Abstract
MicroRNAs (miRNAs) are a class of small regulatory RNAs involved in gene regulation. The regulation is effected by either translational inhibition or transcriptional silencing. In vertebrates, the importance of miRNA in development was discovered from mice and zebrafish dicer knockouts. The miRNA-9 (miR-9) is one of the most highly expressed miRNAs in the early and adult vertebrate brain. It has diverse functions within the developing vertebrate brain. In this article, the role of miR-9 in the developing forebrain (telencephalon and diencephalon), midbrain, hindbrain, and spinal cord of vertebrate species is highlighted. In the forebrain, miR-9 is necessary for the proper development of dorsoventral telencephalon by targeting marker genes expressed in the telencephalon. It regulates proliferation in telencephalon by regulating Foxg1, Pax6, Gsh2, and Meis2 genes. The feedback loop regulation between miR-9 and Nr2e1/Tlx helps in neuronal migration and differentiation. Targeting Foxp1 and Foxp2, and Map1b by miR-9 regulates the radial migration of neurons and axonal development. In the organizers, miR-9 is inversely regulated by hairy1 and Fgf8 to maintain zona limitans interthalamica and midbrain–hindbrain boundary (MHB). It maintains the MHB by inhibiting Fgf signaling genes and is involved in the neurogenesis of the midbrain–hindbrain by regulating Her genes. In the hindbrain, miR-9 modulates progenitor proliferation and differentiation by regulating Her genes and Elav3. In the spinal cord, miR-9 modulates the regulation of Foxp1 and Onecut1 for motor neuron development. In the forebrain, midbrain, and hindbrain, miR-9 is necessary for proper neuronal progenitor maintenance, neurogenesis, and differentiation. In vertebrate brain development, miR-9 is involved in regulating several region-specific genes in a spatiotemporal pattern.
Keywords: miR-9, telencephalon, diencephalon, midbrain, hindbrain, spinal cord
Introduction
MicroRNAs (miRNAs) are endogenously expressed 20–25 nucleotide-long RNA molecules whose identification began with lin-4 in Caenorhabditis elegans, where it was shown to be involved in gene regulation at the larval developmental stage.1 Since then, miRNAs have been reported to regulate multiple biological processes from development to cell proliferation, growth regulation, and apoptosis.2
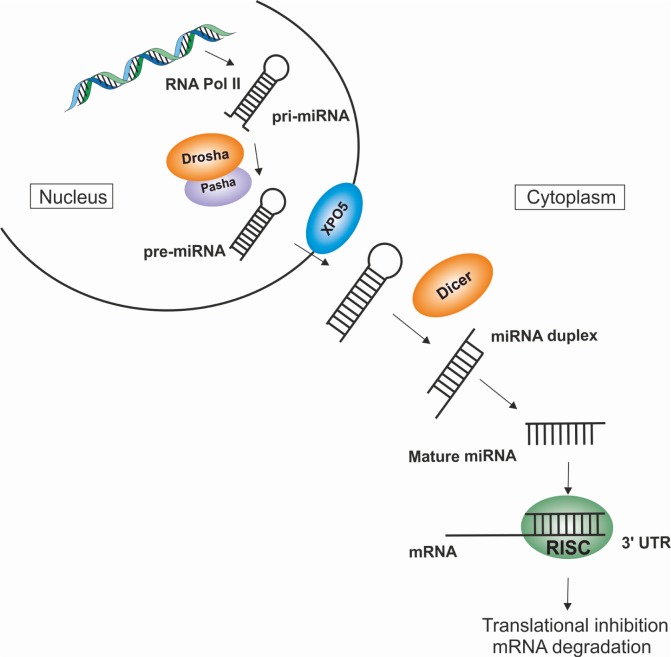
MiRNAs are transcribed by RNA Pol II as primary transcripts (primary miRNA, pri-miRNAs), which are usually several kilobases long and contain local stem-loop structures.3 The maturation of the miRNA begins with the cleavage of larger hairpins into smaller hairpins called the precursor miRNA (pre-miRNA) by Drosha.4 In the case of Drosophila, an additional protein Pasha was identified and was shown to have pri-miRNA-processing capability. Pasha knockouts (KOs) showed the accumulation of pri-miRNA suggesting that Drosha works together with Pasha.5 Post nuclear processing, the pre-miRNAs are transported into the cytoplasm where they are processed again by the enzyme dicer, generating the mature miRNA.6 The miRNA duplexes are incorporated into a protein complex consisting of Argonaute and accessory proteins called the pre-miRNA-induced silencing complex (pre-RISC).7 The removal of the passenger strand from the miRNA duplex results in the formation of the mature RISC complex, which then targets suitable mRNAs resulting in posttranscriptional repression or the destruction of the mRNA target (Fig. 1).8–10
Figure 1.
miRNA biogenesis.
Notes: The schematic figure shows miRNA biogenesis, processing, maturation, and regulation. The miRNAs are transcribed within the nucleus by RNA polymerase II from the noncoding region of the genome. The transcribed pri-miRNA is processed by Drosha/Pasha microprocessor complex into pre-miRNA. The pre-miRNA is exported from the nucleus into the cytoplasm through the nuclear membrane protein, XPO5. In the cytoplasm, the endonuclease dicer processes the pre-miRNA into miRNA duplex. The miRNA duplex is unwound by a helicase and forms the mature miRNA (~18–25 nucleotides). The mature miRNA is incorporated into the RISC with Argonaute protein as a catalytic component. The miRNA–RISC complex binds at the 3′UTR of the mature mRNA and regulates posttranslational gene expression by translation inhibition or mRNA degradation (transcriptional silencing).
Abbreviations: miRNA, microRNA; pre-miRNA, precursor miRNA; pri-miRNA, primary miRNA; RISC, RNA-induced silencing complex; 3′UTR, 3′ untranslated region; XPO5, Exportin 5.
miRNAs in Development
The miRNAs are involved in numerous cellular and biological events.11–13 They are ideal candidates for the fine-tuning of gene expression due to their nature of having specific spatiotemporal expression patterns.14 The importance of miRNAs in development is revealed by the loss of miRNA-processing enzymes and its machinery proteins (dicer, Drosha, dgcr8, Argonaute).15–19
Dicer significance in development
Dicer is essential for the processing of mammalian miRNA. Dicer knockout (KO) studies in mice and zebrafish16,17 have provided insights into the role of miRNAs from a developmental perspective. Dicer ablation results in embryonic lethality in mice16 and zebrafish;17 thus, conditional KO (CKO) serves as a tool to study the role of dicer in the vertebrate system.
Conditional dicer KO in development
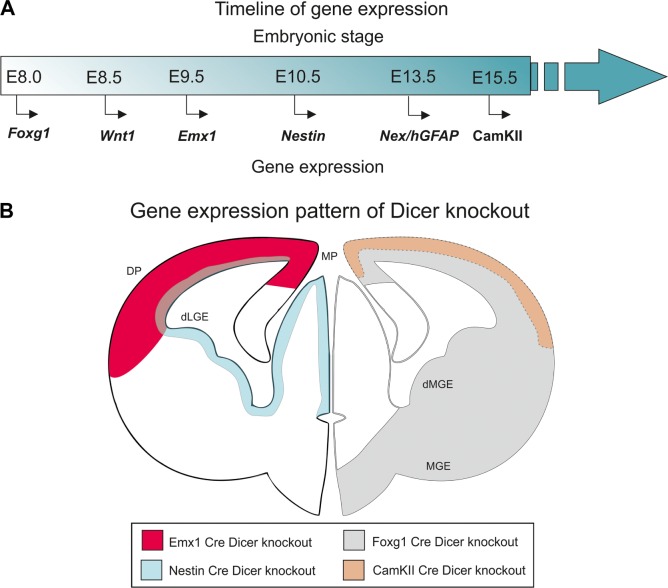
The functional implication for the necessity of miRNA in development comes from the zebrafish maternal–zygotic (MZy) dicer KO embryo, a CKO. The MZy dicer shows abnormalities in brain development with the reduction in the formation of brain vesicles.20 In mice, the dicer CKOs were generated using cre–lox mechanism. The normal expression patterns of the brain-specific genes in which the CKOs were created are as follows: Foxg1-cre21 at E8.0, Wnt1-cre22 at E8.5, Emx1-cre at E9.5,23–26 Nestin-cre at E10.5,27 Nex-cre28,29 and hGFAP-cre30,31 at E13.5, and CaMKII-α-cre at E15.532 (Table 1, Fig. 2A and B).
Table 1.
Summary of dicer Knockout studies.
| SPECIES | KNOCKOUTS/MUTANT | GENE EXPRESSION DURING EMBRYONIC STAGE | EXPRESSION STUDIED STAGE | PHENOTYPE OF THE DICER MUTANTS | DOWNREGULATED miRNA | REFERENCES |
|---|---|---|---|---|---|---|
| Zebrafish | Dicer1 KO | – | – | Homozygous and trans-heterozygous appeared normal till 8 dpf (day post fertilization). Later showed severe developmental growth arrest and died after 14–15 days. | NS | Wienholds et al17 |
| MZy (maternal and zygotic) dicer1 Knockout | – | – | Normal axis formation and differentiate multiple cell types, but shows abnormal morphogenesis during gastrulation, brain formation, somatogenesis and heart development. | NS | Giraldez et al20 | |
| Mice | Dicer1 KO | Dicer expression—E4171 | E7.5–11.5 | At 7.5, embryo appeared small with morphological abnormalities and lacks primitive streak marker, brachyury. Dicer mutant is arrested before the body plan is configured during gastrulation. Lacks Oct 4—a marker for ES cell maintenance and proliferation. E8.5—necrotic embryo. E9.5–11.5—empty and necrotic decidua. |
NS | Bernstein et al16 |
| Foxg1-cre-Dicer1 conditional KO | Foxg1/BF-1 expression—E821 | E10.5–12.5 | 1) At E11.5, the neuroepithelial markers: Prominin1, Notch signaling inhibitor Numb, Sox2 (sex determination region Y box 2) and Numb inhibitor Musashi have not been altered; and the genes involved in forebrain patterning; Foxg1, Pax6, Ngn1, Emx2, Olig2, Dlx2 have also not been altered. 2) The Nestin, Sox9, Erb2 from the radial glial cells of the VZ have been reduced in the dorsal telencephalon by E11.5 and E12.5. 3) E11.5–12.5: The neurons and basal progenitors are greatly affected in dicer KOs. 4) There is an increase in apotopsis from E11.5 onwards. The size of the telencephalon is greatly reduced by E14.5. |
miR-9 and miR-124 is depleted by E11.5 and undetected in the dorsal telencephalon. | Nowakowski et al33 | |
| Wnt1-cre Dicer conditional KO | Wnt1 expression—E8.522 | E12.5, E14.5 and E18.5 | 1) Dicer mutant fetuses exhibited dramatic malformation of the tectum and cerebellum and the eyelids were open. 2) The skeletal structures that are derived from the cranial NC were lost or mostly ablated in Dicer mutant mice. 3) Deletion of Dicer in the NC cells resulted in the malformation of the dorsal root ganglia, enteric nervous system and sympathetic ganglia. 4) Dicer is required for the specification of dopaminergic neurons. |
The expression of miR-9, miR-124 and miR-218 in the midbrain and rostral hind-brain area was mostly eliminated. | Huang et al37 | |
| Emx1-cre-Dicer conditional KO | Emx1 expression—E9.526 | E10.5, E12.5, E13.5, E14.5, E15.5 | 1) The neural progenitor markers have been reduced from E15.5 onwards in the VZ. 2) They have normal karyotype, but have enlarged nuclei. 3) Increased apoptosis. |
The miR-181b and miR-125b expressed in NSCs have been absent in dicer mutant. | Kawase-Koga et al172 | |
| E13.5, E15.5, E16.5, E17.5, E18.5 | 1) Survived till P30. 2) The cortex was significantly smaller and the hippocampus was not detectable due to the lack of neurogenesis. 3) At E17.5, the CP was slightly enlarged and the VZ/SVZ and IZ were greatly reduced. 4) The early born neurons were reduced at E15.5. 5) The Tbr1 cells were observed in the CP and IZ. |
The miR-17, miR-181 was undectable in the cortex of E13.5, E15.5 and P1. | Kawase-Koga et al36 | |||
| E13.5, E14.5, E15.5, E16.5, E18.5 | 1) Has smaller cortex and not distinguishable by P0 showing dicer ablation affecting hippocampal progenitor development. 2) The transcription factor Lhx2 reduced in the hippocampus by E13.5 onwards. 3) The proliferative cells were reduced at E15.5 in hippocampal neuroepithelial cells and DG progenitors. 4) The hippocampal markers Wnt7b, Lef1 have been reduced and Prox1 was absent in the hippocampus by E16.5. 5) Ectopic Reelin was observed in cortex at E18.5 suggesting abnormal CR neuron migration and mature neurons were reduced in the hippocampus at E18.5. 6) Ectopic Tbr2 was observed in the cortical progenitor zone and in hippocampal region at E18.5. |
miR-9 and let-7a was undectable by E16.5. | Li et al28 | |||
| Nestin-cre-Dicer conditional KO | Nestin expression—E10.527 | E18.5 | The dorsal and lateral cortical wall was thinner and the ventricles were larger. The proliferating cells from VZ and SVZ were reduced. The post-mitotic neurons was thinner in the CP, in the IZ was expanded in cortex at E18.5 indicating the late born neurons were reduced. |
– | Kawase-Koga et al36 | |
| E13.5, E14.5, E15.5, E16.5, E18.5 | 1) Died after birth. 2) Hippocampal morphogenesis occurs normally but has reduced hippocampal size. 3) The proliferative cells were reduced at E15.5 in hippocampal neuroepithelial cells, DG progenitors and increased apoptosis observed. 4) Reelin expression was normal in E15.5 and E18.5. 5) The transcription factor Lhx2 was reduced in the hippocampus from E18.5 onwards. 6) The hippocampal marker Prox1 has been reduced in the hippocampus by E18.5. Neurogenesis was affected. 7) Ectopic Tbr2 was observed in the cortical progenitor zone and in hippocampal region at E18.5. |
miR-9 and let-7a was undectable by E14.5. | Li et al28 | |||
| E15.5, E16.5 | 1) Microcephaly, gross enlargement of the lateral ventricles and marked cortical thinning in the embryos. 2) Progenitors were distributed throughout the cortex, instead of confined to the VZ and SVZ. 3) CR neurons are numerous and their distribution is dysmorphic. |
NS | Mcloughlin et al111 | |||
| Nex-cre-Dicer conditional KO | Nex expression—E13.529 | P1, P22 | 1) Died by P23.38 2) The hippocampal morphology was not significantly affected, but has smaller brain. 3) The hippocampal markers Wnt7b have been reduced in the hippocampus by P1. 4) The NeuN was greatly reduced in CA3 pointing to out defects in neurogenesis. 5) Apoptosis was seen in CA3 and DG cells of the hippocampus at P22. |
miR-9 and let-7a was undectable by P22. | Li et al28 | |
| hGFAP-cre Dicer | E13.531 | E15.5, P1 | The brain lipid binding protein (BLBP) is distributed in the VZ/SVZ and CP, rather than in MZ. The RG is translocated in the pial at P1–P5. The retraction of radial process is reduced in the postnatal cerebral cortex. Notch and its ligand Jag2 signaling are enhanced. |
miR-9 and miR-124 reduced by P1 | Zhang et al34 | |
| 1) Phenotype of Bergmann glia (BG) cells was studied. 2) Smaller and underdeveloped cerebellum with aberrant BG morphology. 3) Notch 1 target gene expression affected. |
miR-9 levels decreased. | Kuang et al30 | ||||
| CamkII-cre-Dicer conditional KO | CamKII expression—E15.532 | P0, P15, P21 | 1) Microcephaly, increased ventricle size and reduction of size in white matter. 2) Motor impairments. |
miR-132 (dendritic outgrowth) reduced in the cortex and hippocampus. miR-124 was reduced in the cortex. | Davis et al35 |
Abbreviations: NS, not specified; E, embryonic day; P, postnatal day; VZ, ventricular zone; SVZ, subventricular zone; IZ, intermediate zone; CP, cortical plate; MZ, mantle zone; LGE, lateral ganglionic eminence; DG, dentate gyrus; RG, radial glia.
