865–868. ( 10.1130/G21658.1) [DOI] [Google Scholar]
- 101.Megonigal JP, Hines ME, Visscher PT. 2004. Anaerobic metabolism: linkages to trace gases and aerobic processes. In Biogeochemistry (ed. Schlesinger WH.), pp. 317–424. Oxford, UK: Elsevier-Pergamon. [Google Scholar]
- 102.Pomeroy LR, Williams PJ, Azam F, Hobbie EA. 2007. The microbial loop. Oceanography 20, 28–33. ( 10.5670/oceanog.2007.45) [DOI] [Google Scholar]
- 103.Martin RE, Quigg A, Podkovyrov V. 2008. Marine biodiversification in response to evolving phytoplankton stoichiometry. Palaeogeogr. Palaeoclimatol. Palaeoecol. 258, 277–291. ( 10.1016/j.palaeo.2007.11.003) [DOI] [Google Scholar]
- 104.Laakso TA, Schrag DP. 2014. Regulation of atmospheric oxygen during the Proterozoic. Earth Planet. Sci. Lett. 388, 81–91. ( 10.1016/j.epsl.2013.11.049) [DOI] [Google Scholar]
- 105.Bjerrum CJ, Canfield DE. 2002. Ocean productivity before about 1.9 Gyr ago limited by phosphorus adsorption onto iron oxides. Nature 417, 159–162. ( 10.1038/417159a) [DOI] [PubMed] [Google Scholar]
- 106.Konhauser KO, Lalonde SV, Amskold L, Holland HD. 2007. Was there really an Archean phosphate crisis? Science 315, 1234–1237. ( 10.1126/science.1136328) [DOI] [PubMed] [Google Scholar]
- 107.Jones C, Nomosatryo S, Crowe SA, Bjerrum CJ, Canfield DE. 2015. Iron oxides, divalent cations, silica, and the early earth phosphorus crisis. Geology 43, 135–138. ( 10.1130/G36044.1) [DOI] [Google Scholar]
- 108.Mayer TD, Jarrell WM. 2000. Phosphorus sorption during iron(II) oxidation in the presence of dissolved silica. Water Res. 34, 3949–3959. ( 10.1016/S) [DOI] [Google Scholar]
- 109.Cooray V, Rahman M, Rakov V. 2009. On the NO(x) production by laboratory electrical discharges and lightning. J. Atm. Solar-Terrestr. Physics 71, 1877–1889. ( 10.1016/j.jastp.2009.07.009) [DOI] [Google Scholar]
- 110.Boyd ES, Peters JW. 2013. New insights into the evolutionary history of biological nitrogen fixation. Front. Microbiol. 4, 201. ( 10.3389/fmicb.2013.00201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sanchez-Baracaldo P, Ridgwell A, Raven JA. 2014. A Neoproterozoic transition in the marine nitrogen cycle. Curr. Biol. 24, 652–657. ( 10.1016/j.cub.2014.01.041) [DOI] [PubMed] [Google Scholar]
- 112.Anbar AD, Knoll AH. 2002. Proterozoic ocean chemistry and evolution: a bioorganic bridge? Science 297, 1137–1142. ( 10.1126/science.1069651) [DOI] [PubMed] [Google Scholar]
- 113.Zerkle AL, House CH, Cox RP, Canfield DE. 2006. Metal limitation of cyanobacterial N2 fixation and implications for the Precambrian nitrogen cycle. Geobiology 4, 285–297. ( 10.1111/j.1472-4669.2006.00082.x) [DOI] [Google Scholar]
- 114.Philippot P, Van Zuilen M, Lepot K, Thomazo C, Farquhar J, Van Kranendonk MJ. 2007. Early Archaean microorganisms preferred elemental sulfur, not sulfate. Science 317, 1534–1537. ( 10.1126/science.1145861) [DOI] [PubMed] [Google Scholar]
- 115.Shen Y, Farquhar J, Masterson A, Kaufman AJ, Buick R. 2009. Evaluating the role of microbial sulfate reduction in the early Archean using quadruple isotope systematics. Earth Planet. Sci. Lett. 279, 383–391. ( 10.1016/j.epsl.2009.01.018) [DOI] [Google Scholar]
