Abstract
Glioblastoma multiforme (GBM) is the most common and lethal adult brain tumor. Resistance to standard radiation and chemotherapy is thought to involve survival of GBM cancer stem cells (CSCs). To date, no single marker for identifying GBM CSCs has been able to capture the diversity of CSC populations, justifying the needs for additional CSC markers for better characterization. Employing targeted mass spectrometry, here we present five cell-surface markers HMOX1, SLC16A1, CADM1, SCAMP3 and CLCC1 which were found to be elevated in CSCs relative to healthy neural stem cells (NSCs). Transcriptomic analyses of REMBRANDT and TCGA compendiums also indicated elevated expression of these markers in GBM relative to controls and non-GBM diseases. Two markers SLC16A1 and HMOX1 were found to be expressed among pseudopalisading cells that reside in the hypoxic region of GBM, substantiating the histopathological hallmarks of GBM. In a prospective study (N=8) we confirmed the surface expression of HMOX1 on freshly isolated primary GBM cells (P0). Employing functional assays that are known to evaluate stemness, we demonstrate that elevated HMOX1 expression is associated with stemness in GBM and can be modulated through TGFβ. siRNA-mediated silencing of HMOX1 impaired GBM invasion- a phenomenon related to poor prognosis. In addition, surgical resection of GBM tumors caused declines (18%±5.1SEM) in the level of plasma HMOX1 as measured by ELISA, in 8/10 GBM patients. These findings indicate that HMOX1 is a robust predictor of GBM CSC stemness and pathogenesis. Further understanding of the role of HMOX1 in GBM may uncover novel therapeutic approaches.
Keywords: GBM, CSC, NSC, neurosphere, invasion, pseudopalisading
Graphical abstract
INTRODUCTION
Glioblastoma multiforme (GBM) is the most common adult primary brain tumor, with a median survival of 15 months. In United States alone, 9,500 new GBM cases are registered each year [1] with 13,000 deaths [2]. Despite surgical resection of tumor mass followed by radiation and chemotherapy, GBM recurs in nearly all cases [3]. Evidence indicates that tumor cells called cancer stem cells (CSCs) are responsible for resistance to standard GBM therapies [4-6]. These CSCs are endowed with the properties of normal stem cells such as proliferative and differentiative capabilities with the potential to recapitulate the original human tumor in immune-deficient mice from as few as 100-200 cells [7, 8]. Several recent studies indicate that CSCs are responsible for the genesis, propagation, and recurrence of GBM [9-13]. Thus one of the pressing needs in neuro-oncology is to identify CSC populations effectively so that therapeutic strategies aimed at eradicating these cells could be developed. However, since these CSCs exist as non-dividing and non-proliferative cells for extended periods of time [13], they can be indistinguishable due to intra-tumoral heterogeneity and can pose significant challenges to their isolation. Traditionally, cancer stem cells from GBM are isolated from tumor tissues using the antibody raised against cell-surface marker CD133 [8, 14]. Recently, additional markers such as CD44, CD15, L1CAM, alpha-6 integrin and KIF11 have also been reported [15-19]. Nevertheless, conclusive evidence linking stem cell phenotype to these markers is still equivocal. For example, the facts that normal neural stem cells (NSCs) are known to express CD133 and L1CAM [20-23] and even cells lacking CD133 could also be linked to cancer stemness [24-27] may highlight the inconsistency in the assessment of stemness through CD133 or L1CAM expression alone. Similarly, CD15+/− GBM cells revealed no significant differences in tumor forming abilities [28] and therefore, the utility of this protein as a CSC marker could be ambiguous. Additionally, the functional role of CD133 in tumorigenesis is poorly understood, making it harder to connect functional activities of a cancer stem cell precisely with the surface expression of CD133 protein [29]. Thus we believe, given the heterogeneity of GBM tumor, additional cell surface markers with defined functional role will be useful in the identification and characterization of CSCs.
In an attempt to identify novel stem cell markers in GBM, we developed highly sensitive and precise selected-reaction monitoring (SRM) mass spectrometry assays [30, 31] for a target list of 30 cell-surface transmembrane proteins (CTM). These CTM proteins are selected through literature curation and transcriptomic analyses of REMBRANDT [32] and The Cancer Genome Atlas (TCGA) [33] databases. Since CTM proteins play a pivotal role in cellular signaling that emanate from both the exofacial and the cytoplasmic side of the membrane, aberrant expression of these proteins can disrupt normal cell activities as can happen during the process of neoplastic transformation [34, 35]. In the current study, using SRM mass spectrometry, initially we assessed differentially expressed CTM proteins between GBM cancer stem cells (CSCs) and normal neural stem cells (NSCs) and provided evidence for 5 CTM proteins that are overexpressed in CSCs relative to NSCs. A subset of these CTM proteins were also found to be expressed among a subpopulation of pseudopalisading glioma cells in the hypoxic region of the tumor that is also known to be enriched for stem cells factors. Finally, in conjunction with biological assays related to evaluating stemness, we evaluated the utility of one of these CTM proteins as a putative CSC marker with functional role in invasion. This integrative approach is presented in Figure S1.
MATERIALS AND METHODS
Cell culture and reagents
Primary GBM cells were isolated from freshly resected tumor tissues after taking appropriate patient consent and Institutional Review Board approval. These cells were maintained in NeuroCult® NSA medium (Stem Cell Technologies) with B-27 serum-free supplement (Invitrogen), 20 ng/mL epidermal growth factor (EGF) and 20 ng/mL fibroblast growth factor (FGF-2) as described [36, 37]. Briefly, tissue samples were minced into 1 mm3 fragments and digested with Accutase (Sigma) at 37°C for 15-20 minutes. NSA medium was added to quench Accutase activity and cell suspensions were passed through 70 mm nylon mesh. The suspensions were centrifuged at 500×g for 5 minutes, re-suspended in fresh NSA, and plated into T75 flasks pre-coated with laminin (1:100 in PBS; Sigma). With the exception of prospective study (cells were examined at P0) all primary GBM cells were passaged at 1:5 in every 3 to 5 days and evaluated for neurosphere forming abilities. The human GBM cell lines U-87 MG obtained from ATCC were grown in DMEM high glucose (Life Technologies) culture medium supplemented with 10%FBS (Gibco) and pen strep (Gibco). Isogenic cell lines derived from U-87 MG (viz.U87EGFR, U87vIII, U87EGFR+PTEN, and U87vIII+PTEN) were a gift from Dr.Paul Mischel (Ludwig Institute for Cancer Research, San Diego) and grown as described [38]. Neural stem cells (Millipore) and cancer stem cells (Celprogen) were grown according to suppliers’ specifications.
Brain tissue processing for proteomics
Brain tissues were homogenized in tissue lysis buffer (TLB) composed of 100mM n-octyl-glucoside, 1% NP-40, 150mM NaCl, 1mM PMSF, 2mM sodium orthovanadate and 50mM sodium fluoride in 50mM TEAB, pH8.0. All chemicals were purchased from Sigma if otherwise not mentioned. Tissue homogenate was clarified by centrifugation at 10,000×g for 10 min and the supernatant containing the proteins of interest was preserved at −80 0C till further use. For SRM analysis, tissues were reduced and alkylated and digested with trypsin (Promega) and Glu C (Sigma) o/n in dark. Digestion reaction was quenched with TFA and peptides were lyophilized, C18 purified and solubilized in 1%ACN/0.1% FA for SRM analysis.
Detailed experimental procedures are provided in Supplementary Materials and Methods.
