Abstract
Background
Autoantibodies such as anti-citrullinated protein antibodies (ACPA) are major risk factors for articular bone destruction from the earliest phases of rheumatoid arthritis (RA). The aim of the current study was to determine whether RA-associated autoantibodies also impact on systemic bone loss in patients with early disease.
Methods
Systemic bone mineral density (BMD) was measured in the lumbar spine and the hip in 155 consecutive treatment-naïve patients with early RA (median symptom duration 13 weeks). Demographic and disease-specific parameters, including clinical disease activity, ultrasonographic (US) examination of the hands and wrists, radiographic scoring of joint damage, ACPA and rheumatoid factor (RF) levels were recorded from all patients. Reduced BMD was defined as Z score ≤ -1 SD and analysed in relation to disease-related characteristics and autoantibody subgroups.
Results
Reduced BMD was found in 25.5 % of the patients in the spine and 19.4 % in the hip. Symptom duration, clinical and US disease activity, functional disability and radiographic damage did not significantly impact on spine and hip BMD loss in regression analyses adjusted for possible confounders (age, gender, menopausal status, current smoking, body mass index). In contrast, ACPA positivity (at any level) negatively affected the spine Z-score (adjusted OR (95 % CI) 2.76 (1.19 to 6.42)); the hip Z score was affected by high titres only (adjusted OR (95 % CI) 2.96 (1.15 to 7.66)). The association of ACPA with reduced BMD in the spine was confirmed even at low levels of RF (adjusted OR (95 % CI) 2.65 (1.01 to 7.24)), but was further increased by concomitant high RF (adjusted OR (95 % CI) 3.38 (1.11 to 10.34)). In contrast, Z scores in the hip were significantly reduced only in association with high ACPA and RF (adjusted OR (95 % CI) 4.96 (1.48 to 16.64)).
Conclusions
Systemic BMD in patients with early RA is reduced in relation with ACPA positivity and high RF levels. This finding supports the notion that RA-associated autoimmunity may have a direct causative role in bone remodeling.
Keywords: Early rheumatoid arthritis, Anti-citrullinated protein antibodies, Rheumatoid factor, Bone, Osteoporosis
Background
Rheumatoid arthritis (RA) is a chronic immune-inflammatory disease associated with several forms of skeletal remodeling including peri-articular osteopenia, marginal joint erosions and generalised bone loss. Pro-inflammatory cytokines are traditionally regarded as key drivers of articular and extra-articular bone tissue destruction [1–3]. However, recent experimental evidence indicates that RA-associated autoantibodies, in particular anti-citrullinated protein antibodies (ACPA), can independently stimulate bone remodeling by inducing the differentiation of bone-resorbing osteoclasts [4, 5].
Clinically, the association between ACPA and further progression in joint damage has been reported in several independent studies, [6–8]. This association appears at least partially independent of inflammation. Despite having a similar response to steered treatment strategies, ACPA-positive patients with RA indeed have higher rates of joint damage progression over time [9], and serum receptor activator of nuclear factor kappa B ligand (RANKL) is reported to be increased in ACPA-positive patients independent of acute phase reactants and pro-inflammatory cytokines [10]. More intriguingly, elegant imaging studies have recently demonstrated impairment in the bone microstructure in the metacarpophalangeal joints of ACPA-positive healthy individuals despite no signs of arthritis [11].
As ACPA precede the clinical onset of RA by years and are at least initially produced at extra-articular sites [12], it could be expected that ACPA-positive patients with early RA may already show signs of generalised bone loss in addition to destruction in the joints. However, the pathophysiology of secondary osteoporosis in RA is complex and mostly attributed to long-standing, disabling disease [13, 14]. Accordingly, the few studies available in early RA have reported overall bone mineral density (BMD) almost comparable to that of non-RA controls [15–20], and the potential effect of autoantibodies has not been systematically evaluated.
The Pavia Early Arthritis Clinic is a single-centre inception cohort of patients with recent-onset inflammatory arthritis, who are treatment-naïve at inclusion, and who undergo standardised clinical, laboratory and imaging assessments upon referral [21, 22]. Baseline BMD measurement has been systematically introduced since 2012. Here, we took advantage of this cohort to tackle the question of whether RA-associated autoantibodies affect systemic BMD independent of classical demographic and disease-related risk factors in the initial stages of arthritis.
Methods
Patients
Between 2012 and 2014, all patients newly referred to the Pavia Early Arthritis Clinic with a diagnosis of RA according to the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) 2010 classification criteria [23] were invited to undergo dual-energy x-ray absorptiometry (DXA) at both the hip and the spine. Patients were naïve to glucocorticoids and disease-modifying anti-rheumatic drugs, and had arthritis of short duration (<12 months of symptoms). Patients with definitive diagnoses other than RA, or any suspicion of spondyloarthritis (including personal or familial psoriasis and clinical or imaging evidence enthesitis), were carefully excluded.