- 116.Johnston DT. 2011. Multiple sulfur isotopes and the evolution of Earth's surface sulfur cycle. Earth-Sci. Rev. 106, 161–183. ( 10.1016/j.earscirev.2011.02.003) [DOI] [Google Scholar]
- 117.Crowe SA, et al. 2012. Sulfate was a trace constituent of Archean seawater. Science 346, 735–739. ( 10.1126/science.1258966) [DOI] [PubMed] [Google Scholar]
- 118.Canfield DE, Raiswell R. 1999. The evolution of the sulfur cycle. Am. J. Sci. 299, 97–723 10.2475/ajs.299.7-9.697) [DOI] [Google Scholar]
- 119.Saito MA, Sigman DM, Morel FMM. 2003. The bioinorganic chemistry of the ancient ocean: the co-evolution of cyanobacterial metal requirements and biogeochemical cycles at the Archean-Proterozoic boundary? Inorganica Chim. Acta 356, 308–318. ( 10.1016/S0020-1693(03)00442-0) [DOI] [Google Scholar]
- 120.Zerkle AL, House CH, Brantley SL. 2005. Biogeochemical signatures through time as inferred from whole microbial genomes. Am. J. Sci. 305, 467–502. ( 10.2475/ajs.305.6-8.467) [DOI] [Google Scholar]
- 121.Konhauser KO, Robbins LJ, Pecoits E, Peacock C, Kappler A, Lalonde SV. 2015. The Archean nickel famine revisited. Astrobiology 15, 804–815. ( 10.1089/ast.2015.1301) [DOI] [PubMed] [Google Scholar]
- 122.Frausto da Silva JJR, Williams RJP. 2001. The biological chemistry of the elements. Oxford, UK: Oxford University Press. [Google Scholar]
- 123.Dupont CL, Yang S, Palenik B, Bourne PE. 2006. Modern proteomes contain putative imprints of ancient shifts in trace metal geochemistry. Proc. Natl Acad. Sci. USA 103, 17 822–17 827. ( 10.1073/pnas.0605798103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dupont CL, Butcher A, Valas RE, Bourne PE, Caetano-Anollés G. 2010. History of biological metal utilization inferred through phylogenomic analysis of protein structures. Proc. Natl Acad. Sci. USA 107, 10 567–10 572. ( 10.1073/pnas.0912491107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.David LA, Alm EJ. 2011. Rapid evolutionary innovation during an Archean gene expansion. Nature 469, 93–96. ( 10.1038/natur09649)469(7328):93-6) [DOI] [PubMed] [Google Scholar]
- 126.Cloud P. 1968. Atmospheric and hydrosphere evolution on primitive earth. Science 160, 729–736. ( 10.1126/science.160.3829.729) [DOI] [PubMed] [Google Scholar]
- 127.Rye R, Holland HD. 1998. Paleosols and the evolution of atmospheric oxygen: a critical review. Am. J. Sci. 298, 621–672. ( 10.2475/ajs.298.8.621) [DOI] [PubMed] [Google Scholar]
- 128.Melezhik VA, Fallick AE, Hanski EJ, Kump LR, Lepland A, Prave AR, Strauss H. 2005. Emergence of the aerobic biosphere during the Archean-Proterozoic transition: challenges of future research. GSA Today 15, 4–11. ( 10.1130/1052-5173(2005)015%5B4:EOAABD%5D2.0.CO;2) [DOI] [Google Scholar]
- 129.Bekker A, Karhu JA, Kaufman AJ. 2006. Carbon isotope record for the onset of the Lomagundi carbon isotope excursion in the Great Lakes area, North America. Precambrian Res. 148, 145–180. ( 10.1016/j.precamres.2006.03.008) [DOI] [Google Scholar]
- 130.Johnson JE, Webb SM, Ma C, Fischer WW. 2016. Manganese mineralogy and diagenesis in the sedimentary rock record. Geochim. Cosmochim. Acta 173, 210–231. ( 10.1016/j.gca.2015.10.027) [DOI] [Google Scholar]