Figure 2.
Time line of gene expression and gene expression pattern in the developing brain.
Notes: (A) The time line of gene expression in mouse embryo. (B) The outline of the schematic sketch is adapted from Grant et al.169 Modified and reused under the terms of a CC-BY-NC 3.0 license. This shows that the area will be devoid of dicer in the CKO with the representative genes in the cre/lox system.
Abbreviation: CKOs, conditional knockouts.
The dicer CKOs from Foxg1-cre, Emx1-cre, and Nestin-cre show that miRNAs are necessary in the early forebrain development and Nex-cre, CAMKII-cre, and GFAP-cre in the postnatal forebrain development.28,33–36 The Wnt1-cre dicer demonstrates the necessity of the miRNAs in midbrain and hindbrain development.37 Finally, Wnt1-cre and hGFAP-cre dicer shows the necessity of miRNAs in hindbrain development.30,37 Some of the CKO embryos died early or postnatal.28,35,38 The dicer CKOs provide valuable information on the role of miRNAs in development which include cell proliferation, neurogenesis, migration, maturation, and differentiation. The dicer KO studies are described in detail in the following sections in context with the role of miRNA-9 (miR-9) in brain development.
Role of miR-9 in development
The miR-9 is one of the highly expressed miRNAs in the developing vertebrate brain and involved in several cellular functions and development.39–41 The miR-9 is conserved among the vertebrates and specifically expressed in the central and peripheral nervous system;39–42 it has also been reported to be present in multiple copies (Table 2).41,43 The retention of multiple copies has also been linked to the differential expression of its paralogs and specialization in their function.44 The first evidence of its function in the nervous system comes from mouse,45 where it has been reported to be involved in the patterning of brain structures and in cellular events like proliferation, maturation, and differentiation of neurons in the brain.46–49 The expression pattern of miR-9 in the vertebrates is summarized in Table 3.
Table 2.
miR-9 and their location in chromosomes of different species.
| SPECIES | miR-9 VARIANTS | CHROMOSOMES | EXPRESSION PATTERN | REFERENCES |
|---|---|---|---|---|
| Mouse | miR-9-1 | 3 | Proliferative zone in telencephalon | Shibata et al80 |
| miR-9-2 | 13 | Telencephalon | ||
| miR-9-3 | 7 | Proliferative zone in telencephalon | ||
| Xenopus | miR-9a-1 | GL173063.1 | Forebrain, eye, midbrain and hindbrain | Bonev et al,48 Walker and 126 Harland |
| miR-9a-2 | GL172712.1 | |||
| miR-9b/miR-9-3 | GL172838.1/GL172658.1 | |||
| Zebrafish | miR-9-1 | 16 | Telencephalon | Nepal et al130 |
| miR-9-2 | 10 | Retina, telencephalon | ||
| miR-9-3 | 25 | Telencephalon | ||
| miR-9-4 | 22 | Posterior brain regions, telencephalon | ||
| miR-9-5 | 5 | Telencephalon | ||
| miR-9-6 | 7 | Hypothalamus, telencephalon | ||
| miR-9-7 | 2 | Telencephalon | ||
| Chicken | miR-9-1 | 28 | NS | |
| miR-9-2 | Z | NS |
Abbreviation: NS, not specified.
Table 3.
MiR-9 expression in the developing brain of different species.
| SPECIES | BRAIN VESICLE | miR-9 EXPRESSION | DEVELOPMENTAL STAGE | REFERENCES |
|---|---|---|---|---|
| Mice | Telencephalon | + | E9, E9.5, E10, E11.5, E12.5, E14.5, E15.5, E16.5, E18.5 | 33, 47, 80, 173 |
| Diencephalon | − | E9, E9.5, E10, E11.5 | ||
| Midbrain | NS | |||
| MHB | NS | |||
| Hindbrain | + | E9.5, E10 | ||
| Spinal cord | + | E9.5, E10 | ||
| Xenopus | Forebrain | + | Stage 19, 24, 28, 30, 36 | 48, 126 |
| Midbrain | + | |||
| MHB/ZLI | − | |||
| Hindbrain | + | |||
| Spinal cord | − | |||
| Zebrafish | Telencephalon | + | 20–30 hpf | 39, 43, 46, 128 |
| Diencephalon | + | |||
| Midbrain | + | |||
| MHB | − | 30–35 hpf | ||
| Hindbrain | + | 20–30 hpf | ||
| Spinal cord | + | |||
| Chick | Telencephalon | + | HH18, 20, 22–25 | 42, 83, 132, 167 |
| Diencephalon | + | |||
| Midbrain | + | |||
| MHB | NS | |||
| Hindbrain | + | |||
| Spinal cord | + | HH11–29 |
Abbreviation: NS, not specified.
Apart from its prominent role in development, miR-9 has been correlated with several other cellular roles and pathology.2,50 The most significant of these roles is its association with cancer, where aberrant levels of the miRNA have been identified in tumor formation or progression.51 In the case of gastric cancer, miR-9 knockdowns have shown an inhibitory role in the proliferation of tumor cells.52 Contrastingly, in the case of ovarian cancer cells, the overexpression of miR-9 was shown to be useful in the suppression of tumor proliferation.53 The miR-9 has also been shown to regulate interferon-regulated genes and MHC class I molecules.54 In this review, the role of miR-9 in early brain development is summarized.
Brain Development
The developing central nervous system (CNS) is regionalized into four distinct regions, namely prosencephalon (forebrain), mesencephalon (midbrain), rhombencephalon (hindbrain), and spinal cord. The vertebrate forebrain is further divided anteriorly into telencephalon (prospective cerebral hemisphere) and posteriorly into diencephalon (prospective thalamus and hypothalamus). The rhombencephalon is subdivided into metencephalon (prospective pons and cerebellum) and myelencephalon (prospective medulla oblongata).55,56 The organizers mediate the anterior–posterior (AP) axis and dorsoventral (DV) axis development. The organizers are special group of cells that produce secreted molecules and act as long-lasting coordinators of many cellular events like cell fate, survival, proliferation, maturation, and differentiation. The organizers along the AP axis are anterior neural ridge (ANR)/anterior neural border (ANB), zona limitans interthalamica (ZLI), and isthmus or midbrain–hindbrain boundary (MHB).57,58
Cell biology of neurogenesis
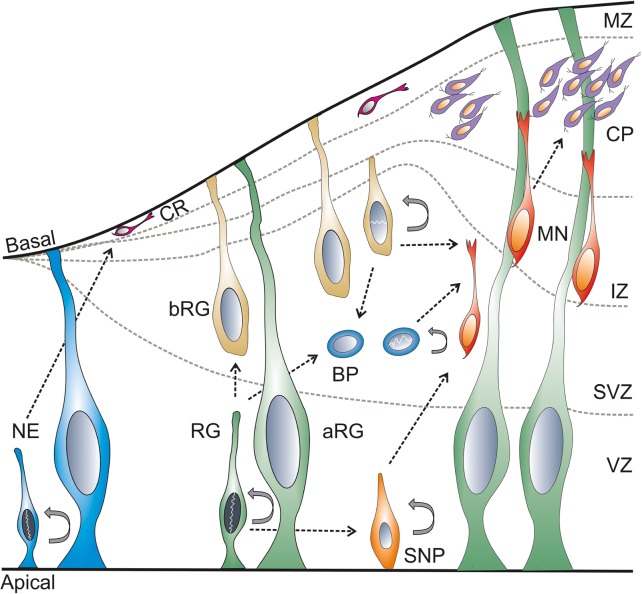
In the early phase of development, the neural plate and neural tube are made of a single layer of neuroepithelial cells. The neuroepithelial cells are primary neural stem cells (NSCs) in the developing neural tube. These NSCs divide symmetrically resulting in massive expansion prior to neurogenesis.59 The telencephalic neurogenesis in mice takes place between E10 and E17.33,60 In chicken, the telencephalic neurogenesis takes place between E4 (HH24) and E10,61 while the neurogenesis of the optic tectum takes place between E3 (HH18) and E20 (HH45).62,63 In zebrafish, the telencephalic formation and the morphogenetic movement take place between 18-hour postfertilization (hpf) and 5 day postfertilization (dpf),64 and the tectal neurogenesis starts by 24 hpf.65 Cortical neurogenesis takes place between stage 22 and 36,66,67 and tectal neurogenesis between stage 35 and 58 in Xenopus.68 In the early phase, the NSCs divide asymmetrically and form the NSC and Cajal–Retzius (CR) neurons. The generation of CR neurons is modulated by miR-9.47,60 The CR neurons69,70 migrate from the ventricular zone (VZ) to the basal lamina that forms the preplate. As the development progresses, the preplate forms the subventricular zone (SVZ), intermediate zone (IZ), the cortical plate (CP), and the marginal/mantle zone (MZ).71 In later stages, the NSCs give rise to the neuronal progenitor cell types: the apical (cells from the VZ) and basal progenitors (cells from the SVZ) and neurons (reside in the MZ). The apical and basal progenitors consist of radial glia and intermediate progenitors.72 These progenitors mature and form the neurons in the MZ (Fig. 3).59,73,74
Figure 3.
Cell biology of neurogenesis.
Notes: During development, at the early stage, the NE divides asymmetrically and forms NE and CR neurons. These CR neurons migrate to the basal region and form the preplate; where at later stages the preplate forms the SVZ, IZ, and CP. At later stages, the RG divides and gives rise to different types of cells, namely SNP and BP. According to the residence of the RG, the cells are known as aRG and bRG cells. The bRG mostly resides in the SVZ. The SNP matures and migrates to CP and MZ. The MNs might also arise from RG and BP. The NE, RG, bRG, BP, and SNP are capable of self-dividing.
Abbreviations: NE, neuroepithelial; CR, Cajal–Retzius; RG, radial glial; aRG, apical radial glia; bRG, basal radial glia; BP, basal/intermediate progenitors; SNP, small neuronal progenitor; SVZ, subventricular zone; MZ, mantle zone; CP, cortical plate; IZ, intermediate zone; MNs, motor neurons.
The miR-9 is expressed throughout the cellular development of neurons, ie, proliferation, neurogenesis, migration, maturation, and differentiation.45,74–78 The miR-9 is also one of the specific markers for neuronal cells.79 It is expressed in the proliferating and differentiating cells in the telencephalon, diencephalon, and optic tectum.39 The miR-9 regulates proliferation by targeting several transcription factors (Foxg1, Meis2, hairy1, hes1, her genes, etc.).46–49,80 The miR-9 suppresses TLX (human homolog of the tailless gene; also known as nuclear receptor subfamily 2, group E member 1 [Nr2e1]) expression to negatively regulate proliferation and accelerate neural differentiation.75 Along with miR-124, miR-9 acts as a key factor in regulating neuronal differentiation.77 Axon development in the developing cortex is regulated by miR-9 via targeting Map1b.81 Neurite outgrowth/migration is also controlled by miR-9 through its regulation of Foxp1 and Foxp2.82,83
Telencephalon development
Telencephalon is derived from the rostral margin of the neural plate.84 During early gastrulation, the formation of the neural plate (neural induction) is promoted by fibroblast growth factor (Fgf) and repressed by transforming growth factor-β and Wnt signaling. Following neural induction, the rostral margin neural plate cells known as ANR in mouse or the ANB in zebrafish which express Fgf8 is essential for the development of telencephalon.85–87
During the early telencephalon development, at the closing end of the neural tube, the telencephalic midline is formed. Within the midline, there are three signaling centers: the dorsal midline, the rostral midline, and the ventral midline. The dorsal midline expresses several bone morphogenetic protein (BMP) genes.88 The ANR/ANB and the nearby area becomes the rostral midline that expresses several Fgf genes including Fgf8.89 The ventral midline is defined by the expression of sonic hedgehog (SHH) signaling.90
The DV patterning of telencephalon is the first step in the formation of the neocortex. The dorsal half forms the pallium, later develops into the cortex, and the ventral half forms the subpallium that later develops into the basal ganglia.57 The pallium is divided into four main subregions: dorsal, medial, lateral, and ventral pallium. The DV patterning is defined by the generation of different cell types from the dorsal and ventral precursor cells; dorsally, they give rise to glutamatergic neurons and form the neocortex and hippocampus, and ventrally they give rise to GABAergic neurons and form the medial ganglionic eminence (MGE) and lateral ganglionic eminence (LGE), which form the striatum and globus pallidus.91 The transcription factor, Pax6, is expressed in the dorsal telencephalon, whereas Gsh2 is expressed in the ventral telencephalon. The counteraction between Pax6 and Gsh2 forms the pallio-subpallial boundary (PSB; Fig. 4).92,93
Figure 4.
