Abstract
Burns are among the most common injuries presenting to the emergency department. While burns, especially large ones, may be associated with significant morbidity and mortality, most are minor and can be managed by emergency practitioners and discharged home with close follow-up. In contrast, patients with large burns require aggressive management of their airway, breathing and circulation in order to reduce mortality and morbidity. While early endotracheal intubation of patients with actual or impending airway compromise and aggressive fluid resuscitation have been emphasized, it appears that the pendulum may have swung a bit too far towards the extreme. The current review will briefly cover the epidemiology, pathogenesis and diagnosis of burn injuries with greater emphasis on airway and fluid management. We will also discuss the local management of the burn wound, which is all that is required for most burn patients in the emergency department.
Keywords: Burns; Emergency service, hospital; Smoke inhalation injury; Diagnosis; Therapy
EPIDEMIOLOGY
The American Burn Association estimates that each year 450,000 burn injuries are treated at medical facilities across the US. Of these, 40,000 require hospital admission, including 30,000 at specialized hospital burn centers. The most common etiologies requiring burn center admission are fire/flame (43%), followed narrowly by scalds (34%), contact with hot objects (9%), electricity (4%), and chemical agents (3%) [1]. Annually, there are 3,400 deaths related to fires, burn and smoke inhalation, with over 2,550 (72%), occurring in the home. The majority of burn patients are males (69%), while children under 16, account for 29%. The overall survival rate of patients admitted to burn centers stands at 96.6% [1]. Despite the large number of burns presenting to the emergency department, the majority do not require admission or surgery and can be managed by emergency practitioners with timely follow-up with a burn specialist in most cases, except for the most minor ones.
PATHOPHYSIOLOGY
The skin with an average surface area of 1.8 m2 is the largest organ of the body, approximating 16% of the total body’s weight. One of the skin’s main functions is to protect the interior body against mechanical, thermal, physical and chemical agents, by creating a barrier against the external environment. Burn injuries result in various local and systemic responses. At the local level, heat causes acute changes such as protein denaturation, disruption in collagen cross-linking, damage to endothelial and epithelial cells and blood vessel occlusion. Additionally, increased permeability of blood vessels occurs leading to edema. Historically, the pathophysiology of burn injuries has been described using Jackson’s 1953 3-dimensional burn wound model [2]. The zone of coagulation, the area nearest the source of heat, is surrounded by a zone of stasis, further surrounded by a zone of hyperemia at the periphery. One of the critical aims in managing burn injuries is to prevent this middle ischemic region from becoming necrotic due to hypoperfusion, edema or infection [3,4]. Apoptosis in the ischemic region may also contribute to irreversible tissue death [5]. The outermost zone of hyperemia is characterized by reversible vasodilation, caused by the production of inflammatory mediators [3,4]. Unless there are complications caused by infection or hypoperfusion, the tissue in this outer region usually recovers fully [6]. In response to injury, there is local and systemic release of a host of inflammatory mediators, reactive oxygen species (ROS) and reactive nitrogen species (RNS) often confounded by local infection. Among the circulating vasoactive and inflammatory mediators are the histamines, prostaglandins, kinins, platelet aggregation factors, angiotensin II, vasopressin, and corticotropin-releasing factors [6] and cell signaling proteins such as cytokines and chemokines [7]. Oxidative stress and recurring cycles of ischemia and reperfusion also play a role in burn injury progression [8]. The massive fluid shifts, especially in burns involving greater than 20% of the total body surface area (TBSA), may result in burn edema and burn shock [9]. Severe burn shock is both distributive and hypovolemic in nature [10], and is manifested by decreases in both urine output and cardiac output and increases in pulmonary and systemic vascular resistances and lowered plasma volume resulting in elevations in hematocrit and hemoglobin values. Hypovolemia can be corrected by effective fluid resuscitation based on the patient’s weight and size of the burn [11]. Even if hypovolemia is alleviated, myocardial depression may continue, leading to multiple organ dysfunction and death [6].
Inhalation injury is caused by heat exposure and inhaling toxic smoke products of incomplete combustion (e.g., carbon monoxide [CO], cyanide, the aldehydes and oxides of sulfur and nitrogen), and particulate matter. The presence of inhalation injury significantly increases mortality [1]. Inhalation injuries are generally classified into three classes: (1) thermal injuries to the upper airways, (2) chemical irritation and injuries to the lower respiratory tract, and (3) systemic toxicity owing to noxious gases such as CO and cyanide [12,13]. Thermal injury below the vocal cords is uncommon owing to a highly efficient heat exchange system in the oro- and nasopharynx and the low heat capacity of the airways and the reflex mechanism of laryngeal closure. Similar to burns of the skin, thermal injuries of the airways damage epithelial cells, denature proteins and initiate a cascade of inflammatory responses, which causes the release of ROS and RNS resulting in widespread damage to cells. Poly(ADP ribose) polymerase (PARP) is also activated, resulting in the depletion of adenosine triphosphate from cells, resulting in further necrosis and apoptosis [13-16]. All these events lead to increased microvascular pressure and enhanced endothelium permeability for proteins, and results in edema formation of the epiglottis and swelling of the tongue causing obstruction of the airways [13,17,18]. Injuries to the lower respiratory tract and the pulmonary parenchyma are mostly caused by toxic chemicals in smoke leading to a reduced ability of the mucociliary transport system to eliminate excess mucus and other secretions reducing bacterial clearance and increasing the likelihood of infection. Furthermore, surfactant loss causes alveolar collapse and atelectasis. Additionally, there may be bronchitis, bronchial swelling, bronchospasm, impairment of lung compliance and increases in dead space ventilation and ventilation-perfusion mismatches [12]. Markedly, there is also the production of a transudate/exudate mixture with a high protein content, which along with de-epithelialized cells, inflammatory cells, fibrin and mucus solidifies to form a pseudomembranous “cast” which may partially or completely block the airways. CO has a much higher (>200 X) affinity for hemoglobin than oxygen and causes a leftward shift in the hemoglobin saturation curve leading to tissue hypoxia. Cyanide, a common component in plastics, inhibits the mitochondrial respiratory chain leading to cytotoxic hypoxia and metabolic acidosis. Additionally, circulating proinflammatory mediators released by the lung may cause the systemic inflammatory response syndrome and multi-organ failure [13].
