Abstract
Use of mesenchymal stem cells (MSCs) found in the stromal vascular fraction (SVF) of equine adipose tissue has promising applications for regenerative therapies. The most commonly used source of equine adipose tissue is the subcutaneous tailhead. The objective of this study was to compare 3 adipose depot sites in horses and determine the viability and cellular yield, capillary density, gene expression for selected markers, and colony-forming unit fibroblasts (CFU-Fs) in adipose tissue taken from these sites. Adipose tissue was excised from the area lateral to the tailhead, the inguinal region, and the small colon mesentery of 6 horses. Lipoaspirate was also collected from the area lateral to the tailhead. Stromal vascular fraction (SVF) was prepared in duplicate from the 3 different adipose tissue depots. The total nucleated and dead cell counts was determined manually using a hemocytometer and percent viability was calculated. Mass and volume of adipose were determined in order to calculate density and factor-VIII immunohistochemical staining was used to determine vascular density in the excisional adipose tissue samples from each horse. Quantitative polymerase chain reaction (qPCR) was used to quantify gene expression for selected cellular markers from each site. There were significant differences in viability, yield of nucleated cells/gram of adipose tissue, vascular density, gene expression, and CFU-Fs among adipose depots. Adipose from the mesentery yielded the highest number of nucleated cells/gram of tissue and the highest vascular density and percentage of CFU-Fs. In the horse, both the anatomical site of collection and the method of tissue collection significantly impact the yield and composition of cells in the SVF. Further study is needed to assess whether one adipose source is superior for harvesting mesenchymal stem cells (MSCs) and whether the differences among sources are clinically relevant for in-vivo treatment of musculoskeletal injuries in horses.
Résumé
L’utilisation de cellules souches mésenchymateuses (CSMs) retrouvées dans la fraction du stroma vasculaire (FSV) du tissu adipeux équin a des applications prometteuses pour les thérapies régénératrices. La source la plus fréquemment utilisée de tissu adipeux équin est le tissu sous-cutané de la base de la queue. L’objectif de la présente étude était de comparer trois sites de dépôts adipeux chez le cheval et de déterminer la viabilité et la récolte cellulaire, la densité capillaire, l’expression génique de marqueurs sélectionnés, et le nombre de fibroblastes formateur des colonies (FFC) dans le tissu adipeux prélevés de ces sites. Le tissu adipeux a été excisé de la région latérale à la base de la queue, de la région inguinale, et du mésentère du petit colon de six chevaux. Des aspirations de lipide ont également été prélevées de la région latérale de la base de la queue. La FSV a été préparée en duplicata à partir de chacun des trois dépôts différents de tissu adipeux. Les dénombrements totaux des cellules nucléées et mortes ont été déterminés manuellement à l’aide d’un hémocytomètre et le pourcentage de viabilité calculé. La masse et le volume de tissu adipeux ont été déterminés afin de calculer la densité et la coloration par immunohistochimie du facteur VIII a été utilisée afin de déterminer la densité vasculaire dans les échantillons de tissu adipeux excisé de chaque cheval. Une réaction d’amplification en chaine par la polymérase quantitative (ACPq) a été utilisée pour quantifier l’expression génique pour des marqueurs cellulaires sélectionnés de chaque site. Il y avait des différences significatives dans la viabilité, le rendement de cellules nucléées/gramme de tissu adipeux, la densité vasculaire, l’expression génique, et les FFCs entre les dépôts adipeux. Le tissu adipeux provenant du mésentère a généré le plus grand nombre de cellules nucléées/gramme de tissu et la plus haute densité vasculaire et pourcentage de FFCs. Chez le cheval, le site anatomique de prélèvement et la méthode de prélèvement du tissu ont un impact significatif sur le rendement et la composition cellulaire dans la FSV. Des études additionnelles sont requises pour évaluer si une source de tissu adipeux est supérieure pour récolter des cellules souches mésenchymateuses et si les différences entre les sources sont cliniquement pertinentes pour le traitement in vivo de blessures morpho-squelettiques chez les chevaux.
(Traduit par Docteur Serge Messier)
Introduction
Regenerative cell-based therapy is a promising tool for treating musculoskeletal injuries in equine athletes and has the potential to regenerate or modulate healing of tissue. Superficial digital flexor tendonitis is an example of a common injury in the horse industry with poor healing, long convalescence periods, loss of earnings, and high reinjury rates either in the same or contralateral limb (1–3). Mesenchymal stem cells (MSCs) have potential for treating musculoskeletal injuries in horses in a wide variety of disciplines (4–7).
There are numerous tissues that contain MSCs, but the most practical and widely used tissues are bone marrow and adipose. There are also many techniques for obtaining MSCs. The most commonly used techniques are culture-expanded bone marrow and adipose-derived stem cells in the stromal vascular fraction (SVF), which contains mesenchymal stem cells and a variety of nucleated cells that can contribute to tissue healing (8–10). Since there are relatively few MSCs in bone marrow, culture expansion is indicated to obtain the desired number of MSCs for implantation (8,10,11). There are data that support MSCs derived from bone marrow for specific applications (12). When the SVF is used for immediate implantation, there is potential for high MSC yield.
Data indicate that up to 222 times more MSCs are found in adipose tissue than in equal amounts of bone marrow in the horse and in humans up to 500 times more MSCs are found in adipose than in bone marrow (8). Because adipose tissue has a relatively high density of MSCs, fresh SVF can be used for immediate implantation without expansion, which is an attractive feature of SVF regenerative therapy (13,14). A number of factors must be considered when choosing the source and technique, including timing of implantation and time required to obtain cells, harvest location, patient morbidity, and efficacy. These factors affect the clinician’s selection of cultured MSCs or fresh SVF. Regardless of the source, the objective is to maximize the MSC harvest.
