Abstract
Objectives:
Long-acting bronchodilators are mainstay treatment for moderate to severe chronic obstructive pulmonary disease. A growing body of evidence indicates an increased risk of cardiovascular events upon initiation of these medications. We hypothesize that this risk is higher in patients with chronic obstructive pulmonary disease who had a preexisting cardiovascular disease regardless of receipt of any cardiovascular medication.
Methods:
A retrospective cohort of patients with a diagnosis of chronic obstructive pulmonary disease based on two outpatient visits or one inpatient visit for chronic obstructive pulmonary disease (International Classification of Diseases, 9th Edition, Clinical Modification codes 491.x, 492.x, 496) in any year between 2001 and 2012 from a commercial insurance database. We then selected those initiating long-acting bronchodilator treatments between April 2001 and September 2012. Each patient had a 1 year look back period to determine history of cardiovascular disease or cardiovascular disease treatment from the time of first prescription of long-acting beta agonist, long-acting muscarinic antagonist, or long-acting beta agonist combined with inhaled corticosteroids. Patients were followed for 90 days for hospitalizations or emergency department visits for cardiovascular event. The cohort was divided into four groups based on the presence of cardiovascular disease (including ischemic heart disease, hypertension, ischemic stroke, heart failure, tachyarrhythmias and artery disease based on International Classification of Diseases, 9th Edition, Clinical Modification codes) and cardiovascular disease treatment defined as acetylsalicylic acid, beta blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, antiplatelet, anticoagulants, calcium channel blockers, nitrate, digoxin, diuretics, antiarrhythmics or statins. Odds of emergency department visit or hospitalization in the 90 days after prescription were examined using multivariable logistic regression models.
Results:
Of 61,651 eligible patients, 36,755 (59.6%) had cardiovascular disease and were on cardiovascular disease treatment (Group 1), 7250 (11.8%) had cardiovascular disease without cardiovascular disease treatment (Group 2), 4715 (7.7%) had no cardiovascular disease but had cardiovascular disease treatment (Group 3) and 12,931 (21%) had no cardiovascular disease and no treatment (Group 4). In these four groups, the unadjusted risk of emergency department visit or hospitalization for cardiovascular disease within 90 days of initiation was 5.45%, 2.95%, 1.55% and 0.96%, respectively. In multivariable analysis, the adjusted odds ratio with 95% confidence interval of emergency department visit/hospitalization for each of the first three groups to those with no cardiovascular disease and no treatment were 3.50 (95% confidence interval, 2.89–4.24), 2.15 (95% confidence interval, 1.71–2.70) and 1.36 (95% confidence interval, 1.01–1.82), respectively.
Conclusion:
The risk of cardiovascular events after initiation of long-acting bronchodilators is highest in patients with baseline cardiovascular disease and on cardiovascular disease medications. Clinicians should be cautious while prescribing these medications in patients with preexisting cardiovascular disease.
Keywords: Chronic obstructive lung disease, long-acting bronchodilators, cardiovascular disease, long-acting beta agonist, long-acting muscarinic antagonist, long-acting beta agonist-inhaled corticosteroids
Introduction
Chronic obstructive pulmonary disease (COPD) is a systematic illness characterized by generalized inflammation that affects multiple organ systems.1,2 Cardiovascular disease (CVD) shares smoking as an important risk factor with COPD and is the most common comorbidity observed in patients with COPD.3 This association carries a poorer prognosis for both conditions.4,5 Studies have shown a variable association between COPD and ischemic heart disease,6 heart failure,7–9 tachyarrhythmia and stroke. Furthermore, both cardiovascular medications and treatments for COPD affect the other conditions differentially, with possible protective effects of angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) for patients with COPD, decreased exacerbation for patient with COPD and CVD on statins10,11 and a modest increase in cardiovascular risk of long-acting bronchodilators (LABDs), especially long-acting beta agonists (LABAs).
LABDs, including long-acting muscarinic antagonists (LAMA), LABAs, combined long-acting beta agonists-inhaled corticosteroids (LABA-ICS) and combined long-acting beta agonists-long-acting muscarinic antagonists, are the cornerstone of the pharmacological management of patients with Global Initiative for Chronic Obstructive Lung disease ( GOLD) classification B, C and D COPD or those with forced expiratory volume in the first second (FEV-1) of less than 50% predicted. Clinical trials conducted in support of the regulatory review and approval of these medications,12,13 observational studies and meta-analyses have shown the efficacy of these medications in improving lung function,14 quality of life15 and exercise tolerance,16,17 as well as in reducing exacerbations18 in moderate to severe COPD, with no effect on mortality.
