Abstract
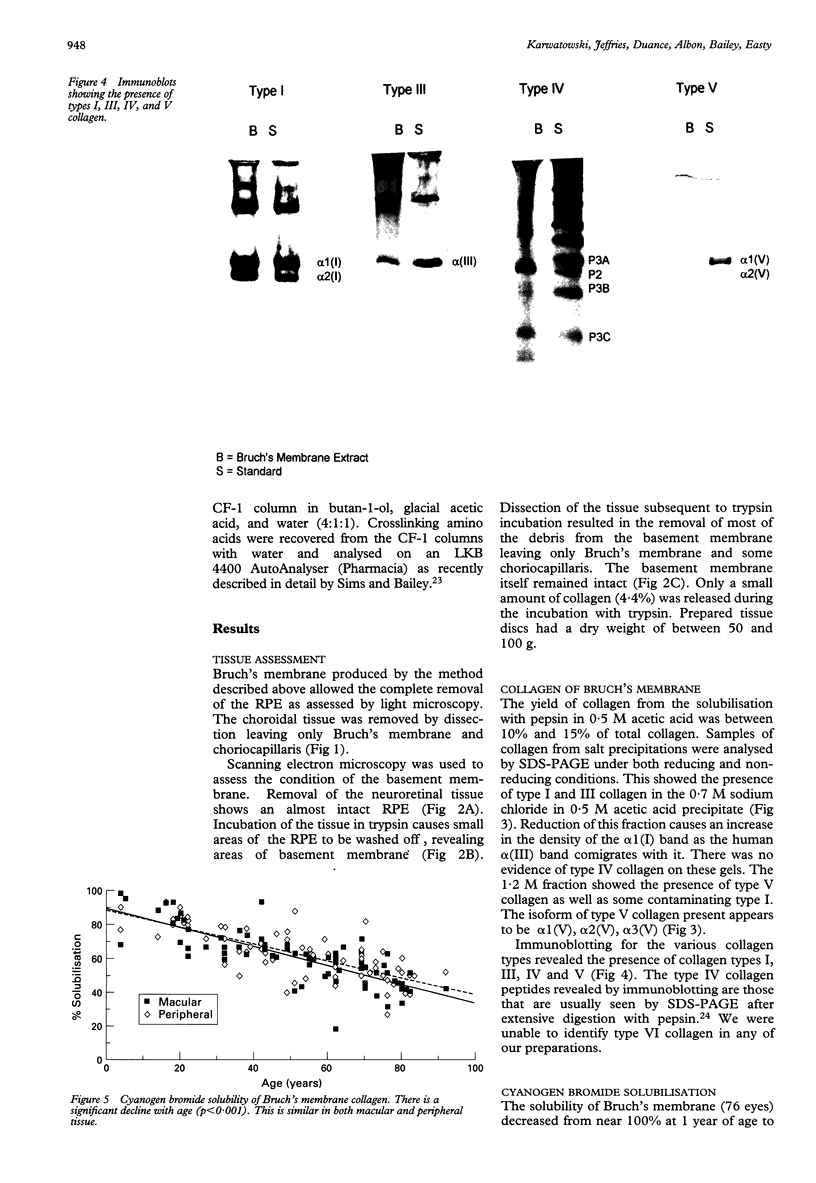
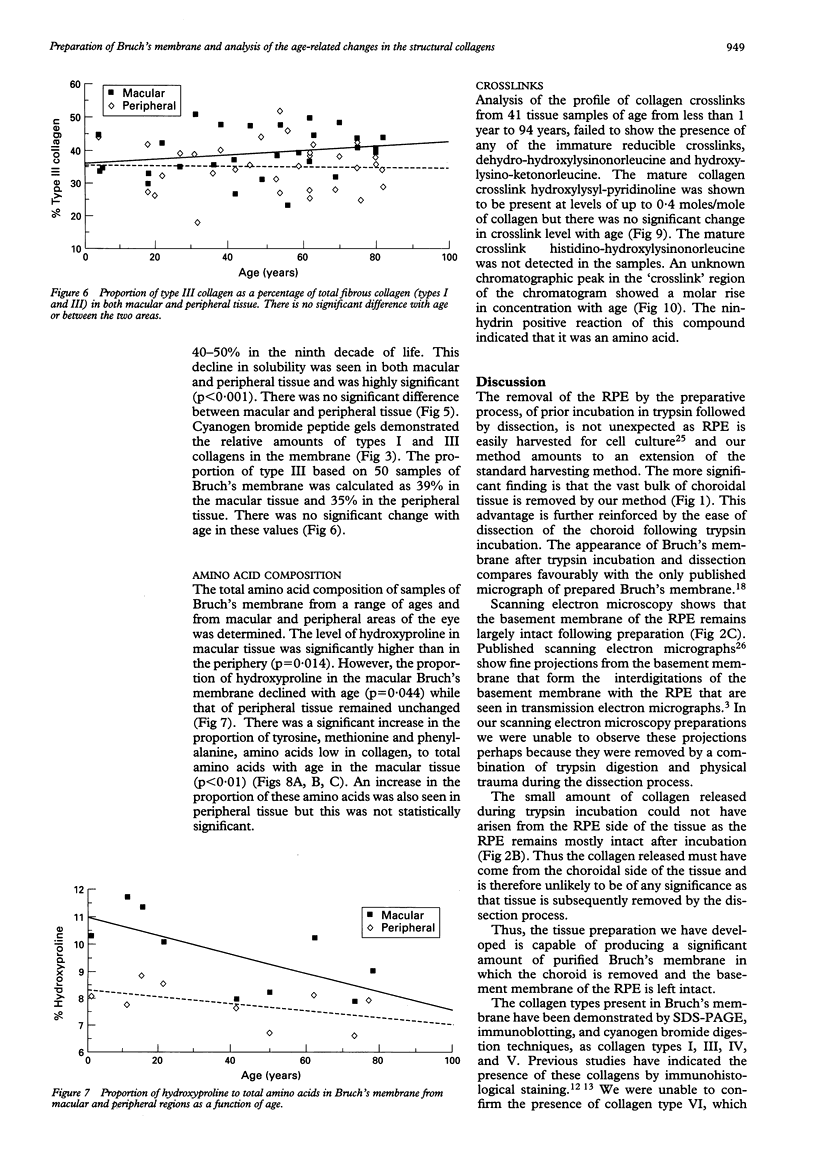
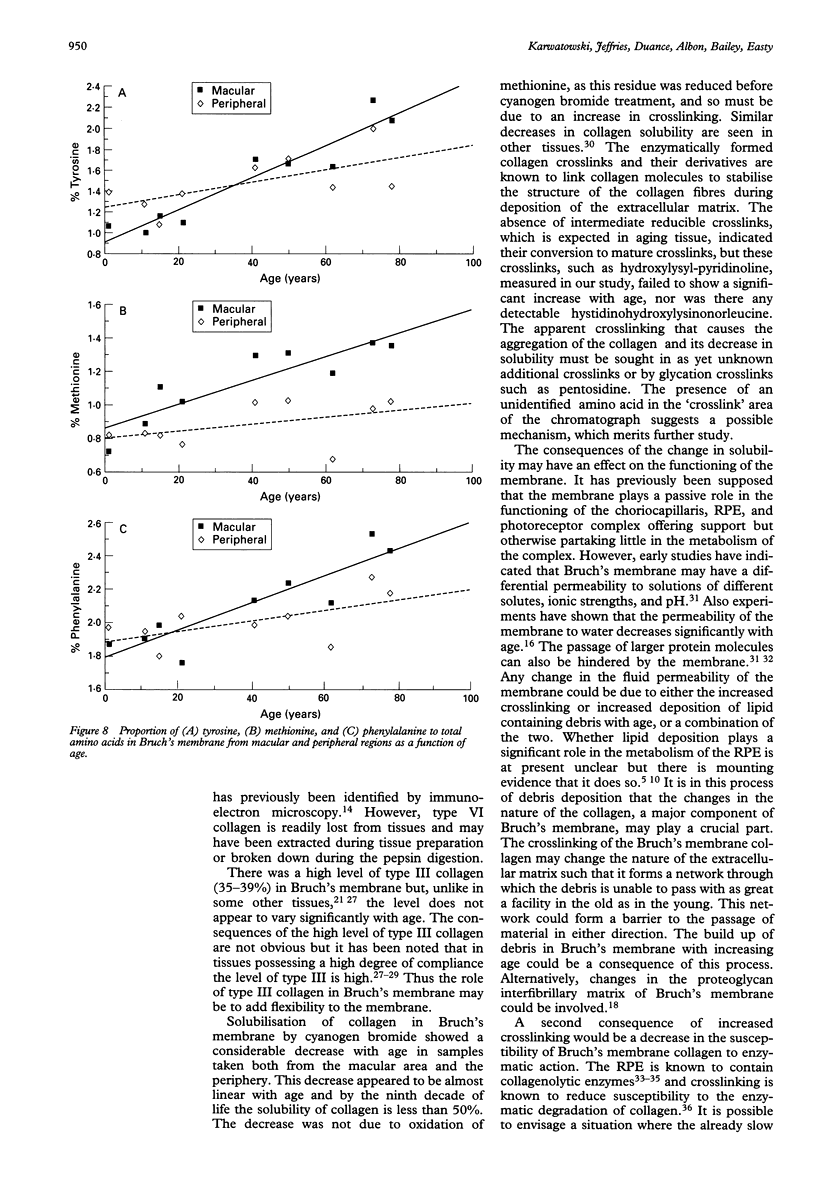
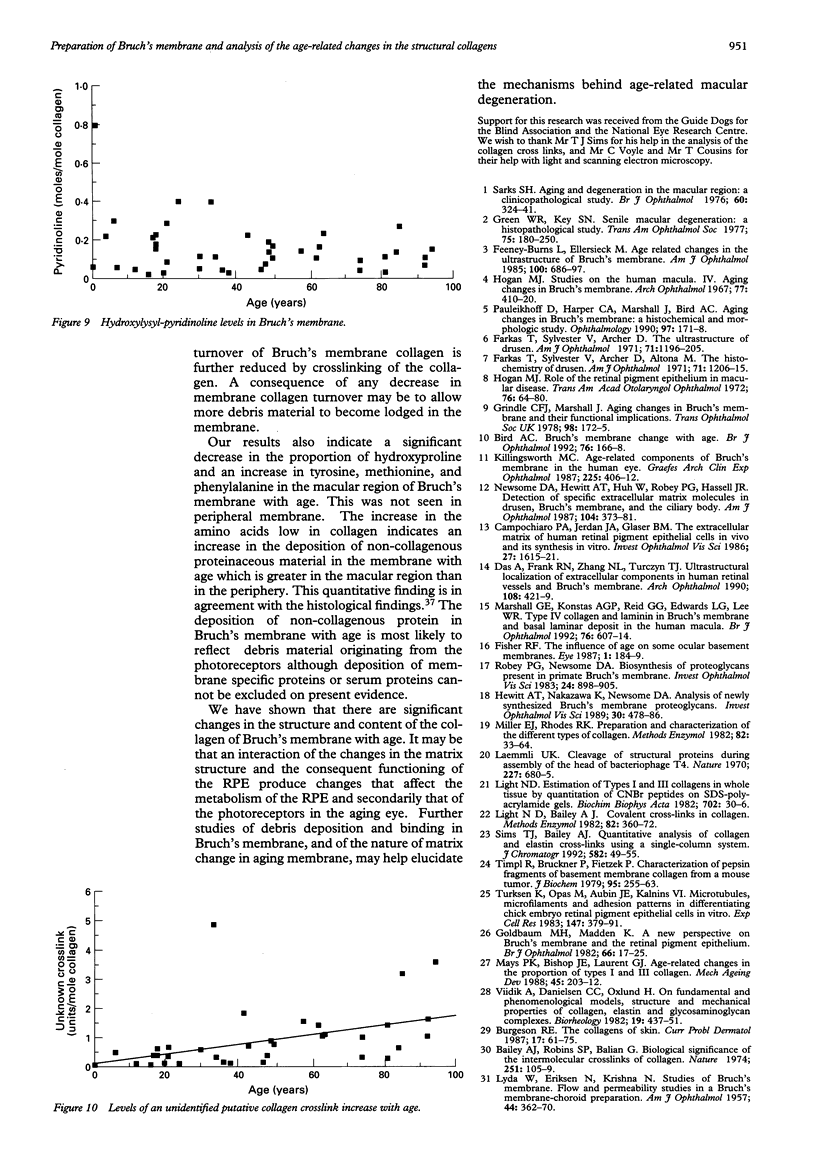
AIMS/BACKGROUND--The morphological changes in Bruch's membrane and its constituent collagen seen during aging have been studied extensively but the chemical nature of the collagen and any aging changes have not previously been evaluated. METHODS--A method for preparing purified Bruch's membrane from human cadaver eyes by dissection preceded by trypsin digestion was developed. Following pepsin digestion, the constituent collagens were analysed by SDS-PAGE and by immunoblotting. Cyanogen bromide digestion was used to ascertain the solubility of the collagen and the proportion of type I to type III collagen. After hydrolysis of Bruch's membrane samples the constituent amino acids and collagen crosslinks were measured. RESULTS--The presence of collagen types I, III, IV, and V in Bruch's membrane was confirmed. The proportion of type III collagen as a percentage of total fibrous collagens was calculated as being between 35% and 39%, with no significant difference between different macular and peripheral sites or with age. There was a highly significant decline in the solubility of Bruch's membrane collagen