Demographic and general characteristics known to affect BMD were obtained by interview and included age, gender, ethnicity, body mass index (BMI), menopausal status, age at menopause, current smoking and alcohol status, risk factors for secondary osteoporosis other than RA, previous clinical fractures, osteoporosis in first-degree relatives, use of calcium and vitamin D supplements, hormone replacement therapy and bisphosphonates (Table 1).
Table 1.
Demographic and clinical characteristics of the study population
| Variable | Value in study participants (n = 155) |
|---|---|
| Demographics | |
| Age, mean (SD), years | 57.8 (13.9) |
| Female gender, n (%) | 115 (74.2) |
| Postmenopausal, n (%) | 70 (60.9) |
| Age at menopause, mean (SD), years | 49.7 (4.2) |
| Premature menopause, n (%) | 3 (4.3) |
| Caucasian, n (%) | 148 (95.5) |
| Body mass index, median (IQR), kg/m2 | 25 (22–28) |
| Current smoker, n (%) | 30 (19.4) |
| Alcohol ≥3 units/day, n (%) | 3 (1.9) |
| Comorbidities, mean number (SD) | 0.4 (0.6) |
| Previous fracture, n (%) | 7 (4.5) |
| Parent fractured hip, n (%) | 15 (9.7) |
| Calcium supplementation, n (%) | 2 (1.3) |
| Vitamin D supplementation, n (%) | 3 (1.9) |
| Bisphosphonate use, n (%) | 2 (1.3) |
| Hormone replacement therapy, n (%) | 5 (3.2) |
| Disease characteristics | |
| Symptom duration, median (IQR), weeks | 12.9 (8.6–25.7) |
| 1987 American College of Rheumatology criteria fulfilled, n (%) | 107 (69) |
| Disease activity score in 28 joints, mean (SD) | 4.42 (1.28) |
| Swollen joint count in 28 joints, median (IQR) | 5 (3–8) |
| Tender joint count in 28 joints, median (IQR) | 6 (3–10) |
| Ultrasound grayscale score, median (IQR), 0–36 score | 5 (2–9) |
| Ultrasound power Doppler score, median (IQR), 0–36 score | 2 (0–7) |
| Erythrocyte sedimentation rate, median (IQR), mm/1 h | 17 (8–33) |
| C-reactive protein, median (IQR), mg/dlL | 0.7 (0.3–1.7) |
| Rheumatoid factor-positive, n (%) | 67 (43.2) |
| Rheumatoid factor titres in positive patients, median (IQR), U/mL | 82 (34.8–195.8) |
| Anti-citrullinated protein antibody-positive, n (%) | 66 (42.6) |
| Anti-citrullinated protein antibody titres in positive patients, median (IQR), U/mL | 237 (77.5–445.8) |
| Erosion Sharp–van der Heijde Score ≥1, n (%) (n = 152) | 32 (21.1) |
| Total Sharp–van der Heijde Score, median (IQR), 0–448 scale (n = 152) | 3 (1.25–7.5) |
| Health Assessment Questionnaire, median (IQR), 0–3 scale | 1 (0.438–1.438) |
| DXA | |
| Spine L1–L4 (n = 153) | |
| Bone mineral density, mean (SD), g/cm2 | 0.936 (0.157) |
| Z score, mean (SD) | 0.013 (1.315) |
| Z score ≤ -1, n (%) | 39 (25.5) |
| - men | 13 (33.3) |
| - premenopausal women | 13 (28.9) |
| - postmenopausal women | 13 (18.1) |
| Total hip (n = 155) | |
| BMD, mean (SD), g/cm2 | 0.728 (0.132) |
| Z score, mean (SD) | -0.128 (1.027) |
| Z score ≤ -1, n (%) | 30 (19.4) |
| - men | 7 (17.5) |
| - premenopausal women | 12 (26.7) |
| - postmenopausal women | 11 (15.7) |
| Either spine L1–L4 or total hip (n = 153) | |
| Z score ≤1, n (%) | 54 (35.3) |
| - men | 16 (41) |
| - premenopausal women | 19 (42.2) |
| - postmenopausal women | 19 (27.5) |
Disease variables
Disease duration was defined as the duration of patient self-reported joint symptoms. Disease activity was assessed using the 28-joint disease activity score (DAS28) based on the number of tender and swollen joints, the visual analogue scale for patient’s global assessment (0–100), and the erythrocyte sedimentation rate. C-reactive protein levels were also recorded. IgM rheumatoid factor (RF) and ACPA were determined by immunonephelometry using a Dimension Vista 1500 system (Siemens, Erlangen, Germany) and by a second-generation Phadia ImmunoCAP 250 EliA CCP assay (Phadia, Freiburg, Germany) respectively, according to the manufacturers’ recommendations. A positive result was defined as any value >20 IU/mL for RF and >10 IU/mL for ACPA. A low antibody level was defined as any value greater than the defined upper limit of normal (ULN) and ≤100 IU/ml, and a high antibody level as a value >100 IU/ml [24]. Different thresholds based on the ULN [23] were not tested because of the very low number of patients with ACPA ≤3 ULN (≤30 IU/mL according to our assay).