When bone marrow is used, it has been shown that different harvest locations vary in nucleated cell counts (15). It is not known, however, if this is also true for adipose harvest sites. Adipose is an abundant source of mesenchymal stem cells, with depots in the horse found lateral to the tailhead, the inguinal region, neck, omentum, and mesentery. Historically, the harvest site of choice for adipose-derived mesenchymal stem cells (MSCs) or stromal vascular fraction (SVF) in horses is lateral to the tailhead (16–18). Fat lateral to the tailhead varies in vascular density, which has in turn been shown to correlate with MSC colony-forming units (CFUs) in vitro (16). There is limited information, however, on the optimal location for harvesting adipose to obtain the SVF in horses and other species.
Certain clinical situations can dictate the harvest site of adipose tissues, including cosmetic appearance, convenience, adequate adipose depot for collection, and positioning of a horse that is already under general anesthesia. The concern with using alternative sites for adipose harvesting is the quality and quantity of cell yield. It has been found in humans that not all fat depot sites exhibit equivalent characteristics and there are differences in surface marker expression, doubling time, and SVF yield among subcutaneous, omental, and intrathoracic fat depot sites (19). Additionally, use of lipoaspiration can speed the adipose recovery process and result in a very small incision lateral to the tailhead, which improves cosmetic outcome and decreases potential morbidity, although the lipoaspiration procedure itself could affect cell yield and viability (18).
The objective of this study was to evaluate the nucleated cell fractions in adipose tissue obtained from the tailhead (excisional and lipoaspirate), inguinal region, and mesentery of the same horse and compare the viability and cellular yield, tissue and vascular density, gene expression for selected markers, and CFU-F in this tissue. We hypothesized that adipose depot sites with higher vascular density would yield a higher concentration of nucleated cells than sites with lower vascular density.
Materials and methods
Animals
Six healthy horses were used in this study, as determined by physical examination and known health histories. It was required that the horses not have any prior history or evidence of intra-abdominal surgery or asymmetry over the gluteal or tailhead region. The median age was 3 y (1 to 15 y) and mean ± standard deviation (SD) weight was 482 ± 86.1 kg (318 to 559 kg). Breed distribution was 5 Quarter Horses and 1 Missouri Fox Trotter. All horses had been euthanized for reasons not associated with this study. Euthanasia procedures were approved by the Iowa State University Institutional Animal Care and Use Committee, which complies with the American Veterinary and Medical Association (AVMA) Guidelines for the Euthanasia of Animals.
Collection of adipose tissue
Following euthanasia, sites over the tailhead, inguinal area, and abdomen were immediately clipped and aseptically prepared. Enough adipose tissue to fill a 50-mL conical tube was harvested from the area lateral to the tailhead, the inguinal region, and the small colon mesentery by surgical excision. Additionally, adipose tissue was collected by lipoaspiration from the contralateral tailhead by making a 1-cm stab incision with a #15 scalpel blade 8 cm from midline adjacent to the tailhead. The adipose tissue was aspirated with a lipoaspirate cannula (3-mm Disposable Cannula; Shippert Medical Technologies, Centennial, Colorado, USA) and a 60-mL luer lock syringe (VacLok; Merit Medical Systems, South Jordan, Utah, USA), without the use of tumescent fluid, to collect approximately 10 to 15 mL of adipose tissue.
Excisional adipose tissue samples from each location were divided into 2 portions, 1 for immunohistochemical (IHC) analysis and the other for cell isolation. The adipose for IHC was fixed in 10% buffered formalin. The lipoaspirate was evaluated for cell isolation only and not used for density or IHC evaluation.
Nucleated cell isolation and counting
The weight of each sample for cell isolation was determined before enzymatic processing. The lipoaspirates were processed in aliquots of approximately 10 g using a commercially available system (ARC System; InGeneron, Houston, Texas, USA) to isolate SVF cells from the adipose tissue. Lipoaspirates were incubated at 37°C and agitated in the processing unit with a proprietary blend of proteolytic enzymes (Matrase reagent; InGeneron) for 30 min to dissociate the tissue and release the nucleated cells, which were then separated from the solid tissue by filtration (100 μm filter, Steriflip; EMD Millipore, Bilerica, Massachusetts, USA). The excisional adipose tissue samples used for cell isolation were minced with Mayo scissors and processed in approximately 10-gram aliquots. Similar to lipoaspirates, the minced excisional adipose samples were processed with Matrase reagent (InGeneron) at 37°C, with the exception that processing time was 60 min, following the manufacturer’s recommendation for excisional adipose tissue.
After processing and filtration, all samples were treated similarly by adding approximately 25 mL of Lactated Ringer’s solution (LRS) to reach a total volume of 50 mL to dilute enzymes and achieve balance and centrifuged for 10 min at 600 × g. The cells were washed twice by centrifugation and resuspension of the cell pellet in 40 mL of LRS each time. The final cell pellet was resuspended in 5 mL of LRS. Samples from each tissue site for each horse were run in duplicate.
To obtain nucleated cell counts, cells were stained with fluorescent nucleic acid stain (SYTO 13; Life Technologies/Thermo Fisher Scientific, Waltham, Massachusetts, USA) using a 1:1 solution and then counted using a hemocytometer and standard light microscope at 40× magnification with a portable imaging cytometer (Bioscope; InGeneron). Non-viable cells were quantified by preparing a 1:1 solution of the cell suspension from each sample in 0.4% Trypan Blue solution. Non-viable cells were counted using a hemocytometer under light microscopy at 40× magnification. Nucleated cell viability was calculated using the following formula: (Nucleated Cells − Dead Cells)/Nucleated Cells × 100.