Observational studies and meta-analyses have reported cardiovascular risks associated with the use of LABDs,19–22 although randomized controlled trials (RCTs) failed to show an increased risk.12,13,23 This discrepancy could be due to selection bias, as these RCTs recruited volunteers who were healthier with fewer comorbidities than patients in other studies and they are unlikely to participate in such trials if they had a prior adverse reaction to these medications. They also had better access to health care services compared to the general population, allowing early adverse reactions to be treated before they develop into events. Furthermore, theses RCTs were not powered enough to detect adverse outcomes and late adverse events that happened after the study period may not have been reported. Comparative studies failed to show any difference in risk between LAMA, LABA and LABA-ICS as well.24–26 In this study, we hypothesize that the cardiovascular risk of LABDs increases in the presence of CVDs at the time of initiation of LABD regardless of treatment with cardiovascular medications.
Methods
We conducted a population-based, retrospective cohort study using a national private insurance company database, Clinformatics™ Data Mart (CDM), a product of Optum, Inc. (Eden Prairie, MN) that covers 53 million Americans nationwide. The data in CDM come from paid claims, which include the diagnoses, medical services, drug prescriptions, cost as well as the date and place associated with the services that enrollees received. CDM consists of (1) administrative data which include the demographics and enrollment information of the enrollees, (2) pharmacy claims data, (3) physician and facility claims data, (4) lab test results data, (5) inpatient confinement data and (6) standard price data. Enrollees in CDM are largely representative of working adults in the United States, with slightly higher representation in the South and Midwest. Due to the nature of private insurance programs, that is, people drop out when they switch to employers that purchase insurance plans from other companies, 64% of the CDM enrollees have at least 12 months of continuous enrollment and 38% have at least 24 months. We selected COPD patients aged 40 years or older who initiated LABD treatment in the period between April 2001 and September 2012. The study was approved by University of Texas Medical Branch Institutional Review Board (IRB). No written informed consent was needed due to the nature of the study.
Cohort selection
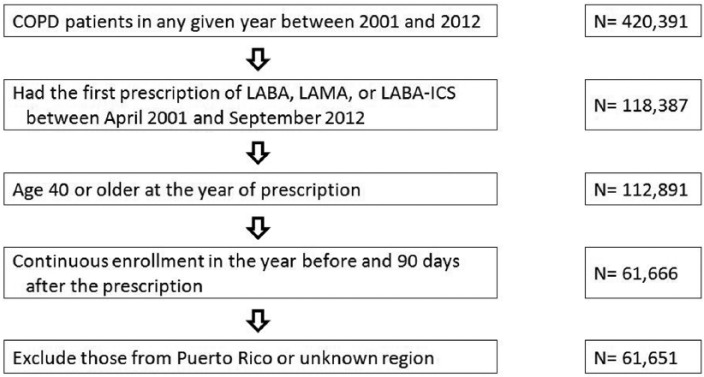
We included patients with COPD based on two outpatient visits or one inpatient visit for COPD, identified by International Classification of Diseases, 9th Edition, Clinical Modification (ICD-9-CM) codes 491.x, 492.x and 496, in any calendar year between 2001 and 2012 (N = 420,391). We identified outpatient visits by Current Procedural Terminology (CPT) codes 99201-99205 and 99211-99215 and inpatient visits by CPT codes 99221-99223, 99231-99233 and 99238-99239. We then selected those who had the first prescription of LABA, LAMA or LABA-ICS between April 2001 and September 2012 (N = 118,387). The first prescription must have been in the same year of COPD diagnosis or a later year. We excluded patients younger than 40 at the first prescription (N = 5496). Finally, we selected those who had continuous enrollment in the prior year and post 90 days of the first prescription, leaving 61,651 patients in the cohort (Figure 1).
Figure 1.
Cohort selection.
Cohort subgroups
Each patient had a 1 year look back period to determine history of CVD or CVD treatment from the time of first prescription of LABA, LAMA or LABA-ICS which was considered the index date. CVD was defined as ischemic heart disease (410.x, 411.x, 412.x, 413.x and 414.x), hypertension (401.x, 402.x, 403.x, 404.x and 405.x), ischemic stroke (433.x, 434.x, 435.x, 436.x, 437.x and 438.x), heart failure (428.x), tachyarrhythmia (427.x) and atherosclerotic arterial disease (440.x, 441.x, 442.x, 443.2x, 443.9 and 444.x) per ICD-9-CM codes. CVD treatment was defined as a prescription for acetylsalicylic acid (ASA), beta blockers (BBs), ACE inhibitors, ARB, antiplatelet, anticoagulants, calcium channel blockers, nitrate, digoxin, diuretics, antiarrhythmics or statins.
Patients were divided into four groups depending on the presence of CVD disease or CVD treatment: Group 1: with CVD and on CVD treatment; Group 2: with CVD but no CVD treatment; Group 3: no CVD disease but on CVD treatment; and Group 4: no CVD disease and no CVD treatment.
Exposure
New initiation of LABDs.
Study outcome
The primary outcome was a hospitalization or emergency department (ED) visit within 3 months from the index date for cardiovascular events including acute coronary syndrome (ACS) (ICD-9-CM codes 410.x, 411.x, and 413.x), ischemic stroke (ICD-9-CM codes 433.x, 434.x, 435.x, and 436.x), heart failure (ICD-9-CM code 428.x) and tachyarrhythmia (ICD-9-CM code 427.x).