CLASSIFICATION AND DIAGNOSIS
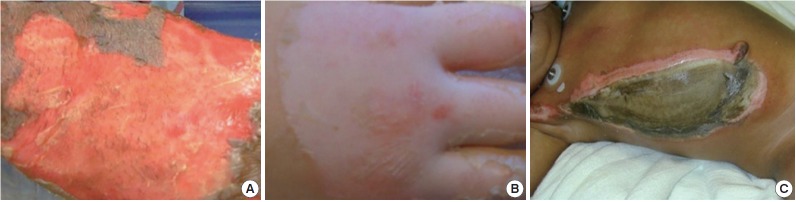
Burn depth is considered one of the more important determinants of outcome. First-degree burns are limited to the outer layer or epidermis of the skin. The skin usually appears red and dry and is very painful to the touch. Healing takes place in 3–5 days. Partial thickness or second-degree burns are further categorized into superficial and deep partial thickness burns. A superficial partial thickness burn extends into the superficial papillary dermis and appears red in color with significant weeping and blisters (Fig. 1A). It will also blanch when pressure is applied and may take between 2–3 weeks to heal. Deep partial thickness burns extend into the reticular dermis and appear yellow or white and dry and may take greater than 3 weeks to heal (Fig. 1B). These burns are extremely painful; however in some cases, the sensation in the deep partial thickness may become diminished. Full thickness or third-degree burns extend through the entire thickness of the dermis. These may appear dry, leathery, black or white and are usually painless since nerves may be destroyed (Fig. 1C). They do not blanch under pressure. Fourth degree burns extend through the entire skin thickness including the fat and underlying tendon, muscle and bone. They also appear charred and black [19]. There are several methods for determining burn depth such as vital dyes, tissue biopsies and ultrasound, that are able to detect dead cells or denatured collagen [20]. Others such as fluorescein perfusion fluoroscopy [21], laser Doppler flowmetry [22] and thermography [23] evaluate changes in blood flow. Nuclear magnetic resonance [24] can also be used to determine burn depth by observing changes such as edema. However, clinical evaluation by an experienced burn practitioner still remains the standard method used to determine burn depth [25]. This method is only 50%–75% accurate and relies on subjective features such as sensation to pin prick, bleeding on needle prick, wound appearance and ability to blanch [26,27]. Since burns tend to progress over the first 2–3 days after injury, burn depth estimation may not be accurate on the initial evaluation. Close monitoring may be required before accurate burn depth determinations are made.
Fig. 1.
Appearance of superficial partial thickness (A), deep partial thickness (B), and full thickness (C) burns.
In addition to burn depth, the extent or TBSA of the burn injury also needs to be evaluated. Estimation of burn area, even by experts, may be inaccurate. As such, clinical rules and charts are used to improve accuracy in estimating TBSA. The three most commonly used methods include the Lund-Brower chart, Wallace rule of nines, and Palmer surface [27]. The Lund-Brower chart is considered the most accurate, if used correctly. It allows for the variation in body proportions with age, and is used especially in children. The Wallace rule of nines is used in adults since it is not accurate in children. With this method, the body is divided into areas of 9% of the body surface area (the head, each upper limb, the front of the trunk, the back of the trunk, the front of each lower extremity, and the back of each lower extremity). The surface area of the patient’s palm (including the fingers), approximately 1% of the TBSA, is used to estimate small (<10% TBSA) burns. However, this method is inaccurate for larger sized burns [27]. Inaccurate estimation of burn size by clinicians is common, often leading to inappropriate patient transfers and excessive fluid resuscitation [28,29]. A chart review of 97 burn referrals to the Danish National Burn Center over a 3 month period found that 30% of the referrals were unnecessary mostly due to overestimation of burn size and depth [30]. Various technologies such as mobile phones applications (apps) [31] and software [32] have been developed to help improve burn size estimation. Introduction of telemedicine among burn centers has been shown to improve the accuracy of burn size estimation [33]. In one study, use of telemedicine reduced air transport of burn patients to a regional burn center from 100% to 44% [34].
EMERGENCY DEPARTMENT MANAGEMENT
Airway management
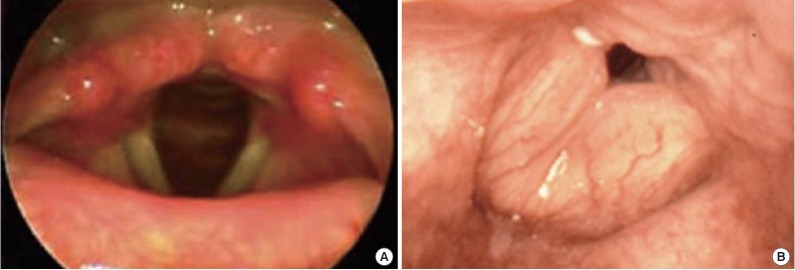
Edema formation occurs when there is direct exposure of the upper airway to superheated gases and toxic fumes. One of the most critical decisions is determining if and when the patient requires protection of their airway, preferably by endotracheal intubation. In some patients the decision to intubate is very clear, such as in the presence of respiratory distress, copious carbonaceous sputum or obvious swelling of the oropharynx. In others, such as in the presence of facial edema, singed nasal vibrissae, or very large burns, the decision is more difficult. The safest approach however has been early intubation. Early endotracheal intubation guided by fiberoptic bronchoscopy should be strongly considered in patients with large burns (>40% TBSA) as edema formation may progress to impair or completely occlude the airways within minutes to hours [35]. Additionally, it avoids the need for surgical cricothyrotomy or needle cricothyrotomy or later difficult intubation and allows for proper management of analgesia and sedation [36]. However, there is now mounting evidence that routine “prophylactic” endotracheal intubation may be harmful to many patients. A recent review by Mackie [37] summarized evidence supporting the hypothesis that mechanical ventilation may be contributing to the pathogenesis of the acute respiratory distress syndrome (ARDS) often seen in patients with presumed inhalation injury. Ventilator associated lung injury is probably the result of the release of inflammatory mediators induced by the pressure and volume changes associated with mechanical ventilation [38]. Ventilator associated pneumonia, with a mortality of roughly 10%, is also seen in 9%–27% of ventilated burn patients. A retrospective study of burn patients with large (>30% TBSA) burns found that the number of patients subjected to mechanical ventilation increased from 38% to 76% between 1987 and 2006 despite similar patient and burn characteristics. However, inhalation injury could not be confirmed in 57% of intubated patients [39]. Another study of 879 burn patients intubated over a 13 year period found that 28% were intubated “prophylactically” with 12% being extubated that same day and 21% on the next day, with none requiring re-intubation suggesting that endotracheal intubation may not have been necessary [40]. If there is any doubt regarding the presence of airway edema or inhalation injury, early visualization of the airway should be performed using fiberoptic or direct laryngoscopy using topical anesthesia (Fig. 2). If the airway appears normal, endotracheal intubation may be deferred. However, such patients may still require close observation or repeated evaluations of their airway if conditions change. If endotracheal intubation is clearly indicated, intubation with the patient awake using topical anesthesia and moderate sedation is recommended. Small intravenous doses of ketamine (10–20 mg) may help facilitate airway evaluation and management. Endotracheal intubation over a fiberoptic bronchoscope or long laryngoscope may be required. Rarely, surgical management (e.g., cricothyrotomy) of the airway may also be required. One significant risk associated with endotracheal intubation is airway obstruction caused by overproduction of mucous. In that case, suctioning should be carried out frequently [35]. In addition, several techniques have been developed to deal with the difficulties associated with securing of the endotracheal tube in burn patients [41].
Fig. 2.
Fiberoptic laryngoscopy images of normal (A) and edematous (B) airway.