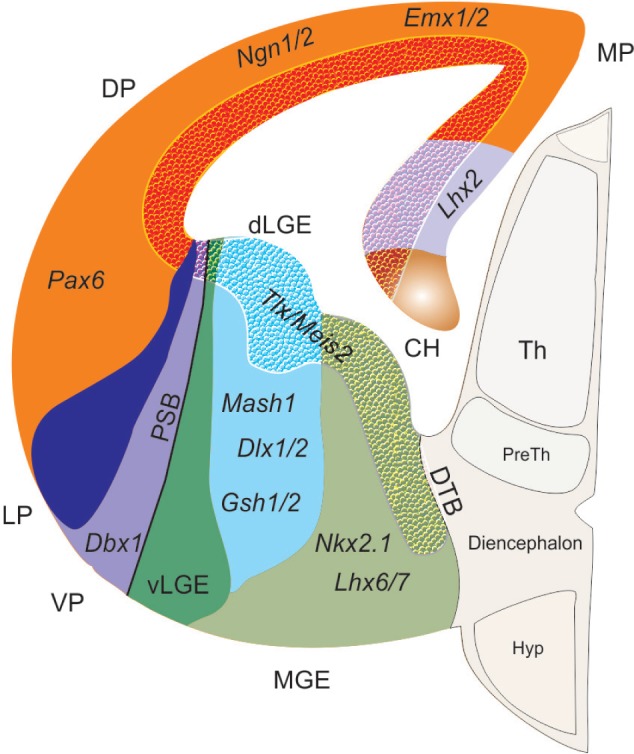
Gene expression in the developing telencephalon.
Notes: The schematic sketch is adapted from Grant et al.169 Modified and reused under the terms of a CC-BY-NC 3.0 license. The telencephalon is divided into dorsal (pallium) and ventral (subpallium) telencephalon. The pallium expresses Pax6 and the subpallium expresses Gsh2. The repression of Pax6 and Gsh2 expression leads to the formation of PSB. The DP progenitor expresses Pax6, Emx1/2, and Ngn1/2. The MP expresses Lhx2, while the VP expresses Dbx1. The ventral subpallium is subdivided into MGE and LGE, where the MGE progenitors express Gsh2, Mash1, and Dlx1/2, and LGE expresses Nkx2.1 and Lhx6/7. The textured area shows Tlx and Meis2 expression in the MP, DP, dLGE and dMGE.
Abbreviations: CH, cortical hem; MP, medial pallium; DP, dorsal pallium; VP, ventral pallium; LP, lateral pallium; dLGE, dorsal lateral ganglionic eminence; vLGE, ventral ganglionic eminence; MGE, medial ganglionic eminence; PSB, pallial-subpallial boundary; DTB, diencephalon–telencephalon boundary; Th, thalamus; preTh, prethalamus; Hyp, hypothalamus.
Overall, the telencephalon is induced by the repression of Wnt signaling. The formation of the telencephalic midline involves the interaction of Shh ventrally, Fgf genes rostrally, Bmp laterally, and Wnt genes dorsally. The signaling centers regulate the expression of Emx2, Pax6, and Gsh2, which is necessary for the regulation of region-specific neurogenic genes such as Ngn1 and Mash1 on dorsal and ventral telencephalon, respectively (Fig. 4).91,94
Role of miR-9 in telencephalon development of mice
In mice, miR-9 has three variants such as miR-9-1, miR-9-2, and miR-9-3 that express the same mature product, miR-9.95 The miR-9-2 is abundantly expressed in the telencephalon, while miR-9-1 and miR-9-3 are observed in the proliferative zone (Table 2).80 The miR-9 expression is first observed at E9.0 stage in the telencephalic primordium. At E10.5, miR-9 is observed in optic vesicles and spinal cord, where they are dorsolaterally graded in the cortex with the subcortex sparsely expressing miR-9. The caudal pallium expresses miR-9 at E10.5; later at E11.5, in the medial pallium, and at E12.5, the expression was restricted to the medial pallium and retained in the MZ of the entire cortex including ventral pallium.47 The hippocampus that is derived from the medial pallium expresses miR-9.28 Histology shows that miR-9 is expressed throughout the proliferative zone of the developing telencephalon80,96 and in some differentiating neurons of the neocortex.49,96,97
The forkhead box protein G1 (Foxg1) is the earliest expression marker in the telencephalon and is expressed at E8.0.98 The Foxg1-cre dicer KO shows that there are no obvious changes in the telencephalic markers: the pan-telencephalic marker such as FoxG1 and dorsal telencephalic markers such as Pax6, Emx2, and Ngn2, and ventral telencephalic markers such as Olig2 and Dlx2. The miR-9 was reduced and undetectable in the telencephalon at E11.5 stage, when neurogenesis occurs. The Nestin (a progenitor marker) in the VZ radial progenitor cells was reduced, with a loss of transcription factor, Sox9 (a co-enhancer of Nestin gene)99,100 and Erb2 receptor (mediator for the formation of radial glial cells via neuregulin1 signaling).101 The postmitotic cells were markedly reduced in E12.5 telencephalon. The CR cells have been depleted in this dicer KO. The proposition of basal progenitor Tbr2 cells has been increased in E11.5 ventral telencephalon and appears at the telencephalic wall, where these cells are not usually found. The telencephalon size was markedly reduced due to apoptosis from E12.5 onward.33
Interestingly, Foxg1 is upregulated and miR-9 is downregulated in the olfactory and GnRH neurons in Dlx5 (distal-less homeobox gene) mutant mice. Thus, proving that miR-9 regulates Foxg1 and Dlx5 facilitates the production of miR-9.102 Dlx5 is one of the earliest markers for the rostral ectoderm. The Dlx5 is expressed in the ANR at E7.5, ventral cephalic epithelium at E8.5 and from E9.5 onward in the telencephalon.103,104 However, in the telencephalon, the interaction between Dlx5 and miR-9 is yet to be established.
The Emx1 (empty spiracles homolog 1) is specifically expressed in the dorsal telencephalon and observed at E9.5.23–25 The Emx1-cre dicer KO shows that the miR-9 is barely detectable from E10.5 telencephalon in the VZ neural progenitors cells. Detailed study indicates that the miR-9 expression is abrogated in the preplate (E11.5), CP (E12.5), and VZ of the telencephalon in comparison to the normal phenotype.96 There is no difference in the apical and basal progenitor population, but there is a reduction in the cortical neuronal layer, which was attributed to apoptosis via depletion of miRNAs. Until E13.5, the apical (Pax6-expressing cells) and basal (Tbr2-expressing cells) progenitor cell division and progression were not affected;96 however, at E14.5, there was a decrease in the mitotic apical and basal progenitors.36,96 At E15, the cortices had higher Tbr1 and Tuj1 expression indicating that early neurogenesis is altered. At E17.5, the cerebral cortices were smaller, and the VZ and IZ were reduced with slightly enlarged CP. The Emx1-cre dicer KO mice survived until postnatal day 30 (P30).36
The Emx1-cre dicer KO also shows severe defects in the hippocampus with a reduction in miR-9 at E16.5. The Emx1-cre has reduced hippocampal neuroepithelial cells and dentate gyrus (DG) granular progenitors.28 The early hippocampal marker, Lhx2, which helps in the expansion of the cortical hem105 where CR neurons are formed,106 was reduced in the hippocampus from E13.5 onward. At E16, the hippocampal marker such as Wnt7b and Lef1 had been reduced in the hippocampus and fimbria and Prox1 in the DG.28 The CA1, CA3, and DG regions of hippocampus are formed at E18.5.107,108 The Tbr2 (progenitor marker) and Prox1 (neuronal marker) expression in the DG was absent. Ectopic expression of Tbr2 was observed in cortical progenitor zone of Emx1-cre dicer KOs. However, mature neurons were reduced in all the three regions.28
The Emx1-cre dicer KO has increased Foxp2 expression in the dorsal telencephalon as well as in some neural progenitors of the VZ,82 which was not observed in the wildtype.109 Similarly in miR-9-2/3 double KO at E18.5, the Foxp2 was increased in the layer VI concluding that Foxp2 expression was increased in the absence of miR-9.80 Foxp2 is a member of forkhead family of transcription factors. Foxp2 is expressed in the ganglionic eminence as early as E12.5, in the CP at E14.5, and from E16.5 onwards as a gradient from lateralhigh to mediallow cortex. They are expressed in the migratory or postmigratory neurons of the striatum.109 The Foxp2 has a 3′ untranslated region (UTR) target site for miR-9. Experimental evidence proved that the increased Foxp2 in the dorsal telencephalon of Emx1-cre dicer is due to the lack of miR-9,82 which is similar to miR-9-2/3 mutant.80 Ectopic Foxp2 expression in the embryonic neocortex impairs radial migration of neurons.82 In the dorsal telencephalon, miR-9 represses Foxp2 expression for proper dorsal telencephalon development that includes radial migration of neurons.
The Nestin expression starts at E9.5 in the developing cerebral cortex,110,111 The Nestin-cre dicer mice did not survive postnatally. At E15, the Tuj1 (a neurofilament marker) was found in IZ, early-born Tbr1 neurons in the CP, and the neurofilament was observed in the axons of IZ. This denotes early neurogenesis being unaffected. No difference in the expression of neuronal progenitor markers such as Pax6 and Tbr2 was observed. At E18, the brain size of the mice appears to be normal. However, histological sections show larger ventricles, reduced dividing cells in the VZ, SVZ and thinning of post-mitotic neurons in the CP. This shows that the late-born neurons are affected, indicating the need for miRNA for proper function.36
The Nestin-cre dicer KO has reduced hippocampal size, and miR-9 was undetectable in the cortex and hippocampus at E14.5. Similar to Emx1-cre, Nestin-cre also has reduced hippocampal neuroepithelial cells and DG granular progenitors. The early hippocampal marker Lhx2 was reduced from E18.5. The DG Tbr2 (progenitor marker) and Prox1 (neuronal marker) expression was reduced. Similar to Emx-cre dicer KO, Tbr2 was ectopically expressed. Early-born neurons expressing Tbr1 were increased in CA1 and DG regions, although mature neurons were reduced in all the three regions of the hippocampus.28
McLoughlin et al111 reported that Nestin-cre dicer mutant has cortical thinning and enlargement of lateral ventricles at E16. At E15.5, the nestin expression was distributed throughout the cortical parenchyma. The neural precursor cell proliferation was greatly reduced in the VZ and SVZ of E15.5 and E16 cortex due to delayed cell division. The thinning of cortex is also due to apoptosis. The CR neuron migration and differentiation was defective, which was observed as more CR neurons in the CP than on the MZ. The premature maturation of neurons was observed by the overproduction of DCX and Rnd2 (a maturation marker). Precocious astrocyte differentiation was also observed in the Nestin-cre dicer KO cortex.111
The Nex expression starts at E13.5 in the hippocampus.28,29 The Nex-cre dicer KO showed smaller brains and died at P23.38 The miR-9 was reduced at P22 in the hippocampus. The CA3 region is severely affected in Nex-cre dicer KO. The mature neurons were reduced in all the three regions.28 The CaMKII-cre dicer KO resulted in microcephaly and reduction in the size of white matter tracts.35 The CaMKII-cre was specific to forebrain structures such as the thalamus, cortex, and hippocampus with little emphasis on cerebellum, medulla, and pons.32,36 The hGFAP starts at E13.5.31 The hGFAP-cre dicer KO shows that miR-9 is necessary for proper cerebral cortex development via Notch1 signaling.34 However, there are no significant abnormalities during embryonic stages in these KOs (Nex-cre, CaMKII-cre, and hGFAP-cre). This might be due to the delayed dicer deletion. These studies indicate that dicer is necessary for the survival of postnatal neurons.
The conditional dicer KOs, namely Foxg1, Emx1, Nestin, Nex, GFAP, and CaMKII, show loss of miR-9 expression in the telencephalon.28,33,34,112 The conditional dicer KOs point out the influence of dicer in early and late neurogenesis in the developing telencephalon. However, it also provides indirect evidence for the necessity of miR-9 in telencephalic development; the role of miR-9 in the development of telencephalon comes from miR-9 KO study. The miR-9-2/3 double KO exhibits severe growth retardation with small cerebral hemispheres and olfactory bulbs, and they died within one week postnatal. Histology of postnatal miR-9-2/3 double KO showed reduction in cortical and VZ, with an expansion of lateral ventricles. The proliferating zone in subpallium is hyperplastic with reduced differentiation. The miR-9-2 or miR-9-3 mutant does not show any defect in neurogenesis in pallium and subpallium; however, the miR-9-2/3 double-mutant does.80 This miR-9-2/3 KO shows that miR-9 is necessary for proper telencephalic development by controlling neural progenitor proliferation and differentiation. Overall, Foxg1-cre, Emx1-cre, Nestin-cre, Nex-cre, and hGFAP-cre dicer KOs point out that miR-9 is necessary for the early and late neurogenesis of the developing cortex and hippocampus.
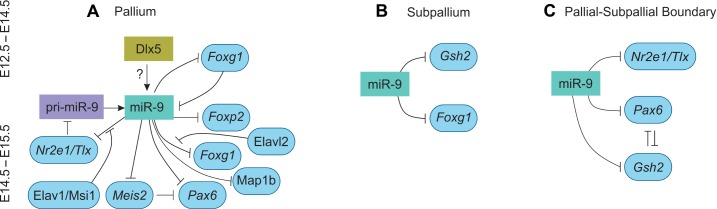
The miR-9-2/3 double mutant shows the direct role of miR-9 in telencephalon development. The telencephalic markers such as Foxg1, Nr2e1/Tlx, Pax6, Meis2 (myeloid ecotropic viral integration site 2), and Gsh2 have miR-9 target binding sites in their 3′UTR.80 The miR-9 targets Foxg1 for the generation of CR neurons in the medial pallium. The miR-9 and Foxg1 genes are expressed in reciprocal gradient along the medial pallium at E11.5.47 The Foxg1/BF1 is necessary for the early development of telencephalon.87,113 Foxg1 mutants displayed delayed cell cycle progression, reduction in proliferative progenitors and increased neurogenesis. Foxg1 controls proliferation through the regulation of Fgf signaling and differentiation through the regulation of BMP signaling.114 The Foxg1-driven dicer mutant also shows that miR-9 is depleted and undetected at E11.5 in the cortex.33 In the early stages of miR-9-2/3 double mutant, Foxg1 was increased in the pallium at E12.5–E14.5, but at E16.5 there was no significant increase. This signifies that miR-9 is necessary for Foxg1 regulation at the early stage for the differentiation of CR and early-born neurons. However, in the later stage, Elavl2 (RNA-binding protein) counteracts the suppression of miR-9 by binding to the U-rich region of Foxg1 3′UTR in the pallium. Both the Foxg1 dicer mutant and miR-9-2/3 double KO studies indicate that miR-9 and Foxg1 negatively regulate each other for proper pallial progenitor proliferation and CR cell differentiation (Fig. 5A).