Baseline characteristics
Patient demographic characteristics (age, gender and region) were obtained from the Clinformatics™ Data Mart member file. The history of short-acting COPD medication, including steroids, roflumilast, theophylline, short-acting beta agonists (SABAs), short-acting muscarinic antagonists (SAMAs) or SABA-SAMA combination, was examined using each patient’s prescriptions in the prior year. Spirometry in the prior year was identified by claims with CPT codes 94010, 94014, 94015, 94016, 94060, 94070 and 94620. Similarly, we identified oxygen use by Healthcare Common Procedure Coding System (HCPCS) codes E1390, E1391 and E1392; influenza vaccine by CPT codes 90656 and 90658, HCPCS codes G0008, G8482, G8483 and G8484 and ICD-9-CM code V04.81; pneumococcal vaccine by CPT code 90732, HCPCS codes G0009 and G8864 and ICD-9-CM codes V03.82 and V06.6. Comorbidities included hyperlipidemia, osteoporosis, diabetes mellitus, liver disease, renal failure, paralysis, neurologic disorder, hypothyroidism, peptic ulcer disease, HIV/AIDS, lymphoma, tumor without metastasis, metastatic cancer, rheumatoid arthritis, obesity, weight loss, alcohol abuse, drug abuse, psychosis and depression. Hyperlipidemia was identified by ICD-9-CM codes 272.0-272.4 and osteoporosis by ICD-9-CM code 733.0. All other comorbidities were determined following an algorithm described by Quan et al.27
Statistical analysis
Patient baseline characteristics in all four groups were summarized for the period 2001–2012 using count and percentage of categorical variables. We calculated the proportions of patients having any heart failure, tachyarrhythmia, stroke, ACS or any of these events in each CVD group. When computing the event type-specific rate, the grouping was different by the event of interest. The medications specific to each event of interest are listed under Table 2. The proportions of patients having any aforementioned outcome events across the three LABD user groups were also calculated. The chi-square test was used to detect any difference in the risk of having the outcome event across these three groups. A multivariable logistic regression model analysis was used to assess the risk of ED visit or hospitalization within 90 days of initiation of LABDs in the four groups, adjusting for patients’ baseline characteristics and comorbidities. We used Group 4 (no CVD and no CVD treatment) as the reference group. All analyses were performed using SAS version 9.3 (SAS Inc., Cary, NC). All reported p-values were two-sided with p < 0.05 considered statistically significant.
Table 2.
Absolute risk of CV event-related hospitalization and/or ED visit within 90 days of initiation of long-acting bronchodilators.
| Cause for hospitalization and/or ED visit | Total |
|||
|---|---|---|---|---|
| N (%) with any event | ||||
| Group 1 (CVD and CVD treatment) | Group 2 (CVD with no CVD treatment) | Group 3 (no CVD but CVD treatment) | Group 4 (no CVD and no CVD treatment) | |
| Heart failure | 32,227 | 11,778 | 2402 | 15,244 |
| 994 (3.08) | 137 (1.16) | 14 (0.58) | 38 (0.25) | |
| Tachyarrhythmia | 23,464 | 20,541 | 1135 | 16,511 |
| 564 (2.40) | 206 (1.00) | 7 (0.62) | 67 (0.41) | |
| Stroke | 21,605 | 22,400 | 2803 | 14,843 |
| 140 (0.65) | 95 (0.42) | 3 (0.11) | 22 (0.15) | |
| Acute coronary syndrome | 25,606 | 18,399 | 3128 | 14,518 |
| 328 (1.28) | 116 (0.63) | 14 (0.45) | 49 (0.34) | |
| Any of the above | 36,755 | 7250 | 4715 | 12,931 |
| 2003 (5.45) | 214 (2.95) | 73 (1.55) | 124 (0.96) | |
CV: cardiovascular; ED: emergency department; CVD: cardiovascular disease; ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blocker; CVD treatment: cardiovascular disease treatment with medications specific to the event of interest as listed below.
Heart failure: ACE inhibitors, ARBs, beta blockers, diuretics, nitrates, digoxin.
Tachyarrhythmia: antiarrhythmics, beta blockers, calcium channel blockers.
Stroke: statins, antiplatelet agents, anticoagulants.
Acute coronary syndrome: statins, beta blockers, antiplatelet agents.
Any CVD: ACE inhibitors, ARBs, acetylsalicylic acid, antiarrhythmics, anticoagulants, antiplatelet agents, beta blockers, calcium channel blockers, digoxin, diuretics, nitrates, statins.
Results
Subject characteristics
A total of 61,651 patients with COPD met the inclusion criteria. Of these, 36,755 (59.6%) had CVD and were on treatment (Group 1), 7250 (11.8%) had CVD but were not on treatment (Group 2), 4715 (7.6%) were on cardiovascular medication but had no prior history of CVD (Group 3) and 12,931 (21%) had neither CVD nor were on any cardiovascular medication in the year prior to new prescription of LABD (Group 4).