The remaining cells not used for counting were centrifuged at 600 × g for 10 min and the supernatant removed. The final stromal vascular fraction (SVF) was suspended in 2 mL of LRS and aliquots were mixed with an equal volume of Trizol reagent (TRIzol; Life Technologies/Thermo Fisher Scientific) to lyse cells and preserve the ribonucleic acid (RNA) and then frozen at −80°C until gene expression was determined or cryopreserved in cryopreservation media containing 5% (v/v) dimethyl sulfoxide (DMSO) for later analyses in cell culture (CryoStor CS 5; BioLife Solutions, Bothell, Washington, USA).
Adipose tissue density
The mass of each sample was determined using a digital electronic scale (PB303; Mettler-Toledo, Columbus, Ohio, USA) and the samples were then submerged in a known volume of deionized water at a temperature of 37°C in a graduated cylinder. A stylet from a 20-gauge, 8-in spinal needle was used to submerge the adipose tissue and this was used each time for consistency. The volume of water displaced by the sample was used to determine the density of the sample, using the formula Density = Mass/Volume.
Immunohistochemistry
The fixed adipose tissues were embedded in paraffin and routinely sectioned at ~5 to 8 μm, then mounted on glass slides for IHC factor-VIII staining, which was done manually. A series of degrading alcohol steps was used to dehydrate the sections. The blocking step with 10% goat serum was carried out before incubation with primary antibody. The slides were incubated for 120 min with 1:100 rabbit anti-human polyclonal factor-VIII antibodies (Factor VIII; Dako North America, Carpenteria, California, USA), which have been shown to cross-react with equine tissue. After incubation, slides were rinsed and anti-rabbit mouse immunoglobulin G (IgG) secondary antibody (HK638-UK; BioGenex, Fremont, California, USA) was applied and allowed to incubate for 15 min. Horseradish peroxidase (Life Technologies/Thermo Fisher Scientific) was applied to tissue sections for 15 min. NovaRED peroxidase (Vector Laboratories, Burlingame, California, USA) was applied to the sections for 5 min and the sections were then washed with deionized water and counterstained with hematoxylin for 2 min. The factor VIII-stained adipose tissue was evaluated under light microscopy by 1 evaluator who was blinded (GM). The factor VIII-stained capillaries were manually counted at 40× power in 3 fields of view that were randomly selected.
Quantitative PCR
The relative gene expression of MSC surface markers CD44, CD73, CD90, CD105, CD146, CD166, hematopoietic markers CD34 and CD45, endothelial cell marker CD31, along with genes associated with adipogenic (PPARG2), osteogenic (OSTEOCALCIN) lineages, as well as type-1 collagen (Col 1A2) was measured in total RNA from each adipose source.
For each condition tested, 2 replicates of total RNA were isolated from freshly isolated cells and measured using spectrophotometry (Nanodrop ND-100; Thermo Fisher Scientific, Wilmington, Delaware, USA). First strand complementary deoxyribonucleic acid (cDNA) synthesis (First Strand Kit; Qiagen, Chatsworth, California, USA) was carried out using 0.500 μg of total RNA, according to the manufacturer’s instructions. All individual samples were analyzed in duplicate. Each plate contained both a no-reverse transcriptase (RT) control and no-template control and the gene used as constitutive baseline was β-actin. Quantitative PCR was carried out using a thermal cycler (iCycler; Bio-Rad, Hercules, California, USA) and a complete amplification mix (Green Supermix; Bio-Rad), with primer sequences listed in Appendix A (Sigma Genosys; Sigma-Aldrich, St. Louis, Missouri, USA).
Appendix A.
The primer sequences utilized in this study.
| Gene | Forward 5′ | Reverse 5′ |
|---|---|---|
| CD44 | CGCAGGGGTATTCCATGTG | CCCTTCTATGAACCCGTACCTG |
| CD73 | GGGATTGTTGGATACACTTCAAAAG | GCTGCAACGCAGTGATTTCA |
| CD90 | CATCGCTCTCCTGCTGACA | GGTGTGGCGGTGGTATTCTC |
| CD105 | CGACGCCAATCACAACATAC | GTAGCCAGGGATGTTCCTCTC |
| CD146 | GTGTGGAAATCAGGTGTTTGGC | CATCCGTTGTCTCTTCCTCCATC |
| CD166 | GAACACGATGAGGCAGACGAG | CAACGACGATTCCCACGATG |
| CD34 | GCACAAGTCTGACCTGAGCA | GGAATAGTGCTGGTGGCTCTC |
| CD45 | CTGATGTGATGATTGCTGCTC | GGCTTCCTGGTCTCCACTC |
| CD31 | TCTAGAACGGAAGGCTCCCT | TGGGAGCAGGGCAGGTTCA |
| PPARG2 | GACATCAAGCCCTTCACTACTG | CTGAATAATAAGGTGGGGATGC |
| OSTEO | CAGTGAGGTGGTGAAGAGATTC | CTCACACACCTCCCTCCTG |
| COL2A1 | AGCAGGAATTTGGTGTGGAC | TCTGCCCAGTTCAGGTCTCT |
| β-actin | CCAGCACGATGAAGATCAAG | GTGGACAATGAGGCCAGAAT |
| GAPDH | CCAGAACATCATCCCTGCTT | CGTATTTGGCAGCTTTCTCC |
Colony-forming unit fibroblast assay
The assay for colony-forming unit fibroblasts (CFU-Fs) was adapted from an established method (20). Cells were plated and CFU-Fs were induced after first passage. For each adipose sample, cells from the first passage were plated at 3 cell densities: 250 000 cells, 50 000 cells, and 10 000 cells per 35-mm diameter well and repeated twice for each density. Cells were incubated for 14 d in standard growth media with 10% horse serum. Media were changed twice weekly. The cells were fixed with 4% formalin, stained with hematoxylin for 10 min, washed with phosphate-buffered saline (PBS), and pictures were obtained from 5 randomly chosen sections in each well for counts using a 2.5× objective on a light microscope. Two different experienced investigators, blinded to cell source, counted all visible colonies individually. A colony-forming unit was defined as a cluster containing at least 10 fibroblast-like, fusiform cells. After counting, the density of 10 000 cells per well was selected for further evaluation. The CFU-Fs were expressed as CFU-F% per 1 × 106 adherent cells.