Figure 5.
Role of miR-9 in mice brain development.
Notes: The schematic figure shows the mechanism of gene regulation in telencephalon by miR-9. (A) In pallium, miR-9 regulates several genes, namely Foxg1, Foxp2, Pax6, Meis2, Nr2e1/Tlx, and Map1b. At E12.5–E14.5, the Foxg1 is inhibited by miR-9 for the differentiation of CR neurons. However, at E14.5–15.5, there is no reduction in Foxg1 in the pallium of miR-9-2/3 KO mice. This is due to the Elavl2, an RNA-binding protein inhibit the binding of miR-9 to the 3′UTR of Foxg1. Likewise, Elavl1/Msi1 inhibits Nr2e1/Tlx regulation by miR-9. The axonal development is regulated by miR-9 by targeting Map1b. The interaction between Pax6, Meis2, Nr2e1, and miR-9 leads to proper neuronal proliferation in the pallium. The migration of CR and early-born neurons is facilitated by miR-9 interaction with Foxp2. (B) The miR-9 regulates Foxg1 and Gsh2 for proper subpallium development. (C) The miR-9 regulates Pax6, Gsh2, and Nr2e1/Tlx to form the proper PSB.
Abbreviations: CR, Cajal–Retzius; KO, knockout; miR-9, miRNA-9; PSB, pallial-subpallial boundary; 3′UTR, 3′ untranslated region.
The Foxg1 and Pax6 are expressed in the progenitors of the developing telencepahlon.87,115 Foxg1 regulates telencephalic progenitor proliferation by an autonomous mechanism as well as region-specific progenitor proliferation through the regulation of Pax6.116 In E14.5, miR-9-2/3 double mutant, Pax6 is reduced in the pallium with an increase in Meis2.80 Meis2 belongs to three amino acid loop extension superclass family of homeobox genes.117 Meis2 is first observed at E10.5 in the ventrolateral telencephalon (ie, LGE and MGE), and at E12.5, it is observed in the VZ of the entire telencephalon. At E16.5, Meis2 is observed in the striatum and CP.118 Meis2 acts as an essential Pax6 cofactor in adult olfactory bulb neurogenesis119 and is known to regulate Pax6 expression.120 It can be postulated that at later stages, miR-9 regulates Pax6 via Meis2 for progenitor proliferation in VZ and SVZ of the developing pallium.
However, Nr2e1 has 3′UTR binding site for miR-9; at E13.5, the Nr2e1 mRNA was upregulated in miR-9-2/3 double KO.80 The introduction of miR-9 or let-7d into the VZ of E13.5 embryonic cortex results in the reduction in proliferative cells, and they migrate into the CP from the VZ at E15.5. The Tlx null mutant has upregulated pri-miR-9-1 and pri-miR-9-2.75,76 The let-7d inhibits TLX and promotes miR-9 expression.76 This suggests that miR-9 helps in neuronal migration and differentiation through loop regulation of Tlx and miR-9 at early stages (E12.5–15.5). At E16.5, there is no reduction of Nr2e1 in the miR-9-2/3 double mutant. The presence of RNA-binding protein, Elav1 and Msi1, in the pallium may attenuate the suppression of Nr2e1 by miR-9.80 Thus, miR-9 plays a role in the development of pallium by regulating Foxg1, Pax6, Nr2e1, and Meis2 (Fig. 5A).
In the E15.5 miR-9-2/3 mutant subpallium, Gsh2 and Foxg1 are increased.80 The mechanistic role of Foxg1 is not clearly understood in subpallial progenitor proliferation and differentiation.114,121 Gsh2 is necessary for the identification of early LGE progenitor cells.122,123 The increase in Gsh2 and Foxg1 results in hyperplastic, where the proliferating cells expand into differentiating areas of the subpallium. This shows that miR-9 is necessary for proper subpallial development by regulating Gsh2 and Foxg1 expression (Fig. 5B).
Pax6 and Gsh2 have a role in specifying dorsal and ventral telencephalon,92 and these cells by counteraction form the PSB.93,124 The Tlx/Nr2e1 is expressed in the VZ and spans along the PSB, with the exception of the dorsomedial and ventromedial regions. The Tlx mutant is similar to Pax6 mutants. The Tlx/Nr2e1 along with Pax6 regulates the establishment of PSB.125 Similar to Tlx/Nr2e1 mutant,125 the miR-9-2/3 double-mutant subpallium80 shows that the development of basal ganglia including striatum and globus pallidus was suppressed, the PSB shifted dorsally, and the ventral pallidum was lost. This was substantiated by the inhibition of Pax6 via Meis2 and upregulation of Gsh2 and Nr2e1. Overall, this suggests that miR-9 is necessary for proper PSB formation by regulating Pax6, Gsh2, and Nr2e1 as well as for normal DV telencephalic development (Fig. 5C).
Role of miR-9 in telencephalon development of Xenopus
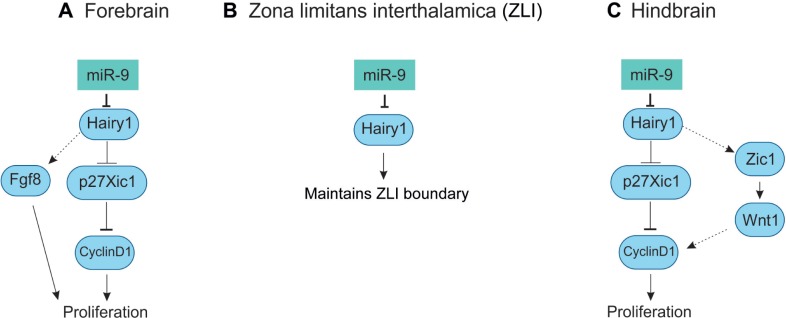
In Xenopus tropicalis, miR-9 is expressed from four variants that produce mature miR-9. The pre-miR-9 expression is similar in all the transcripts in comparison to the mature miR-9 expression. At stages 19, 30, and 36, the pre-miR-9 transcripts were observed in the forebrain, eye, midbrain, and hindbrain, but not observed in MHB (Table 2). The miR-9 expression is evident from stage 18/19 in the anterior neural plate stage of the prospective forebrain. At stage 23/24, mature miR-9 was observed in the forebrain, eye and retina, midbrain, and hindbrain with the exception of MHB.48,126 In the forebrain, miR-9 expression was widespread, in both neural progenitors (VZ) and postmitotic neurons (MZ). Morpholino (MO) miR-9 studies indicate the miR-9 was necessary for the survival of progenitors and neuronal differentiation. The miR-9 knockdown results in the inhibition of neurogenesis via apoptosis. It has been proposed that hairy1 is the main target for miR-9, where it affects proliferation via Fgf8 in the forebrain (Fig. 6A).48 Zebrafish Her6, mammalian Hes1, Xenopus hairy1, and chicken hairy1 belong to the family of transcription factor, hairy/enhancer of split. The hairy/enhancer of split belongs to basic helix-loop-helix proteins of DNA-binding transcription factors.127
Figure 6.
Role of miR-9 in Xenopus brain development.
Notes: (A) This shows the mechanism of miR-9 in forebrain development. In the forebrain, miR-9 inhibits hairy1 and mediates the effects through Fgf8 signaling for the proliferation of cells. (B) In ZLI, miR-9 inhibits hairy1 for the proper maintenance of the boundary. (C) In the hindbrain, the miR-9 inhibits hairy1 for the proliferation of cells and mediates the effects through Wnt1 signaling.
Abbreviation: miR-9, miRNA-9.
Role of miR-9 in telencephalon development of zebrafish
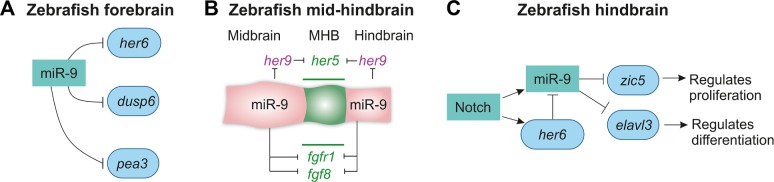
In zebrafish, miR-9 expression is observed in the telencephalon by 24 hpf.46,128 The zebrafish have seven miR-9 variants that express the same mature product, miR-9.41 Each pri-miR-9 variant shows differential expression pattern (Table 2). At Prim6 stage129 (25 hpf), the mature miR-9 is expressed only in the telencephalon. However, the pri-miR-9 variants 1–7 are expressed in the telencephalon with subtle differences; pri-miR-9-2 is expressed in the developing retina, pri-miR-9-4 in the posterior brain regions, and pri-miR-9-6 in the hypothalamus.130 At 30 hpf, the miR-9 is expressed in the entire CNS, but spared MHB.46,128 At 48 hpf, all pri-miR-9 variants expressed throughout the CNS, but always spared MHB. Similarly, the mature miR-9 is expressed throughout the entire CNS.130 In the telencephalon, miR-9 is regulated by her6 via feedback mechanism in the proliferative cells, but not in the differentiated cells.128 The miR-9 regulates Fgf target genes (dusp6 and Pea3) in the telencephalon46 (Fig. 7A). Limited information is available on the role of miR-9 in zebrafish telencephalon development; however, studies are needed to know how miR-9 is regulated, influences gene regulation and thereby promotes cellular changes in the zebrafish telencephalon.
Figure 7.
Role of miR-9 in zebrafish brain development.
Notes: (A) Zebrafish forebrain: In the forebrain, miR-9 inhibits proneural gene her6 and targets Fgf signaling pathway genes (dusp6, pea3). However, detailed study is not available on this account. (B) Zebrafish midbrain and hindbrain: The miR-9 regulates MHB by limiting Fgf8 and its receptor Fgfr1 expression within the MHB. The miR-9 regulates the progenitor state in the MH by regulating her5 and her9. (C) Zebrafish hindbrain: The miR-9 is regulated by Notch signaling and its target gene, her6. The miR-9 regulates proliferation by inhibiting Zic5 and differentiation by inhibiting elavl3.
Abbreviations: MH, midbrain–hindbrain; MHB, MH boundary; miR-9, miRNA-9.
Role of miR-9 in telencephalon development of chick embryo
The early expression of miR-9 is observed in the area opaca at HH (Hamburger Hamilton stage)131 4–5. At HH18, miR-9 is diversely expressed in all the developing brain vesicles and in the spinal cord. At HH20, it is expressed in the telencephalic vesicle, hindbrain, and spinal cord. From HH22-25 onward, miR-9 is expressed in the telencephalic vesicle, diencephalon, mid-brain, hindbrain, and spinal cord (for detailed information, see GEISHA website).42,132 The chick has two pre-miRs (miR-9-1 and miR-9-2), which are processed by the internal dicer to produce the same mature product, miR-9.83 So far no research reports are available on the role of miR-9 in chick telencephalon.
Diencephalon development
The diencephalon is the posterior part of the developing forebrain and develops into the thalamus and hypothalamus.56–58 The ZLI acts as an organizer for diencephalic development.133–136 It is a narrow strip of Shh-expressing cells in the diencephalon, transecting between prethalamus and thalamus (Fig. 8).21 The neural tissues anterior to ZLI and in between MHB and ZLI can induce Shh.135 The ZLI is formed at the interface between the anterior Zinc finger proteins of the Fez family and posterior homeodomain protein of Iroquois (Irx) family.137,138 Like Gbx2 in the MHB, Irx3 positions the ZLI in the posterior forebrain.139 The Six3 and Irx3 mutually repress each other and form the ZLI boundary.140
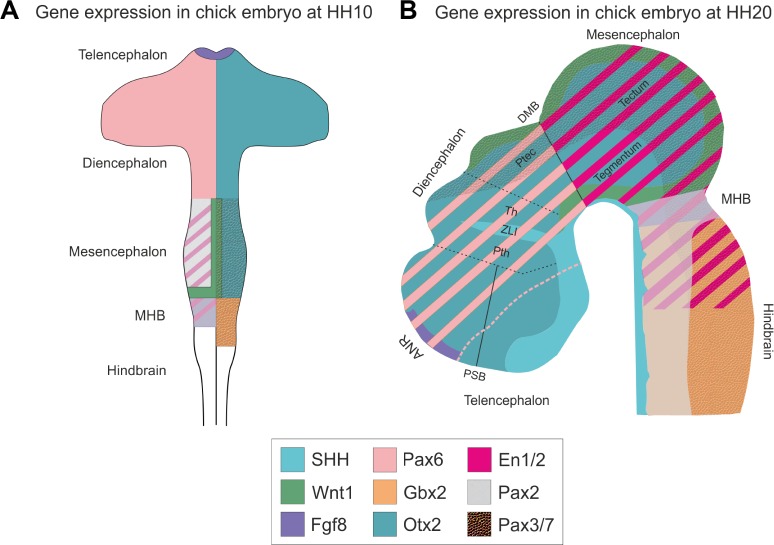
Figure 8.
Gene expression in developing chick embryo.
Notes: The schematic figure shows the gene expression in developing chick embryo at HH10 (A) and at HH20 (B). (A) This figure is adapted from Nakamura et al, with permission from the author.170 At HH10, the MHB is formed by the repressive action of Otx2 and Gbx2. Fgf8 is expressed in the ANR and MHB. DMB is formed by the repressive action between Pax2/En1 and Pax6. Pax3/7 expression in the midbrain/mesencephalon tectum ensures the tectal differentiation. The Pax2, En1/2, and Fgf8 overlap with each other. (B) Shh is secreted in the entire floor plate in developing CNS. Cell lineage restrictions are observed in the PSB, regions anterior and posterior to the ZLI and DMB. The Pax2, En1/2, Wnt1, and Fgf8 overlap each other in the MHB and are necessary for the maintenance of MHB. The two signaling centers are ANR and MHB, where ANR is necessary for the maintenance of forebrain identity and MHB is necessary for the development of midbrain and hindbrain structures. The gene expressions shown are Shh (turquoise), Otx2 (dark turquoise), Gbx2 (orange), Wnt1 (Spring green), Fgf8 (purple), En1/2 (magenta), Pax6 (pink), Pax2 (pale white), and Pax3/7 (textured).