Table 1 presents the baseline demographics of these four groups. Patients in Group 1 were older, more likely to be male, with higher percent of patients with a spirometry and on oxygen therapy compared to other groups. The overall use of short-acting bronchodilators was low in Group 2 compared to all other groups. Up to one-third of patients had a prescription for oral steroids in the preceding year. As expected, patients in Groups 1 and 2 had a higher comorbidity burden compared to those in Groups 3 and 4. The most commonly prescribed COPD medication was LABA-ICS combination followed by LAMA.
Table 1.
Baseline characteristics of patients with COPD with preexisting cardiovascular disease and/or cardiovascular medication from 2001 to 2011.
| Patient characteristics | N (column %) |
|||
|---|---|---|---|---|
| Group 1 (CVD and CVD treatment) | Group 2 (CVD with no CVD treatment) | Group 3 (no CVD but CVD treatment) | Group 4 (no CVD and no CVD treatment) | |
| Overall | 36,755 (100) | 7250 (100) | 4715 (100) | 12,931 (100) |
| Age | ||||
| 40–49 | 2546 (6.9) | 889 (12.3) | 660 (14.0) | 2968 (23.0) |
| 50–59 | 9703 (26.4) | 2134 (29.4) | 1654 (35.1) | 5086 (39.3) |
| 60–69 | 13,082 (35.6) | 2278 (31.4) | 1630 (34.6) | 3613 (27.9) |
| Above 70 | 11,424 (31.1) | 1949 (26.9) | 771 (16.4) | 1264 (9.8) |
| Gender | ||||
| Female | 17,863 (48.6) | 3571 (49.3) | 2832 (60.1) | 6935 (53.6) |
| Male | 18,892 (51.4) | 3679 (50.7) | 1883 (39.9) | 5996 (46.4) |
| First long-acting bronchodilators | ||||
| LABA | 3260 (8.9) | 703 (9.7) | 434 (9.2) | 1117 (8.6) |
| LABA-ICS | 22,468 (61.1) | 4413 (60.9) | 3013 (63.9) | 8467 (65.5) |
| LAMA | 11,027 (30.0) | 2134 (29.4) | 1268 (26.9) | 3347 (25.9) |
| Cardiovascular diseasea | ||||
| Ischemic heart disease | 15,349 (41.8) | 1934 (26.7) | 0 (0) | 0 (0) |
| Hypertension | 32,834 (89.3) | 4722 (65.1) | 0 (0) | 0 (0) |
| Cerebrovascular disease | 6027 (16.4) | 920 (12.7) | 0 (0) | 0 (0) |
| Arrhythmia | 9636 (26.2) | 1553 (21.4) | 0 (0) | 0 (0) |
| Heart failure | 9333 (25.4) | 1121 (15.5) | 0 (0) | 0 (0) |
| Artery disease | 7235 (19.7) | 1125 (15.5) | 0 (0) | 0 (0) |
| Any of the above | 36,755 (100) | 7250 (100) | 0 (0) | 0 (0) |
| Cardiovascular medicationa | ||||
| ACE inhibitors | 14,500 (39.5) | 0 (0) | 568 (12.0) | 0 (0) |
| ARBs | 7904 (21.5) | 0 (0) | 274 (5.8) | 0 (0) |
| Acetylsalicylic acid | 254 (0.7) | 0 (0) | 82 (1.7) | 0 (0) |
| Antiarrhythmics | 1732 (4.7) | 0 (0) | 14 (0.3) | 0 (0) |
| Anticoagulants | 4145 (11.3) | 0 (0) | 194 (4.1) | 0 (0) |
| Antiplatelet agents | 5112 (13.9) | 0 (0) | 99 (2.1) | 0 (0) |
| Beta blockers | 15,545 (42.3) | 0 (0) | 696 (14.8) | 0 (0) |
| Calcium channel blockers | 12,806 (34.8) | 0 (0) | 511 (10.8) | 0 (0) |
| Digoxin | 2581 (7.0) | 0 (0) | 68 (1.4) | 0 (0) |
| Diuretics | 16,420 (44.7) | 0 (0) | 1267 (26.9) | 0 (0) |
| Nitrates | 5380 (14.6) | 0 (0) | 144 (3.1) | 0 (0) |
| Statins | 18,441 (50.2) | 0 (0) | 2607 (55.3) | 0 (0) |
| Any of the above | 36,755 (100) | 0 (0) | 4715 (100) | 0 (0) |
| COPD specifics | ||||
| Spirometry | 15,032 (40.9) | 2855 (39.4) | 1708 (36.2) | 4617 (35.7) |
| Oxygen therapy | 5730 (15.6) | 981 (13.5) | 450 (9.5) | 822 (6.4) |
| Influenza vaccination | 8913 (24.2) | 1362 (18.8) | 937 (19.9) | 1888 (14.6) |
| Pneumococcal vaccination | 2607 (7.1) | 477 (6.6) | 312 (6.6) | 832 (6.4) |
| Short-acting COPD medicationa | ||||
| Inhaled steroids | 4576 (12.5) | 633 (8.7) | 729 (15.5) | 1526 (11.8) |
| Oral steroids | 11,132 (30.3) | 1688 (23.3) | 1393 (29.5) | 3690 (28.5) |
| Roflumilast | 6 (0.0) | 1 (0.0) | 0 (0) | 1 (0.0) |
| SABA | 14,430 (39.3) | 2210 (30.5) | 2015 (42.7) | 5321 (41.1) |
| SABA-SAMA | 7631 (20.8) | 1105 (15.2) | 922 (19.6) | 2255 (17.4) |
| SAMA | 2974 (8.1) | 420 (5.8) | 367 (7.8) | 854 (6.6) |
| Theophylline | 1527 (4.2) | 233 (3.2) | 205 (4.3) | 387 (3.0) |
| Any of the above | 23,244 (63.2) | 3506 (48.4) | 3062 (64.9) | 7765 (60.0) |
| Comorbiditiesb | ||||
| 0 | 10,843 (29.5) | 3387 (46.7) | 2313 (49.1) | 9370 (72.5) |