Statistical analysis
Data were entered into a spreadsheet (Microsoft Office Excel; Microsoft, Redmond, Washington, USA) and imported into a statistical software package for analysis (SAS; SAS Institute, Cary, North Carolina, USA). Responses were analyzed using linear-mixed models, with location and age as the fixed effects and horse as the random effect. For variables found to be significant in the model, comparisons among locations were assessed using an F-test, followed by Tukey pairwise comparisons. The qPCR data were normalized to β-actin and quantitated with the delta-delta cycle threshold (CT) method. There were 3 groups for comparison of density and immunohistochemistry (IHC), because the physical material of the lipoaspiration did not permit determination of these variables. The CFU-F data were evaluated with a 1-way analysis of variance (ANOVA) using tissue source as the main effect and a Tukey’s post-hoc test. A P of ≤ 0.05 was considered significant.
Results
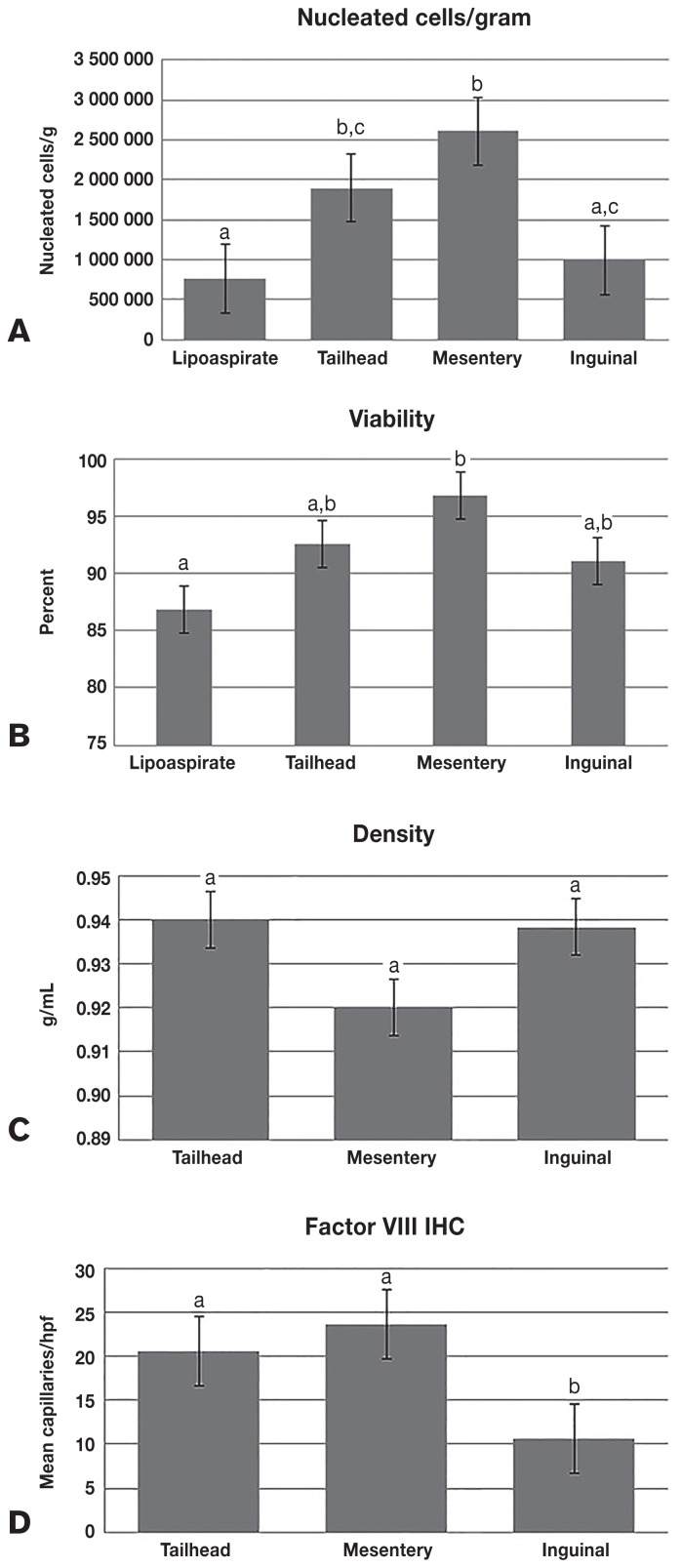
The source of the adipose resulted in significantly different nucleated cells/gram of tissue {P = 0.0001 [mean values ± standard error (SE): lipoaspirate = 761 667 ± 227 348; tailhead = 1 900 000 ± 227 348; mesentery = 2 608 333 ± 227 348; inguinal = 992 333 ± 227 348]} and percent viable [P = 0.0150 (lipoaspirate = 86.8 ± 3.3; tailhead = 92.5 ± 3.3; mesentery = 96.8 ± 3.3, inguinal = 91.1 ± 3.3)], but not for tissue density (g/cm3) [P = 0.0666 (tailhead = 0.94 ± 0.008; mesentery = 0.92 ± 0.008; inguinal = 0.938 ± 0.008)] (Figure 1). The IHC showed a significantly different capillary density (capillaries/hpf) among locations, as shown by factor-VIII staining [P < 0.0001 (tailhead = 20.6 ± 1.56; mesentery = 23.6 ± 1.56; inguinal = 10.6 ± 1.56)] (Figure 1).