RESULTS
Evaluation of differentially expressed CTM proteins in cancer stem cell relative to normal neural stem cell
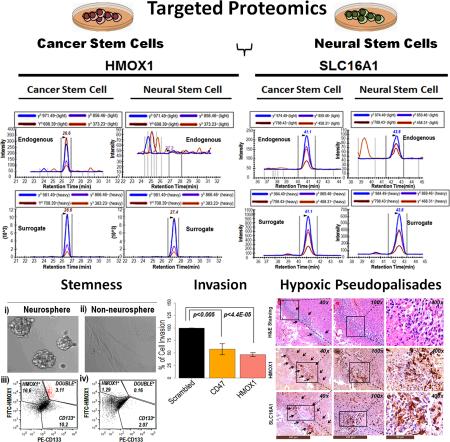
A growing body of evidence indicates that NSCs or progenitors can undergo mutational changes and give rise to GBM CSCs with sustained self-renewal capabilities that can induce tumor growth, drug resistance and recurrence [39-45]. Thus NSCs were used as a control to evaluate differentially expressed CTMs in CSC population. We employed highly sensitive SRM mass spectrometry assays to detect CTM proteins (N=30) since these transmembrane proteins are low abundant representing only 1/3 of the whole human genome [46, 47]. To optimize SRM assays, we obtained representatives of synthetic peptides for each CTM protein labeled (13C15N) C-terminally with either lysine (K) or arginine (R). These synthetic peptides act as the surrogates of endogenous peptides and improved the accuracy in the detection of endogenous peptides in complex biological isolates. The peak areas of surrogate peptides and endogenous peptides were quantified through skyline [48, 49] and presented as a ratio of H (surrogate)/L (endogenous). Each cell type was analyzed four times and the results from these runs were averaged and presented as a bar diagram in figure 1A. The majority CTM proteins exhibited unique patterns of expression differentiating CSC and NSC populations including five CTM proteins SLC16A1, HMOX1, CADMI, SCAMP3 and CLCC1 that exhibited higher CSC than NSC expression levels, and thus may be useful as putative CSC marker. Surface expressions of two CTM proteins SLC16A1 and HMOX1 were further verified by an alternate method flow cytometry (Fig. 1C-E). We isolated primary GBM cells from a GBM tumor (GT-1) and enriched CSCs by growing the primary cells in stem cell permissive conditions that ensured the enrichment of nestin and CD133 cells. Cells grown in such stem-cell mimicking conditions exhibited elevated expressions of putative CSC markers SLC16A1 and HMOX1 on the surface of primary GBM cells relative to NSCs. In essence both flow cytometry analyses and SRM results revealed the potential of a subset of CTM proteins that can distinguish CSCs and NSCs.
Figure 1. Identification and characterization of putative cancer stem cell markers.
A) targeted proteomic analyses of cancer stem cells (CSCs) and neural stem cells (NSCs) revealed the identity of five (denoted with red ‘*’) cell-surface proteins HMOX1, SLC16A1, CADM1, SCAMP3, and CLCC1 that are elevated (log2 expression values) in CSCs relative to NSCs; B) Selected reaction monitoring (SRM) mass spectrometry traces of i) HMOX1 and ii) SLC16A1 indicate elevated expressions in CSCs relative to NSCs. Each SRM trace is composed of surrogate (bottom panel) and endogenous (upper panel) peptides that are co-eluted on the chromatographic gradient; C) flow cytometry analysis of primary GBM cells (obtained from GT-1 tumor) grown in stem cell permissive condition for i) HMOX1 and ii) SLC16A1 expressions; D) flow cytometry analysis of NSCs for i) HMOX1 and ii) SLC16A1 expressions; E) bar diagram highlights elevated surface expressions of HMOX1and SLC16A1 on CSCs relative to NSCs. Results from four experiments are averaged and presented with indicated p values.
Integrated omics analyses of putative CSC markers
First we integrated SRM derived CSC marker (HMOX1, SLC16A1, CLCC1, SCAMP3, and CADM1) expression data with the transcriptomic compendiums of REMBRANDT comprised of 228 GBM, 148 astrocytoma, 67 oligodendroma, and 16 non-tumor brain specimens. We observed that all 5 putative CSC markers were elevated in GBM relative to control and non-GBM brain tumor specimens (Fig. 2A). Similar directionality of expression was also evident in completely independent TCGA dataset (547 GBM specimens and 10 non-tumor brain specimens) (Fig. 2B), indicating commonality in expression of putative CSC markers in GBM across platforms. In the next phase, we compared the RNASeq expression data of 10 GBM tumors across different anatomical structures (7 regions per tumor as determined through H&E staining) of tumor. After identification through H&E staining, each anatomical structure was isolated by laser microdissection and subjected to RNA extraction for RNASeq analysis. This work was done in collaboration with our group and Allen Institute for Brain Science and hosted freely at http://glioblastoma.alleninstitute.org. We observed that these putative CSC markers were differentially regulated between different anatomical structures of the tumors (Fig. 2C-D). Especially, HMOX1 and SLC16A1 expressions were clearly elevated in the areas of GBM tumors that are rich in stem cell factors such as POU5F1, SOX2 and CD133. This region is also hypoxic and characterized by the presence of tumor cells palisading [50] the necrotic areas. Enrichment pattern of these markers is further highlighted in the heatmap and the principal component analysis (PCA) diagram (Fig. 2C-D). The pattern of enrichment of SLC16A1 and HMOX1 in pseudopalisading glioma cells was further validated independently by immunohistochemistry analysis (Fig. 2E) of a GBM tumor. Pseudopalisading and perivascular zones inside the tumor were identified from H&E staining and were subsequently confirmed for SLC16A1 and HMOX1 expressions. Enrichment of SLC16A1 and HMOX1 in the subpopulations of pseudopalisading glioma cells is in good agreement with the RNASeq data. Taken together, our observation highlights that the elevated levels of putative CSC markers were rather regulated through the penetrance of GBM and are independent of the source of specimens being collected, since all tumor and non-tumor specimens were non-overlapping. Finally, we confirmed the expression of these markers at tissue proteome levels by SRM mass spectrometry. Three GBM tissues and two non-tumor brains (retrospective collection) were processed for SRM analysis. We detected 4 of 5 putative cancer markers by SRM mass spectrometry. Our results indicate that all putative markers were elevated in GBM relative to non-tumor brains especially HMOX1 (5.4-fold) and SLC16A1 (5.3-fold) exhibited highest levels of expression in GBM relative to non-tumor brains (Fig. 2F-H). We selected these two proteins (HMOX1 and SLC16A1) to investigate their association with stemness subsequently.
Figure 2. Integrated omics analysis of putative CSC markers.
A) transcriptomic analyses of REMBRANDT compendium for 228 GBM tumors, 9 controls, 148 astrocytomas, 67 oligodendromas indicates elevated expressions of putative CSC markers in GBM. Each column of the heatmap is presented as an average expression value for a specified gene. CNT: non-tumor brain, AST: astrocytoma, and OLI: oligodendroma. B) independent transcriptomic data from TCGA for 547 GBM tumors and 10 controls also revealed elevated expression of putative CSC markers in GBM relative to controls; C) heatmap showing RNA sequencing data of different anatomical structures of GBM tumors (n=10) for putative CSC markers. After identifying major anatomical structures (7 structures per tumor) of a tumor through H&E staining, RNA was isolated from these structures through laser microdissection. LE: leading edge, IT:infiltrating tumor, CT: cellular tumor, MP: microvascular proliferation, HPV: hyperplastic blood vessels, PC: pseudopalisading cells, PZ: perinectrotic Zone; D) principal component analysis (PCA) of the same RNA sequencing data of anatomical structures of GBM tumors highlight enrichment of HMOX1 and SLC16A1 in the hypoxic region of tumors characterized by pseudopalisading cells (PC) (colored in red) encircling the necrotic zone- a histopathological hallmark of GBM. PCs are also enriched for known stem cell factors such as SOX2, CD133, and POU5F1. Elliptical boundaries indicate 95% confidence interval; E) immunohistochemistry analysis of an independent GBM tumor similarly highlights enrichment of SLC16A1 and HMOX1 among a subpopulation of pseudopalisading (highlighted by arrows) glioma cells near perivascular niche; scale bars (brown) for 40X, 100X, and 400X images are appended below each panel. F) SRM mass spectrometry analysis of 3 GBM tumors and 2 non-tumor brains provide confirmatory evidence of putative CSC marker expression at the protein level. SRM traces of G) HMOX1 and H) SLC16A1 expression in GBM and controls. Elevated expressions of putative CSC markers at both RNA and protein levels in GBM relative to controls may highlight systemic role of these proteins in GBM pathogenesis.