Functional ability was measured by the Health Assessment Questionnaire Disability Index. Ultrasound (US) examination was performed by a single experienced operator using a Logiq 9 scanner (General Electrics Medical Systems) with a multifrequency linear array transducer (8–15 MHz), according to EULAR guidelines [25]. The US assessment included transverse and longitudinal scanning of the medial and lateral dorsal aspects of both wrists and the first to fifth metacarpophalangeal joints, as previously described [21, 22]. Grayscale (GS) and power Doppler (PD) signals were assigned to each joint in accordance with semi-quantitative 0–3 scales, and an overall US score for GS and PD was calculated as the sum of either GS or PD signal scores obtained from each joint (range 0–36). Radiographic joint damage according to the Sharp–van der Heijde score (SHS) [26] was rated independently by two experienced physicians, and the mean of the scores of the two assessors was used for analysis.
BMD measurements
Out of 167 eligible patients, 155 agreed to undergo BMD measurement, with no significant differences between those who did or did not consent (not shown). BMD measurements in the left hip (femoral neck and total hip) and lumbar spine, vertebrae L1–4 were performed using the same DXA equipment (Hologic, Vilvoorde, Belgium). All procedures were performed by two trained technicians in accordance with the manufacturer’s standardised procedures. Due to logistic reasons, spine measurement was not performed in two patients. Z score estimations were computed through age-adjusted and gender-adjusted BMD according to locally used reference populations provided by the manufacturer. The Z score was used in order to correct for the heterogeneous age and gender of our cohort and to directly estimate the contribution of RA-related factors beyond classical determinants of primary osteoporosis [27, 28]. Reduced BMD was defined as a Z score ≤ -1 SD [13, 14, 20].
Statistical analyses
Data were described as mean and standard deviation (SD) or median and interquartile range (IQR) if continuous and as counts and percent if categorical. Disease-related variables in relation to the occurrence of reduced BMD (Z score ≤ -1 SD) were analysed by regression analyses adjusted for possible confounders (age, gender, menopausal status, current smoking and BMI). Comparisons between autoantibody subgroups were evaluated using the independent samples t test or analysis of variance (ANOVA) with Bonferroni post hoc testing. Statistical analyses were performed using MedCalc® Version 12.7.0.0, and the level of significance was set at 0.05.
Results
Characteristics of the study population
Table 1 shows the characteristics of the 155 patients with early RA for whom baseline BMD was available. The study population mainly consisted of women (74.2 %), of whom 60.9 % were postmenopausal. Mean (SD) age was 58 (14) years, and 76.1 % of the subjects were <70 years of age.
Patients were treatment-naïve with a short history of RA (median (IQR) 12.9 (8.6–25.7) weeks). Sixty-nine percent also fulfilled the 1987 ACR classification criteria for RA [29]. All had active disease (mean (SD) DAS28 4.42 (1.28)), 64.8 % had PD-positive synovitis (PD score >0), and 21.1 % had evidence of radiographic erosions (erosion SHS >0). Sixty-seven patients (43.2 %) were RF-positive, 66 (42.6 %) were ACPA-positive, and 58 (37.4 %) were double-positive.
Prevalence and distribution of reduced BMD
Mean (SD) Z scores were 0.01 ± 1.31 in the spine and -0.13 ± 1.03 in the hip. BMD was below the expected range for gender and age (Z score ≤ -1) in the spine in 25.5 % of the patients, in the hip in 19.4 % of patients, and in either site in 35.3 % of patients. Reduced BMD tended to be more common in men and premenopausal women in the spine, and in premenopausal women in the hip (Table 1).
BMD in relation to disease duration and activity
The relationship between reduced BMD and disease variables is shown in Fig. 1. Fulfillment of the 1987 ACR criteria, symptom duration and clinical, laboratory or imaging parameters of inflammation were not significantly associated with Z score ≤ -1 in the measured sites. Similarly, the very low levels of functional disability and radiographic damage characterising our cohort did not appear to impact on systemic BMD.
Fig. 1.