with age, from near 100% in the first decade of life to 40-50% in the ninth decade at both macular and peripheral sites. There was no significant change in the amount of enzymatically formed collagen crosslinks with age. Amino acid analysis indicated a significant increase in the amount of non-collagen protein with age in macular but not peripheral sites. CONCLUSION--Changes in the constituent collagens may contribute to the accumulation of debris in Bruch's membrane with age and interfere with the function of the retinal pigment epithelium, with subsequent consequences for the overlying photoreceptors.
Full text
PDF

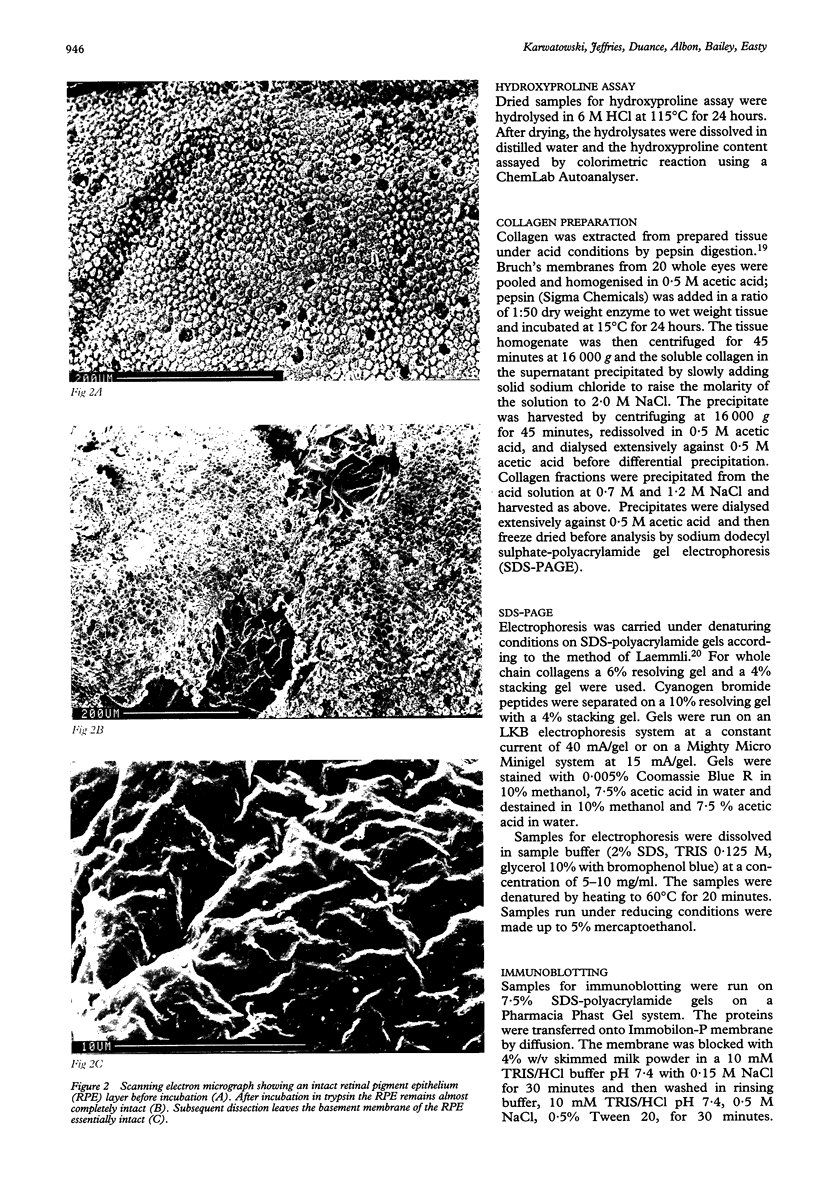
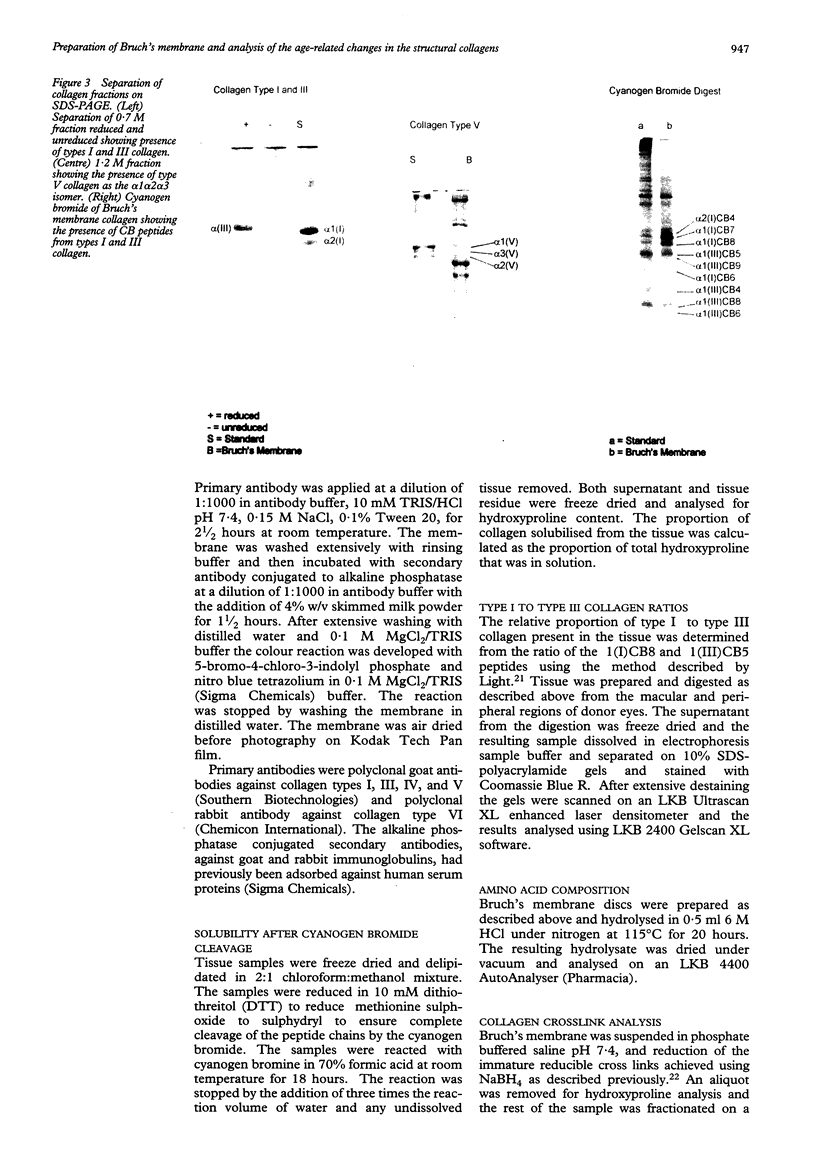

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. P., Bradley J. M., Gabourel J. D., Acott T. S. Expression of matrix metalloproteinases and inhibitor by human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1990 Dec;31(12):2520–2528. [PubMed] [Google Scholar]
- Bailey A. J., Robins S. P., Balian G. Biological significance of the intermolecular crosslinks of collagen. Nature. 1974 Sep 13;251(5471):105–109. doi: 10.1038/251105a0. [DOI] [PubMed] [Google Scholar]