| 1 | 12,507 (34.0) | 2285 (31.5) | 1460 (31.0) | 2586 (20.0) |
| 2 | 8080 (22.0) | 1024 (14.1) | 696 (14.8) | 746 (5.8) |
| 3+ | 5325 (14.5) | 554 (7.6) | 246 (5.2) | 229 (1.8) |
COPD: chronic obstructive pulmonary disease; CVD: cardiovascular disease; ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blocker; SABA: short-acting beta agonist; SAMA: short-acting muscarinic antagonist; LABA: long-acting beta agonist; LABA-ICS: long-acting beta agonist-inhaled corticosteroid; LAMA: long-acting muscarinic antagonist.
A patient can have more than one condition/medication.
Comorbidities included hyperlipidemia, diabetes mellitus, liver disease, renal failure, paralysis, neurologic disorder, hypothyroidism, peptic ulcer disease, HIV/AIDS, lymphoma, tumor without metastasis, metastatic cancer, rheumatoid arthritis, osteoporosis, obesity, weight loss, alcohol abuse, drug abuse, psychosis, and depression.
Cardiovascular risk associated with initiation of LABDs
The unadjusted risk of ED visit or hospitalization for CVD disease within 90 days of initiation of LABDs for Group 1, Group 2, Group 3 and Group 4 was 5.45%, 2.95%, 1.55% and 0.96%, respectively (Table 2). The most common cardiovascular (CV) event in Group 1 leading to ED visit and/or hospitalization within 90 days of initiation of LABD was heart failure (3.08%) followed by tachyarrhythmia (2.40%).
Table 3 presents the risk of cardiovascular events by class of medication used. The overall risk of CV event within 90 days of initiation of LABD was higher in the LABA alone group compared to the LAMA alone group (4.84% vs 3.74%), which was not different from that of the LABA-ICS group (3.86%). The most common CV event in patients on new initiation of LABA, LAMA or LABA-ICS was heart failure. However, this risk differed across the three drug categories (p < 0.001, chi-square test).
Table 3.
Cardiovascular events within 90 days of prescription by class of medications initiated.
| Cause for hospitalization and/or ED visit | N (%) with any event |
p value, chi-square test among three types of LABDs users | ||
|---|---|---|---|---|
| LABA |
LABA-ICS |
LAMA |
||
| N = 5514 | N = 38,361 | N = 17,776 | ||
| Heart failurea | 144 (2.61) | 738 (1.92) | 301 (1.69) | <0.001 |
| Arrhythmia | 94 (1.70) | 500 (1.30) | 250 (1.41) | 0.050 |
| Stroke | 21 (0.38) | 161 (0.42) | 78 (0.44) | 0.841 |
| Acute coronary syndrome | 53 (0.96) | 313 (0.82) | 141 (0.79) | 0.471 |
| Any of the abovea | 267 (4.84) | 1482 (3.86) | 665 (3.74) | 0.008 |
ED: emergency department; LABA: long-acting beta agonist; LABA-ICS: long-acting beta agonist-inhaled corticosteroid; LAMA: long-acting muscarinic antagonist; LABD: long-acting bronchodilator.
The rates among LABA and LAMA users were significantly different (p < 0.001, chi-square test).
Table 4 shows the results of multivariable analysis examining the risk of a CVD event among these four groups adjusting for age, gender, region, the type and year of the first LABD, COPD specifics, medications and comorbidities. The odds of having an ED visit or a hospitalization due to a CV event was 3.5-fold higher in Group 1 with preexisting CVD and CVD treatment (odds ratio (OR), 3.50, 95% confidence interval (CI), 2.89–4.24) compared to Group 4. This risk was also higher in Groups 2 and 3 compared to Group 4. Group 2, who had CVD and were not on treatment, had the second highest risk (OR = 2.15, 95% CI = 1.71–2.70). Patients in Group 3, with no CVD but receiving treatment, were at moderate risk (OR = 1.36, 95% CI = 1.01–1.82) compared to Group 4, who had no CVD and no treatment. Our finding suggests that preexisting CVD remains the single most important determinant for future cardiovascular event. Patients on oxygen therapy and those with a greater comorbidity burden had higher odds of a CV event. There was a stepwise increase in the risk of cardiovascular event with increasing age category. Patients with COPD on LABA or LABA-ICS combination were at higher risk of cardiovascular events compared to those on LAMA.