General measures for moderate to severe burns
Humidified oxygen should be administered either by mechanical ventilation or through a high flow facemask to maintain oxygen saturations greater than 92%. For fluid administration and drug infusion in patients with severe burns, intravenous access should be obtained via large peripheral veins, preferably through non-burned skin. Patients should also be monitored for urine output and evaluated for rhabdomyolysis and myoglobinuria using an indwelling urinary catheter. A double lumen nasogastric tube should also be used with large burns as patients may experience gastroparesis and possible vomiting, which may compromise ventilation and put them at risk of aspiration. Patients should also undergo cardiac monitoring and assessment of arterial oxygenation and body core temperature. Pulmonary artery catheterization should be considered in patients suffering from burn shock and with significant comorbidities such as heart failure, as this may allow for improved fluid resuscitation [36].
Diagnosis of inhalation injury
The diagnosis of inhalation injury can be highly subjective and has traditionally been based on a combination of clinical findings such as patient history, physical examination and carboxyhemoglobin levels. Patient history should include the duration of exposure, whether the patient was found in an enclosed space, and the amount of inhaled irritants and type of toxins involved. Other factors such as advanced age, large TBSA, the presence of carbonaceous sputum, facial burns and oropharyngeal edema, all correlate with a greater likelihood of suffering inhalation injury. Diagnostic certainty however, is improved by fiberoptic bronchoscopy, which has been reported to have a sensitivity of 0.79 and specificity of 0.94 when performed within 24 hours of admission [42]. This “gold standard” is useful in identifying the presence of soot, edema, inflammation and airway necrosis [43]. There have also been attempts to develop scoring systems based on bronchoscopic evaluation in order to predict the development of ARDS [44,45]. Fiberoptic bronchoscopy may underestimate the presence of parenchymal disease [46]. In that case, Xenon scanning has been suggested [47], however most hospital settings do not have access to this technology [48]. Another technique that has potential clinical value for assessing inhalation injury, is the use of thermal and dye dilution measurements to estimate extravascular lung water [49,50].
CO poisoning is one of the most common causes of death from inhalation injury. Immediate management involves the administration of 100% normobaric oxygen delivered via a non-rebreather reservoir facemask. This should continue until a carboxyhemoglobin level of <5% is achieved [12]. The use of hyperbaric oxygen remains controversial and is beyond the scope of this review [12]. Cyanide is also a common toxic inhalation irritant causing inhalation injury, however because of its short blood half-life (~1 hour), accurate determination of cyanide poisoning is hampered by delayed blood sampling. Blood CO concentration is highly correlated with cyanide and as such, may be considered as an indicator of cyanide poisoning [51]. Two antidotes are approved for treatment of cyanide poisoning in the US: the traditional cyanide antidote kit (consisting of amyl nitrite, 10% sodium nitrite, 25% sodium thiosulfate) and hydroxocobalamin, the latter which appears to be more tolerable, especially in patients with pre-existing hypotension, those who are pregnant or those found in enclosed spaces [52]. There are no specific treatment strategies for mechanical ventilation. As such, clinicians are guided by the American College of Chest Physicians recommendations [53]. There are several mechanical ventilator techniques, each with their advantages and disadvantages, providing alternate options for inhalation injury patients [46]. The mode chosen should complement the clinical team’s experience and should support oxygenation and ventilation. Achieving an oxygen saturation level >92% and limiting plateau pressures to 35 cmH2O or less will help minimize the incidence of barotrauma. Positive end-respiratory pressure, which supports airways patency by maintaining the positive pressure in the lungs at the end of expiration, is also used to control oxygenation and should be reassessed regularly. Permissive hypercapnia, where blood carbon dioxide partial pressure (PCO2) is allowed to rise (<60 mmHg) should be considered to limit plateau pressures. Studies have shown that smaller tidal volumes (4–6 mL/kg) are associated with lower mortality in patients with ARDS [46,54], and are therefore also recommended in burn patients. Noninvasive ventilation (NIV) methods, such as continuous positive airway pressure and pressure support ventilation may be considered in patients who are awake and cooperative [12,55]. NIV avoids the trauma associated with endotracheal intubation, allows patients to communicate better with the clinicians, requires less sedation and allows better maintenance of oral hygiene [12]. Although not in routine clinical use, there are several promising experimental pharmaceutical adjuncts that address physiologic changes associated with inhalation injury. Aerosolized racemic epinephrine serves as a bronchodilator, vasoconstrictor and mucolytic agent to alleviate wheezing and bronchospasm caused by chemical tracheobronchitis [46]. An aerosolized N-acetylcysteine/heparin combination therapy which acts as both an oxygen free radical scavenger and a mucolytic agent has also been successfully used in children and adults with inhalation injury. This combination was found to reduce re-intubation rates, atelectasis and mortality [56-58]. Holt et al. [59], however, did not find any significant improvements in a follow up study done in patients treated with this combination. Several clinical trials have also been conducted using β-agonists to treat acute lung injury or ARDS, pathological conditions similar to inhalation injury [60-63]. Several studies in humans and animals suggest that nitric oxide may also improve outcomes [64-67].
Circulation and fluid resuscitation
Burns are associated with significant fluid shifts and losses due to local and systemic changes in vascular permeability, which may result in hypovolemic shock. Fluid resuscitation should balance the need to restore organ perfusion while avoiding fluid overload, which is often referred to as “fluid creep.” [68] Overly aggressive fluid administration may result in pulmonary edema, abdominal or extremity compartment syndromes, as well as the extension of the burn injury due to excessive local edema. Factors that contribute to “fluid creep” include overestimation of the burn size, emphasis on achieving supra-physiological hemodynamic targets, and excessive vasodilatation associated with excessive use of opioids. Equally important has been the general hesitancy for reducing the rate of fluid administration in patients with evidence of adequate tissue perfusion such as a urine output greater than 30–50 mL/hr in adults or greater than 0.5–1.0 mL/kg in children under 30 kg in weight. Whichever fluid resuscitation formula is chosen, this should only be used as a starting point with the need to closely monitor and titrate fluid administration based on clinical parameters such as mental status, urine output and vital signs.