Abbreviations: Pth, prethalamus; ZLI, zona limitans interthalamica; Th, thalamus; ptec, pretectum; ANR, anterior neural ridge; PSB, pallial-subpallial boundary; DMB, di-mesencephalic boundary; MHB, midbrain–hindbrain boundary; CNS, central nervous system.
Role of miR-9 in the diencephalon development of mice
The miR-9 is not expressed in the diencephalon of E11.5 mouse.33 No reports are available with regard to gene regulation by miR-9 expression in diencephalon.
Role of miR-9 in the diencephalon development of Xenopus
In Xenopus tropicalis, miR-9 is not expressed in the MHB and ZLI, where hairy1 and Fgf8 are expressed. The miR-9 MO and hairy1 target protection assay leads to the expansion of hairy1- and Fgf8-positive domain,48 which indicates that miR-9 inversely regulates hairy1 expression via non-cell autonomous signaling pathway in ZLI (Fig. 6B). Another study has shown that hes1 in mouse, similar to hairy1 in Xenopus, inversely regulates miR-9 biogenesis.49
Role of miR-9 in the diencephalon development of zebrafish
The miR-9 expression is observed in the diencephalon.46,128,141 However, there are no research reports available on gene regulation by miR-9.
Role of miR-9 in the diencephalon development of chick
There are no reports on the role of miR-9 on chick embryonic diencephalon. However, the miR-9 expression is observed in the diencephalon42 from HH22 onward.
Mesencephalon/midbrain development
The MHB plays an important role for the AP development of midbrain. The midbrain consists of two main components along the DV axis: the dorsal tectum and the ventral tegmentum. The tectum develops from the alar plate, and the tegmentum develops from the basal plate.142 The MHB arises from the morphological constriction between the midbrain and hindbrain. The organization of MHB has been divided into four stages: (1) positioning and establishment, (2) induction, (3) maintenance,55 and (4) morphogenesis.143 The Otx2 and Gbx2 are the critical factors for position and establishment of the MHB.144,145 These signals refine the position of MHB.146–148 The Otx2 and Gbx2 repress each other, from where the Fgf8 is expressed.149 In zebrafish, her5 has been postulated to establish the midbrain and hindbrain cells,150 and Pou2 is necessary for the establishment of MHB.151 The induction of MHB is regulated by the members of Pax, Fgf, Wnt, and En families, where Fgf8 plays a crucial role.152 In Xenopus, Xiro along with Otx2/Gbx2 is necessary for the induction of Fgf8 at MHB.153 The maintenance of MHB is achieved by interdependent regulation of genes, namely Pax2/5, Wnt1, En1/2, and Fgf8 (Fig. 8A and B).55,143 Recently, it was shown that the maintenance and morphogenesis of MHB in zebrafish was governed by the transcription factor Grhl2b (grainy head-like 2b).154
Role of miR-9 in the mesencephalon/midbrain development of mice
Foxg1 is also expressed in the MHB at E10.5.98 However, there is no report on the role of miRNAs in the MHB of Foxg1-cre dicer KO.
The observation in Emx1-cre dicer KO shows that there is no change in midbrain structure and miR-9 expression in the mesencephalon,96 which is obvious since Emx1 expression is observed only in the telencephalon.26
The Pax2-cre dicer KO and Wnt1-cre dicer KO have similar impaired growth in the midbrain and hindbrain structures.37,155 These Pax2-cre mice died at E18.5. Pax2 is expressed in the MHB156 and Wnt1 is expressed in the midbrain at E8.157 The Wnt1-cre dicer KO shows several face malformation. The size of the tectum, rostral (superior colliculus), and caudal (inferior colliculus) tectum was clearly reduced, and the cerebellum was ablated in these KOs. The AP pattern was unaffected. The expression of genes such as Otx2, Gbx2, Wnt1, Fgf8, and Lmx1b was not affected, and the MHB was correctly positioned. In comparison to the wild type, the miR-9, miR-124, and miR-218 were greatly eliminated in tectum, ventral midbrain, and rostral hindbrain of the Wnt1-cre dicer mutant. The dicer mutant points out that the TH expression was markedly reduced in the ventral midbrain. The TH and Nurr1-positive cells failed to properly differentiate into mDA (mesencephalic dopaminergic) neurons.37 This shows that dicer mutant have mDA precursor cells, but needs miRNAs to differentiate into proper mDA neurons. It appears that the miRNAs (miR-9, miR-124, miR-218) eliminated in Wnt1-cre dicer KOs are necessary for the proper development of mDA neurons.
Role of miR-9 in the mesencephalon/midbrain development in Xenopus
At stages 30–36, the miR-9 is expressed in the midbrain as well as in the hindbrain with the exception of MHB. The miR-9 expression in the midbrain and hindbrain is restricted to the proliferating progenitor cells of the VZ. The primary miR-9 (miR-9a-1, miR-9a-2, miR-9-3) is expressed in the midbrain (Table 2).48,126 The hairy1 is expressed in the ventral midbrain that coincides with the expression of miR-9a-1.48 It might be possible that miR-9 regulates hairy1 for neurogenesis and differentiation in the Xenopus midbrain.
Role of miR-9 in the mesencephalon/midbrain development in zebrafish
In zebrafish, miR-9 is not expressed in the MHB.46,128 The gain of function (GOF) of miR-9 experiment shows that overexpression leads to the loss of MHB and MHB marker gene expression. The GOF and loss of function (LOF) studies show that miR-9 has influence on the Fgf signaling pathway genes (Fgf8, Fgfr1, and canopy1) and its target genes such as Pea3 and dusp6 are downregulated. Experimental evidence proves that Her5, her9, canopy1, fgf8, and fgfr1 are in vivo targets of miR-9. Independent of Fgf signaling interaction, miR-9 also promotes midbrain–hindbrain neurogenesis and neuronal differentiation in vivo via regulating her (her5 and her9) genes (Fig. 7B).46 Overall, miR-9 is involved in neurogenesis, Fgf signaling, and maintenance of MHB.
Role of miR-9 in the mesencephalon/midbrain development in chick
The miR-9 is expressed in the midbrain;42 however, no research reports are available on the role of miR-9 in gene regulation of midbrain and MHB genes.
Hindbrain development
The developing hindbrain is formed from the rudimentary segments of rhombomeres, where the cells move freely between rhombomeres before the boundary formation and after the boundary formation they expands within the boundaries.158 The development of hind-brain depends on the retinoic acid gradient signals, tyrosine kinases, and several transcription factors along the rhombomere boundaries.159–161 In zebrafish, several Wnt genes are expressed in the boundaries between the rhombomeres.162,163 In chick, Hox genes play a main role in the formation of rhombomere boundaries.159,160
Role of miR-9 in the hindbrain development of mice
In Wnt1-cre dicer mutant, notable defects were observed in the hindbrain that included the ablation of cerebellum. The miR-9 along with miR-124 and miR-218 expression was ablated in rostral hindbrain of the Wnt1-cre dicer mutant. There was no difference in the expression of Gbx2 expression in the hindbrain. The mitotic progenitors in the hindbrain were similar to the control,37 indicating dicer KO does not affect proliferation in mice hindbrain. Further research is needed to pinpoint the role of miR-9 in the developing hindbrain.
In hGFAP-cre dicer mutant, the activity was observed as early as E13.5 at the rhombic lip of the cerebellar plate and in postnatal cerebellar cells including the Bergmann glia. Defective cerebellar foliation was observed at E16.5. In the Bergmann glia, miR-9 is needed for the maintenance of these cells via Notch1 signaling.30
Role of miR-9 in the hindbrain development of Xenopus
Bonev et al reported that in Xenopus, miR-9 expression was restricted to the Sox3 (neural progenitor marker)-positive domain, ie, VZ cells of the developing hindbrain. The miR-9 is involved in the hindbrain development through hairy1 on proliferation via Wnt signaling (Fig. 6C). The MO experiment also shows that miR-9 is necessary for the differentiation of neuron. In comparison to forebrain, miR-9 is necessary for limiting progenitor proliferation in hindbrain and promotes the onset of neurogenesis.48
Role of miR-9 in the hindbrain development in zebrafish
In zebrafish, at 30 hpf, the miR-9 expression is observed in the hindbrain with the absence of MHB and rhombomere boundary.41,46,128 In the hindbrain, radial glial cells in the VZ express miR-9, where the expression is segmentally pattern and stronger near the rhombomere boundaries. The miR-9 expression is also observed in the neurons of the MZ. The miR-9 regulates hindbrain neurogenesis by maintaining neural progenitor cells through zic5 and her6 and neuronal differentiation by targeting elavl3/HuC (Fig. 7C). The miR-9 was positively regulated by Notch signaling and negatively regulated by Notch target Her6.128 The miR-9 inhibits Her9, thereby regulates neuronal progenitor maintenance.46 Overall, miR-9 is involved in proper neuronal progenitor maintenance and neurogenesis in zebrafish.
Role of miR-9 in the hindbrain development of chick
From HH20 onward, the miR-9 expression is observed in the hindbrain. At HH24, it is strongly expressed in the margin of the roof plate and absent in the floor plate.42 However, there are no research reports on gene regulation by miR-9 in the hindbrain.
Spinal cord development
The spinal cord develops from the posterior neural tube by the induction of cues from the dorsal ectoderm. The regional differentiation occurs along the DV and rostrocaudal axis. The retinoid synthesis by RALDH2 (retinaldehyde dehydrogenase 2) is critical for establishing the distinction of spinal cord neural cells.164 The dorsally positioned neurons are induced by BMP signaling, and the ventrally positioned neurons are induced by the graded expression of SHH. The Hox genes govern the patterning of spinal cord.165 The motor neurons (MNs) and interneurons (IN) are generated by the graded action of SHH. The graded SHH repress the class I homeodomain proteins (Pax6/7, Irx3, DBX1/2) and class II proteins (Nkx2.2 and Nkx6.1) for the generation of five distinct class of neurons (ventral IN—V0-3 and MN) from the ventral progenitor cells along DV axis of the ventral neural tube.166 The combinatorial action of Nkx6.1, Nkx2.2, and Irx3 restricts the generation of MN to a single progenitor domain.164,166 The median motor column (MMC) neurons innervate axial muscles and lateral motor column (LMC) innervate limb muscles.164
Role of miR-9 in the spinal cord development of chick
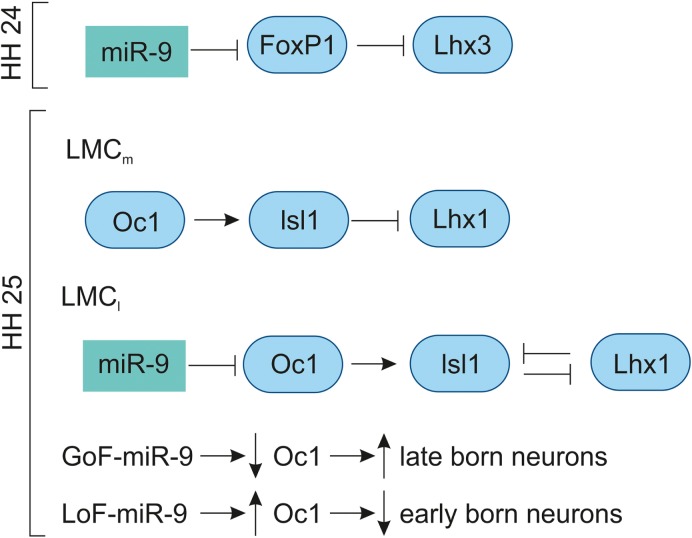
The miR-9 is expressed in the VZ of developing chick spinal cord from HH11 to HH29.42,83 In the MN, the miR-9 expression was low at HH20, increased at HH24 and undetectable at HH29. At HH24, miR-9 overlaps with the MN markers (FoxP1 and Isl1/2) in the LMC; however, they do not overlap with MN markers (Lhx3, HB9) of the MMC neurons. The overexpression of miR-9 results in switching of LMC into MMC neurons by repressing the production of LMC neurons by downregulating FoxP1 and Isl1/2 and promoting the differentiation of MMC neurons by upregulating Lhx3 and HB9. The overlapping expression of miR-9 and FoxP1 along with experimental evidence shows that miR-9 regulates FoxP1 expression in LMC in a dose-dependent manner. In chick, preganglionic motor column (PGC) is positioned along the thoracic level innervate axons to the sympathetic target. The overexpression of miR-9 shows that there is a reduced expression of PGC MN marker, Smad and Isl1/2.83 In the LMC, the GoF-miR-9 shows that FoxP1 is reduced and vice versa in the LoF-miR-9. Likewise, the reduction and increase in FoxP1 are inversely proportional to the Lhx3 MN marker in the LMC.167 Overall, miR-9 finely tunes FoxP1 expression for normal LMC (Fig. 9) and PGC neuron specification.
Figure 9.
Role of miR-9 in chick spinal cord development.
Notes: At HH24, miR-9 fine-tunes Foxp1 for LMC MN specification via inhibiting Lhx3. At HH25, miR-9 regulates OC1 protein for the proper development of early- and late-born MN neurons in the LMC. The GOF and LOF with miR-9 indicate that miR-9 inversely regulates OC1 for early- and late-born MNs. The OC1 acts upstream of Isl1, controls the specific neuronal subtypes within LMC. OC1 has stronger expression with no miR-9 expression in the LMCm, thereby regulates LMCm MNs by promoting Isl1 expression and inhibiting Lhx1 expression. However, in the LMCl the expression of miR-9 expression inhibits OC1 expression and promotes Lhx1 expression in the LMCl MNs.
Abbreviations: GOF, gain of function; LMC, lateral motor column; LOF, loss of function; miR-9, miRNA-9; MN, motor neuron; OC1, Onecut 1.
At HH25, the miR-9 expression is increased in the intermediate and VZs of the spinal cord. At HH28, miR-9 is expressed in specific subpopulations of the MNs; the MN migration is completed by this stage, where the MMC (marker: Lhx3), medial LMC (marker: Isl1/FoxP1), and lateral LMC (marker: Lhx1/Hb9) can be clearly distinguished. The transcription factor OC1 (Onecut 1) is expressed in the lateral aspect of the ventral horn. At HH25, the OC1 is co-localized with Isl1/Lhx3 of early-born neurons of MMC, in the medial LMC with Isl1/FoxP1 and in the lateral LMC with Lhx1/Hb9. At HH27, the lateral MMC shows less OC1 compared to the medial MMC, while the miR-9 is reversed in strength. The medial LMC has strong OC1 expression with no miR-9, and lateral LMC has strong miR-9 with reduced OC1. The GoF-miR-9 results in the reduction in lateral MMC and medial LMC, while the medial MMC and lateral LMC are increased. The opposite reactions are observed in LoF-miR-9. The GoF-miR-9 results in the downregulation of OC1 and an increase in late-born neurons. Likewise, LOF-miR-9 results in the upregulation of OC1 and an increase in early-born neurons.168 Overall, miR-9 regulates Onecut 1 to promote the switching of early-born to late-born MN population (Fig. 9).