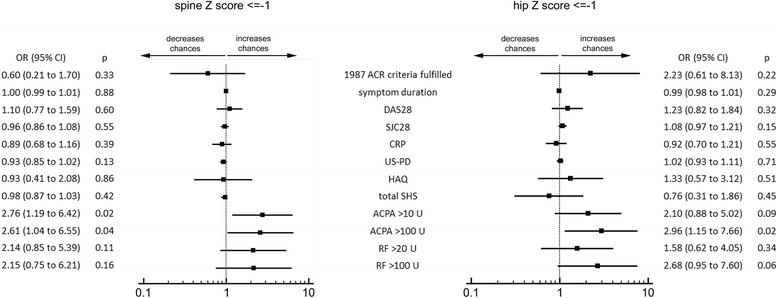
Systemic bone mineral density in relation to disease-related variables. Regression analysis of reduced bone mineral density (Z score ≤ -1) in the spine and the hip. Odds ratios (OR) are presented with corresponding 95 % CI levels. All variables were adjusted for age, gender, menopausal status, body mass index and current smoking. ACR American College of Rheumatology, DAS28 28-joint disease activity score, SJC28 swollen joint count in 28 joints, CRP C-reactive protein, US-PD ultrasonographic power Doppler score, HAQ Health Assessment Questionnaire, SHS Sharp–van der Heijde score, ACPA anti-citrullinated protein antibodies, RF rheumatoid factor
BMD in relation to autoantibody positivity and levels
ACPA-positive patients had significantly lower Z scores in the spine compared to ACPA-negative patients (mean -0.35 ± 1.19 vs 0.30 ± 1.34, p = 0.003), and ACPA emerged as independent predictors of reduced BMD with an adjusted OR (95 % CI) of 2.76 (1.19–6.42) (Fig. 1). The difference in hip Z scores was borderline significant (mean -0.28 ± 1.03 vs 0.00 ± 1.00, p = 0.09), whilst RF per se did not impact on either spine or hip BMD loss (adjusted ORs (95 % CI) 2.14 (0.85 to 5.39) and 1.58 (0.62 to 4.05) respectively) (Fig. 1).
We further questioned whether ACPA and RF levels (rather than dichotomous positivity) had different effects on BMD, and whether there is any interaction between the two autoantibody systems. We thus compared Z scores in patients with negative ACPA (<10 U), and in those with low (10–100 U) and high (>100 U) values [24]. On ANOVA, these subgroups differed significantly in both the spine and the hip (Fig. 2a, b). For spine Z scores, differences between ACPA-positive patients with low and high values were not significant, and both subgroups had similarly reduced BMD compared with ACPA-negative patients (Fig. 2a). In contrast, reductions in Z scores in the hip were restricted to patients with high ACPA values (Fig. 2b), and ACPA >100 U predicted hip BMD loss with an adjusted OR (95 % CI) of 2.96 (1.15–7.66) (Fig. 1). The relationship between RF and BMD loss was instead clearly dose-dependent in both the spine and the hip, with significant differences being observed for patients with high values only (Fig. 2c, d).
Fig. 2.
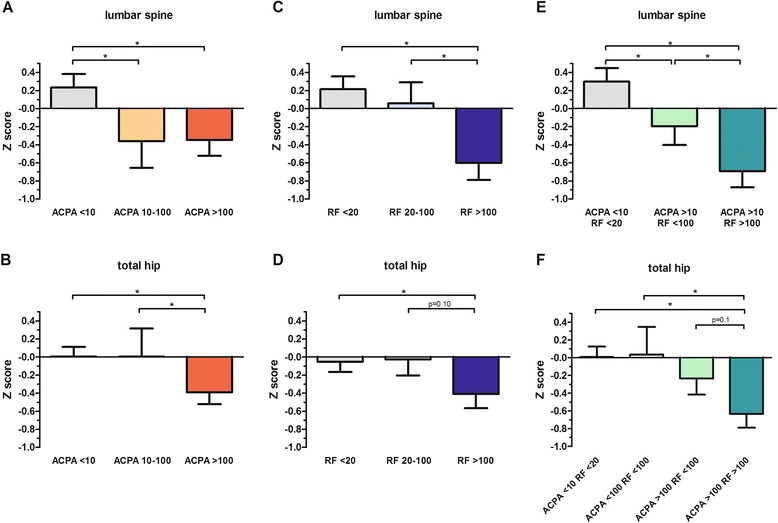
Association between systemic bone mineral density and anti-citrullinated protein antibodies and/or rheumatoid factor. Mean Z scores in the lumbar spine, vertebrae L1-L4 (a, c, e) and total hip (b, d, f) in subgroups of patients stratified according to anti-citrullinated protein antibody (ACPA) titres (a, b), rheumatoid factor (RF) titres (c, d) and the combination of ACPA and RF (e, f). *Significant differences (p <0.05)
When combining ACPA with RF titres, it was evident that the presence of ACPA negatively affected the Z score in the spine even in the presence of low levels of RF (difference in means (Δmeans) 0.50, SE 0.26 compared to seronegative patients), but RF >100 U conferred additional risk (Δmeans 0.50, SE 0.30 compared to patients who were ACPA-positive RF-low) (Fig. 2e). Accordingly, the adjusted OR (95 % CI) of ACPA in predicting spine Z score ≤ -1 increased from 2.65 (1.01–7.24) in the presence of concomitant RF ≤100 to 3.38 (1.11–10.34) for RF >100. In contrast, Z scores in the hip were significantly reduced only in association with high ACPA and RF. (Fig. 2f). ACPA >100 and RF >100 predicted reduced BMD in the hip with an adjusted OR (95 %) of 4.96 (1.48–16.64).