Evaluation of HMOX1 and SLC16A1 expressions in proliferative and differentiative conditions in primary GBM cells
Primary GBM cells from the GBM tumor (GT-1) that exhibited highest levels of expression for HMOX1 and SLC16A1 in tissue SRM analysis were grown in culture conditions that are known to be permissive for stem cell proliferation [36]. In parallel, we also grew NSCs as healthy controls in identical conditions. Such stemness permissive culture conditions allowed the enrichment of cells (>80%) expressing nestin- a known stem cell marker in both primary GBM cells (PrGBM) and healthy NSCs (Fig.3A). HMOX1 and SLC16A1 expressions (Fig. 3D-E) were also found very prominent (>90%) among PrGBM populations as was CD133 expression (Fig. 3B). However, unlike HMOX1, low CD133 expression was also visible among NSCs, highlighting the broader-specificity of CD133 expression across both healthy and cancer stem cells. To assess whether HMOX1 and SLC16A1 expression were related to stemness, we forced differentiation in primary GBM cells and NSCs by withdrawing growth factors. The differentiating cells were identified from the rest by increased expression of GFAP and diminished surface expression of known CSC marker CD133 (Fig. 3C) and known stem cell marker nestin. Similarly, HMOX1 and SLC16A1 expression were invisible in differentiating conditions (Fig. 3D-E). These observations raised the possibility that the expressions of HMOX1 and SLC16A1 could be associated with stemness that diminished once these cells were differentiated. In subsequent experiments we investigated if HMOX1 expression was associated with stemness. We selected HMOX1 over other putative CSC markers since the later was mostly elevated in CSCs relative to NSCs (Fig. 1B-C), highly expressed at both mRNA (Fig. 2A-B) and protein level (Fig. 2F-G), and by pseudopalisading cells in the hypoxic region of the tumor (Fig.2E).
Figure 3. Modular expression of putative CSC markers during proliferation and differentiation.
Primary GBM cells (PrGBM) obtained from GT-1 tumor and healthy NSCs were grown in cell culture conditions that are known to be permissive to stemness as evident from the enrichment of A) nestin (green) and B) CD133 (red) expression. Such expression of stem cell factors were diminished when cells were allowed to differentiate as evident in C) from the expression of differentiating marker GFAP expression; enrichment of putative CSC markers D) HMOX1 and E) SLC16A1 in proliferating conditions and subsequent diminishing expression in differentiating conditions are similar to that of known stem cell factors nestin and CD133. This observation may highlight the association of putative CSC marker expression with stemness. Nuclei were stained with DAPI. Images were captured at 10X magnification.
Prospective tumor collection and evaluation of HMOX1 expression during early passage (P0) of tumor cells
Since cell-surface expression of proteins could be subjected to variation introduced through culturing methods, we verified HMOX1 expression on freshly isolated primary tumor cells (P0) from GBM tumor after surgery. Primary cells were isolated from 8 GBM tumors (as confirmed by histopathology) and were allowed to acclimatize for a few days in culture conditions (at passage zero- P0) before flow cytometry analysis. Since CD133 has been known traditionally as a CSC marker in GBM [8, 14], all flow cytometry analyses for the test subject (HMOX1) also accompanied CD133 expression assessment. Three of eight GBM tumors were stained for both CD133 and HMOX1 in single analysis (Fig. 4A-C) while the rest of the five GBM tumors were assessed for HMOX1 and CD133 expressions in parallel (Fig. 4D-H). All tumors (8/8) were found positive for HMOX1, while 7/8 tumors were positive for CD133 expression. Among CD133 positive tumors, an average of 3.8% cells were positive. HMOX1 expression, on the other hand was much more abundant with 12% cells were found to be positive (Fig. 4A-H). A low percentage (2.5%) of cells expressing both CD133 and HMOX1 were visible, suggesting the rarity of stem cell populations as such. In essence, our results indicate that HMOX1 expression on primary GBM cells is not from any artifact of culturing conditions since zero passaged primary GBM cells exhibited a strong pattern of HMOX1 expression similar to a known CSC marker CD133.
Figure 4. Prospective analysis of putative CSC marker HMOX1 expression on early passaged (P0) primary GBM cells.
All primary GBM cells isolated from 8 different GBM tumors, were assessed for known CSC marker CD133 expression along with the test subject HMOX1 by flow cytometry. Primary GBM cells from A) TN-8, B) TG-11, and C) TG-16 were examined for cells double positive for CD133 and HMOX1 while D) TG-12, E) TN-7, F) TG-14, G) TG-15 cells were examined for individual marker expression. Cells double positive for both CD133 and HMOX1 were found to be rare (average 2.4% of the population). H) boxplot showing the distribution of HMOX1 and CD133 expression. As evident, percentage of primary GBM cells expressing HMOX1 (average 12%) are higher than CD133 positive cells (average 3.8%). Additionally, one of eight GBM tumors did have poor or no expression of CD133 expression but exhibited strong HMOX1 expression (data not shown).
Assessment of HMOX1 expression through neurosphere and self-renewal assay
Neurosphere formation in culture is a defining feature for both neural stem cells and cancer stem cells and has been employed for the evaluation of stemness in GBM [17, 51-53]. Here we have investigated whether HMOX1 expression is regulated through neurosphere forming ability of primary GBM cells. This assay was performed for 5 independent GBM tumors. Primary cells from these tumors were isolated and grown in parallel in culture conditions that favored neurosphere (NR) formation or inhibited its formation (nNR). Keeping the limitations of neurosphere formation [54] in mind, we optimized cell culture conditions that ensured the enrichment of known stem cell factor nestin (Fig.S2) among neurospheres relative to cells grown under nNR conditions. Percentage of CD133 and HMOX1 positive cells among NR populations relative to nNR were assessed by flow cytometry (Fig. 5A-G). We observed a 7-fold increase of CD133+ cells in neurosphere culture relative to cells grown in nNR conditions (p<0.009) (Fig. 5F). Neurosphere cells expressing both markers (HMOX1+/CD133+) were also increased by 20-fold (p<0.009) relative to nNR cells (Fig. 5G), suggesting possible regulation of HMOX1 through cancer stemness as much as the condition favors for the enrichment of CD133+ cells. In addition to HMOX1+/CD133+ cells, HMOX1+ cells alone in neurosphere conditions were increased by 9-fold (p<0.02) relative to nNR conditions (Fig. 5F). To evaluate whether HMOX1+ cells can inform us about stemness, we performed qPCR analysis for known stem cell factor SOX2 on cells sorted based on HMOX1 or CD133 from neurosphere culture. We observed 50% enrichment of SOX2 transcripts among HMOX1+ cells (Fig. 5H) relative to starting populations, suggesting possible isolation of putative cancer stem cells through HMOX1 selection. Self-renewability of CSCs through HMOX1 selection was also investigated. Neurosphere forming cells from GT-8 tumor were dissociated and sorted based on HMOX1 expression. Sorted cells (HMOX1+/HMOX1−) and starting populations (unsorted cells) were subjected to limiting dilution sphere forming assay [17] by plating with various seeding densities (50-5000 cells/well in a 96 well plate) and assessing for neurosphere forming abilities within 2 weeks. As evident (Fig.5I-J) HMOX1+ cells can self-renew and form neurosphere with as few as 50 cells/well unlike HMOX1− or unsorted cells that required much higher number of cells to form neurosphere on similar time frame. This observation highlights the fact that HMOX1 selection may support the enrichment of CSC population.