Conclusion
In the developing brain, miR-9 influences gene expression, proliferation, neurogenesis, maturation, migration, and differentiation in a spatiotemporal pattern. The studies point out that miR-9 is expressed in neuronal cells which are pro-liferative and differentiated cells. The endogenous miR-9 expression pattern also differs from species to species; in zebrafish and Xenopus, miR-9 is excluded from the MHB, but in mice and chicken there is no such distinction in the expression pattern. The miR-9 is necessary for the maintenance of boundaries: PSB, ZLI, and MHB.
Although the studies on dicer KO mice show the lack of miR-9, one cannot ignore the plethora of other miRNAs involvement in brain development. At the same time, we could not reject the experimental evidence showing the necessity of miR-9 and its role in vertebrate brain development. The other dicer KOs have similar developmental defects in telencephalon in comparison to miR-9-2/3 double mutant. Combining these facts with other experimental evidence like GOF/LOF, we can conclude that miR-9 plays a role in the vertebrate telencephalon development in mice. One among such facts is the regulation of CR neurons by miR-9. The miR-9 modulates the generation, maturation, differentiation, and migration of CR neurons in the developing telencephalon. The miR-9 is also governed by several genes, like Notch, Hes/hairy1, Tlx, Dlx5, etc. There are also feedback loop mechanisms for Tlx, Foxg1, and Foxp1 with miR-9 that regulates cellular events within the developing vertebrate brain.
Several hairy/enhancer of split genes (Xenopus hairy1, zebrafish her5, her9, her6) are regulated by miR-9. Most of these are proneural genes involve in the regulation of progenitor pools. In these studies, we found that miR-9 regulates proliferation and differentiation via targeting her genes. Even within the species, the miR-9 is differentially regulated. For example, hairy1 mediates the effects of miR-9 on proliferation through Fgf8 signaling in the forebrain and via Wnt signaling in the hindbrain.
Further studies are required to completely understand the role of miR-9 in vertebrate brain development. For instance, miR-9 expression has been observed in the chick diencephalon from HH22, and reports on the role of miR-9 in the embryonic diencephalon are lacking. Similarly, miR-9 expression has been reported in the zebrafish diencephalon, but data on its gene regulatory role are not yet available. In the case of the chick midbrain and hindbrain, no data are available on the role of miR-9 in both these regions. This situation is also prevalent in the case of the chick telencephalon, allowing much more scope for understanding the role of miR-9. This may also provide an insight into the role played by miR-9 from an evolutionary standpoint. To conclude, miR-9 is necessary for the development of the embryonic brain.
Footnotes
ACADEMIC EDITOR: Lora Talley Watts, Editor in Chief
PEER REVIEW: Peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1242 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived and designed the review, analyzed the data, contributed equally in writing the manuscript: BR and AAPA. Agree with manuscript results and conclusions: BR and AAPA. Drawn figures for the manuscript using graphic software: BR. Jointly developed the structure and arguments for the paper: BR and AAPA. Made critical revisions and approved final version: AAPA. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Ambros V, Lee RC, Lavanway A, Williams PT, Jewell D. MicroRNAs and other tiny endogenous RNAs in C. elegans. Curr Biol. 2003;13(10):807–818. doi: 10.1016/s0960-9822(03)00287-2. [DOI] [PubMed] [Google Scholar]
- 2.Sun E, Shi Y. MicroRNAs: small molecules with big roles in neurodevelopment and diseases. Exp Neurol. 2015;268:46–53. doi: 10.1016/j.expneurol.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Saini HK, Griffiths-Jones S, Enright AJ. Genomic analysis of human microRNA transcripts. Proc Natl Acad Sci U S A. 2007;104(45):17719–17724. doi: 10.1073/pnas.0703890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee Y, Ahn C, Han J, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425(6956):415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 5.Liu Q, Paroo Z. Biochemical principles of small RNA pathways. Annu Rev Biochem. 2010;79:295–319. doi: 10.1146/annurev.biochem.052208.151733. [DOI] [PubMed] [Google Scholar]
- 6.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10(2):126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 7.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 8.Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13(4):271–282. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- 9.Kim VN. Small RNAs: classification, biogenesis, and function. Mol Cells. 2005;19(1):1–15. [PubMed] [Google Scholar]
- 10.Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6(5):376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- 11.Friedman JM, Jones PA. MicroRNAs: critical mediators of differentiation, development and disease. Swiss Med Wkly. 2009;139(33–34):466–472. doi: 10.4414/smw.2009.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malone CD, Hannon GJ. Small RNAs as guardians of the genome. Cell. 2009;136(4):656–668. doi: 10.1016/j.cell.2009.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;9(3):219–230. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 14.Meza-Sosa KF, Pedraza-Alva G, Perez-Martinez L. microRNAs: key triggers of neuronal cell fate. Front Cell Neurosci. 2014;8:175. doi: 10.3389/fncel.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babiarz JE, Hsu R, Melton C, Thomas M, Ullian EM, Blelloch R. A role for noncanonical microRNAs in the mammalian brain revealed by phenotypic differences in Dgcr8 versus Dicer1 knockouts and small RNA sequencing. RNA. 2011;17(8):1489–1501. doi: 10.1261/rna.2442211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernstein E, Kim SY, Carmell MA, et al. Dicer is essential for mouse development. Nat Genet. 2003;35(3):215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 17.Wienholds E, Koudijs MJ, van Eeden FJ, Cuppen E, Plasterk RH. The microRNA-producing enzyme Dicer1 is essential for zebrafish development. Nat Genet. 2003;35(3):217–218. doi: 10.1038/ng1251. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet. 2007;39(3):380–385. doi: 10.1038/ng1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Carmell MA, Rivas FV, et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305(5689):1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 20.Giraldez AJ, Cinalli RM, Glasner ME, et al. MicroRNAs regulate brain morphogenesis in zebrafish. Science. 2005;308(5723):833–838. doi: 10.1126/science.1109020. [DOI] [PubMed] [Google Scholar]
- 21.Shimamura K, Hartigan DJ, Martinez S, Puelles L, Rubenstein JL. Longitudinal organization of the anterior neural plate and neural tube. Development. 1995;121(12):3923–3933. doi: 10.1242/dev.121.12.3923. [DOI] [PubMed] [Google Scholar]
- 22.Bally-Cuif L, Alvarado-Mallart RM, Darnell DK, Wassef M. Relationship between Wnt-1 and En-2 expression domains during early development of normal and ectopic met-mesencephalon. Development. 1992;115(4):999–1009. doi: 10.1242/dev.115.4.999. [DOI] [PubMed] [Google Scholar]
- 23.Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci. 2002;22(15):6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cecchi C, Boncinelli E. Emx homeogenes and mouse brain development. Trends Neurosci. 2000;23(8):347–352. doi: 10.1016/s0166-2236(00)01608-8. [DOI] [PubMed] [Google Scholar]
- 25.Simeone A, Gulisano M, Acampora D, Stornaiuolo A, Rambaldi M, Boncinelli E. Two vertebrate homeobox genes related to the Drosophila empty spiracles gene are expressed in the embryonic cerebral cortex. EMBO J. 1992;11(7):2541–2550. doi: 10.1002/j.1460-2075.1992.tb05319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boncinelli E, Gulisano M, Broccoli V. Emx and Otx homeobox genes in the developing mouse brain. J Neurobiol. 1993;24(10):1356–1366. doi: 10.1002/neu.480241008. [DOI] [PubMed] [Google Scholar]
- 27.Dahlstrand J, Lardelli M, Lendahl U. Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res Dev Brain Res. 1995;84(1):109–129. doi: 10.1016/0165-3806(94)00162-s. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Bian S, Hong J, et al. Timing specific requirement of microRNA function is essential for embryonic and postnatal hippocampal development. PLoS One. 2011;6(10):e26000. doi: 10.1371/journal.pone.0026000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goebbels S, Bormuth I, Bode U, Hermanson O, Schwab MH, Nave KA. Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis. 2006;44(12):611–621. doi: 10.1002/dvg.20256. [DOI] [PubMed] [Google Scholar]
- 30.Kuang Y, Liu Q, Shu X, et al. Dicer1 and MiR-9 are required for proper Notch1 signaling and the Bergmann glial phenotype in the developing mouse cerebellum. Glia. 2012;60(11):1734–1746. doi: 10.1002/glia.22392. [DOI] [PubMed] [Google Scholar]
- 31.Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31(2):85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]
- 32.Dragatsis I, Zeitlin S. CaMKIIalpha-Cre transgene expression and recombination patterns in the mouse brain. Genesis. 2000;26(2):133–135. doi: 10.1002/(sici)1526-968x(200002)26:2<133::aid-gene10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 33.Nowakowski TJ, Mysiak KS, Pratt T, Price DJ. Functional dicer is necessary for appropriate specification of radial glia during early development of mouse telencephalon. PLoS One. 2011;6(8):e23013. doi: 10.1371/journal.pone.0023013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang C, Ge X, Liu Q, Jiang M, Li MW, Li H. MicroRNA-mediated non-cell-autonomous regulation of cortical radial glial transformation revealed by a Dicer1 knockout mouse model. Glia. 2015;63(5):860–876. doi: 10.1002/glia.22789. [DOI] [PubMed] [Google Scholar]
- 35.Davis TH, Cuellar TL, Koch SM, et al. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J Neurosci. 2008;28(17):4322–4330. doi: 10.1523/JNEUROSCI.4815-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawase-Koga Y, Otaegi G, Sun T. Different timings of Dicer deletion affect neurogenesis and gliogenesis in the developing mouse central nervous system. Dev Dyn. 2009;238(11):2800–2812. doi: 10.1002/dvdy.22109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang T, Liu Y, Huang M, Zhao X, Cheng L. Wnt1-cre-mediated conditional loss of Dicer results in malformation of the midbrain and cerebellum and failure of neural crest and dopaminergic differentiation in mice. J Mol Cell Biol. 2010;2(3):152–163. doi: 10.1093/jmcb/mjq008. [DOI] [PubMed] [Google Scholar]
- 38.Hong J, Zhang H, Kawase-Koga Y, Sun T. MicroRNA function is required for neurite outgrowth of mature neurons in the mouse postnatal cerebral cortex. Front Cell Neurosci. 2013;7:151. doi: 10.3389/fncel.2013.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kapsimali M, Kloosterman WP, de Bruijn E, Rosa F, Plasterk RH, Wilson SW. MicroRNAs show a wide diversity of expression profiles in the developing and mature central nervous system. Genome Biol. 2007;8(8):R173. doi: 10.1186/gb-2007-8-8-r173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun AX, Crabtree GR, Yoo AS. MicroRNAs: regulators of neuronal fate. Curr Opin Cell Biol. 2013;25(2):215–221. doi: 10.1016/j.ceb.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coolen M, Katz S, Bally-Cuif L. miR-9: a versatile regulator of neurogenesis. Front Cell Neurosci. 2013;7:220. doi: 10.3389/fncel.2013.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darnell DK, Kaur S, Stanislaw S, Konieczka JH, Yatskievych TA, Antin PB. MicroRNA expression during chick embryo development. Dev Dyn. 2006;235(11):3156–3165. doi: 10.1002/dvdy.20956. [DOI] [PubMed] [Google Scholar]
- 43.Yuva-Aydemir Y, Simkin A, Gascon E, Gao FB. MicroRNA-9: functional evolution of a conserved small regulatory RNA. RNA Biol. 2011;8(4):557–564. doi: 10.4161/rna.8.4.16019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berezikov E. Evolution of microRNA diversity and regulation in animals. Nat Rev Genet. 2011;12(12):846–860. doi: 10.1038/nrg3079. [DOI] [PubMed] [Google Scholar]
- 45.Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microRNA array reveals extensive regulation of microRNAs during brain development. RNA. 2003;9(10):1274–1281. doi: 10.1261/rna.5980303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leucht C, Stigloher C, Wizenmann A, Klafke R, Folchert A, Bally-Cuif L. MicroRNA-9 directs late organizer activity of the midbrain-hindbrain boundary. Nat Neurosci. 2008;11(6):641–648. doi: 10.1038/nn.2115. [DOI] [PubMed] [Google Scholar]
- 47.Shibata M, Kurokawa D, Nakao H, Ohmura T, Aizawa S. MicroRNA-9 modulates Cajal-Retzius cell differentiation by suppressing Foxg1 expression in mouse medial pallium. J Neurosci. 2008;28(41):10415–10421. doi: 10.1523/JNEUROSCI.3219-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonev B, Pisco A, Papalopulu N. MicroRNA-9 reveals regional diversity of neural progenitors along the anterior-posterior axis. Dev Cell. 2011;20(1):19–32. doi: 10.1016/j.devcel.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonev B, Stanley P, Papalopulu N. MicroRNA-9 modulates Hes1 ultradian oscillations by forming a double-negative feedback loop. Cell Rep. 2012;2(1):10–18. doi: 10.1016/j.celrep.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meza-Sosa KF, Valle-Garcia D, Pedraza-Alva G, Perez-Martinez L. Role of microRNAs in central nervous system development and pathology. J Neurosci Res. 2012;90(1):1–12. doi: 10.1002/jnr.22701. [DOI] [PubMed] [Google Scholar]
- 51.Ma L, Young J, Prabhala H, et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12(3):247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rotkrua P, Akiyama Y, Hashimoto Y, Otsubo T, Yuasa Y. MiR-9 downregulates CDX2 expression in gastric cancer cells. Int J Cancer. 2011;129(11):2611–2620. doi: 10.1002/ijc.25923. [DOI] [PubMed] [Google Scholar]