- Bird A. C. Bruch's membrane change with age. Br J Ophthalmol. 1992 Mar;76(3):166–168. doi: 10.1136/bjo.76.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgeson R. E. The collagens of skin. Curr Probl Dermatol. 1987;17:61–75. [PubMed] [Google Scholar]
- Campochiaro P. A., Jerdon J. A., Glaser B. M. The extracellular matrix of human retinal pigment epithelial cells in vivo and its synthesis in vitro. Invest Ophthalmol Vis Sci. 1986 Nov;27(11):1615–1621. [PubMed] [Google Scholar]
- Das A., Frank R. N., Zhang N. L., Turczyn T. J. Ultrastructural localization of extracellular matrix components in human retinal vessels and Bruch's membrane. Arch Ophthalmol. 1990 Mar;108(3):421–429. doi: 10.1001/archopht.1990.01070050119045. [DOI] [PubMed] [Google Scholar]
- Farkas T. G., Sylvester V., Archer D., Altona M. The histochemistry of drusen. Am J Ophthalmol. 1971 Jun;71(6):1206–1215. doi: 10.1016/0002-9394(71)90964-0. [DOI] [PubMed] [Google Scholar]
- Farkas T. G., Sylvester V., Archer D. The ultrastructure of drusen. Am J Ophthalmol. 1971 Jun;71(6):1196–1205. doi: 10.1016/0002-9394(71)90963-9. [DOI] [PubMed] [Google Scholar]
- Feeney-Burns L., Ellersieck M. R. Age-related changes in the ultrastructure of Bruch's membrane. Am J Ophthalmol. 1985 Nov 15;100(5):686–697. doi: 10.1016/0002-9394(85)90625-7. [DOI] [PubMed] [Google Scholar]