Figure 5. Association of HMOX1 expression with stemness.
Primary GBM cells isolated from five different GBM tumors were grown in neurosphere (NR) or non-neurosphere (nNR) conditions. HMOX1 expression was assessed along with CD133 expression for A) GT-5, B) GT-6, C) GT-7, D) GT-8, and E) GT-9 NR (i) or nNR (ii) forming cells; F) bar diagram shows the enrichment of HMOX1 or CD133 positive cells alone F) and G) double positive cells in NR and n-NR conditions. Student t-test was performed to analyze significance of expression. Individual p values are indicated in the figure; H) q-PCR analysis of sorted cells (based on HMOX1 and CD133 expression) from neurosphere populations (obtained from GT-8 tumor) revealed sox2 expression in both HMOX1 positive cells alone or cells double positive for HMOX1 and CD133; I) limiting dilution sphere forming assay was performed for GT-8 neurosphere forming cells after sorting based on HMOX1 expression. Sorted cells (HMOX1+, HMOX1−, and unsorted cells) were plated with various seeding densities (50-5000 cells/well in a 96 well plate) and assessed for neurosphere forming abilities within 2 weeks. Neurospheres are indicated with red arrows. As evident from the dilution curve in J) HMOX1+ cells can self-renew and form neurosphere with as few as 50 cells/well unlike HMOX1− or unsorted cells that required much higher number of cells to form neurosphere on similar time frame. Scale (white) represents 100μm.
Biological regulation of HMOX1 expression on tumor cells could be modulated through TGFβ
Since acquisition of EMT phenotype provides a means for the enrichment of CSCs [55, 56], it was anticipated that the expressions of CSC cell-surface markers including ours could be regulated by this process. Among the known mediators of EMT, TGFβ plays a dual role in oncogenesis as it can propel the growth of the tumor or can inhibit the proliferation of normal epithelial cells and astrocytes [57-60]. TGFβ-induced EMT process and CSC enrichment could also be regulated through additional modes of endogenous control mechanisms [61]. For example, in conjunction with EGFR or its mutant form EGFRvIII, TGFβ can augment stemness or suppress it through the activities of tumor suppressor PTEN [62-66]. This multimodal regulation of TGFβ may add additional elements of complexity in the development of CSC niche and may also impact the expression of CSC markers. Since TGFβ is known to promote stemness in GBM [67], we hypothesized that HMOX1 being highly expressed on CSC, could also be regulated through TGFβ signaling network. To test the hypothesis, we treated U-87 MG cells with/without TGFβ that ensured C-terminal phosphorylation of SMAD2 or diminished it respectively (Fig. 6A). C-terminal phosphorylation of SMAD2 indicated the activation of TGFβ signaling in our optimized experimental condition. Changes in HMOX1 expression following TGFβ treatment was measured by SRM mass spectrometry. Expression of a known TGFβ inducible CTM protein TGFBI (BIGH3) was also measured simultaneously as a positive marker. Our results (Fig. 6B-C) indicated that the directionality of HMOX1 expression (2.5-fold increase) following TGFβ treatment relative to control was similar to that of a known TGFβ inducible protein TGFBI (5-fold increase), highlighting a possible regulatory mechanism of HMOX1 expression through TGFβ signaling. In the next step, we investigated if TGFβ mediated regulation of HMOX1 surface expression could also be modulated through signaling pathways that are known to interact with TGFβ network and can augment stemness such as EGFR/EGFRvIII and pathway that suppresses stemness such as PTEN. We used isogenic U-87 MG cell line that was engineered to overexpress EGFR or EGFRvIII alone or in combination with the tumor suppressor PTEN. These cells were treated with or without TGFβ, and the surface expression of HMOX1 was evaluated by flow cytometry. TGFβ treatment induced >2.5folds increase in HMOX1+ cells in EGFR or EGFRvIII isogenics relative to U-87 MG cell lines (Fig. 6D). However, HMOX1 expression was abrogated (~4-fold and ~1.5-fold respectively) when EGFR or EGFRvIII isogenic cell lines also overexpressed the tumor suppressor PTEN (Fig. 6D). These results highlight that HMOX1 surface expression could be regulated through TGFβ signaling, and that the endogenous activator (e.g. EGFR or EGFRvIII) and inhibitor (e.g. PTEN) of TGFβ signaling might also interfere with the expression of HMOX1.
Figure 6. HMOX1 expression is associated with TGFβ responsiveness and GBM invasion.
A) Addition of TGFβ in the medium of U-87 MG cells grown in serum free culture conditions induced TGFβ signaling as evident from the c-terminal phosphorylation of SMAD2 in TGFβ treated cells relative to untreated cells. Such treatment left little or no effect on un-phosphorylated SMAD2. GAPDH was used as loading controls; B) TGFβ treatment induced the expression (5fold changes) of a known TGFβ inducible protein TGFBI (BIGH3) relative to untreated cells as measured by SRM mass spectrometry; C) TGFβ treatment also induced the expression (2.5fold) of HMOX1 as evident from the SRM traces; D) flow cytometry analysis of U-87 MG isogenic cell lines expressing EGFR or EGFRvIII in combination with or without PTEN were treated with TGFβ. Percentage increase in HMOX1+ populations following TGFβ treatment was evaluated relative to control (untreated) cells and are presented with indicated p values. As evident, TGFβ responsiveness of HMOX1 can be modulated through tumor suppressor PTEN; E) flow cytometry analysis of si-RNA mediated inhibition of HMOX1 and CD47 synthesis revealed reduced surface expression of these proteins; F) si-RNA treatment against HMOX1 reduced GBM cell invasion (at indicated p values) similar to that of a known invasive proteins CD47 (at indicated p values).
HMOX1 expression is associated with GBM invasiveness and poor prognosis
Since HMOX1 is regulated through TGFβ signaling, we sought to determine if HMOX1 in conjunction with TGFβ might regulate tumor invasion. To test the hypothesis, we inhibited HMOX1 expression through siRNA mediated silencing in astrocytoma cell line U-87 MG and measured the ability of these cells to invade through extracellular matrix. The efficiency of siRNA mediated gene silencing was evaluated by both qPCR-at the transcript level (Fig. 6E) and by flow cytometry- on the cell-surface (Fig. 6E). Results from gene silencing experiments revealed greater than two-fold reduced expression of HMOX1 in comparison to non-targeting RNAs (Fig. 6E). To evaluate the impact of the knock downs on cell migration and invasion, siRNA or non-targeting RNA treated cells were seeded in transwell chambers and the degree of cell invasion was evaluated as percentage of cells invaded in comparison to non-siRNA targeting cells. siRNA-mediated inhibition of known invasive protein CD47 was used as a positive control. The resultant cell invasion from three independent experiments is presented in figure 6F. As evident, the silencing of HMOX1 resulted 46.76% ± 2.27SEM, reduced cell invasion similar to the known invasive protein CD47 (57.74% ± 6.32SEM reduced cell invasion). In essence, our results indicate that HMOX1 expression favors tumor invasion and could be regulated through TGFβ signaling.