Among the numerous fluid resuscitation formulas available, the Parkland is most commonly used which calls for 4 mL/kg/%TBSA of Ringer’s Lactate (RL) solution given over the first 24 hours, half of which is given within the first 8 hours from the time of injury. A recent study demonstrated that when the Parkland formula was used, patients received an average of 6.3 mL/kg/%TBSA over the first 24 hours [69]. Excessive fluid administration was primarily related to inadequate reduction in fluid infusion in response to excessive urine output. The Brooke formula that calls for initial administration of RL 2 mL/kg/%TBSA over the first 24 hours with frequent adjustments based on clinical response may be preferred in order to reduce the likelihood of “fluid creep.” The simplest method for calculating the initial fluid requirement proposed by the United States Armed Forces Institute of Surgical Research is the “Rule of Ten.” Based on this rule the patient should receive 10 mL RL per hour for every %TBSA with hourly adjustments based on urine output and clinical response [70]. For every 10 kg above 80 kg, an additional 100 mL of fluid should be given. With this method the fluid rate may be over-estimated in patients weighing <40 kg, and under estimated in patients weighing >140 kg. The Burn Resuscitation Index (BRI), in which a burn score is assigned based on the patient’s weight and estimated burn size, may also be used to help improve the accuracy of fluid estimation [71]. When compared with the Parkland formula, the BRI significantly improved the percentage of emergency medicine residents who correctly calculated fluid rates [71]. Several smartphone software apps have been developed for use in healthcare, among them uBurn and MerseyBurns, which can be used to calculate fluid requirement using the Parkland formula [72]. Monitoring urine output, 0.5 mL/kg/hr in adults and 0.5–1.0 mL/kg/hr in children less than 30 kg in weight, remains one of the primary means of determining the adequacy of fluid resuscitation [10]. Deciding on the best fluid to use, has generated widespread debate and controversy—the crystalloid versus colloid debate [73]. Because of their low cost and availability, isotonic crystalloids have preferentially been used for early resuscitation in burn victims. In the US, RL solution, which most closely approximates normal body fluid, has been the principal resuscitation fluid utilized [74]. Colloids have the potential to increase oncotic pressure and thereby reduce fluid shifts and losses. However, due to an increase in vascular permeability, colloids often do not stay within the intravascular space and may actually increase tissue and pulmonary edema. A recent large multicenter study demonstrated that colloids do not reduce mortality when compared with crystalloids [74]. Colloids may be beneficial after the first 12–24 hours at which time most patients will no longer be in the emergency department. The evidence supporting the use of hypertonic saline is controversial [75-78], and its use should be limited to centers and practitioners experienced in its use [79]. There have also been appeals for alternatives to IV fluid resuscitation in the case of mass burn casualties or austere conditions where IV therapy may be limited. One suggestion has been to use the oral route with World Health Organization oral rehydration solutions in patients with burns smaller than 30% TBSA [80,81].
LOCAL BURN WOUND CARE
Local care of burns generally involves stopping the burning process, cleansing, debridement, and application of topical ointments and/or dressings to support healing. It is well established that initial cooling of the burn surface has many benefits such as alleviating pain, ending the progression of tissue necrosis caused by elevated temperature and possibly aiding in wound healing [82]. What are controversial however are the ideal cooling agent, optimal timing, duration and temperature of cooling agent. Tap water (12°C–18°C) for 30 minutes has been shown in animal studies to reduce necrosis and enhance healing [83]. It is necessary to remove all burned clothing, belts, watches, rings etc. and begin cleansing and debridement utilizing soap and water or a mild skin disinfectant. Non-adherent necrotic skin should be removed and the wound covered with a topical antimicrobial agent and overlying simple non-adherent dressing or an advanced occlusive dressing until the wound is completely reepithelialized. First-degree burns generally do not require topical therapies, however topical application of anesthetics, aloe vera and nonsteroidal anti-inflammatory drugs [84,85] may be considered. Furthermore, analgesics may be given orally. Because of their tendency to progress and heal with significant scarring, deep partial thickness and full thickness burns generally require consultation with a burn specialist for possible excision and grafting. Until then the burns should be covered with a topical antimicrobial agent.
The management of burn blisters, which are mostly seen in superficial partial thickness burns, remains controversial. Those in favor of leaving blisters intact argue that the blister functions as a biological dressing reducing the risk of contamination and infection while providing an optimal moist environment for healing. Those in favor of deroofing and debriding the blisters argue that the blister fluid contains substances detrimental to wound healing (such as thromboxane A2) and that the fluid provides media for bacterial growth. A practical compromise is to aspirate or deroof large or tense blisters and those over joints, which are likely to rupture anyway [86]. Patients may also require a tetanus toxoid booster (diphtheria and tetanus) if they have not received one in the last 5 years. For patients who have never received primary immunization, passive tetanus immune globulin should be given.
Topical wound therapies
In general there are two methods used to treat partial thickness burns. In the “open method,” an antimicrobial containing topical cream or ointment covered by a simple dressing (except on the face) is used. In the “closed method,” an advanced occlusive burn dressing (many of which contain silver), is used (Table 1). The advantage of topical creams, and especially ointments, is their ability to maintain a moist wound environment as well as providing antimicrobial activity to reduce local wound infection. They are also less expensive than advanced dressings. Use of topical antimicrobial agents is especially helpful in heavily weeping, contaminated or infected burns. Their major disadvantage is the need for frequent (once or twice daily) and painful dressing changes. The advanced burns dressings are more expensive, but may be left on the burn for up to a week, reducing the need for painful dressing changes. Many of these dressings are also designed to absorb exudate and maintain a moist healing environment. Since they reduce the need for dressing changes, advanced dressings, such as the silver based Mepilex Ag (Molnlycke Health Care, Gothenburg, Sweden) and Aquacel Ag (ConvaTec, Skillman, NJ, USA), are more cost effective than silver sulfadiazine (SSD) [87].
Table 1.
Representative topical agents and burn dressings for partial thickness burns
| Description | Advantage | Disadvantage | |
|---|---|---|---|
| Topical agents | |||
| Triple antibiotic ointment/bacitracin | Topical antimicrobial ointment | Inexpensive, painless, maintains moist environment | Requires frequent daily applications, messy, does not penetrate eschar |
| Silver sulfadiazine | Water based cream containing silver salt | Wide antimicrobial coverage, painless | Delays healing, stains tissues, contains sulfa, may cause leukopenia |
| Mafenide acetate | Water based cream | Penetrates eschar, wide antibacterial coverage, can be used on face | May be painful to apply, associated with metabolic acidosis |
| Advanced dressings | |||
| Mepilex Ag | Silver impregnated, silicone coated foam | May be left for 7 days, absorptive, broad antimicrobial coverage, comfortable, easy to remove | Expensive, do not use with magnetic resonance imaging |
| Aquacel Ag | Nylon, silver impregnated dressing | May be left for 7 days, broad antimicrobial coverage, painless | Expensive, not compatible with oil-based products |
| Duoderm | Hydrocolloid | May be left for 7 days, painless, facilitates autolytic debridement | Not for large, heavily exudating burns |
| Mepitel | Silicone | May be left for up to 14 days, painless | Expensive, non absorptive, no antimicrobial activity |
SSD was introduced nearly 50 years ago and has led to a significant reduction in the incidence of burn wound sepsis. Indeed, SSD is still the most commonly used topical therapy for burn wounds. However, there is growing evidence that SSD is no longer a good choice since it may delay healing. A systemic review of 30 randomized control trials found that hydrocolloids, silver containing antimicrobial dressings, polyurethane film and biosynthetic dressings appear to be beneficial to wound healing, while SSD was consistently associated with poor healing [88]. In vitro studies have also shown that SSD is toxic to keratinocytes and fibroblasts [89,90]. As such, SSD no longer should be routinely used to treat non-infected burns. A comparison of topical agents and dressings is presented in Table 1. A silicone surfaced foam dressing that slowly releases silver (Mepilex Ag), adheres to dry skin, but is non-adhesive to burn surfaces, is easy to use and very comfortable for patients [91].