- 53.Laios A, O’Toole S, Flavin R, et al. Potential role of miR-9 and miR-223 in recurrent ovarian cancer. Mol Cancer. 2008;7:35. doi: 10.1186/1476-4598-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao F, Zhao ZL, Zhao WT. miR-9 modulates the expression of interferon-regulated genes and MHC class I molecules in human nasopharyngeal carcinoma cells. Biochem Biophys Res Commun. 2013;431(3):610–616. doi: 10.1016/j.bbrc.2012.12.097. [DOI] [PubMed] [Google Scholar]
- 55.Wurst W, Bally-Cuif L. Neural plate patterning: upstream and downstream of the isthmic organizer. Nat Rev Neurosci. 2001;2(2):99–108. doi: 10.1038/35053516. [DOI] [PubMed] [Google Scholar]
- 56.Altmann CR, Brivanlou AH. Neural patterning in the vertebrate embryo. Int Rev Cytol. 2001;203:447–482. doi: 10.1016/s0074-7696(01)03013-3. [DOI] [PubMed] [Google Scholar]
- 57.Kiecker C, Lumsden A. The role of organizers in patterning the nervous system. Annu Rev Neurosci. 2012;35:347–367. doi: 10.1146/annurev-neuro-062111-150543. [DOI] [PubMed] [Google Scholar]
- 58.Rhinn M, Brand M. The midbrain—hindbrain boundary organizer. Curr Opin Neurobiol. 2001;11(1):34–42. doi: 10.1016/s0959-4388(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 59.Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6(10):777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- 60.Volvert ML, Rogister F, Moonen G, Malgrange B, Nguyen L. MicroRNAs tune cerebral cortical neurogenesis. Cell Death Differ. 2012;19(10):1573–1581. doi: 10.1038/cdd.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsai HM, Garber BB, Larramendi LM. 3H-thymidine autoradiographic analysis of telencephalic histogenesis in the chick embryo: I. Neuronal birthdates of telencephalic compartments in situ. J Comp Neurol. 1981;198(2):275–292. doi: 10.1002/cne.901980207. [DOI] [PubMed] [Google Scholar]
- 62.Lever M, Brand-Saberi B, Theiss C. Neurogenesis, gliogenesis and the developing chicken optic tectum: an immunohistochemical and ultrastructural analysis. Brain Struct Funct. 2014;219(3):1009–1024. doi: 10.1007/s00429-013-0550-6. [DOI] [PubMed] [Google Scholar]
- 63.Puelles L, Kuwana E, Puelles E, et al. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol. 2000;424(3):409–438. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 64.Folgueira M, Bayley P, Navratilova P, Becker TS, Wilson SW, Clarke JD. Morphogenesis underlying the development of the everted teleost telencephalon. Neural Dev. 2012;7:32. doi: 10.1186/1749-8104-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ross LS, Parrett T, Easter SS., Jr Axonogenesis and morphogenesis in the embryonic zebrafish brain. J Neurosci. 1992;12(2):467–482. doi: 10.1523/JNEUROSCI.12-02-00467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hartenstein V. Early pattern of neuronal differentiation in the Xenopus embryonic brainstem and spinal cord. J Comp Neurol. 1993;328(2):213–231. doi: 10.1002/cne.903280205. [DOI] [PubMed] [Google Scholar]
- 67.Pannese M, Lupo G, Kablar B, Boncinelli E, Barsacchi G, Vignali R. The Xenopus Emx genes identify presumptive dorsal telencephalon and are induced by head organizer signals. Mech Dev. 1998;73(1):73–83. doi: 10.1016/s0925-4773(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 68.Rapacioli M, Palma V, Flores V. Morphogenetic and histogenetic roles of the temporal-spatial organization of cell proliferation in the vertebrate corticogenesis as revealed by inter-specific analyses of the optic tectum cortex development. Front Cell Neurosci. 2016;10:67. doi: 10.3389/fncel.2016.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martinez-Cerdeno V, Noctor SC, Cajal Retzius. Cajal-Retzius cells. Front Neuroanat. 2014;8:48. doi: 10.3389/fnana.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hevner RF, Neogi T, Englund C, Daza RA, Fink A. Cajal-Retzius cells in the mouse: transcription factors, neurotransmitters, and birthdays suggest a pallial origin. Brain Res Dev Brain Res. 2003;141(1–2):39–53. doi: 10.1016/s0165-3806(02)00641-7. [DOI] [PubMed] [Google Scholar]
- 71.Dehay C, Kennedy H. Cell-cycle control and cortical development. Nat Rev Neurosci. 2007;8(6):438–450. doi: 10.1038/nrn2097. [DOI] [PubMed] [Google Scholar]
- 72.Florio M, Huttner WB. Neural progenitors, neurogenesis and the evolution of the neocortex. Development. 2014;141(11):2182–2194. doi: 10.1242/dev.090571. [DOI] [PubMed] [Google Scholar]
- 73.Huttner WB, Kosodo Y. Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr Opin Cell Biol. 2005;17(6):648–657. doi: 10.1016/j.ceb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 74.Barca-Mayo O, De Pietri Tonelli D. Convergent microRNA actions coordinate neocortical development. Cell Mol Life Sci. 2014;71(16):2975–2995. doi: 10.1007/s00018-014-1576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao C, Sun G, Li S, Shi Y. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol. 2009;16(4):365–371. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao C, Sun G, Ye P, Li S, Shi Y. MicroRNA let-7d regulates the TLX/microRNA-9 cascade to control neural cell fate and neurogenesis. Sci Rep. 2013;3:1329. doi: 10.1038/srep01329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krichevsky AM, Sonntag KC, Isacson O, Kosik KS. Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells. 2006;24(4):857–864. doi: 10.1634/stemcells.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gao FB. Context-dependent functions of specific microRNAs in neuronal development. Neural Dev. 2010;5:25. doi: 10.1186/1749-8104-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoo AS, Sun AX, Li L, et al. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476(7359):228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shibata M, Nakao H, Kiyonari H, Abe T, Aizawa S. MicroRNA-9 regulates neurogenesis in mouse telencephalon by targeting multiple transcription factors. J Neurosci. 2011;31(9):3407–3422. doi: 10.1523/JNEUROSCI.5085-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dajas-Bailador F, Bonev B, Garcez P, Stanley P, Guillemot F, Papalopulu N. microRNA-9 regulates axon extension and branching by targeting Map1b in mouse cortical neurons. Nat Neurosci. 2012;15(5):697–699. doi: 10.1038/nn.3082. [DOI] [PubMed] [Google Scholar]
- 82.Clovis YM, Enard W, Marinaro F, Huttner WB, De Pietri Tonelli D. Convergent repression of Foxp2 3′UTR by miR-9 and miR-132 in embryonic mouse neo-cortex: implications for radial migration of neurons. Development. 2012;139(18):3332–3342. doi: 10.1242/dev.078063. [DOI] [PubMed] [Google Scholar]
- 83.Otaegi G, Pollock A, Hong J, Sun T. MicroRNA miR-9 modifies motor neuron columns by a tuning regulation of FoxP1 levels in developing spinal cords. J Neurosci. 2011;31(3):809–818. doi: 10.1523/JNEUROSCI.4330-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rubenstein JL, Beachy PA. Patterning of the embryonic forebrain. Curr Opin Neurobiol. 1998;8(1):18–26. doi: 10.1016/s0959-4388(98)80004-4. [DOI] [PubMed] [Google Scholar]
- 85.Hoch RV, Rubenstein JL, Pleasure S. Genes and signaling events that establish regional patterning of the mammalian forebrain. Semin Cell Dev Biol. 2009;20(4):378–386. doi: 10.1016/j.semcdb.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 86.Wilson SW, Rubenstein JL. Induction and dorsoventral patterning of the telencephalon. Neuron. 2000;28(3):641–651. doi: 10.1016/s0896-6273(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 87.Shimamura K, Rubenstein JL. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124(14):2709–2718. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- 88.Furuta Y, Piston DW, Hogan BL. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124(11):2203–2212. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- 89.Crossley PH, Martinez S, Ohkubo Y, Rubenstein JL. Coordinate expression of Fgf8, Otx2, Bmp4, and Shh in the rostral prosencephalon during development of the telencephalic and optic vesicles. Neuroscience. 2001;108(2):183–206. doi: 10.1016/s0306-4522(01)00411-0. [DOI] [PubMed] [Google Scholar]
- 90.Ericson J, Muhr J, Placzek M, Lints T, Jessell TM, Edlund T. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell. 1995;81(5):747–756. doi: 10.1016/0092-8674(95)90536-7. [DOI] [PubMed] [Google Scholar]
- 91.Hebert JM. Unraveling the molecular pathways that regulate early telencephalon development. Curr Top Dev Biol. 2005;69:17–37. doi: 10.1016/S0070-2153(05)69002-3. [DOI] [PubMed] [Google Scholar]
- 92.Yun K, Potter S, Rubenstein JL. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development. 2001;128(2):193–205. doi: 10.1242/dev.128.2.193. [DOI] [PubMed] [Google Scholar]
- 93.Toresson H, Potter SS, Campbell K. Genetic control of dorsal-ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development. 2000;127(20):4361–4371. doi: 10.1242/dev.127.20.4361. [DOI] [PubMed] [Google Scholar]
- 94.Marin O, Rubenstein JL. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2(11):780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- 95.Griffiths-Jones S. miRBase: the microRNA sequence database. Methods Mol Biol. 2006;342:129–138. doi: 10.1385/1-59745-123-1:129. [DOI] [PubMed] [Google Scholar]
- 96.De Pietri Tonelli D, Pulvers JN, Haffner C, Murchison EP, Hannon GJ, Huttner WB. miRNAs are essential for survival and differentiation of newborn neurons but not for expansion of neural progenitors during early neurogenesis in the mouse embryonic neocortex. Development. 2008;135(23):3911–3921. doi: 10.1242/dev.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.De Pietri Tonelli D, Calegari F, Fei JF, et al. Single-cell detection of microRNAs in developing vertebrate embryos after acute administration of a dual-fluorescence reporter/sensor plasmid. Biotechniques. 2006;41(6):727–732. doi: 10.2144/000112296. [DOI] [PubMed] [Google Scholar]
- 98.Hebert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol. 2000;222(2):296–306. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- 99.Stolt CC, Lommes P, Sock E, Chaboissier MC, Schedl A, Wegner M. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003;17(13):1677–1689. doi: 10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Scott CE, Wynn SL, Sesay A, et al. SOX9 induces and maintains neural stem cells. Nat Neurosci. 2010;13(10):1181–1189. doi: 10.1038/nn.2646. [DOI] [PubMed] [Google Scholar]
- 101.Yau HJ, Wang HF, Lai C, Liu FC. Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cereb Cortex. 2003;13(3):252–264. doi: 10.1093/cercor/13.3.252. [DOI] [PubMed] [Google Scholar]
- 102.Garaffo G, Conte D, Provero P, et al. The Dlx5 and Foxg1 transcription factors, linked via miRNA-9 and -200, are required for the development of the olfactory and GnRH system. Mol Cell Neurosci. 2015;68:103–119. doi: 10.1016/j.mcn.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang L, Zhang H, Hu G, Wang H, Abate-Shen C, Shen MM. An early phase of embryonic Dlx5 expression defines the rostral boundary of the neural plate. J Neurosci. 1998;18(20):8322–8330. doi: 10.1523/JNEUROSCI.18-20-08322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eisenstat DD, Liu JK, Mione M, et al. DLX-1, DLX-2, and DLX-5 expression define distinct stages of basal forebrain differentiation. J Comp Neurol. 1999;414(2):217–237. doi: 10.1002/(sici)1096-9861(19991115)414:2<217::aid-cne6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 105.Bulchand S, Grove EA, Porter FD, Tole S. LIM-homeodomain gene Lhx2 regulates the formation of the cortical hem. Mech Dev. 2001;100(2):165–175. doi: 10.1016/s0925-4773(00)00515-3. [DOI] [PubMed] [Google Scholar]
- 106.Lavdas AA, Grigoriou M, Pachnis V, Parnavelas JG. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci. 1999;19(18):7881–7888. doi: 10.1523/JNEUROSCI.19-18-07881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kitazawa A, Kubo K, Hayashi K, Matsunaga Y, Ishii K, Nakajima K. Hippocampal pyramidal neurons switch from a multipolar migration mode to a novel “climbing” migration mode during development. J Neurosci. 2014;34(4):1115–1126. doi: 10.1523/JNEUROSCI.2254-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smart IH. Radial unit analysis of hippocampal histogenesis in the mouse. J Anat. 1982;135(pt 4):763–793. [PMC free article] [PubMed] [Google Scholar]
- 109.Ferland RJ, Cherry TJ, Preware PO, Morrisey EE, Walsh CA. Characterization of Foxp2 and Foxp1 mRNA and protein in the developing and mature brain. J Comp Neurol. 2003;460(2):266–279. doi: 10.1002/cne.10654. [DOI] [PubMed] [Google Scholar]
- 110.Dubois NC, Hofmann D, Kaloulis K, Bishop JM, Trumpp A. Nestin-Cre transgenic mouse line Nes-Cre1 mediates highly efficient Cre/loxP mediated recombination in the nervous system, kidney, and somite-derived tissues. Genesis. 2006;44(8):355–360. doi: 10.1002/dvg.20226. [DOI] [PubMed] [Google Scholar]
- 111.McLoughlin HS, Fineberg SK, Ghosh LL, Tecedor L, Davidson BL. Dicer is required for proliferation, viability, migration and differentiation in corticoneurogenesis. Neuroscience. 2012;223:285–295. doi: 10.1016/j.neuroscience.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nowakowski TJ, Mysiak KS, O’Leary T, Fotaki V, Pratt T, Price DJ. Loss of functional Dicer in mouse radial glia cell-autonomously prolongs cortical neurogenesis. Dev Biol. 2013;382(2):530–537. doi: 10.1016/j.ydbio.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hanashima C, Shen L, Li SC, Lai E. Brain factor-1 controls the proliferation and differentiation of neocortical progenitor cells through independent mechanisms. J Neurosci. 2002;22(15):6526–6536. doi: 10.1523/JNEUROSCI.22-15-06526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Martynoga B, Morrison H, Price DJ, Mason JO. Foxg1 is required for specification of ventral telencephalon and region-specific regulation of dorsal telencephalic precursor proliferation and apoptosis. Dev Biol. 2005;283(1):113–127. doi: 10.1016/j.ydbio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 115.Manuel M, Price DJ. Role of Pax6 in forebrain regionalization. Brain Res Bull. 2005;66(4–6):387–393. doi: 10.1016/j.brainresbull.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 116.Manuel MN, Martynoga B, Molinek MD, et al. The transcription factor Foxg1 regulates telencephalic progenitor proliferation cell autonomously, in part by controlling Pax6 expression levels. Neural Dev. 2011;6:9. doi: 10.1186/1749-8104-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Burglin TR. Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Res. 1997;25(21):4173–4180. doi: 10.1093/nar/25.21.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Toresson H, Parmar M, Campbell K. Expression of Meis and Pbx genes and their protein products in the developing telencephalon: implications for regional differentiation. Mech Dev. 2000;94(1–2):183–187. doi: 10.1016/s0925-4773(00)00324-5. [DOI] [PubMed] [Google Scholar]