- Fisher R. F. The influence of age on some ocular basement membranes. Eye (Lond) 1987;1(Pt 2):184–189. doi: 10.1038/eye.1987.35. [DOI] [PubMed] [Google Scholar]
- Goldbaum M. H., Madden K. A new perspective on Bruch's membrane and the retinal pigment epithelium. Br J Ophthalmol. 1982 Jan;66(1):17–25. doi: 10.1136/bjo.66.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green W. R., Key S. N., 3rd Senile macular degeneration: a histopathologic study. Trans Am Ophthalmol Soc. 1977;75:180–254. [PMC free article] [PubMed] [Google Scholar]
- Grindle C. F., Marshall J. Ageing changes in Bruch's membrane and their functional implications. Trans Ophthalmol Soc U K. 1978 Apr;98(1):172–175. [PubMed] [Google Scholar]
- Hayasaka S. Lysosomal enzymes in ocular tissues and diseases. Surv Ophthalmol. 1983 Jan-Feb;27(4):245–258. doi: 10.1016/0039-6257(83)90125-x. [DOI] [PubMed] [Google Scholar]
- Hewitt A. T., Nakazawa K., Newsome D. A. Analysis of newly synthesized Bruch's membrane proteoglycans. Invest Ophthalmol Vis Sci. 1989 Mar;30(3):478–486. [PubMed] [Google Scholar]
- Hogan M. J., Alvarado J. Studies on the human macula. IV. Aging changes in Bruch's membrane. Arch Ophthalmol. 1967 Mar;77(3):410–420. doi: 10.1001/archopht.1967.00980020412022. [DOI] [PubMed] [Google Scholar]