Discussion
We show here that, overall, systemic BMD is preserved in patients with early RA, and classic disease-related risk factors, including disease duration, inflammatory activity and functional ability, have negligible impact on measurable bone remodeling at this stage of the disease. However, ACPA appear associated with significantly reduced BMD, and the concomitant presence of high levels of RF further enhances the risk of bone loss. Whilst the effect of ACPA on the trabecular bone of the spine is readily appreciable, changes in the cortical bone of the hip require higher levels of autoantibodies.
The small proportion of patients with reduced BMD in our study is consistent with earlier reports [15–20] and confirms that overt secondary osteoporosis in RA mostly develops in association with chronic, disabling disease. The short symptom duration and the low levels of functional impairment and radiographic damage in our contemporary cohort diagnosed according to the 2010 criteria may explain the lack of association between disease-related factors and BMD, as compared with historical groups of patients with early RA with longer disease duration and variable treatment [15, 19, 20].
Whilst synovial inflammation may thus take longer to produce systemic effects at joint-remote sites [30], we show here that RA-associated autoimmunity appears coupled with reduced BMD from the early stages of clinical disease. Although functional verification of the pathogenic effects of autoantibodies on extra-articular bone in our study is lacking, our data fit with recent evidence linking ACPA to bone changes in vitro and in vivo [4–6]. Relevantly, the association between ACPA and BMD loss was enhanced by high levels of RF. Sensitive imaging techniques have shown that RF dose-dependently influences the size of bone erosions on an ACPA background [24], and the combined presence of ACPA and RF mediates increased pro-inflammatory cytokine production in vitro [31, 32]. The immune-complex activity of RF may have thus elicited a subclinical inflammatory milieu able to enhance ACPA-mediated osteoclast activation also at joint-remote sites. The different associations between autoantibody levels and spine and hip BMD loss might be explained by the higher rate of turnover in trabecular compared to cortical bone [33].
Our study has some limitations. Although we cannot exclude RA over-diagnosis according to the 2010 criteria [34], differential diagnoses were carefully evaluated and applying the 1987 criteria did not affect the results. The frequency of autoantibodies was lower than expected [35–37], but still comparable with other early arthritis cohorts from similar geographical areas [38], including the Etude et Suivi des POlyarthrites Indifférenciées Récentes (ESPOIR) cohort, in which 46–49 % of the patients fulfilling the RA classification criteria at inclusion are reported as ACPA-positive [39–41]. Irrespective of the prevalence of autoantibodies, however, we believe that the observed differences in BMD according to autoantibody levels clearly confirm the specific association between RA autoimmunity and systemic bone loss. Although the small number of single-positive patients hampers definitive conclusions on the independent associations with ACPA and RF, spine BMD was significantly reduced in ACPA-positive patients with low RF (Fig. 1e) in the absence of the definitive effects of low RF (Fig. 1c). This finding indirectly supports the notion that ACPA are key drivers of bone damage, and RF becomes important when ACPA are also present [24]. Also, the cross-sectional character of this study hampers definition of the potential effect of autoantibodies on the progression of BMD loss. DXA follow up is ongoing to define whether treatment can halt autoantibody-dependent systemic bone remodeling. It is also equally important to emphasise that additional measures of bone quality, including micro-architecture, mineralisation and turnover [42–44], might help better define the net impact of autoantibodies and autoantibody levels on bone. Finally, it is important to recall that vitamin-D is a key determinant of bone health [45], and patients with RA have significantly lower vitamin D serum levels compared to healthy controls [46]. As vitamin D deficiency might be more prevalent in ACPA-positive patients [47], we cannot exclude that possibility that the observed association between autoantibodies and reduced BMD might be at least partly mediated by lower vitamin D levels.
Conclusions
In summary, our data suggest that ACPA are associated with systemic bone loss from the earliest stages of RA, and high levels of RF further increase the risk. ACPA-RF-positive patients with early RA should thus be carefully monitored for the development of generalised osteoporosis beyond the assessment of traditional risk factors.
Acknowledgments
Funding
This study was supported in part by funding from the IRCCS Policlinico San Matteo Foundation, Pavia, Italy.
Availability of data and materials
The datasets analysed in the current study are available from the corresponding author on reasonable request.
Authors’ contributions
SB designed the study, collected and analysed the data and drafted the manuscript. LB collected the data and helped to revise the manuscript. AM participated in the design of the study and helped to draft the manuscript. BV performed laboratory assays and helped to revise the manuscript. CM designed the study and critically revised the manuscript. RC critically revised the manuscript and provided final approval. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests with regard to this study.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The current study was approved by the local Ethics Committee of the IRCCS Policlinico San Matteo Foundation, Pavia, Italy, and written informed consent was obtained from all patients.