Escharotomy
The necrotic eschar that forms with deep burns is often stiff and unyielding having leather-like mechanical properties. When the eschar overlies vital structures, especially when circumferential, it may compromise circulation (in the extremities) or breathing (when over the thorax or neck). Increases in tissue pressure may be exacerbated during fluid resuscitation, causing dangerous elevations in tissue pressure. This may result in ischemia leading to tissue necrosis [92] if not recognized and treated aggressively by performing an escharotomy. An escharotomy is performed by making an incision, either with a surgical blade or electric cautery, through the eschar to release the underlying pressure. The incision should be made all the way down to the fat resulting in splitting of the stiff eschar shell. Since there is little pain with full thickness burns, escharotomies may be performed with very little analgesia or sedation. Placement of the incisions should avoid injury to underlying structures such as nerves and vessels. With extremity eschars, incisions should be made over the medial and lateral aspects of the extremity. With eschars of the thorax incisions should be made along the anterior axillary lines, costal margins, and below and parallel to the clavicles in a V shape. There is insufficient scientific data to support any specific standard or objective test to help identify early signs of compartment syndrome to aid the clinician in deciding on whether an escharotomy is warranted [92,93]. Traditionally, deciding on performing an escharotomy has been based on decreased or absent palpable pulses, pulse oximetry signals [94] or Doppler signals [95]. Increased pain (especially with passive motion), pallor, motor weakness, and loss of sensation may also be associated with a compartment syndrome [92]. Difficult ventilation or airway obstruction may indicate the need for an escharotomy of the thorax and neck respectively.
PAIN MANAGEMENT
Effective pain management for severely burned victims can be problematic [96]. During the early emergent phase, potent opioids such as morphine sulfate, hydromorphone and fentanyl should be given intravenously and titrated based on patient response [96,97]. For moderate pain, an oral oxycodone/acetaminophen mixture may be used while oral non-steroidal anti-inflammatory drugs and acetaminophen can be administered for minor pain [97]. Antidepressants, anticonvulsants, and antianxiety agents have also been found to be useful adjuvants in pain management of burn patients [98]. Ketamine, at sub-dissociative doses of 0.1–0.2 mg/kg can be given intravenously, especially in patients who are resistant to large doses of opioids.
PATIENT REFERRAL
The American Burn Association has established criteria for referral to a specialized burn center that include both patient and burn characteristics such as size, depth, and etiology (Table 2) [99].
Table 2.
Criteria for referral to a burn center [99]
| Partial thickness burns greater than 10% total body surface area. |
| Burns that involve the face, hands, feet, genitalia, perineum, or major joints. |
| Third degree burns in any age group. |
| Electrical burns, including lightning injury. |
| Chemical burns. |
| Inhalation injury. |
| Burn injury in patients with preexisting medical disorders that could complicate management, prolong recovery, or affect mortality. |
| Any patient with burns and concomitant trauma (such as fractures) in which the burn injury poses the greatest risk of morbidity or mortality. |
| Burned children in hospitals without qualified personnel or equipment for the care of children. |
| Burn injury in patients who will require special social, emotional, or rehabilitative intervention. |
Capsule Summary
What is already known
Early endotracheal intubation has been encouraged to prevent rapid deterioration of the airway and aggressive fluid resuscitation based on the Parkland formula is widespread. Silver sulfadiazine remains the most common topical therapy for burns.
What is new in the current study
Prophylactic endotracheal intubation in patients with a normal appearing airway on laryngoscopy is discouraged and may worsen outcomes. Overly aggressive fluid resuscitation may lead to “fluid creep” and may be avoided by close monitoring of urine output and clinical response. Silver sulfadiazine delays healing and should not be routinely used in burn wounds. A number of advanced dressings that release silver simplify local wound care.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Bessey PQ, Phillips BD, Lentz CW, et al. Synopsis of the 2013 annual report of the national burn repository. J Burn Care Res. 2014;35 Suppl 2:S218–34. doi: 10.1097/BCR.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 2.Jackson DM. The diagnosis of the depth of burning. Br J Surg. 1953;40:588–96. doi: 10.1002/bjs.18004016413. [DOI] [PubMed] [Google Scholar]
- 3.Hettiaratchy S, Dziewulski P. ABC of burns: pathophysiology and types of burns. 2004;328:1427–9. doi: 10.1136/bmj.328.7453.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sterling JP, Heimbach DM, Gibran NS. Management of the burn wound. In: ACS surgery: principles and practice. Hamilton, ON: Decker; 2010. http://dx.doi.org/10.2310/7800.S07C15. [Google Scholar]
- 5.Gravante G, Palmieri MB, Esposito G, et al. Apoptotic cells are present in ischemic zones of deep partial-thickness burns. J Burn Care Res. 2006;27:688–93. doi: 10.1097/01.BCR.0000238101.94950.EC. [DOI] [PubMed] [Google Scholar]
- 6.Keck M, Herndon DH, Kamolz LP, Frey M, Jeschke MG. Pathophysiology of burns. Wien Med Wochenschr. 2009;159:327–36. doi: 10.1007/s10354-009-0651-2. [DOI] [PubMed] [Google Scholar]
- 7.Shupp JW, Nasabzadeh TJ, Rosenthal DS, Jordan MH, Fidler P, Jeng JC. A review of the local pathophysiologic bases of burn wound progression. J Burn Care Res. 2010;31:849–73. doi: 10.1097/BCR.0b013e3181f93571. [DOI] [PubMed] [Google Scholar]
- 8.Jaskille AD, Jeng JC, Sokolich JC, Lunsford P, Jordan MH. Repetitive ischemia-reperfusion injury: a plausible mechanism for documented clinical burn-depth progression after thermal injury. J Burn Care Res. 2007;28:13–20. doi: 10.1097/BCR.0b013E31802CB82C. [DOI] [PubMed] [Google Scholar]
- 9.Haberal M, Sakallioglu Abali AE, Karakayali H. Fluid management in major burn injuries. Indian J Plast Surg. 2010;43:S29–36. doi: 10.4103/0970-0358.70715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latenser BA. Critical care of the burn patient: the first 48 hours. Crit Care Med. 2009;37:2819–26. doi: 10.1097/CCM.0b013e3181b3a08f. [DOI] [PubMed] [Google Scholar]