Figure 1.
The mean (± SE) yield of nucleated cells/gram of tissue (A) and percent viability (B) for lipoaspirate, tailhead, inguinal, and mesenteric adipose. The density (C) and factor-VIII immunohistochemistry (IHC) (D) were obtained for tailhead, inguinal, and mesenteric adipose only because the lipoaspiration from the tailhead was represented by the solid sample from the tailhead and lipoaspiration was not physically conducive to obtaining these data. Columns with different superscript letters denote a significant difference.
For the qPCR outcome data, adipose source was significantly related to differences found for CD 44 (P = 0.0095), CD 45 (P = 0.0006), CD 73 (P ≤ 0.0001), CD 90 (P ≤ 0.0001), CD 105 (P = 0.007), CD 146 (P = 0.0041), Col1A2 (P ≤ 0.0001), and CD 34 (P = 0.0067) (Table I). Osteocalcin was the only outcome variable significantly related to age (P = 0.048). The mean osteocalcin expression of the 15-year-old horse was 10.2 compared to 11.5 for all the remaining samples.
Table I.
The relative expression of each gene for the stromal vascular fraction (SVF) from each of the 4 sources
| Gene | Lipoaspiration | Tailhead | Mesentery | Inguinal | P-value |
|---|---|---|---|---|---|
| CD44 | 4.7a | 3.66b,c | 4.06a,c | 3.78b,c | 0.0095 |
| CD73 | 7.02a | 5.75b | 5.21b | 5.05b | < 0.0001 |
| CD90 | 4.35a | 2.87b,c | 3.14b | 2.25c | < 0.0001 |
| CD105 | 4.16a | 4.32a | 4.89b,c | 4.61a,c | 0.007 |
| CD146 | 4.5a | 4.85a | 5.79b,c | 5.09a,c | 0.0041 |
| CD166 | 6.26 | 6.26 | 6.23 | 5.75 | 0.423 |
| CD34 | 2.97a | 2.91a | 3.40a,b | 2.38a,c | 0.0067 |
| CD45 | 6.65a | 5.93a | 5.06b | 5.58a | 0.0006 |
| CD31 | 2.05 | 2.29 | 2.88 | 2.08 | 0.1764 |
| PPARG2 | 4.92 | 4.48 | 5.17 | 4.36 | 0.1934 |
| OSTEO | 11.11 | 11.73 | 11.29 | 10.78 | 0.4789 |
| Col 1A2 | 2.07a | 0.57b | 3.7c | 2.12a | < 0.0001 |
The qPCR data were normalized to β-actin and quantitated with the delta-delta cycle threshold (CT) method. As the data in the table are the expression of the gene relative to β-actin, for the CD44 lipoaspirate sample, the expression was 4.7 times that of the housekeeping gene β-actin.
P-value in each row shows that the significance for the linear-mixed model and of the values with different superscript letters in the same row are significantly different based on Tukey pairwise comparison. Significance was set at P ≤ 0.05. The superscript letters denote significance.
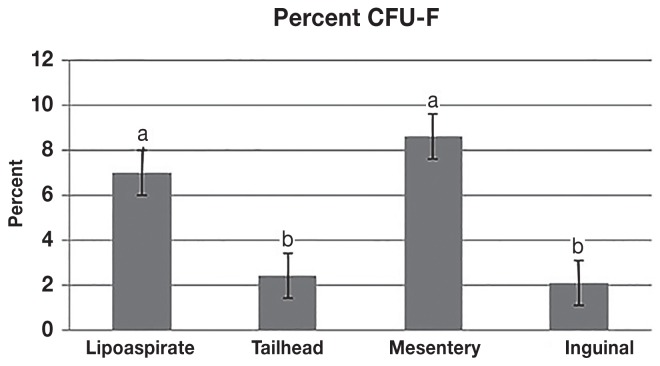
The CFU-F assay also resulted in significantly different frequencies of CFU-F formation among the various adipose sources. The mean (± SD) CFU-F% for the liposuction was 7.0% (± 0.11) and for the mesentery was 8.59% (± 0.11), both of which were significantly higher than the excisional adipose from the tailhead (2.42% ± 0.04) and inguinal (2.11% ± 0.06) locations (Figure 2).
Figure 2.
The mean (± SD) for the colony-forming unit fibroblasts (CFU-Fs) from each source. The data are presented as CFU-F% per 1 × 106 adherent cells. Cells collected from the mesentery and by lipoaspiration had a 3 to 4 times greater amount of CFU-Fs than cells isolated from the tailhead and inguinal adipose depots. Columns with different superscript letters denote a significant difference.
A subjective finding not included as an outcome parameter but identified during the study was that adipose obtained by liposuction and from the mesentery could easily be filtered after enzymatic processing, whereas excisional adipose from the tailhead occasionally required a second filter because of clogging from fibrous tissue. All the inguinal samples required multiple filters, which suggests a denser connective tissue matrix.
Discussion
The data obtained in this study confirmed our hypothesis that the more vascular, dense adipose tissue would yield a higher concentration of nucleated cells. We found that there are significant differences among locations for nucleated cell yield per gram of adipose, viability, gene expression, and CFU-F%. We also found that fewer capillaries, as identified by factor-VIII IHC in the inguinal adipose, corresponded to the lowest yield of nucleated cells/gram, which is similar to results of previous studies (16).