- Hogan M. J. Role of the retinal pigment epithelium in macular disease. Trans Am Acad Ophthalmol Otolaryngol. 1972 Jan-Feb;76(1):64–80. [PubMed] [Google Scholar]
- Killingsworth M. C. Age-related components of Bruch's membrane in the human eye. Graefes Arch Clin Exp Ophthalmol. 1987;225(6):406–412. doi: 10.1007/BF02334166. [DOI] [PubMed] [Google Scholar]
- LYDA W., ERIKSEN N., KRISHNA N. Studies of Bruch's membrane; flow and permeability studies in a Bruch's membrane-choroid preparation. Am J Ophthalmol. 1957 Nov;44(5 Pt 2):362–370. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Light N. D., Bailey A. J. Covalent cross-links in collagen. Methods Enzymol. 1982;82(Pt A):360–372. doi: 10.1016/0076-6879(82)82073-9. [DOI] [PubMed] [Google Scholar]
- Marshall G. E., Konstas A. G., Reid G. G., Edwards J. G., Lee W. R. Type IV collagen and laminin in Bruch's membrane and basal linear deposit in the human macula. Br J Ophthalmol. 1992 Oct;76(10):607–614. doi: 10.1136/bjo.76.10.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays P. K., Bishop J. E., Laurent G. J. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988 Nov 30;45(3):203–212. doi: 10.1016/0047-6374(88)90002-4. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Rhodes R. K. Preparation and characterization of the different types of collagen. Methods Enzymol. 1982;82(Pt A):33–64. doi: 10.1016/0076-6879(82)82059-4. [DOI] [PubMed] [Google Scholar]