Table 4.
Multivariable analysisa of odds of cardiovascular event within 90 days of initiation of long-acting bronchodilators.
| Patient characteristics | Odds ratio (95% confidence interval) |
|---|---|
| Treatment group | |
| Group 1 (CVD and CVD treatment) | 3.50 (2.89, 4.24) |
| Group 2 (CVD with no CVD treatment) | 2.15 (1.71, 2.70) |
| Group 3 (no CVD but CVD treatment) | 1.36 (1.01, 1.82) |
| Group 4 (no CVD and no CVD treatment) | 1.00 |
| Age | |
| 40–49 | 1.00 |
| 50–59 | 1.45 (1.16, 1.80) |
| 60–69 | 1.90 (1.54, 2.35) |
| Above 70 | 3.29 (2.66, 4.07) |
| Gender | |
| Female | 1.00 |
| Male | 1.36 (1.25, 1.48) |
| First long-acting bronchodilator | |
| LAMA | 1.00 |
| LABA | 1.33 (1.13, 1.57) |
| LABA-ICS | 1.13 (1.03, 1.25) |
| COPD specifics, yes versus no | |
| Spirometry | 0.92 (0.85, 1.00) |
| Oxygen therapy | 1.37 (1.23, 1.52) |
| Influenza vaccination | 0.94 (0.85, 1.04) |
| Pneumococcal vaccination | 0.81 (0.68, 0.98) |
| Comorbidities | |
| 0 | 1.00 |
| 1 | 1.14 (1.01, 1.27) |
| 2 | 1.65 (1.46, 1.86) |
| 3+ | 2.53 (2.23, 2.87) |
| Short-acting COPD medication, yes versus no | |
| Inhaled steroids | 0.78 (0.67, 0.90) |
| Oral steroids | 1.04 (0.95, 1.15) |
| SABA | 0.91 (0.83, 1.00) |
| SABA-SAMA | 0.90 (0.80, 1.00) |
| SAMA | 1.14 (0.97, 1.33) |
| Theophylline | 0.87 (0.70, 1.09) |
CVD: cardiovascular disease; COPD: chronic obstructive pulmonary disease; LAMA: long-acting muscarinic antagonist; LABA: long-acting beta agonist; LABA-ICS: long-acting beta agonist-inhaled corticosteroid; SABA: short-acting beta agonist; SAMA: short-acting muscarinic antagonist.
This analysis was also adjusted for region of patients’ residence and the year of the first long-acting bronchodilator prescription.
Discussion
Our findings can be summarized as follows: patients with COPD who had a history of CVD and were on any cardiovascular medication are at a 3.5-fold higher risk of having a CV event leading to ED visit and/or hospitalization within 90 days of a new prescription of LABD. For the most common CV event of heart failure, the risk was higher for LABA and LABA-ICS combination compared to LAMA.
Prior observational studies showed increased cardiovascular risk with LABDs. Gershon et al.,25 in a nested case control analysis of a retrospective cohort study, found increased cardiovascular events in patients with newly prescribed inhaled anticholinergics and beta agonists. Salpeter et al.,19 in a meta-analysis of 33 placebo-controlled RCTs, demonstrated an increased risk of cardiovascular events with SABA and LABA in patients with COPD and asthma. Singh et al.,20,28 in a meta-analysis of 17 RCTs, showed an increased risk of a composite end point of cardiovascular death, myocardial infarction or stroke for ipratropium and tiotropium compared to placebo. However, reanalysis of Singh et al.29 in 2008 when UPLIFT trial data was added failed to show an increased risk. Another meta-analysis of five RCTs by Singh et al.21 showed an increased risk of mortality with tiotropium delivered by a soft mist inhaler (Respimat) when compared to placebo. The Tiotropium Safety and Performance in Respimat Trial (TIOSPIR) that followed showed no increased risk with both Respimat and Handihaler tiotropium.30,31
The pathophysiology of the cardiovascular risk for LABDs remains unclear but several possible mechanisms may be responsible. Beta agonist acts through direct stimulation of beta receptors and anticholinergics by inhibition of the vagal tone; together, they create a state of increased sympathetic stimulation on the heart and blood vessels with resultant vasoconstriction and ischemia. Similar mechanisms are responsible for the development of tachyarrhythmia. Beta agonists may also cause hypokalemia that increases the risk of arrhythmia and the accompanying increased heart rate may contribute to heart failure.19,32 Our results showed that heart failure and tachyarrhythmias were common CV events leading to ED visits or hospitalization. Tiotropium, found to increase interleukin (IL)-8 in sputum and IL-8 level in serum, is also associated with increased myocardial infarction, potentially indicating that anticholinergics add to the systemic inflammatory state of COPD which in turn increases the cardiovascular risk.33
The finding of highest risk for CV event in Group 1 patients who had CVD and were on CVD treatment can be explained by selection bias as patients in this group are older and have more comorbidities than those in the other groups. However, these findings are relevant for clinical decision making. The effect of CVD medications on COPD patients is thought to be protective in terms of mortality and exacerbations. For example, Ekström et al.,11 in a prospective national study in COPD patients on oxygen in Sweden, found decreased mortality with antiplatelet agents and a similar but statistically insignificant trend for ACE inhibitors, ARBs and statins, while BBs were associated with increased mortality. However, a meta-analysis by Etminan et al.34 showed a decrease in all-cause mortality in COPD patients on BBs. The STATCOPE, a multicenter RCT, showed no decrease in exacerbations in COPD patients using simvastatin 40 compared to placebo while observational studies showed decrease in exacerbations in patients with COPD and coexisting CVD.10,35 One possible explanation of our finding is the significantly high risk at baseline in the group with preexisting CVD, such that cardiovascular medications failed to mitigate the added risk. Moreover, the initiation of LABDs that have sympathomimetic and anticholinergic effects tips the balance to unmasking the risk which is not ameliorated by CV medications. We also expect that the proper administration of inhaler medications is important to avoid systemic effects.