In general, invasive phenotype is associated with poor prognosis in cancer. Since HMOX1 was found to be involved in invasion directly, we sought to investigate if tumor resection would reflect changes in the level of HMOX1 that is possibly related to prognosis. While many cell-surface proteins with transmembrane domain/s (CTM) are known to be secreted or released in the blood stream by various mechanisms [68, 69], it was investigational for us to determine if HMOX1 is released in the blood of GBM patients. As a precursory step, we employed SRM mass spectrometry assays and determined the level of HMOX1 in the blood of GBM patients (Fig. S3). The observations that HMOX1 is in the blood of GBM patients and is associated with invasion provided us the right impetus to further study if HMOX1 concentration in the blood is predictive of prognosis. In a prospective study we collected blood plasmas from 10 GBM patients at preoperative stage, and continued collection longitudinally over a week post-surgery. Blood circulating HMOX1 was measured by ELISA at pre and post-operative stages for each patient. Our results (Table S3) indicate (Fig.S4) that circulating HMOX1 level was decreased (18%±5.1SEM) within 10 days post-surgery for 8 of 10 GBM patients that might be related to improved prognosis. However, additional follow-ups of patients will be required in the future to predict progression-free survival that is currently ongoing. Nevertheless, post-surgery decline of HMOX1- a protein, which is associated with stemness and invasion in GBM, may also highlight its potential as a putative blood biomarker.
DISCUSSION
The presence of CSCs in various cancers offers a plausible explanation to the genesis, progression, and therapeutic refractoriness of the disease. CSCs have the ability to self-renew and differentiate into multiple distinct populations, thereby generating heterogeneous cell types [10]. In GBM, various CSCs with proliferative and differentiative capabilities have been identified that can also recapitulate the original tumor in immune-compromised mice [8, 17, 18, 70]. It is believed that these CSCs may arise from NSCs, which share common stem cell factors and have similar proliferative and differentiative capabilities [39, 40, 42, 45, 71, 72]. Because of such commonality of marker expression between healthy NSCs and CSCs, development of targeted therapeutic strategies that are aimed at eradicating CSCs should also be specific enough to spare healthy cells so that non-specific damages caused to healthy cells due to treatments could be minimized. However, inconsistency in identifying CSCs through CD133, which is also expressed in healthy NSCs [21-23], renders the development of such targeted treatment options unrealistic and highlights the need for additional CSC markers. To identify uniquely expressed CSC markers, we filtered out those cell-surface protein markers, that are commonly expressed by both NSCs and CSCs. Using targeted proteomics we identified five cell-surface markers SLC16A1, HMOX1, CADM1, SCAMP3 and CLCC1, that are elevated in CSCs relative to NSCs (Fig. 1A-G).
Comparative transcriptomic analysis with independent datasets from REMBRANDT and TCGA also revealed elevated expressions of all five putative CSC markers in GBM relative to controls. These markers were poorly expressed in lower grade brain tumors (Fig. 2A), suggesting these markers may have specific roles in the genesis of GBM. SRM mass spectrometry of GBM tissues also revealed elevated expressions of these markers at the protein level relative to non-tumor brain controls (Fig. 2F-H). Taken together, our results indicate that the expression of these putative CSC markers is systemic across the GBM specimens tested, at both the transcriptome and proteome levels and such expression might be regulated through the emergent properties and penetrance of GBM.
Through prospective flow cytometric analysis of eight GBM tumors, first we demonstrated that HMOX1 was predominantly expressed on the surface of primary GBM cells relative to tumor cells expressing CD133 (Fig. 4A-H). This is an important observation since isolating CSCs using CD133 alone remains challenging [25, 26, 73, 74]. Despite the abundance of HMOX1 expression on primary GBM cells, we found cells expressing both CD133 and HMOX1 (HMOX+/CD133+) were rare. Given the heterogeneity of GBM, the existence of multiple CSC markers in solid tumors [18, 75, 76], and the fact that HMOX1+/CD133− GBM cells also express stem cell factor sox2 (Fig. 5H), it is possible that HMOX1+ cells may also exist as isolated populations and could still inform about stemness independent of CD133+ populations [18, 75, 76]. The hypothesis that HMOX1 is associated with stemness is further supported by our in vitro studies (Figures 3D, 4A-C, and 5A-G), which demonstrate higher HMOX1 expression under stem cell culture conditions. There was also increase in the number of HMOX1+/CD133+ populations grown in NR culture relative to cells grown in nNR condition (Fig 5A-G), suggesting possible synergistic regulation of these stem cell populations similar to what we observed in prospective study (Fig. 4A-C). On differentiation of primary GBM cells (GT-1), which were prior grown in stem cell condition, we observed reduction in HMOX1 expression relative to stem-cell mimicking condition (Fig. 3D), which might suggest additionally that HMOX1 expression could be associated with stemness.
Expression of putative CSC markers that we report here clearly captures the histopathology of GBM. For example, localization of two putative CSC markers SLC16A1 and HMOX1 to the areas of GBM tumor comprised of densely-packed tumor cells palisading the necrotic region - a histopathological hallmark of GBM may further substantiate the role of these proteins in GBM pathogenesis. Principal component analysis and heatmap (Fig. 2C-D) of RNAseq data obtained from laser microdissection of H&E-defined GBM anatomical structures (n=10 patients each with 7 anatomical structures of tumor) clearly highlighted the enrichment pattern of SLC16A1 and HMOX1 among pseudopalisading cells similar to that of known stem cell factors such as POU5F1, SOX2, and CD133. This observation was independently validated through immunohistochemistry analysis (Fig. 2E) of a GBM tumor that also supported the pattern of SLC16A1 and HMOX1 enrichment among a subpopulation of pseudopalisading glioma cells-presumably CSCs. This region of tumor is the cynosure of active growth and may support the development and maintenance of a stem cell niche. Since the pseudopalisading region is hypoxic [50, 77], we suspect that elevated HMOX1 expression on tumor cells may point towards the protective effect of this protein against hypoxia induced damages and inflammatory response in tumor cells [78]. For example, in squamous cell carcinoma, Sokolowska et al observed that mouse expressing HMOX1+/+ favored the progression of invasive carcinoma, linking oxidative stress response and growth factor accumulation with tumor progression [79]. Although mechanistic underpinnings as to how HMOX1 can promote tumor survival is an unresolved issue, it is possible that enzymatic activity of HMOX1 may play a crucial role in promoting tumor survival. One testable hypothesis is that since HMOX1 generates carbon monoxide (CO) through enzymatic reaction, it can alter the metabolic state of GBM cells and offset cellular respiration under hypoxic conditions, which would promote the survival and maintenance of CSCs in a non-differentiated state. In general CO has a cytoprotective effect [80, 81] since it limits excessive production of toxic reactive oxygen species (ROS) in mitochondria by inducing a mild-uncoupling state [82-86]. The cytoprotective effect of HMOX1 through CO generation could be adopted by CSCs to reduce ROS levels and survive in the hypoxic region of the tumor. If so, understanding the mechanism for ROS regulation in CSCs may help in the development of powerful therapeutic strategies in future. We observed differential regulation of HMOX1 among primary GBM cells grown in stem cell-mimicking conditions relative to differentiated (Fig. 3D) conditions. The fact that HMOX1 expression was diminished following differentiation of CSCs (Fig. 3D) or in non-neurosphere conditions (Fig. 5A-H) may highlight the requirements for different levels of HMOX1 activity and diverse metabolic demands set forth by tumor cells in these states. However, HMOX1 plays a complex role in the growth and progression of tumor and may vary in different cancers. For example, Weigel et al noticed that in prostate cancer cells HMOX1 is localized in the nucleus in an inactive state that accelerated DNA stress and damage as an early event of carcinogenesis [84]. On the contrary, in GBM we found HMOX1 is predominantly localized on the cell-surface of CSCs with poor or no expression on healthy NSCs (Fig. 1B-C). Thus we believe the activity and regulation of HMOX1 in GBM tumor cells would be different from that in prostate cancer cells. With cytoprotective effect, HMOX1 expression may ensure greater survival and maintenance of CSCs in the hypoxic zone of the tumor.