The mesenteric adipose tissue provided the highest nucleated cells/gram of tissue and viability, which corresponds with the IHC vascular density findings among the tissue sites. The nucleated cells/gram in the excisional adipose from the tailhead were statistically similar to those in the mesentery and were significantly higher in both sites than in the lipoaspirate from the tailhead. The viability and yield of the lipoaspirated adipose tissue from the tailhead were the lowest of the 4 groups. Physical destruction during the lipoaspiration process can contribute to lower recovery and viability. It is well-established for human subcutaneous adipose tissue that harvest method can affect cell yield (21). The lower yield associated with mechanical trituration of adipose tissue caused by lipoaspiration has been previously described (18) and was similarly seen in this study.
We chose not to use tumescent fluid in this study because its analgesic properties were unnecessary, as the tissues were harvested from euthanized horses and we could accurately determine nucleated cells/gram of tissue. This could cause decreased yield and viability, however, as the tumescent fluid acts as a lubricant and buffer from physical damage during the aspiration process and speeds up the harvest of the adipose, which decreases the tissue damage created by the motion of the cannula. In a study comparing adipose collection techniques in humans, however, liposuction with tumescent fluid and adipose collected by resection were not significantly different in terms of viability and cellular yield of MSCs, while doubling times were significantly longer with ultrasound-assisted liposuction (22).
Tissue density was determined because it was initially thought that adipose with a greater percentage of adipose vacuoles and therefore a lower density would contain less vasculature and fewer nucleated cells. We found the opposite in that the tissue with the higher density had lower nucleated cells/gram because it was higher in dense fibrous connective tissue. Although the differences in densities were not significant, the mesenteric adipose tissue was much more easily digested and had less fibrous tissue. The lipoaspirated adipose tissue from the tailhead behaved similarly to the mesenteric adipose tissue during digestion and filtration. There was more connective tissue within the inguinal adipose during its harvest, mincing, and filtering. This was also evident histologically and corresponded with the higher density of the inguinal tissue. It is intuitive to think that the inguinal adipose tissue generated lower nucleated cells/gram due to the fibrous connective tissue, which would lower cellular yield.
It is known that most of the MSCs in bone marrow are in the initial segment of the collection and a small percentage of nucleated cells are MSCs (15,23). An advantage of any of the adipose sources is that there can be large deposits with a consistent yield of cells/gram of tissue that do not decline like bone marrow sources. In order to gain more MSCs from adipose, more adipose can be harvested and processed. The results of this study showed a similar cell yield for the lipoaspirate from the tailhead (7.65 × 105) as in a previous study (7.9 × 105) (18). The previous study demonstrated a 23-times increase in adherent cells and 50 times as many CFU-Fs compared to an equal number of nucleated cells derived from bone marrow.
The value of adipose-derived stromal vascular fraction (SVF) for regenerative medicine is emphasizing the need to further characterize the SVF. It is recognized that there are changes in surface markers between the SFV and cultured adipose-derived stem cells (24). The data presented here are the first data obtained from equine adipose and provide information about surface markers on the equine SVF that can be used in future studies. Differences in gene expression were found among the sources. The SVF from all tissue sources expressed CD73, CD90, and CD105 cell surface markers, which according to the International Society for Cellular Therapy, denotes the presence of MSCs (25). Although there is no specific surface marker group for equine stem cells, in those used for this study, the lipoaspirate sample showed the significantly highest expression for CD44, CD73, and CD90. This indicates that, despite somewhat lower cell yields, there may be a higher percentage of MSCs in the SVF obtained by lipoaspiration. The CFU-F data also demonstrated that there was a higher percentage of CFU-Fs in the lipoaspirate and the mesenteric samples.
Since these cells are from the SVF with multiple cell types present, we expected expression of hematopoietic markers (25). The hematopoietic markers were highest in the tailhead (CD45) and the mesentery (CD34) and lower in the inguinal region, similar to the IHC factor-VIII staining for capillaries. The niche concept, where stem cells are located close to the vasculature, is consistent with our findings (26,27). As the perivascular environment may provide the conditions that maintain the population of undifferentiated MSCs, the SVFs with the maximal MSC yield are likely to contain numerous cells positive for CD34 and CD45.
It is difficult to compare our results for gene expression with other studies of equine SVF because of the variation in isolation methods and cellular surface markers being studied. Some of the many types of cells present in the SVF can also stimulate healing, similar to MSCs. The stromal vascular fraction contains fibroblasts, endothelial cells, leukocytes, and very small embryonic-like cells with additional cytokine production and healing capabilities that are not found in culture-expanded stem cells (28–30). In addition to the cells being transplanted when using the SVF, a recent study showed the ability of the SVF to induce or enhance MSC migration and proliferation in vitro (14). It was speculated that mediators released from the SVF resulted in the marked migration of the MSCs in the culture.
The objective of this study was to make a relative comparison of adipose depots for harvesting SVF in the horse using qPCR to determine the relative gene expression. While immunophenotyping of cells by flow cytometry could be complementary to the data obtained in this study, quantitative PCR was used in this study for a number of reasons. In studies where both methods have been used, outcomes have been similar (31,32). As there is no uniformly accepted definitive phenotype or surface marker for isolating MSCs from uncultured samples, they have been phenotypically identified retrospectively (33). Cell surface markers change with culture and at this time we do not have a composite phenotype of freshly isolated MSCs or a single marker that solely identifies native MSCs (34). In 1 study in which fresh bone marrow aspirate was followed over time, gene expression followed the same pattern as cellular protein expression at each time point (31). There are relatively few antibodies that have been validated in the horse and the relative expression of qPCR data has provided a viable comparison when antibodies have failed to bind equine cells (20,35).