- 131.Rasmussen B, Buick R. 1999. Redox state of the Archean atmosphere: evidence from detrital heavy minerals in ca. 3250–2750 Ma sandstones from the Pilbara Craton, Australia. Geology 27, 115–118. ( 10.1130/0091-7613(1999)027%3C0115:RSOTAA%3E2.3.CO;2) [DOI] [Google Scholar]
- 132.Bekker A, Holland HD, Wang PL, Rumble D III, Stein HJ, Hannah JL, Coetzee LL, Beukes NJ. 2004. Dating the rise of atmospheric oxygen. Nature 427, 117–120. ( 10.1038/nature02260) [DOI] [PubMed] [Google Scholar]
- 133.Guo Q, et al. 2009. Reconstructing Earth's surface oxidation across the Archean-Proterozoic transition. Geology 37, 399–402. ( 10.1130/G25423A.1) [DOI] [Google Scholar]
- 134.Reinhard CT, Planavsky NJ, Lyons TW. 2013. Long-term sedimentary recycling of rare sulphur isotope anomalies. Nature 497, 100–103. ( 10.1038/nature12021) [DOI] [PubMed] [Google Scholar]
- 135.Zahnle KJ, Catling DC, Claire MW. 2013. The rise of oxygen and the hydrogen hourglass. Chem. Geol. 362, 26–34. ( 10.1016/j.chemgeo.2013.08.004) [DOI] [Google Scholar]
- 136.Bekker A, Holland HD. 2012. Oxygen overshoot and recovery during the early Paleoproterozoic. Earth Planet. Sci. Lett. 317–318, 295–304. ( 10.1016/j.epsl.2011.12.012) [DOI] [Google Scholar]
- 137.Canfield DE. 1998. A new model for Proterozoic ocean chemistry. Nature 396, 450–453. ( 10.1038/24839) [DOI] [Google Scholar]
- 138.Poulton SW, Canfield DE. 2011. Ferruginous conditions: a dominant feature of the ocean through Earth's history. Elements 7, 107–112. ( 10.2113/gselements.7.2.107) [DOI] [Google Scholar]
- 139.Nealson KH. 1997. Sediment bacteria: who's there, what are they doing, and what's new? Annu. Rev. Earth Planet. Sci. 25, 403–434. ( 10.1146/annurev.earth.25.1.403) [DOI] [PubMed] [Google Scholar]
- 140.Raymond J, Segre D. 2006. The effect of oxygen on biochemical networks and the evolution of complex life. Science 311, 1764–1767. ( 10.1126/science.1118439) [DOI] [PubMed] [Google Scholar]
- 141.Reinhard CT, Planavsky NJ, Robbins LJ, Partin CA, Gill BC, Lalonde SV, Bekker A, Konhauser KO, Lyons TW. 2013. Proterozoic ocean redox and biogeochemical stasis. Proc. Natl Acad. Sci. USA 110, 5357–5362. ( 10.1073/pnas.1208622110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Javaux E, Knoll AH, Walter MR. 2001. Ecological and morphological complexity in early eukaryotic ecosystems. Nature 412, 66–69. ( 10.1038/35083562) [DOI] [PubMed] [Google Scholar]
- 143.Müller M, et al. 2012. Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiol. Mol. Biol. Rev. 76, 444–495. ( 10.1128/MMBR.05024-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Knoll AH, Lahr DJG. 2016. Fossils, feeding and the evolution of complex multicellularity. In The origins and consequences of multicellularity (eds Niklas K, Neumann S), pp. 3–16. Cambridge, MA: MIT Press. [Google Scholar]
- 145.Cavalier-Smith T. 2013. Early evolution of eukaryote feeding modes, cell structural diversity, and classification of the protozoan phyla Loukozoa, Sulcozoa, and Choanozoa. Eur. J. Protistol. 49, 115–178. ( 10.1016/j.ejop.2012.06.001) [DOI] [PubMed] [Google Scholar]