Abbreviations
- ACPA
Anti-citrullinated protein antibodies
- ACR
American College of Rheumatology
- ANOVA
Analysis of variance
- BMD
Bone mineral density
- BMI
Body mass index
- CI
Confidence interval
- DAS28
28-joint disease activity score
- DXA
Dual-energy x-ray absorptiometry
- EULAR
European League Against Rheumatism
- GS
Grayscale
- IQR
Interquartile range
- OR
Odds ratio
- PD
Power Doppler
- RA
Rheumatoid arthritis
- RF
Rheumatoid factor
- SD
Standard deviation
- SHS
Sharp–van der Heijde score
- ULN
Upper limit of normal
- US
Ultrasonography
Contributor Information
Serena Bugatti, Email: serena.bugatti@unipv.it.
Laura Bogliolo, Email: l.bogliolo@smatteo.pv.it.
Barbara Vitolo, Email: barbara.vitolo@virgilio.it.
Antonio Manzo, Email: antonio.manzo@unipv.it.
Carlomaurizio Montecucco, Phone: +39 0382 501878, Email: montecucco@smatteo.pv.it.
Roberto Caporali, Email: caporali@smatteo.pv.it.
References
- 1.Walsh NC, Gravallese EM. Bone remodeling in rheumatic disease: a question of balance. Immunol Rev. 2010;233:301–12. doi: 10.1111/j.0105-2896.2009.00857.x. [DOI] [PubMed] [Google Scholar]
- 2.Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol. 2012;8:656–64. doi: 10.1038/nrrheum.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bugatti S, Manzo A, Caporali R, Montecucco C. Assessment of synovitis to predict bone erosions in rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2012;4:235–44. doi: 10.1177/1759720X12453092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harre U, Georgess D, Bang H, Bozec A, Axmann R, Ossipova E, et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest. 2012;122:1791–802. doi: 10.1172/JCI60975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnamurthy A, Joshua V, Haj Hensvold A, Jin T, Sun M, Vivar N, et al. Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Ann Rheum Dis. 2016;75:721–9. doi: 10.1136/annrheumdis-2015-208093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rönnelid J, Wick MC, Lampa J, Lindblad S, Nordmark B, Klareskog L, et al. Longitudinal analysis of citrullinated protein/peptide antibodies (anti-CP) during 5 year follow up in early rheumatoid arthritis: anti-CP status predicts worse disease activity and greater radiological progression. Ann Rheum Dis. 2005;64:1744–9. doi: 10.1136/ard.2004.033571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Helm-van Mil AH, Verpoort KN, Breedveld FC, Toes RE, Huizinga TW. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther. 2005;7:R949–58. doi: 10.1186/ar1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Syversen SW, Gaarder PI, Goll GL, Ødegård S, Haavardsholm EA, Mowinckel P, et al. High anti-cyclic citrullinated peptide levels and an algorithm of four variables predict radiographic progression in patients with rheumatoid arthritis: results from a 10-year longitudinal study. Ann Rheum Dis. 2008;67:212–7. doi: 10.1136/ard.2006.068247. [DOI] [PubMed] [Google Scholar]
- 9.van den Broek M, Dirven L, Klarenbeek NB, Molenaar TH, Han KH, Kerstens PJ, et al. The association of treatment response and joint damage with ACPA-status in recent-onset RA: a subanalysis of the 8-year follow-up of the BeSt study. Ann Rheum Dis. 2012;71:245–8. doi: 10.1136/annrheumdis-2011-200379. [DOI] [PubMed] [Google Scholar]
- 10.Hensvold AH, Joshua V, Li W, Larkin M, Qureshi F, Israelsson L, et al. Serum RANKL levels associate with anti- citrullinated protein antibodies in early untreated rheumatoid arthritis and are modulated following methotrexate. Arthritis Res Ther. 2015;17:239. doi: 10.1186/s13075-015-0760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleyer A, Finzel S, Rech J, Manger B, Krieter M, Faustini F, et al. Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann Rheum Dis. 2014;73:854–60. doi: 10.1136/annrheumdis-2012-202958. [DOI] [PubMed] [Google Scholar]
- 12.Catrina AI, Joshua V, Klareskog L, Malmström V. Mechanisms involved in triggering rheumatoid arthritis. Immunol Rev. 2016;269:162–74. doi: 10.1111/imr.12379. [DOI] [PubMed] [Google Scholar]
- 13.Haugeberg G, Uhlig T, Falch JA, Halse JI, Kvien TK. Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: results from 394 patients in the Oslo County Rheumatoid Arthritis register. Arthritis Rheum. 2000;43:522–30. doi: 10.1002/1529-0131(200003)43:3<522::AID-ANR7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 14.Lodder MC, de Jong Z, Kostense PJ, Molenaar ET, Staal K, Voskuyl AE, et al. Bone mineral density in patients with rheumatoid arthritis: relation between disease severity and low bone mineral density. Ann Rheum Dis. 2004;63:1576–80. doi: 10.1136/ard.2003.016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laan RF, Buijs WC, Verbeek AL, Draad MP, Corstens FH, van de Putte LB, et al. Bone mineral density in patients with recent onset rheumatoid arthritis: influence of disease activity and functional capacity. Ann Rheum Dis. 1993;52:21–6. doi: 10.1136/ard.52.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shenstone BD, Mahmoud A, Woodward R, Elvins D, Palmer R, Ring F, et al. Bone mineral density in nonsteroid treated early rheumatoid arthritis. Ann Rheum Dis. 1994;53:681–4. doi: 10.1136/ard.53.10.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gough AK, Lilley J, Eyre S, Holder RL, Emery P. Generalised bone loss in patients with early rheumatoid arthritis. Lancet. 1994;344:23–7. doi: 10.1016/S0140-6736(94)91049-9. [DOI] [PubMed] [Google Scholar]