Quantitative PCR has been used previously for cell surface markers of MSCs from multiple equine tissues (32,35–37). The expression of the surface markers CD90 and CD105 as characteristic of equine MSCs has also been determined by qPCR (37). In the heterogenous population of the SVF, the relative expression of the genes evaluated provides information about differences in the cell populations at the different locations. Because of potential issues with cellular debris and the multiple cell types present in fresh SVF, the question of the validity for multiple antibodies in the horse, and the lack of previous data using flow cytometry in fresh SVF of the horse, qPCR provided the most valuable information for this study.
The CFU-F is the standard for defining the number of progenitor cells in the SVF (24). The frequency of CFU-F from human adipose ranges from 1% to 10%. Our data from the horse fall into this range, with inguinal SVF at 2.11% and mesenteric SVF at 8.9%. The SVF from the mesentery and lipoaspirate was statistically similar and both were higher than that from the solid adipose from the tailhead and inguinal region. The mesenteric adipose had the highest cell yield, viability, and factor-VIII staining and the lowest density of CFU-Fs.
We selected locations that would represent abundant adipose depots and flexibility in harvesting during different recumbencies. The mesentery was included as a harvest site because many athletic horses have limited subcutaneous deposits. Furthermore, the other adipose tissues were of similar subcutaneous origin and the abdomen represented a source that may be expected to be different based on location and gross appearance. Mesenteric adipose can be harvested with the horse in dorsal recumbency during a surgical procedure when it is elected to obtain a SVF or it can be obtained in other horses with limited invasiveness by laparoscopy.
This study was designed to compare autogenous cell yield from different locations within a horse to determine if there is an optimum source. We did not select horses based on age, but there was a horse that was 15 y old in the study. No relationship was found between cell yield and age. It has been shown that age can affect the number of MSCs obtained from bone marrow, as the ilium is found to deplete with age in equids, which makes it in an unreliable source of bone-marrow collection in older horses. In humans, the CFU-F numbers also significantly decrease in older age groups (38,39).
A potential limitation of this study is that the multipotency of the mesenchymal stem cells (MSCs) present in the stromal vascular fraction (SVF) was not evaluated. As previous studies verified the multipotency of adipose-derived MSCs (18), however, it was not pursued in this study. Additionally, a larger group of horses with a more extensive age range could allow the effect of age on MSC yield to be further evaluated.
In this study, significant differences were found in viability, cellular yield, vascular density, gene expression, and CFU-F among adipose tissue depots in the horse. The clinical application of this information indicates that there are multiple sources of adipose in horses that can be used to collect SVF. Further work is needed to determine whether the differences among adipose sources observed in this study are clinically relevant for in-vivo treatment of musculoskeletal injuries in horses.
Acknowledgments
The authors thank Lisa Leake, Dr. Michael Coleman, and Maria P. Almario for their technical support. This study was supported in kind by Ingeneron, Inc.
Footnotes
Conflict of interest
Rudy Martinez, who completed the polymerase chain reaction (PCR) and colony-forming unit fibroblast (CFU-F) assay for this study, is employed by InGeneron. The authors who completed the remainder of the study and compiled the data have no financial interests or holdings in InGeneron.
References
- 1.Dyson SJ. Medical management of superficial digital flexor tendonitis: A comparative study in 219 horses (1992–2000) Equine Vet J. 2004;36:415–419. doi: 10.2746/0425164044868422. [DOI] [PubMed] [Google Scholar]
- 2.Avella CS, Ely ER, Verheyen KL, Price JS, Wood JL, Smith RK. Ultrasonographic assessment of the superficial digital flexor tendons of National Hunt racehorses in training over two racing seasons. Equine Vet J. 2009;41:449–454. doi: 10.2746/042516409x391042. [DOI] [PubMed] [Google Scholar]
- 3.Ross MW, Genovese RL, Dyson SJ, Jorgensen JS. Superficial digital flexor tendonitis. In: Ross MW, Dyson SJ, editors. Diagnosis and Management of Lameness in the Horse. 2nd ed. St Louis, Missouri: Saunders Elsevier; 2011. pp. 706–726. [Google Scholar]
- 4.Pacini S, Spinabella S, Trombi L, et al. Suspension of bone marrow-derived undifferentiated mesenchymal stromal cells for repair of superficial digital flexor tendon in race horses. Tissue Eng. 2007;13:2949–2955. doi: 10.1089/ten.2007.0108. [DOI] [PubMed] [Google Scholar]
- 5.Smith RK. Mesenchymal stem cell therapy for equine tendinopathy. Disabil Rehabil. 2008;30:1752–1758. doi: 10.1080/09638280701788241. [DOI] [PubMed] [Google Scholar]
- 6.Godwin EE, Young NJ, Dudhia J, Beamish IC, Smith RK. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J. 2012;44:25–32. doi: 10.1111/j.2042-3306.2011.00363.x. [DOI] [PubMed] [Google Scholar]
- 7.Renzi S, Ricco S, Dotti S, et al. Autologous bone marrow mesenchymal stromal cells for regeneration of injured equine ligaments and tendons: A clinical report. Res Vet Sci. 2013;95:272–277. doi: 10.1016/j.rvsc.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Riordan NH, Ichim TE, Min WP, et al. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med. 2009;7:1–9. doi: 10.1186/1479-5876-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gentile P, Orlandi A, Scioli MG, Di Pasquali C, Bocchini I, Cervelli V. Concise review: Adipose-derived stromal vascular fraction cells and platelet-rich plasma: Basic and clinical implications for tissue engineering therapies in regenerative surgery. Stem Cells Transl Med. 2012;1:230–236. doi: 10.5966/sctm.2011-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strem BM, Hicok KC, Zhu M, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–141. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 12.Frisbie DD, Smith RKW. Clinical update on the use of mesenchymal stem cells in equine orthopaedics. Equine Vet J. 2010;42:86–89. doi: 10.2746/042516409X477263. [DOI] [PubMed] [Google Scholar]