Our study has several limitations. First, we defined COPD based on billing data not on the basis of degree of obstruction on spirometry, as it is not available from claims. Second, we were not able to demonstrate whether the cardiovascular risk would vary with COPD severity, which can potentially affect the outcome as administrative data does not allow us to identify severity. However, one measure of severity that can be captured in claims data is receipt of oxygen supplement; we used this variable in the risk adjustment model as a proxy for greater COPD severity. Third, our population is extracted from one large private insurance database and the results may not be applicable to uninsured or underinsured patients with COPD. Fourth, the clinical indication for use of some of the cardiovascular medications can be for a non-cardiovascular condition and cannot be captured in the administrative database. Fifth, the high risk of cardiovascular event in patients with preexisting cardiovascular condition after an initiation of LABA/LAMA does not imply cause and effect. Finally, we did not examine the type of beta-blocker used (selective or non-selective). The benefit of beta-blocker in patients with COPD is not a class effect. It may not be protective in patients with preexisting CV disease who started LABD.
In conclusion, the risk of cardiovascular events after initiation of LABDs is highest in patients with preexisting CVD and is not ameliorated by CVD medications. Clinicians should be cautious when prescribing these medications in patients with preexisting CVD.
Acknowledgments
All authors had access to the data and contributed in writing the manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from IRB at University of Texas Medical Branch (IRB-Temp-0853).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding was provided by the Division of Pulmonary, Critical Care and Sleep Medicine at University of Texas Medical Branch.
Informed consent: Not applicable as data were obtained retrospectively from a private insurance company database.
References
- 1. Aubier M, Marthan R, Berger P, et al. COPD and inflammation: statement from a French expert group: inflammation and remodelling mechanisms. Rev Mal Respir 2010; 27: 1254–1266. [DOI] [PubMed] [Google Scholar]
- 2. Wang ZL. Evolving role of systemic inflammation in comorbidities of chronic obstructive pulmonary disease. Chin Med J 2010; 123: 3467–3478. [PubMed] [Google Scholar]
- 3. Curkendall SM, DeLuise C, Jones JK, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol 2006; 16: 63–70. [DOI] [PubMed] [Google Scholar]
- 4. Akramova É, Khamitova RIa. Cardiac comorbidity in patients with chronic obstructive pulmonary disease: diagnosis and economics. Ter Arkh 2014; 86: 24–27. [PubMed] [Google Scholar]
- 5. Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest 2005; 128: 2640–2646. [DOI] [PubMed] [Google Scholar]
- 6. Głuszek J. Ischaemic heart disease and hypertension in patients with chronic obstructive pulmonary disease and obstructive sleep apnoea. Pneumonol Alergol Pol 2013; 81: 567–574. [PubMed] [Google Scholar]
- 7. Freixa X, Portillo K, Paré C, et al. Echocardiographic abnormalities in patients with COPD at their first hospital admission. Eur Respir J 2013; 41: 784–791. [DOI] [PubMed] [Google Scholar]
- 8. Portillo K, Abad-Capa J, Ruiz-Manzano J. Chronic obstructive pulmonary disease and left ventricle. Arch Bronconeumol. Epub ahead of print 9 May 2014. DOI: 10.1016/j.arbres.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 9. De Miguel Díez J, Chancafe Morgan J, Jiménez García R. The association between COPD and heart failure risk: a review. Int J Chron Obstruct Pulmon Dis 2013; 8: 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ingebrigtsen TS, Marott JL, Nordestgaard BG, et al. Statin use and exacerbations in individuals with chronic obstructive pulmonary disease. Thorax 2015; 70: 33–40. [DOI] [PubMed] [Google Scholar]
- 11. Ekström MP, Hermansson AB, Ström KE. Effects of cardiovascular drugs on mortality in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187: 715–720. [DOI] [PubMed] [Google Scholar]
- 12. Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356: 775–789. [DOI] [PubMed] [Google Scholar]