In addition to the hypoxic environment, a multitude of signaling events including those emanating from endothelial and stromal cells may impact the composition and dynamics of the microenvironment and add elements of complexity to the regulation of cancer stemness [56, 87-90]. Several growth factors, chemokines, colony stimulating factors and cytokines have been reported in the microenvironment that can drive tumor proliferation and invasion [91, 92]. Among cytokines, TGFβ has been found to be elevated in high grade gliomas that confers poor prognosis [93, 94], and additional studies have shown that TGFβ can enhance self-renewal properties of CSCs [67, 95]. Additionally, in embryonic stem cells (ESCs), the convergence of TGFβ signaling with the hypoxia mediated signaling has also been elucidated [96], highlighting the likelihood of TGFβ playing similar roles in stemness for both normal and CSCs. However, the mechanistic regulation of stemness through TGFβ signaling will be different between healthy and cancer cells [64]. Here, we have demonstrated that the expression of putative CSC marker HMOX1 can be regulated through canonical TGFβ (Fig.6C) signaling, which is in good agreement with prior reports of promoting stemness through TGFβ [67]. Likewise, endogenous activators of TGFβ signaling such as EGFR or EGFRvIII increased HMOX1 expression but concomitant expression of tumor suppressor PTEN diminished such effects (Fig. 6D). The antagonistic effect of TGFβ and PTEN is also reported in other cancers [63,64]. Thus we believe that the induction of HMOX1 through TGFβ and its regulation through the endogenous activators and inhibitors of TGFβ may be indicative of the role that TGFβ plays to enhance stemness and tumor growth- the regulation of which in normal cells would certainly be different. It is well established that cancer progression towards malignancy is accompanied by increased cell migration, invasion and resistance to apoptosis. We observed reduced cell invasion (46.76% ± 2.27SEM) when GBM cells were treated with siRNA directed against HMOX1 (Fig. 6F), pointing towards the direct involvement of HMOX1 in GBM cell invasion. It is therefore likely that elevated expression of HMOX1 on CSCs may confer these cells with the ability to metastasize and populate distant regions of the brain. Since metastatic mechanisms maneuver signaling external to the tumor it might have also played a role in the regulation of invasion-mediating proteins including HMOX1, which we found in the blood of GBM patients (Fig.S3). Thus it was anticipated that low level of HMOX1 would be indicative of good prognosis. In fact, decline of circulating HMOX1 level (18%±5.1SEM) in the blood for majority of the GBM patients tested (8 out of 10 patients) within 10 days post-surgery may support such a notion (Supplementary Fig. S4). However, translation of HMOX1 as a prognostic indicator or therapeutic target into clinics will require a significant amount of work and resources. Nevertheless, the lessons we have learned from the current study may spur the development of future applications.
Conclusion
In this study we have demonstrated that HMOX1 expression is associated with stemness and invasion in GBM. Being expressed among pseudopalisading cells that also express a number of known stem cell factors, HMOX1 expression substantiates GBM histopathological hallmarks nicely. The fact that HMOX1 expression is uniquely elevated in CSCs which predominantly reside in the hypoxic region of GBM tumor and the observation that disruption of its expression inhibits GBM cell invasion, suggests HMOX1 could be a potential target for anti-GBM therapy.
Supplementary Material
SIGNIFICANCE STATEMENT.
Cancer stem cell plays an important role in the genesis and therapeutic refractoriness of GBM. Identification and characterization of cancer stem cells may help in the development of targeted therapeutics aimed at the elimination of these cells. Here, using targeted mass spectrometry and functional assays we have shown that elevated expression of HMOX1 is associated with stemness and invasion in GBM, highlighting the potential of HMOX1 as a target for therapeutic development.
ACKNOWLEDGEMNTS
We are grateful to Dr.Paul Mischel of UC San Diego for providing us with the U87 isogenic cell lines. We are also thankful to Dr.Cory Funk and Katherine Rouleau of Institute for Systems Biology, Seattle for critical discussions and suggestions. Mass spectrometry and flow cytometry facilities at ISB, Seattle, and flow cytometry resource at Fred Hutchinson Cancer Research Center, Seattle are duly acknowledged. The work was supported by NIH/NCI NanoSystems Biology Cancer Center grant U54 CA151819A (LH) and NIH R01 NS070289 (CC).
Abbreviations
- CSC
Cancer Stem Cell
- NSC
Neural Stem Cell
- CTM
Cell-surface proteins with transmembrane domains
- PCA
Principal Component Analysis
Footnotes
AUTHOR CONTRIBUTIONS
D.G. designed the study, performed experiments and prepared the manuscript; D.G., I.U., L.P, and K.U. performed experiments; P.H. and L.P developed GBM cell lines; C.C. performed craniotomy and collected plasmas; L.E.H., S.R., and C.C. performed IHC; all authors edited and revised the manuscript; L.H. and C.C. provided funding supports and supervised research.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicate no potential conflicts of interest.
References
- 1.Dolecek TA, Propp JM, Stroup NE, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neurooncology. 2012;14(Suppl 5):v1–49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mrugala MM AJ, Kiem HP. Outside the box--novel therapeutic strategies for glioblastoma. Cancer J. 2012;18:51–58. doi: 10.1097/PPO.0b013e318243f785. [DOI] [PubMed] [Google Scholar]
- 3.Weller M, Cloughesy T, Perry JR, et al. Standards of care for treatment of recurrent glioblastoma--are we there yet? Neuro-oncology. 2013;15:4–27. doi: 10.1093/neuonc/nos273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 5.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 6.Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Brien CA, Pollett A, Gallinger S, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 8.Singh SK CI, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 9.Vaillant F, Asselin-Labat ML, Shackleton M, et al. The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res. 2008;68:7711–7717. doi: 10.1158/0008-5472.CAN-08-1949. [DOI] [PubMed] [Google Scholar]
- 10.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 11.Lathia JD, Mack SC, Mulkearns-Hubert EE, et al. Cancer stem cells in glioblastoma. Genes & development. 2015;29:1203–1217. doi: 10.1101/gad.261982.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson M, Hassiotou F, Nowak A. Glioblastoma stem-like cells: at the root of tumor recurrence and a therapeutic target. Carcinogenesis. 2015;36:177–185. doi: 10.1093/carcin/bgu243. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Li Y, Yu TS, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh SK, Venugopal C, Hallett R, et al. Pyrvinium targets CD133 in human glioblastoma brain tumor-initiating cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015 doi: 10.1158/1078-0432.CCR-14-3147. [DOI] [PubMed] [Google Scholar]
- 15.Bao S, Wu Q, Li Z, et al. Targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer Res. 2008;68:6043–6048. doi: 10.1158/0008-5472.CAN-08-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anido J, Saez-Borderias A, Gonzalez-Junca A, et al. TGF-beta Receptor Inhibitors Target the CD44(high)/Id1(high) Glioma-Initiating Cell Population in Human Glioblastoma. Cancer Cell. 2010;18:655–668. doi: 10.1016/j.ccr.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 17.Son MJ, Woolard K, Nam DH, et al. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell stem cell. 2009;4:440–452. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lathia JD, Gallagher J, Heddleston JM, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell stem cell. 2010;6:421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venere M, Horbinski C, Crish JF, et al. The mitotic kinesin KIF11 is a driver of invasion, proliferation, and self-renewal in glioblastoma. Science translational medicine. 2015;7:304ra143. doi: 10.1126/scitranslmed.aac6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nature neuroscience. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- 21.Sykes AM, Huttner WB. Prominin-1 (CD133) and the Cell Biology of Neural Progenitors and Their Progeny. Advances in experimental medicine and biology. 2013;777:89–98. doi: 10.1007/978-1-4614-5894-4_6. [DOI] [PubMed] [Google Scholar]