- 11.Scheulen JJ, Munster AM. The Parkland formula in patients with burns and inhalation injury. J Trauma. 1982;22:869–71. doi: 10.1097/00005373-198210000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Dries DJ, Endorf FW. Inhalation injury: epidemiology, pathology, treatment strategies. Scand J Trauma Resusc Emerg Med. 2013;21:31. doi: 10.1186/1757-7241-21-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rehberg S, Maybauer MO, Enkhbaatar P, Maybauer DM, Yamamoto Y, Traber DL. Pathophysiology, management and treatment of smoke inhalation injury. Expert Rev Respir Med. 2009;3:283–97. doi: 10.1586/ERS.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pacher P, Szabo C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol. 2008;173:2–13. doi: 10.2353/ajpath.2008.080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lobo SM, Orrico SR, Queiroz MM, et al. Pneumonia-induced sepsis and gut injury: effects of a poly-(ADP-ribose) polymerase inhibitor. J Surg Res. 2005;129:292–7. doi: 10.1016/j.jss.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Gero D, Szabo C. Poly(ADP-ribose) polymerase: a new therapeutic target? Curr Opin Anaesthesiol. 2008;21:111–21. doi: 10.1097/ACO.0b013e3282f63c15. [DOI] [PubMed] [Google Scholar]
- 17.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–80. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 18.Toon MH, Maybauer MO, Greenwood JE, Maybauer DM, Fraser JF. Management of acute smoke inhalation injury. Crit Care Resusc. 2010;12:53–61. [PubMed] [Google Scholar]
- 19.Singer AJ, Brebbia J, Soroff HH. Management of local burn wounds in the ED. Am J Emerg Med. 2007;25:666–71. doi: 10.1016/j.ajem.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Kalus AM, Aindow J, Caulfield MR. Application of ultrasound in assessing burn depth. Lancet. 1979;1:188–9. doi: 10.1016/s0140-6736(79)90583-x. [DOI] [PubMed] [Google Scholar]
- 21.Gatti JE, LaRossa D, Silverman DG, Hartford CE. Evaluation of the burn wound with perfusion fluorometry. J Trauma. 1983;23:202–6. doi: 10.1097/00005373-198303000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Gill P. The critical evaluation of laser Doppler imaging in determining burn depth. Int J Burns Trauma. 2013;3:72–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Medina-Preciado JD, Kolosovas-Machuca ES, Velez-Gomez E, Miranda-Altamirano A, Gonzalez FJ. Noninvasive determination of burn depth in children by digital infrared thermal imaging. J Biomed Opt. 2013;18:061204. doi: 10.1117/1.JBO.18.6.061204. [DOI] [PubMed] [Google Scholar]
- 24.Koruda MJ, Zimbler A, Settle RG, et al. Assessing burn wound depth using in vitro nuclear magnetic resonance (NMR) J Surg Res. 1986;40:475–81. doi: 10.1016/0022-4804(86)90218-0. [DOI] [PubMed] [Google Scholar]
- 25.Heimbach D, Engrav L, Grube B, Marvin J. Burn depth: a review. World J Surg. 1992;16:10–5. doi: 10.1007/BF02067108. [DOI] [PubMed] [Google Scholar]
- 26.Monstrey S, Hoeksema H, Verbelen J, Pirayesh A, Blondeel P. Assessment of burn depth and burn wound healing potential. Burns. 2008;34:761–9. doi: 10.1016/j.burns.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Amirsheybani HR, Crecelius GM, Timothy NH, Pfeiffer M, Saggers GC, Manders EK. The natural history of the growth of the hand: I. Hand area as a percentage of body surface area. Plast Reconstr Surg. 2001;107:726–33. doi: 10.1097/00006534-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Freiburg C, Igneri P, Sartorelli K, Rogers F. Effects of differences in percent total body surface area estimation on fluid resuscitation of transferred burn patients. J Burn Care Res. 2007;28:42–8. doi: 10.1097/BCR.0B013E31802C88B2. [DOI] [PubMed] [Google Scholar]
- 29.Collis N, Smith G, Fenton OM. Accuracy of burn size estimation and subsequent fluid resuscitation prior to arrival at the Yorkshire Regional Burns Unit: a three year retrospective study. Burns. 1999;25:345–51. doi: 10.1016/s0305-4179(99)00007-8. [DOI] [PubMed] [Google Scholar]
- 30.Reiband HK, Lundin K, Alsbjorn B, Sorensen AM, Rasmussen LS. Optimization of burn referrals. Burns. 2014;40:397–401. doi: 10.1016/j.burns.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Shokrollahi K, Sayed M, Dickson W, Potokar T. Mobile phones for the assessment of burns: we have the technology. Emerg Med J. 2007;24:753–5. doi: 10.1136/emj.2007.046730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haller HL, Dirnberger J, Giretzlehner M, Rodemund C, Kamolz L. “Understanding burns”: research project BurnCase 3D--overcome the limits of existing methods in burns documentation. Burns. 2009;35:311–7. doi: 10.1016/j.burns.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Holt B, Faraklas I, Theurer L, Cochran A, Saffle JR. Telemedicine use among burn centers in the United States: a survey. J Burn Care Res. 2012;33:157–62. doi: 10.1097/BCR.0b013e31823d0b68. [DOI] [PubMed] [Google Scholar]
- 34.Saffle JR, Edelman L, Theurer L, Morris SE, Cochran A. Telemedicine evaluation of acute burns is accurate and cost-effective. J Trauma. 2009;67:358–65. doi: 10.1097/TA.0b013e3181ae9b02. [DOI] [PubMed] [Google Scholar]
- 35.Cancio LC. Airway management and smoke inhalation injury in the burn patient. Clin Plast Surg. 2009;36:555–67. doi: 10.1016/j.cps.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Gueugniaud PY, Carsin H, Bertin-Maghit M, Petit P. Current advances in the initial management of major thermal burns. Intensive Care Med. 2000;26:848–56. doi: 10.1007/s001340051273. [DOI] [PubMed] [Google Scholar]
- 37.Mackie DP. Inhalation injury or mechanical ventilation: which is the true killer in burn patients? Burns. 2013;39:1329–30. doi: 10.1016/j.burns.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Pinhu L, Whitehead T, Evans T, Griffiths M. Ventilator-associated lung injury. Lancet. 2003;361:332–40. doi: 10.1016/S0140-6736(03)12329-X. [DOI] [PubMed] [Google Scholar]
- 39.Mackie DP, van Dehn F, Knape P, Breederveld RS, Boer C. Increase in early mechanical ventilation of burn patients: an effect of current emergency trauma management? J Trauma. 2011;70:611–5. doi: 10.1097/TA.0b013e31821067aa. [DOI] [PubMed] [Google Scholar]
- 40.Eastman AL, Arnoldo BA, Hunt JL, Purdue GF. Pre-burn center management of the burned airway: do we know enough? J Burn Care Res. 2010;31:701–5. doi: 10.1097/BCR.0b013e3181eebe4f. [DOI] [PubMed] [Google Scholar]