- 18.Keller C, Hafström I, Svensson B. Bone mineral density in women and men with early rheumatoid arthritis. Scand J Rheumatol. 2001;30:213–20. doi: 10.1080/030097401316909558. [DOI] [PubMed] [Google Scholar]
- 19.Forslind K, Keller C, Svensson B, Hafström I, BARFOT Study Group Reduced bone mineral density in early rheumatoid arthritis is associated with radiological joint damage at baseline and after 2 years in women. J Rheumatol. 2003;30:2590–6. [PubMed] [Google Scholar]
- 20.Güler-Yüksel M, Bijsterbosch J, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Ronday HK, Peeters AJ, et al. Bone mineral density in patients with recently diagnosed, active rheumatoid arthritis. Ann Rheum Dis. 2007;66:1508–12. doi: 10.1136/ard.2007.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montecucco C, Todoerti M, Sakellariou G, Sciré CA, Caporali R. Low-dose oral prednisone improves clinical and ultrasonographic remission rates in early rheumatoid arthritis: results of a 12-month open-label randomised study. Arthritis Res Ther. 2012;14:R112. doi: 10.1186/ar3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bugatti S, Manzo A, Benaglio F, Klersy C, Vitolo B, Todoerti M, et al. Serum levels of CXCL13 are associated with ultrasonographic synovitis and predict power Doppler persistence in early rheumatoid arthritis treated with non-biological disease-modifying anti-rheumatic drugs. Arthritis Res Ther. 2012;14:R34. doi: 10.1186/ar3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–8. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 24.Hecht C, Englbrecht M, Rech J, Schmidt S, Araujo E, Engelke K, et al. Additive effect of anti-citrullinated protein antibodies and rheumatoid factor on bone erosions in patients with RA. Ann Rheum Dis. 2015;74:2151–6. doi: 10.1136/annrheumdis-2014-205428. [DOI] [PubMed] [Google Scholar]
- 25.Backhaus M, Burmester GR, Gerber T, Grassi W, Machold KP, Swen WA, et al. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis. 2001;60:641–9. doi: 10.1136/ard.60.7.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Heijde DM, van Riel PL, Nuver-Zwart IH, Gribnau FW. vad de Putte LB. Effects of hydroxychloroquine and sulphasalazine on progression of joint damage in rheumatoid arthritis. Lancet. 1989;1:1036–8. doi: 10.1016/S0140-6736(89)92442-2. [DOI] [PubMed] [Google Scholar]
- 27.Leslie WD, Adler RA, El-Hajj Fuleihan G, Hodsman AB, Kendler DL, McClung M, et al. Application of the 1994 WHO classification to populations other than postmenopausal Caucasian women: the 2005 ISCD Official Positions. J Clin Densitom. 2006;9:22–30. doi: 10.1016/j.jocd.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Rossini M, Viapiana O, Idolazzi L, Ghellere F, Fracassi E, Troplini S, et al. Higher level of Dickkopf-1 is associated with low bone mineral density and higher prevalence of vertebral fractures in patients with ankylosing spondylitis. Calcif Tissue Int. 2016;98:438–45. doi: 10.1007/s00223-015-0093-3. [DOI] [PubMed] [Google Scholar]
- 29.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 30.Güler-Yüksel M, Allaart CF, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, van Groenendael JH, Mallée C, et al. Changes in hand and generalised bone mineral density in patients with recent-onset rheumatoid arthritis. Ann Rheum Dis. 2009;68:330–6. doi: 10.1136/ard.2007.086348. [DOI] [PubMed] [Google Scholar]
- 31.Sokolove J, Johnson DS, Lahey LJ, Wagner CA, Cheng D, Thiele GM, et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:813–21. doi: 10.1002/art.38307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laurent L, Anquetil F, Clavel C, Ndongo-Thiam N, Offer G, Miossec P, et al. IgM rheumatoid factor amplifies the inflammatory response of macrophages induced by the rheumatoid arthritis-specific immune complexes containing anticitrullinated protein antibodies. Ann Rheum Dis. 2015;74:1425–31. doi: 10.1136/annrheumdis-2013-204543. [DOI] [PubMed] [Google Scholar]