- 22.Uchida N, Buck DW, He D, et al. Direct isolation of human central nervous system stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee A, Kessler JD, Read TA, et al. Isolation of neural stem cells from the postnatal cerebellum. Nature neuroscience. 2005;8:723–729. doi: 10.1038/nn1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beier D, Hau P, Proescholdt M, et al. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 25.Meng X, Li M, Wang X, et al. Both CD133+ and CD133− subpopulations of A549 and H446 cells contain cancer-initiating cells. Cancer science. 2009;100:1040–1046. doi: 10.1111/j.1349-7006.2009.01144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Sakariassen PO, Tsinkalovsky O, et al. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. International journal of cancer Journal international du cancer. 2008;122:761–768. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- 27.Lottaz C, Beier D, Meyer K, et al. Transcriptional profiles of CD133+ and CD133- glioblastoma-derived cancer stem cell lines suggest different cells of origin. Cancer Res. 2010;70:2030–2040. doi: 10.1158/0008-5472.CAN-09-1707. [DOI] [PubMed] [Google Scholar]
- 28.Kenney-Herbert E, Al-Mayhani T, Piccirillo SG, et al. CD15 Expression Does Not Identify a Phenotypically or Genetically Distinct Glioblastoma Population. Stem cells translational medicine. 2015;4:822–831. doi: 10.5966/sctm.2014-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horst D, Scheel SK, Liebmann S, et al. The cancer stem cell marker CD133 has high prognostic impact but unknown functional relevance for the metastasis of human colon cancer. The Journal of pathology. 2009;219:427–434. doi: 10.1002/path.2597. [DOI] [PubMed] [Google Scholar]
- 30.Picotti P, Aebersold R. Selected reaction monitoring-based proteomics: workflows, potential, pitfalls and future directions. Nature methods. 2012;9:555–566. doi: 10.1038/nmeth.2015. [DOI] [PubMed] [Google Scholar]
- 31.Schiess R, Wollscheid B, Aebersold R. Targeted proteomic strategy for clinical biomarker discovery. Molecular oncology. 2009;3:33–44. doi: 10.1016/j.molonc.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madhavan S, Zenklusen JC, Kotliarov Y, et al. Rembrandt: helping personalized medicine become a reality through integrative translational research. Molecular cancer research : MCR. 2009;7:157–167. doi: 10.1158/1541-7786.MCR-08-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cancer Genome Atlas Research N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okumura S, Baba H, Kumada T, et al. Cloning of a G-protein-coupled receptor that shows an activity to transform NIH3T3 cells and is expressed in gastric cancer cells. Cancer science. 2004;95:131–135. doi: 10.1111/j.1349-7006.2004.tb03193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teh JL, Chen S. Glutamatergic signaling in cellular transformation. Pigment cell & melanoma research. 2012;25:331–342. doi: 10.1111/j.1755-148X.2012.00983.x. [DOI] [PubMed] [Google Scholar]
- 36.Pollard SM, Yoshikawa K, Clarke ID, et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell stem cell. 2009;4:568–580. doi: 10.1016/j.stem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Ulasov IV, Shah N, Kaverina NV, et al. Tamoxifen improves cytopathic effect of oncolytic adenovirus in primary glioblastoma cells mediated through autophagy. Oncotarget. 2015;6:3977–3987. doi: 10.18632/oncotarget.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang MY, Lu KV, Zhu S, et al. Mammalian target of rapamycin inhibition promotes response to epidermal growth factor receptor kinase inhibitors in PTEN-deficient and PTEN-intact glioblastoma cells. Cancer Res. 2006;66:7864–7869. doi: 10.1158/0008-5472.CAN-04-4392. [DOI] [PubMed] [Google Scholar]
- 39.Alcantara Llaguno S, Chen J, Kwon CH, et al. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009;15:45–56. doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alcantara Llaguno SR, Chen J, Parada LF. Signaling in malignant astrocytomas: role of neural stem cells and its therapeutic implications. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:7124–7129. doi: 10.1158/1078-0432.CCR-09-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim DA, Cha S, Mayo MC, et al. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro-oncology. 2007;9:424–429. doi: 10.1215/15228517-2007-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Y, Guignard F, Zhao D, et al. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005;8:119–130. doi: 10.1016/j.ccr.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hollon TC, Price RL, Kwon CH, et al. Mutations in glioblastoma oncosuppressive pathways pave the way for oncomodulatory activity of cytomegalovirus. Oncoimmunology. 2013;2:e25620. doi: 10.4161/onci.25620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price RL, Song J, Bingmer K, et al. Cytomegalovirus contributes to glioblastoma in the context of tumor suppressor mutations. Cancer Res. 2013;73:3441–3450. doi: 10.1158/0008-5472.CAN-12-3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goffart N, Kroonen J, Rogister B. Glioblastoma-initiating cells: relationship with neural stem cells and the micro-environment. Cancers. 2013;5:1049–1071. doi: 10.3390/cancers5031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fagerberg L, Jonasson K, von Heijne G, et al. Prediction of the human membrane proteome. Proteomics. 2010;10:1141–1149. doi: 10.1002/pmic.200900258. [DOI] [PubMed] [Google Scholar]
- 47.Ghosh D, Lippert D, Krokhin O, et al. Defining the membrane proteome of NK cells. Journal of mass spectrometry : JMS. 2010;45:1–25. doi: 10.1002/jms.1696. [DOI] [PubMed] [Google Scholar]
- 48.Bereman MS, MacLean B, Tomazela DM, et al. The development of selected reaction monitoring methods for targeted proteomics via empirical refinement. Proteomics. 2012;12:1134–1141. doi: 10.1002/pmic.201200042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maclean B, Tomazela DM, Abbatiello SE, et al. Effect of collision energy optimization on the measurement of peptides by selected reaction monitoring (SRM) mass spectrometry. Analytical chemistry. 2010;82:10116–10124. doi: 10.1021/ac102179j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rong Y, Durden DL, Van Meir EG, et al. 'Pseudopalisading' necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. Journal of neuropathology and experimental neurology. 2006;65:529–539. doi: 10.1097/00005072-200606000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Laks DR, Masterman-Smith M, Visnyei K, et al. Neurosphere formation is an independent predictor of clinical outcome in malignant glioma. Stem Cells. 2009;27:980–987. doi: 10.1002/stem.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 53.Reynolds BA, Rietze RL. Neural stem cells and neurospheres--re-evaluating the relationship. Nature methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- 54.Pastrana E, Silva-Vargas V, Doetsch F. Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell stem cell. 2011;8:486–498. doi: 10.1016/j.stem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells - what challenges do they pose? Nature reviews Drug discovery. 2014;13:497–512. doi: 10.1038/nrd4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johns LD, Babcock G, Green D, et al. Transforming growth factor-beta 1 differentially regulates proliferation and MHC class-II antigen expression in forebrain and brainstem astrocyte primary cultures. Brain research. 1992;585:229–236. doi: 10.1016/0006-8993(92)91211-v. [DOI] [PubMed] [Google Scholar]
- 58.Rich JN, Zhang M, Datto MB, et al. Transforming growth factor-beta-mediated p15(INK4B) induction and growth inhibition in astrocytes is SMAD3-dependent and a pathway prominently altered in human glioma cell lines. The Journal of biological chemistry. 1999;274:35053–35058. doi: 10.1074/jbc.274.49.35053. [DOI] [PubMed] [Google Scholar]