- 41.Helvig B, Mlcak R, Nichols RJ., Jr Anchoring endotracheal tubes on patients with facial burns: review from Shriners Burns Institute, Galveston, Texas. J Burn Care Rehabil. 1987;8:236–7. [PubMed] [Google Scholar]
- 42.Masanes MJ, Legendre C, Lioret N, Maillard D, Saizy R, Lebeau B. Fiberoptic bronchoscopy for the early diagnosis of subglottal inhalation injury: comparative value in the assessment of prognosis. J Trauma. 1994;36:59–67. doi: 10.1097/00005373-199401000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Moylan JA, Adib K, Birnbaum M. Fiberoptic bronchoscopy following thermal injury. Surg Gynecol Obstet. 1975;140:541–3. [PubMed] [Google Scholar]
- 44.Woodson LC. Diagnosis and grading of inhalation injury. J Burn Care Res. 2009;30:143–5. doi: 10.1097/BCR.0b013e3181923b71. [DOI] [PubMed] [Google Scholar]
- 45.Endorf FW, Gamelli RL. Inhalation injury, pulmonary perturbations, and fluid resuscitation. J Burn Care Res. 2007;28:80–3. doi: 10.1097/BCR.0B013E31802C889F. [DOI] [PubMed] [Google Scholar]
- 46.Mlcak RP, Suman OE, Herndon DN. Respiratory management of inhalation injury. Burns. 2007;33:2–13. doi: 10.1016/j.burns.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Moylan JA, Jr, Wilmore DW, Mouton DE, Pruitt BA., Jr Early diagnosis of inhalation injury using 133 xenon lung scan. Ann Surg. 1972;176:477–84. doi: 10.1097/00000658-197210000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hassan Z, Wong JK, Bush J, Bayat A, Dunn KW. Assessing the severity of inhalation injuries in adults. Burns. 2010;36:212–6. doi: 10.1016/j.burns.2009.06.205. [DOI] [PubMed] [Google Scholar]
- 49.Meredith JW, Martin MB, Poole GV, Jr, Kon ND, Breyer RH, Mills SA. Measurement of extravascular lung water in sheep during colloid and crystalloid resuscitation from smoke inhalation. Am Surg. 1983;49:637–41. [PubMed] [Google Scholar]
- 50.Brown LM, Liu KD, Matthay MA. Measurement of extravascular lung water using the single indicator method in patients: research and potential clinical value. Am J Physiol Lung Cell Mol Physiol. 2009;297:L547–58. doi: 10.1152/ajplung.00127.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baud FJ, Barriot P, Toffis V, et al. Elevated blood cyanide concentrations in victims of smoke inhalation. N Engl J Med. 1991;325:1761–6. doi: 10.1056/NEJM199112193252502. [DOI] [PubMed] [Google Scholar]
- 52.Hamel J. A review of acute cyanide poisoning with a treatment update. Crit Care Nurse. 2011;31:72–81. doi: 10.4037/ccn2011799. [DOI] [PubMed] [Google Scholar]
- 53.Slutsky AS. Mechanical ventilation: American College of Chest Physicians’ Consensus Conference. Chest. 1993;104:1833–59. doi: 10.1378/chest.104.6.1833. [DOI] [PubMed] [Google Scholar]
- 54.Oba Y, Salzman GA. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury. N Engl J Med. 2000;343:813. [PubMed] [Google Scholar]
- 55.Endorf FW, Dries DJ. Noninvasive ventilation in the burned patient. J Burn Care Res. 2010;31:217–28. doi: 10.1097/BCR.0b013e3181d0f62c. [DOI] [PubMed] [Google Scholar]
- 56.Desai MH, Mlcak R, Richardson J, Nichols R, Herndon DN. Reduction in mortality in pediatric patients with inhalation injury with aerosolized heparin/N-acetylcystine therapy. J Burn Care Rehabil. 1998;19:210–2. doi: 10.1097/00004630-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Miller AC, Rivero A, Ziad S, Smith DJ, Elamin EM. Influence of nebulized unfractionated heparin and N-acetylcysteine in acute lung injury after smoke inhalation injury. J Burn Care Res. 2009;30:249–56. doi: 10.1097/BCR.0b013e318198a268. [DOI] [PubMed] [Google Scholar]
- 58.Yip LY, Lim YF, Chan HN. Safety and potential anticoagulant effects of nebulised heparin in burns patients with inhalational injury at Singapore General Hospital Burns Centre. Burns. 2011;37:1154–60. doi: 10.1016/j.burns.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 59.Holt J, Saffle JR, Morris SE, Cochran A. Use of inhaled heparin/N-acetylcystine in inhalation injury: does it help? J Burn Care Res. 2008;29:192–5. doi: 10.1097/BCR.0b013e31815f596b. [DOI] [PubMed] [Google Scholar]
- 60.Palmieri TL. Use of beta-agonists in inhalation injury. J Burn Care Res. 2009;30:156–9. doi: 10.1097/BCR.0b013e3181923bc3. [DOI] [PubMed] [Google Scholar]
- 61.Perkins GD, McAuley DF, Thickett DR, Gao F. The beta-agonist lung injury trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med. 2006;173:281–7. doi: 10.1164/rccm.200508-1302OC. [DOI] [PubMed] [Google Scholar]
- 62.Gao Smith F, Perkins GD, Gates S, et al. Effect of intravenous β-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet. 2012;379:229–35. doi: 10.1016/S0140-6736(11)61623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gates S, Perkins GD, Lamb SE, et al. Beta-Agonist Lung injury TrIal-2 (BALTI-2): a multicentre, randomised, double-blind, placebo-controlled trial and economic evaluation of intravenous infusion of salbutamol versus placebo in patients with acute respiratory distress syndrome. Health Technol Assess. 2013;17:v-vi, 1-87. doi: 10.3310/hta17380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taylor RW, Zimmerman JL, Dellinger RP, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004;291:1603–9. doi: 10.1001/jama.291.13.1603. [DOI] [PubMed] [Google Scholar]
- 65.Lundin S, Mang H, Smithies M, Stenqvist O, Frostell C. Inhalation of nitric oxide in acute lung injury: results of a European multicentre study. The European Study Group of Inhaled Nitric Oxide. Intensive Care Med. 1999;25:911–9. doi: 10.1007/s001340050982. [DOI] [PubMed] [Google Scholar]
- 66.Dellinger RP, Zimmerman JL, Taylor RW, et al. Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled Nitric Oxide in ARDS Study Group. Crit Care Med. 1998;26:15–23. doi: 10.1097/00003246-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 67.Soejima K, Traber LD, Schmalstieg FC, et al. Role of nitric oxide in vascular permeability after combined burns and smoke inhalation injury. Am J Respir Crit Care Med. 2001;163:745–52. doi: 10.1164/ajrccm.163.3.9912052. [DOI] [PubMed] [Google Scholar]
- 68.Pruitt BA., Jr Protection from excessive resuscitation: “pushing the pendulum back”. J Trauma. 2000;49:567–8. doi: 10.1097/00005373-200009000-00030. [DOI] [PubMed] [Google Scholar]
- 69.Cartotto R, Zhou A. Fluid creep: the pendulum hasn’t swung back yet! J Burn Care Res. 2010;31:551–8. doi: 10.1097/BCR.0b013e3181e4d732. [DOI] [PubMed] [Google Scholar]