- Newsome D. A., Hewitt A. T., Huh W., Robey P. G., Hassell J. R. Detection of specific extracellular matrix molecules in drusen, Bruch's membrane, and ciliary body. Am J Ophthalmol. 1987 Oct 15;104(4):373–381. doi: 10.1016/0002-9394(87)90227-3. [DOI] [PubMed] [Google Scholar]
- Pauleikhoff D., Harper C. A., Marshall J., Bird A. C. Aging changes in Bruch's membrane. A histochemical and morphologic study. Ophthalmology. 1990 Feb;97(2):171–178. [PubMed] [Google Scholar]
- Pino R. M., Essner E. Permeability of rat choriocapillaris to hemeproteins. Restriction of tracers by a fenestrated endothelium. J Histochem Cytochem. 1981 Feb;29(2):281–290. doi: 10.1177/29.2.7252121. [DOI] [PubMed] [Google Scholar]
- Robey P. G., Newsome D. A. Biosynthesis of proteoglycans present in primate Bruch's membrane. Invest Ophthalmol Vis Sci. 1983 Jul;24(7):898–905. [PubMed] [Google Scholar]
- Sarks S. H. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976 May;60(5):324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarks S. H. Council Lecture. Drusen and their relationship to senile macular degeneration. Aust J Ophthalmol. 1980 May;8(2):117–130. doi: 10.1111/j.1442-9071.1980.