- 33.Onal M, Xiong J, Chen X, Thostenson JD, Almeida M, Manolagas SC, et al. Receptor activator of nuclear factor kB ligand (RANKL) protein expression by B lymphocytes contributes to ovariectomy-induced bone loss. J Biol Chem. 2012;287:29851–60. doi: 10.1074/jbc.M112.377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Linden MP, Knevel R, Huizinga TW, van der Helm-van Mil AH. Classification of rheumatoid arthritis: comparison of the 1987 American College of Rheumatology criteria and the 2010 American College of Rheumatology/European League Against Rheumatism criteria. Arthritis Rheum. 2011;63:37–42. doi: 10.1002/art.30100. [DOI] [PubMed] [Google Scholar]
- 35.Norli ES, Brinkmann GH, Kvien TK, Bjørneboe O, Haugen AJ, Nygaard H, et al. Self-limiting arthritis among patients fulfilling the 2010 ACR/EULAR classification criteria for rheumatoid arthritis in a very early arthritis cohort. Semin Arthritis Rheum. 2016;16:30119–6. doi: 10.1016/j.semarthrit.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Nieuwenhuis WP, de Wit MP, Boonen A, van der Helm-van Mil AH. Changes in the clinical presentation of patients with rheumatoid arthritis from the early 1990s to the years 2010: earlier identification but more severe patient reported outcomes. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2016-209949. [DOI] [PubMed] [Google Scholar]
- 37.Cader MZ, Filer A, Hazlehurst J, de Pablo P, Buckley CD, Raza K. Performance of the 2010 ACR/EULAR criteria for rheumatoid arthritis: comparison with 1987 ACR criteria in a very early synovitis cohort. Ann Rheum Dis. 2011;70:949–55. doi: 10.1136/ard.2010.143560. [DOI] [PubMed] [Google Scholar]
- 38.Gremese E, Salaffi F, Bosello SL, Ciapetti A, Bobbio-Pallavicini F, Caporali R, et al. Very early rheumatoid arthritis as a predictor of remission: a multicentre real life prospective study. Ann Rheum Dis. 2013;72:858–62. doi: 10.1136/annrheumdis-2012-201456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durán J, Combe B, Niu J, Rincheval N, Gaujoux-Viala C, Felson DT. The effect on treatment response of fibromyalgic symptoms in early rheumatoid arthritis patients: results from the ESPOIR cohort. Rheumatology. 2015;54:2166–70. doi: 10.1093/rheumatology/kev254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruyssen-Witrand A, van Steenbergen HW, van Heemst J, Gourraud PA, Nigon D, Lukas C, et al. A new classification of HLA-DRB1 alleles based on acid-base properties of the amino acids located at positions 13, 70 and 71: impact on ACPA status or structural progression, and meta-analysis on 1235 patients with rheumatoid from two cohorts (ESPOIR and EAC cohort) RMD Open. 2015;1 doi: 10.1136/rmdopen-2015-000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fautrel B, Combe B, Rincheval N, Dougados M. ESPOIR Scientific Committee. Level of agreement of the 1987 ACR and 2010 ACR/EULAR rheumatoid arthritis classification criteria: an analysis based on ESPOIR cohort data. Ann Rheum Dis. 2012;71:386–9. doi: 10.1136/annrheumdis-2011-200259. [DOI] [PubMed] [Google Scholar]
- 42.Mawatari T, Miura H, Hamai S, Shuto T, Nakashima Y, Okazaki K, et al. Vertebral strength changes in rheumatoid arthritis patients treated with alendronate, as assessed by finite element analysis of clinical computed tomography scans: a prospective randomized clinical trial. Arthritis Rheum. 2008;58:3340–9. doi: 10.1002/art.23988. [DOI] [PubMed] [Google Scholar]
- 43.Koumakis E, Avouac J, Winzenrieth R, Toth E, Payet J, Kahan A, et al. Trabecular bone score in female patients with systemic sclerosis: comparison with rheumatoid arthritis and influence of glucocorticoid exposure. J Rheumatol. 2015;42:228–35. doi: 10.3899/jrheum.140752. [DOI] [PubMed] [Google Scholar]
- 44.Coiffier G, Bouvard B, Chopin F, Biver E, Funck-Brentano T, Garnero P, et al. Common bone turnover markers in rheumatoid arthritis and ankylosing spondylitis: a literature review. Joint Bone Spine. 2013;80:250–7. doi: 10.1016/j.jbspin.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Carmeliet G, Dermauw V, Bouillon R. Vitamin D signaling in calcium and bone homeostasis: a delicate balance. Best Pract Res Clin Endocrinol Metab. 2015;29:621–31. doi: 10.1016/j.beem.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Lin J, Liu J, Davies ML, Chen W. Serum vitamin D level and rheumatoid arthritis disease activity: review and meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerr GS, Sabahi I, Richards JS, Caplan L, Cannon GW, Reimold A, et al. Prevalence of vitamin D insufficiency/deficiency in rheumatoid arthritis and associations with disease severity and activity. J Rheumatol. 2011;38:53–9. doi: 10.3899/jrheum.100516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed in the current study are available from the corresponding author on reasonable request.