- 70.Alvarado R, Chung KK, Cancio LC, Wolf SE. Burn resuscitation. Burns. 2009;35:4–14. doi: 10.1016/j.burns.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 71.Kahn SA, Schoemann M, Lentz CW. Burn resuscitation index: a simple method for calculating fluid resuscitation in the burn patient. J Burn Care Res. 2010;31:616–23. doi: 10.1097/BCR.0b013e3181e4d6ee. [DOI] [PubMed] [Google Scholar]
- 72.Morris R, Javed M, Bodger O, Hemington Gorse S, Williams D. A comparison of two smartphone applications and the validation of smartphone applications as tools for fluid calculation for burns resuscitation. Burns. 2014;40:826–34. doi: 10.1016/j.burns.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 73.Williams C. Fluid resuscitation in burn patients 1: using formulas. Nurs Times. 2008;104:28–9. [PubMed] [Google Scholar]
- 74.Tricklebank S. Modern trends in fluid therapy for burns. Burns. 2009;35:757–67. doi: 10.1016/j.burns.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 75.Huang PP, Stucky FS, Dimick AR, Treat RC, Bessey PQ, Rue LW. Hypertonic sodium resuscitation is associated with renal failure and death. Ann Surg. 1995;221:543–54. doi: 10.1097/00000658-199505000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kien ND, Antognini JF, Reilly DA, Moore PG. Small-volume resuscitation using hypertonic saline improves organ perfusion in burned rats. Anesth Analg. 1996;83:782–8. doi: 10.1097/00000539-199610000-00022. [DOI] [PubMed] [Google Scholar]
- 77.Kuroda T, Harada T, Tsutsumi H, Kobayashi M. Hypernatremia deepens the demarcating borderline of leukocytic infiltration in the burn wound. Burns. 1997;23:432–7. doi: 10.1016/s0305-4179(97)00016-8. [DOI] [PubMed] [Google Scholar]
- 78.Monafo WW, Halverson JD, Schechtman K. The role of concentrated sodium solutions in the resuscitation of patients with severe burns. Surgery. 1984;95:129–35. [PubMed] [Google Scholar]
- 79.Pham TN, Cancio LC, Gibran NS; American Burn Association. American Burn Association practice guidelines burn shock resuscitation. J Burn Care Res. 2008;29:257–66. doi: 10.1097/BCR.0b013e31815f3876. [DOI] [PubMed] [Google Scholar]
- 80.Brown TL, Hernon C, Owens B. Incidence of vomiting in burns and implications for mass burn casualty management. Burns. 2003;29:159–62. doi: 10.1016/s0305-4179(02)00211-5. [DOI] [PubMed] [Google Scholar]
- 81.Kramer GC, Michell MW, Oliveira H, et al. Oral and enteral resuscitation of burn shock the historical record and implications for mass casualty care. Eplasty. 2010;10 [PMC free article] [PubMed] [Google Scholar]
- 82.Jandera V, Hudson DA, de Wet PM, Innes PM, Rode H. Cooling the burn wound: evaluation of different modalites. Burns. 2000;26:265–70. doi: 10.1016/s0305-4179(99)00133-3. [DOI] [PubMed] [Google Scholar]
- 83.Venter TH, Karpelowsky JS, Rode H. Cooling of the burn wound: the ideal temperature of the coolant. Burns. 2007;33:917–22. doi: 10.1016/j.burns.2006.10.408. [DOI] [PubMed] [Google Scholar]
- 84.Magnette J, Kienzler JL, Alekxandrova I, et al. The efficacy and safety of low-dose diclofenac sodium 0.1% gel for the symptomatic relief of pain and erythema associated with superficial natural sunburn. Eur J Dermatol. 2004;14:238–46. [PubMed] [Google Scholar]
- 85.Maenthaisong R, Chaiyakunapruk N, Niruntraporn S, Kongkaew C. The efficacy of aloe vera used for burn wound healing: a systematic review. Burns. 2007;33:713–8. doi: 10.1016/j.burns.2006.10.384. [DOI] [PubMed] [Google Scholar]
- 86.Sargent RL. Management of blisters in the partial-thickness burn: an integrative research review. J Burn Care Res. 2006;27:66–81. doi: 10.1097/01.bcr.0000191961.95907.b1. [DOI] [PubMed] [Google Scholar]
- 87.Sheckter CC, Van Vliet MM, Krishnan NM, Garner WL. Cost-effectiveness comparison between topical silver sulfadiazine and enclosed silver dressing for partial-thickness burn treatment. J Burn Care Res. 2014;35:284–90. doi: 10.1097/BCR.0b013e3182a36916. [DOI] [PubMed] [Google Scholar]
- 88.Wasiak J, Cleland H, Campbell F. Dressings for superficial and partial thickness burns. Cochrane Database Syst Rev. 2008;(4):CD002106. doi: 10.1002/14651858.CD002106.pub3. [DOI] [PubMed] [Google Scholar]
- 89.Lee AR, Moon HK. Effect of topically applied silver sulfadiazine on fibroblast cell proliferation and biomechanical properties of the wound. Arch Pharm Res. 2003;26:855–60. doi: 10.1007/BF02980032. [DOI] [PubMed] [Google Scholar]
- 90.McCauley RL, Li YY, Poole B, et al. Differential inhibition of human basal keratinocyte growth to silver sulfadiazine and mafenide acetate. J Surg Res. 1992;52:276–85. doi: 10.1016/0022-4804(92)90086-f. [DOI] [PubMed] [Google Scholar]
- 91.Silverstein P, Heimbach D, Meites H, et al. An open, parallel, randomized, comparative, multicenter study to evaluate the cost-effectiveness, performance, tolerance, and safety of a silver-containing soft silicone foam dressing (intervention) vs silver sulfadiazine cream. J Burn Care Res. 2011;32:617–26. doi: 10.1097/BCR.0b013e318236fe31. [DOI] [PubMed] [Google Scholar]
- 92.Orgill DP, Piccolo N. Escharotomy and decompressive therapies in burns. J Burn Care Res. 2009;30:759–68. doi: 10.1097/BCR.0b013e3181b47cd3. [DOI] [PubMed] [Google Scholar]
- 93.Saffle J. Practice guidelines for burn care. Boston, MA: American Burn Association; 2001. [Google Scholar]
- 94.Bardakjian VB, Kenney JG, Edgerton MT, Morgan RF. Pulse oximetry for vascular monitoring in burned upper extremities. J Burn Care Rehabil. 1988;9:63–5. doi: 10.1097/00004630-198801000-00015. [DOI] [PubMed] [Google Scholar]
- 95.Moylan JA, Jr, Inge WW, Jr, Pruitt BA., Jr Circulatory changes following circumferential extremity burns evaluated by the ultrasonic flowmeter: an analysis of 60 thermally injured limbs. J Trauma. 1971;11:763–70. doi: 10.1097/00005373-197109000-00007. [DOI] [PubMed] [Google Scholar]
- 96.Summer GJ, Puntillo KA, Miaskowski C, Green PG, Levine JD. Burn injury pain: the continuing challenge. J Pain. 2007;8:533–48. doi: 10.1016/j.jpain.2007.02.426. [DOI] [PubMed] [Google Scholar]
- 97.Norman AT, Judkins KC. Pain in the patient with burns. Contin Educ Anaesth Crit Care Pain. 2004;4:57–61. [Google Scholar]
- 98.Richardson P, Mustard L. The management of pain in the burns unit. Burns. 2009;35:921–36. doi: 10.1016/j.burns.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 99.American College of Surgeons . Resources for optimal care of the injured patient 2006. Chicago, IL: American College of Surgeons; 2007. [Google Scholar]