- 13.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2010;21:2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 14.Kol A, Walker NJ, Galuppo LD, et al. Autologous point-of-care cellular therapies variably induce equine mesenchymal stem cell migration, proliferation and cytokine expression. Equine Vet J. 2013;45:193–198. doi: 10.1111/j.2042-3306.2012.00600.x. [DOI] [PubMed] [Google Scholar]
- 15.Adams MK, Goodrich LR, Rao S, et al. Equine bone marrow-derived mesenchymal stromal cells (BMDMSCs) from the ilium and sternum: Are there differences? Equine Vet J. 2012;45:372–375. doi: 10.1111/j.2042-3306.2012.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meirelles LS, Sand TT, Harman RJ, Lennon RJ, Caplan A. MSC frequency correlates with blood vessel density in equine adipose tissue. Tissue Eng Part A. 2009;15:221–229. doi: 10.1089/ten.tea.2008.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burk J, Ribitsch I, Gittel C, et al. Growth and differentiation characteristics of equine mesenchymal stromal cells derived from different sources. Vet J. 2013;195:98–106. doi: 10.1016/j.tvjl.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Bruno I, Martinez R, Sanchez A, Friddle C, McClure SR. Characterization of nucleated cells from equine adipose tissue and bone marrow aspirate processed for point-of-care use. J Equine Vet Sci. 2014;34:1118–1127. [Google Scholar]
- 19.Russo V, Yu C, Belliveau P, Hamilton A, Flynn LE. Comparison of human adipose-derived stem cells isolated from subcutaneous, omental, and intrathoracic adipose tissue depots for regenerative applications. Stem Cells Transl Med. 2014;3:206–217. doi: 10.5966/sctm.2013-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun J, Hack A, Weis-Klemm M, et al. Evaluation of the osteogenic and chondrogenic differentiation capacities of equine adipose tissue-derived mesenchymal stem cells. Am J Vet Res. 2010;71:1228–1236. doi: 10.2460/ajvr.71.10.1228. [DOI] [PubMed] [Google Scholar]
- 21.Banyard D, Salibian A, Widgerow AD, Evans GRD. Implications for human adipose-derived stem cells in plastic surgery. J Cell Mol Med. 2015;19:21–30. doi: 10.1111/jcmm.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M, et al. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy. 2006;8:166–177. doi: 10.1080/14653240600621125. [DOI] [PubMed] [Google Scholar]
- 23.Caplan AL. The mesengenic process. Clin Plast Surg. 1994;21:429–435. [PubMed] [Google Scholar]
- 24.Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics (IFATS) and Science and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dominic M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stem cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 26.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: Regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart MC, Steward AA. Mesenchymal stem cells: Characteristics, sources, and mechanisms of action. Vet Clin North Am Equine Pract. 2011;27:243–261. doi: 10.1016/j.cveq.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Xu H, Barns GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ratajczak MZ, Zuba-Surma EK, Wysoczynski M, Ratajczak J, Kucia M. Very small embryonic-like stem cells: Characterization, developmental origin, and biological significance. Exp Hematol. 2008;36:742–751. doi: 10.1016/j.exphem.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radcliffe CH, Flaminio M, Fortier LA. Temporal analysis of equine bone marrow aspirate during establishment of putative mesenchymal progenitor cell populations. Stem Cells Dev. 2010;19:269–282. doi: 10.1089/scd.2009.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohanty N, Gulati BR, Kumar R, et al. Immunophenotypic characterization and tenogenic differentiation of mesenchymal stromal cells isolated from equine umbilical cord blood. In Vitro Cell Dev Biol Anim. 2014;50:538–548. doi: 10.1007/s11626-013-9729-7. [DOI] [PubMed] [Google Scholar]
- 33.Liu Z, Zhuge Y, Velazquez OC. Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem. 2009;106:984–991. doi: 10.1002/jcb.22091. [DOI] [PubMed] [Google Scholar]
- 34.Baer PC. Adipose-derived mesenchymal stromal/stem cells: An update on their phenotype in vivo and in vitro. World J Stem Cells. 2015;6:256–265. doi: 10.4252/wjsc.v6.i3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ranera B, Ordovás L, Lyahyai J, et al. Comparative study of equine bone marrow and adipose tissue-derived mesenchymal stromal cells. Equine Vet J. 2012;44:33–42. doi: 10.1111/j.2042-3306.2010.00353.x. [DOI] [PubMed] [Google Scholar]
- 36.Lange-Consiglio A, Corradetti B, Rutigliano L, Cremonesi F, Bizzaro D. In vitro studies of horse umbilical cord matrix-derived cells: From characterization to labeling for magnetic resonance imaging. Open Tissue Eng Regen Med J. 2011;4:120–133. [Google Scholar]
- 37.Raabe O, Shell K, Würtz A, Reich CM, Wenisch S, Arnhold S. Further insights into the characterization of equine adipose tissue-derived mesenchymal stem cells. Vet Res Commun. 2011;35:355–365. doi: 10.1007/s11259-011-9480-z. [DOI] [PubMed] [Google Scholar]
- 38.Stolzing J, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Delling U, Lindner K, Ribitsch I, Jülke H, Brehm W. Comparison of bone marrow aspiration at the sternum and the tuber coxae in middle-aged horses. Can J Vet Res. 2012;76:52–56. [PMC free article] [PubMed] [Google Scholar]