- 59.Aigner L, Bogdahn U. TGF-beta in neural stem cells and in tumors of the central nervous system. Cell and tissue research. 2008;331:225–241. doi: 10.1007/s00441-007-0466-7. [DOI] [PubMed] [Google Scholar]
- 60.Golestaneh N. B. M. TGF-beta, neuronal stem cells and glioblastoma. Oncogene. 2005;24:5722–5730. doi: 10.1038/sj.onc.1208925. [DOI] [PubMed] [Google Scholar]
- 61.Massague J. TGFbeta signalling in context. Nature reviews Molecular cell biology. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li DM, Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res. 1997;57:2124–2129. [PubMed] [Google Scholar]
- 63.Chow JY, Quach KT, Cabrera BL, et al. RAS/ERK modulates TGFbeta-regulated PTEN expression in human pancreatic adenocarcinoma cells. Carcinogenesis. 2007;28:2321–2327. doi: 10.1093/carcin/bgm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hjelmeland AB, Hjelmeland MD, Shi Q, et al. Loss of phosphatase and tensin homologue increases transforming growth factor beta-mediated invasion with enhanced SMAD3 transcriptional activity. Cancer Res. 2005;65:11276–11281. doi: 10.1158/0008-5472.CAN-05-3016. [DOI] [PubMed] [Google Scholar]
- 65.Chen W, Zhong X, Wei Y, et al. TGF-beta Regulates Survivin to Affect Cell Cycle and the Expression of EGFR and MMP9 in Glioblastoma. Molecular neurobiology. 2015 doi: 10.1007/s12035-015-9121-6. [DOI] [PubMed] [Google Scholar]
- 66.Pala A, Karpel-Massler G, Kast RE, et al. Epidermal to Mesenchymal Transition and Failure of EGFR-Targeted Therapy in Glioblastoma. Cancers. 2012;4:523–530. doi: 10.3390/cancers4020523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Penuelas S, Anido J, Prieto-Sanchez RM, et al. TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell. 2009;15:315–327. doi: 10.1016/j.ccr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 68.Shao H, Chung J, Balaj L, et al. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nature medicine. 2012;18:1835–1840. doi: 10.1038/nm.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li XJ, Hayward C, Fong PY, et al. A blood-based proteomic classifier for the molecular characterization of pulmonary nodules. Science translational medicine. 2013;5:207ra142. doi: 10.1126/scitranslmed.3007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lathia JD, Li M, Sinyuk M, et al. High-throughput flow cytometry screening reveals a role for junctional adhesion molecule a as a cancer stem cell maintenance factor. Cell reports. 2014;6:117–129. doi: 10.1016/j.celrep.2013.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y, Yang J, Zheng H, et al. Expression of mutant p53 proteins implicates a lineage relationship between neural stem cells and malignant astrocytic glioma in a murine model. Cancer Cell. 2009;15:514–526. doi: 10.1016/j.ccr.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng H, Ying H, Yan H, et al. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455:1129–1133. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beier D HP, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP. CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 74.Kemper K, Sprick MR, de Bree M, et al. The AC133 epitope, but not the CD133 protein, is lost upon cancer stem cell differentiation. Cancer Res. 2010;70:719–729. doi: 10.1158/0008-5472.CAN-09-1820. [DOI] [PubMed] [Google Scholar]
- 75.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell stem cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Piccirillo SG, Combi R, Cajola L, et al. Distinct pools of cancer stem-like cells coexist within human glioblastomas and display different tumorigenicity and independent genomic evolution. Oncogene. 2009;28:1807–1811. doi: 10.1038/onc.2009.27. [DOI] [PubMed] [Google Scholar]
- 77.Brat DJ, Castellano-Sanchez AA, Hunter SB, et al. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64:920–927. doi: 10.1158/0008-5472.can-03-2073. [DOI] [PubMed] [Google Scholar]
- 78.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Was H, Sokolowska M, Sierpniowska A, et al. Effects of heme oxygenase-1 on induction and development of chemically induced squamous cell carcinoma in mice. Free radical biology & medicine. 2011;51:1717–1726. doi: 10.1016/j.freeradbiomed.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vieira HL, Alves PM, Vercelli A. Modulation of neuronal stem cell differentiation by hypoxia and reactive oxygen species. Progress in neurobiology. 2011;93:444–455. doi: 10.1016/j.pneurobio.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 81.Almeida AS, Figueiredo-Pereira C, Vieira HL. Carbon monoxide and mitochondria-modulation of cell metabolism, redox response and cell death. Frontiers in physiology. 2015;6:33. doi: 10.3389/fphys.2015.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lo Iacono L, Boczkowski J, Zini R, et al. A carbon monoxide-releasing molecule (CORM-3) uncouples mitochondrial respiration and modulates the production of reactive oxygen species. Free radical biology & medicine. 2011;50:1556–1564. doi: 10.1016/j.freeradbiomed.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 83.Queiroga CS, Almeida AS, Vieira HL. Carbon monoxide targeting mitochondria. Biochemistry research international. 2012;2012:749845. doi: 10.1155/2012/749845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wegiel B, Gallo D, Csizmadia E, et al. Carbon monoxide expedites metabolic exhaustion to inhibit tumor growth. Cancer Res. 2013;73:7009–7021. doi: 10.1158/0008-5472.CAN-13-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Diehn M, Cho RW, Lobo NA, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dong XZ, Zhang M, Wang K, et al. Sanguinarine inhibits vascular endothelial growth factor release by generation of reactive oxygen species in MCF-7 human mammary adenocarcinoma cells. BioMed research international. 2013;2013:517698. doi: 10.1155/2013/517698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2009;23:43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
- 88.Lu J, Ye X, Fan F, et al. Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1. Cancer Cell. 2013;23:171–185. doi: 10.1016/j.ccr.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010;10:138–146. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hossain A, Gumin J, Gao F, et al. Mesenchymal Stem Cells Isolated From Human Gliomas Increase Proliferation and Maintain Stemness of Glioma Stem Cells Through the IL-6/gp130/STAT3 Pathway. Stem Cells. 2015;33:2400–2415. doi: 10.1002/stem.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Witz IP. The tumor microenvironment: the making of a paradigm. Cancer microenvironment : official journal of the International Cancer Microenvironment Society. 2009;2(Suppl 1):9–17. doi: 10.1007/s12307-009-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Persano L, Rampazzo E, Basso G, et al. Glioblastoma cancer stem cells: role of the microenvironment and therapeutic targeting. Biochemical pharmacology. 2013;85:612–622. doi: 10.1016/j.bcp.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 93.Rich JN. The role of transforming growth factor-beta in primary brain tumors. Frontiers in bioscience : a journal and virtual library. 2003;8:e245–260. doi: 10.2741/992. [DOI] [PubMed] [Google Scholar]
- 94.Joseph JV, Balasubramaniyan V, Walenkamp A, et al. TGF-beta as a therapeutic target in high grade gliomas - promises and challenges. Biochemical pharmacology. 2013;85:478–485. doi: 10.1016/j.bcp.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 95.Imamura T, Hikita A, Inoue Y. The roles of TGF-beta signaling in carcinogenesis and breast cancer metastasis. Breast cancer. 2012;19:118–124. doi: 10.1007/s12282-011-0321-2. [DOI] [PubMed] [Google Scholar]
- 96.Wierenga AT, Vellenga E, Schuringa JJ. Convergence of hypoxia and TGFbeta pathways on cell cycle regulation in human hematopoietic stem/progenitor cells. PloS one. 2014;9:e93494. doi: 10.1371/journal.pone.0093494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cobbs CS, Matlaf L, Harkins LE. Methods for the detection of cytomegalovirus in glioblastoma cells and tissues. Methods in molecular biology. 2014;1119:165–196. doi: 10.1007/978-1-62703-788-4_11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.