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Sims T. J., Bailey A. J. Quantitative analysis of collagen and elastin cross-links using a single-column system. J Chromatogr. 1992 Nov 6;582(1-2):49–55. doi: 10.1016/0378-4347(92)80301-6. [DOI] [PubMed] [Google Scholar]
- Timpl R., Bruckner P., Fietzek P. Characterization of pepsin fragments of basement membrane collagen obtained from a mouse tumor. Eur J Biochem. 1979 Apr 2;95(2):255–263. doi: 10.1111/j.1432-1033.1979.tb12961.x. [DOI] [PubMed] [Google Scholar]
- Turksen K., Opas M., Aubin J. E., Kalnins V. I. Microtubules, microfilaments and adhesion patterns in differentiating chick retinal pigment epithelial (RPE) cells in vitro. Exp Cell Res. 1983 Sep;147(2):379–391. doi: 10.1016/0014-4827(83)90220-3. [DOI] [PubMed] [Google Scholar]
- Vater C. A., Harris E. D., Jr, Siegel R. C. Native cross-links in collagen fibrils induce resistance to human synovial collagenase. Biochem J. 1979 Sep 1;181(3):639–645. doi: 10.1042/bj1810639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viidik A., Danielson C. C., Oxlund H. On fundamental and phenomenological models, structure and mechanical properties of collagen, elastin and glycosaminoglycan complexes. Biorheology. 1982;19(3):437–451. doi: 10.3233/bir-1982-19305. [DOI] [PubMed] [Google Scholar]