- 13. Antoniu SA. UPLIFT Study: the effects of long-term therapy with inhaled tiotropium in chronic obstructive pulmonary disease. Evaluation of: Tashkin DP, Celli B, Senn S et al.: a 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med (2008) 359(15):1543–1554. Expert Opin Pharmacother 2009; 10: 719–722. [DOI] [PubMed] [Google Scholar]
- 14. Bateman ED, van Dyk M, Sagriotis A. Comparable spirometric efficacy of tiotropium compared with salmeterol plus fluticasone in patients with COPD: a pilot study. Pulm Pharmacol Ther 2008; 21: 20–25. [DOI] [PubMed] [Google Scholar]
- 15. Kurashima K, Hara K, Yoneda K, et al. Changes in lung function and health status in patients with COPD treated with tiotropium or salmeterol plus fluticasone. Respirology 2009; 14: 239–244. [DOI] [PubMed] [Google Scholar]
- 16. Aguilaniu B. Impact of bronchodilator therapy on exercise tolerance in COPD. Int J Chron Obstruct Pulmon Dis 2010; 5: 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jayaram L, Wong C, McAuley S, et al. Combined therapy with tiotropium and formoterol in chronic obstructive pulmonary disease: effect on the 6-minute walk test. COPD 2013; 10: 466–472. [DOI] [PubMed] [Google Scholar]
- 18. Oba Y, Lone NA. Comparative efficacy of inhaled corticosteroid and long-acting beta agonist combinations in preventing COPD exacerbations: a Bayesian network meta-analysis. Int J Chron Obstruct Pulmon Dis 2014; 9: 469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest 2004; 125: 2309–2321. [DOI] [PubMed] [Google Scholar]
- 20. Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA 2008; 300: 1439–1450. [DOI] [PubMed] [Google Scholar]
- 21. Singh S, Loke YK, Enright PL, et al. Mortality associated with tiotropium mist inhaler in patients with chronic obstructive pulmonary disease: systematic review and meta-analysis of randomised controlled trials. BMJ 2011; 342: d3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Au DH, Curtis JR, Every NR, et al. Association between inhaled beta-agonists and the risk of unstable angina and myocardial infarction. Chest 2002; 121: 846–851. [DOI] [PubMed] [Google Scholar]
- 23. Nelson HS, Gross NJ, Levine B, et al. Cardiac safety profile of nebulized formoterol in adults with COPD: a 12-week, multicenter, randomized, double-blind, double-dummy, placebo- and active-controlled trial. Clin Ther 2007; 29: 2167–2178. [DOI] [PubMed] [Google Scholar]
- 24. Gershon A, Croxford R, To T, et al. Comparison of inhaled long-acting β-agonist and anticholinergic effectiveness in older patients with chronic obstructive pulmonary disease: a cohort study. Ann Intern Med 2011; 154: 583–592. [DOI] [PubMed] [Google Scholar]
- 25. Gershon A, Croxford R, Calzavara A, et al. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med 2013; 173: 1175–1185. [DOI] [PubMed] [Google Scholar]
- 26. Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011; 364: 1093–1103. [DOI] [PubMed] [Google Scholar]
- 27. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 28. Singh S, Loke YK, Enright P, et al. Republished: pro-arrhythmic and pro-ischaemic effects of inhaled anticholinergic medications. Postgrad Med J 2014; 90: 205–207. [DOI] [PubMed] [Google Scholar]
- 29. Oba Y, Zaza T, Thameem DM. Safety, tolerability and risk benefit analysis of tiotropium in COPD. Int J Chron Obstruct Pulmon Dis 2008; 3: 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wise RA, Anzueto A, Calverley P, et al. The Tiotropium Safety and Performance in Respimat Trial (TIOSPIR), a large scale, randomized, controlled, parallel-group trial-design and rationale. Respir Res 2013; 14: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Halpin DM, Dahl R, Hallmann C, et al. Tiotropium HandiHaler(®) and Respimat(®) in COPD: a pooled safety analysis. Int J Chron Obstruct Pulmon Dis 2015; 10: 239–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003; 107: 1514–1519. [DOI] [PubMed] [Google Scholar]
- 33. Boekholdt SM, Peters RJ, Hack CE, et al. IL-8 plasma concentrations and the risk of future coronary artery disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol 2004; 24: 1503–1508. [DOI] [PubMed] [Google Scholar]
- 34. Etminan M, Jafari S, Carleton B, et al. Beta-blocker use and COPD mortality: a systematic review and meta-analysis. BMC Pulm Med 2012; 12: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Criner GJ, Connett JE, Aaron S, et al. Simvastatin for the prevention of exacerbations in moderate-to-severe COPD. N Engl J Med 2014; 370: 2201–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]