- 119.Agoston Z, Heine P, Brill MS, et al. Meis2 is a Pax6 co-factor in neurogenesis and dopaminergic periglomerular fate specification in the adult olfactory bulb. Development. 2014;141(1):28–38. doi: 10.1242/dev.097295. [DOI] [PubMed] [Google Scholar]
- 120.Agoston Z, Schulte D. Meis2 competes with the Groucho co-repressor Tle4 for binding to Otx2 and specifies tectal fate without induction of a secondary midbrain-hindbrain boundary organizer. Development. 2009;136(19):3311–3322. doi: 10.1242/dev.037770. [DOI] [PubMed] [Google Scholar]
- 121.Manuel M, Martynoga B, Yu T, West JD, Mason JO, Price DJ. The transcription factor Foxg1 regulates the competence of telencephalic cells to adopt subpallial fates in mice. Development. 2010;137(3):487–497. doi: 10.1242/dev.039800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yun K, Garel S, Fischman S, Rubenstein JL. Patterning of the lateral ganglionic eminence by the Gsh1 and Gsh2 homeobox genes regulates striatal and olfactory bulb histogenesis and the growth of axons through the basal ganglia. J Comp Neurol. 2003;461(2):151–165. doi: 10.1002/cne.10685. [DOI] [PubMed] [Google Scholar]
- 123.Hebert JM, Fishell G. The genetics of early telencephalon patterning: some assembly required. Nat Rev Neurosci. 2008;9(9):678–685. doi: 10.1038/nrn2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Carney RS, Cocas LA, Hirata T, Mansfield K, Corbin JG. Differential regulation of telencephalic pallial-subpallial boundary patterning by Pax6 and Gsh2. Cereb Cortex. 2009;19(4):745–759. doi: 10.1093/cercor/bhn123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stenman J, Yu RT, Evans RM, Campbell K. Tlx and Pax6 co-operate genetically to establish the pallio-subpallial boundary in the embryonic mouse telencephalon. Development. 2003;130(6):1113–1122. doi: 10.1242/dev.00328. [DOI] [PubMed] [Google Scholar]
- 126.Walker JC, Harland RM. Expression of microRNAs during embryonic development of Xenopus tropicalis. Gene Expr Patterns. 2008;8(6):452–456. doi: 10.1016/j.gep.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Davis RL, Turner DL. Vertebrate hairy and enhancer of split related proteins: transcriptional repressors regulating cellular differentiation and embryonic patterning. Oncogene. 2001;20(58):8342–8357. doi: 10.1038/sj.onc.1205094. [DOI] [PubMed] [Google Scholar]
- 128.Coolen M, Thieffry D, Drivenes O, Becker TS, Bally-Cuif L. miR-9 controls the timing of neurogenesis through the direct inhibition of antagonistic factors. Dev Cell. 2012;22(5):1052–1064. doi: 10.1016/j.devcel.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 129.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203(3):253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 130.Nepal C, Coolen M, Hadzhiev Y, et al. Transcriptional, post-transcriptional and chromatin-associated regulation of pri-miRNAs, pre-miRNAs and moRNAs. Nucleic Acids Res. 2016;44(7):3070–3081. doi: 10.1093/nar/gkv1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88(1):49–92. [PubMed] [Google Scholar]
- 132.Darnell DK, Kaur S, Stanislaw S, et al. GEISHA: an in situ hybridization gene expression resource for the chicken embryo. Cytogenet Genome Res. 2007;117(1–4):30–35. doi: 10.1159/000103162. [DOI] [PubMed] [Google Scholar]
- 133.Kiecker C, Lumsden A. Hedgehog signaling from the ZLI regulates diencephalic regional identity. Nat Neurosci. 2004;7(11):1242–1249. doi: 10.1038/nn1338. [DOI] [PubMed] [Google Scholar]
- 134.Scholpp S, Wolf O, Brand M, Lumsden A. Hedgehog signalling from the zona limitans intrathalamica orchestrates patterning of the zebrafish diencephalon. Development. 2006;133(5):855–864. doi: 10.1242/dev.02248. [DOI] [PubMed] [Google Scholar]
- 135.Vieira C, Garda AL, Shimamura K, Martinez S. Thalamic development induced by Shh in the chick embryo. Dev Biol. 2005;284(2):351–363. doi: 10.1016/j.ydbio.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 136.Vue TY, Bluske K, Alishahi A, et al. Sonic hedgehog signaling controls thalamic progenitor identity and nuclei specification in mice. J Neurosci. 2009;29(14):4484–4497. doi: 10.1523/JNEUROSCI.0656-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Scholpp S, Foucher I, Staudt N, Peukert D, Lumsden A, Houart C. Otx1l, Otx2 and Irx1b establish and position the ZLI in the diencephalon. Development. 2007;134(17):3167–3176. doi: 10.1242/dev.001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hirata T, Nakazawa M, Muraoka O, Nakayama R, Suda Y, Hibi M. Zinc-finger genes Fez and Fez-like function in the establishment of diencephalon subdivisions. Development. 2006;133(20):3993–4004. doi: 10.1242/dev.02585. [DOI] [PubMed] [Google Scholar]
- 139.Braun MM, Etheridge A, Bernard A, Robertson CP, Roelink H. Wnt signaling is required at distinct stages of development for the induction of the posterior forebrain. Development. 2003;130(23):5579–5587. doi: 10.1242/dev.00685. [DOI] [PubMed] [Google Scholar]
- 140.Kobayashi D, Kobayashi M, Matsumoto K, Ogura T, Nakafuku M, Shimamura K. Early subdivisions in the neural plate define distinct competence for inductive signals. Development. 2002;129(1):83–93. doi: 10.1242/dev.129.1.83. [DOI] [PubMed] [Google Scholar]
- 141.Pappalardo-Carter DL, Balaraman S, Sathyan P, Carter ES, Chen WJ, Miranda RC. Suppression and epigenetic regulation of MiR-9 contributes to ethanol teratology: evidence from zebrafish and murine fetal neural stem cell models. Alcohol Clin Exp Res. 2013;37(10):1657–1667. doi: 10.1111/acer.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li N, Hornbruch A, Klafke R, Katzenberger B, Wizenmann A. Specification of dorsoventral polarity in the embryonic chick mesencephalon and its presumptive role in midbrain morphogenesis. Dev Dyn. 2005;233(3):907–920. doi: 10.1002/dvdy.20434. [DOI] [PubMed] [Google Scholar]
- 143.Dworkin S, Jane SM. Novel mechanisms that pattern and shape the midbrain-hindbrain boundary. Cell Mol Life Sci. 2013;70(18):3365–3374. doi: 10.1007/s00018-012-1240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hidalgo-Sanchez M, Millet S, Bloch-Gallego E, Alvarado-Mallart RM. Specification of the mesoisthmo-cerebellar region: the Otx2/Gbx2 boundary. Brain Res Brain Res Rev. 2005;49(2):134–149. doi: 10.1016/j.brainresrev.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 145.Hidalgo-Sanchez M, Millet S, Simeone A, Alvarado-Mallart RM. Comparative analysis of Otx2, Gbx2, Pax2, Fgf8 and Wnt1 gene expressions during the formation of the chick midbrain/hindbrain domain. Mech Dev. 1999;81(1–2):175–178. doi: 10.1016/s0925-4773(98)00224-x. [DOI] [PubMed] [Google Scholar]
- 146.Acampora D, Gulisano M, Simeone A. Otx genes and the genetic control of brain morphogenesis. Mol Cell Neurosci. 1999;13(1):1–8. doi: 10.1006/mcne.1998.0730. [DOI] [PubMed] [Google Scholar]
- 147.Acampora D, Mazan S, Lallemand Y, et al. Forebrain and midbrain regions are deleted in Otx2−/− mutants due to a defective anterior neuroectoderm specification during gastrulation. Development. 1995;121(10):3279–3290. doi: 10.1242/dev.121.10.3279. [DOI] [PubMed] [Google Scholar]
- 148.Millet S, Bloch-Gallego E, Simeone A, Alvarado-Mallart RM. The caudal limit of Otx2 gene expression as a marker of the midbrain/hindbrain boundary: a study using in situ hybridisation and chick/quail homotopic grafts. Development. 1996;122(12):3785–3797. doi: 10.1242/dev.122.12.3785. [DOI] [PubMed] [Google Scholar]
- 149.Katahira T, Sato T, Sugiyama S, et al. Interaction between Otx2 and Gbx2 defines the organizing center for the optic tectum. Mech Dev. 2000;91(1–2):43–52. doi: 10.1016/s0925-4773(99)00262-2. [DOI] [PubMed] [Google Scholar]
- 150.Tallafuss A, Bally-Cuif L. Tracing of her5 progeny in zebrafish transgenics reveals the dynamics of midbrain-hindbrain neurogenesis and maintenance. Development. 2003;130(18):4307–4323. doi: 10.1242/dev.00662. [DOI] [PubMed] [Google Scholar]
- 151.Belting HG, Hauptmann G, Meyer D, et al. spiel ohne grenzen/pou2 is required during establishment of the zebrafish midbrain-hindbrain boundary organizer. Development. 2001;128(21):4165–4176. doi: 10.1242/dev.128.21.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Crossley PH, Marinez S, Martin G. Midbrain development induced by FGF8 in the chick embryo. Nature. 1996;380:66–68. doi: 10.1038/380066a0. [DOI] [PubMed] [Google Scholar]
- 153.Glavic A, Gomez-Skarmeta JL, Mayor R. The homeoprotein Xiro1 is required for midbrain-hindbrain boundary formation. Development. 2002;129(7):1609–1621. doi: 10.1242/dev.129.7.1609. [DOI] [PubMed] [Google Scholar]
- 154.Dworkin S, Darido C, Georgy SR, et al. Midbrain-hindbrain boundary patterning and morphogenesis are regulated by diverse grainy head-like 2-dependent pathways. Development. 2012;139(3):525–536. doi: 10.1242/dev.066522. [DOI] [PubMed] [Google Scholar]
- 155.Barritt LC, Miller JM, Scheetz LR, et al. Conditional deletion of the human ortholog gene Dicer1 in Pax2-Cre expression domain impairs orofacial development. Indian J Hum Genet. 2012;18(3):310–319. doi: 10.4103/0971-6866.107984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pfeffer PL, Payer B, Reim G, di Magliano MP, Busslinger M. The activation and maintenance of Pax2 expression at the mid-hindbrain boundary is controlled by separate enhancers. Development. 2002;129(2):307–318. doi: 10.1242/dev.129.2.307. [DOI] [PubMed] [Google Scholar]
- 157.Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8(24):1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- 158.Fraser S, Keynes R, Lumsden A. Segmentation in the chick embryo hindbrain is defined by cell lineage restrictions. Nature. 1990;344(6265):431–435. doi: 10.1038/344431a0. [DOI] [PubMed] [Google Scholar]
- 159.Lumsden A. Segmentation and compartition in the early avian hindbrain. Mech Dev. 2004;121(9):1081–1088. doi: 10.1016/j.mod.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 160.Kiecker C, Lumsden A. Compartments and their boundaries in vertebrate brain development. Nat Rev Neurosci. 2005;6(7):553–564. doi: 10.1038/nrn1702. [DOI] [PubMed] [Google Scholar]
- 161.Lumsden A, Sprawson N, Graham A. Segmental origin and migration of neural crest cells in the hindbrain region of the chick embryo. Development. 1991;113(4):1281–1291. doi: 10.1242/dev.113.4.1281. [DOI] [PubMed] [Google Scholar]
- 162.Riley BB, Chiang MY, Storch EM, Heck R, Buckles GR, Lekven AC. Rhombomere boundaries are Wnt signaling centers that regulate metameric patterning in the zebrafish hindbrain. Dev Dyn. 2004;231(2):278–291. doi: 10.1002/dvdy.20133. [DOI] [PubMed] [Google Scholar]
- 163.Amoyel M, Cheng YC, Jiang YJ, Wilkinson DG. Wnt1 regulates neurogenesis and mediates lateral inhibition of boundary cell specification in the zebrafish hindbrain. Development. 2005;132(4):775–785. doi: 10.1242/dev.01616. [DOI] [PubMed] [Google Scholar]
- 164.Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1(1):20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 165.Carpenter EM. Hox genes and spinal cord development. Dev Neurosci. 2002;24(1):24–34. doi: 10.1159/000064943. [DOI] [PubMed] [Google Scholar]
- 166.Briscoe J, Pierani A, Jessell TM, Ericson J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101(4):435–445. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- 167.Otaegi G, Pollock A, Sun T. An optimized sponge for microRNA miR-9 affects spinal motor neuron development in vivo. Front Neurosci. 2011;5:146. doi: 10.3389/fnins.2011.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Luxenhofer G, Helmbrecht MS, Langhoff J, Giusti SA, Refojo D, Huber AB. MicroRNA-9 promotes the switch from early-born to late-born motor neuron populations by regulating Onecut transcription factor expression. Dev Biol. 2014;386(2):358–370. doi: 10.1016/j.ydbio.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 169.Grant E, Hoerder-Suabedissen A, Molnar Z. Development of the corticothalamic projections. Front Neurosci. 2012;6:53. doi: 10.3389/fnins.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nakamura H, Sato T, Suzuki-Hirano A. Isthmus organizer for mesencephalon and metencephalon. Dev Growth Differ. 2008;50:S113–S118. doi: 10.1111/j.1440-169X.2008.00995.x. [DOI] [PubMed] [Google Scholar]
- 171.Cheong AW, Pang RT, Liu WM, Kottawatta KS, Lee KF, Yeung WS. MicroRNA Let-7a and dicer are important in the activation and implantation of delayed implanting mouse embryos. Hum Reprod. 2014;29(4):750–762. doi: 10.1093/humrep/det462. [DOI] [PubMed] [Google Scholar]
- 172.Kawase-Koga Y, Low R, Otaegi G, et al. RNAase-III enzyme Dicer maintains signaling pathways for differentiation and survival in mouse cortical neural stem cells. J Cell Sci. 2010;123(Pt 4):586–594. doi: 10.1242/jcs.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Deo M, Yu JY, Chung KH, Tippens M, Turner DL. Detection of mammalian microRNA expression by in situ hybridization with RNA oligonucleotides. Dev Dyn. 2006;235(9):2538–2548. doi: 10.1002/dvdy.20847. [DOI] [PubMed] [Google Scholar]