Abstract
Background
Despite the high frequency of hypotension during spinal anesthesia with proper sedation, no previous report has compared the hemodynamic effects of dexmedetomidine and midazolam sedation during spinal anesthesia. We compared the effects of bispectral index (BIS)-guided intravenous sedation using midazolam or dexmedetomidine on hemodynamics and recovery profiles in patients who underwent spinal anesthesia.
Material/Methods
One hundred and sixteen adult patients were randomly assigned to receive either midazolam (midazolam group; n=58) or dexmedetomidine (dexmedetomidine group; n=58) during spinal anesthesia. Systolic, diastolic, and mean arterial pressures; heart rates; peripheral oxygen saturations; and bispectral index scores were recorded during surgery, and Ramsay sedation scores and postanesthesia care unit (PACU) stay were monitored.
Results
Hypotension occurred more frequently in the midazolam group (P<0.001) and bradycardia occurred more frequently in the dexmedetomidine group (P<0.001). Mean Ramsay sedation score was significantly lower in the dexmedetomidine group after arrival in the PACU (P=0.025) and PACU stay was significantly longer in the dexmedetomidine group (P=0.003).
Conclusions
BIS-guided dexmedetomidine sedation can attenuate intraoperative hypotension, but induces more bradycardia, prolongs PACU stay, and delays recovery from sedation in patients during and after spinal anesthesia as compared with midazolam sedation.
MeSH Keywords: Anesthesia, Spinal; Bradycardia; Dexmedetomidine; Hypotension; Midazolam
Background
Despite the benefits of spinal anesthesia, which include its greater cost effectiveness and less postoperative pain than in general anesthesia [1], it has been associated with a high rate of hemodynamic instability. A previous analysis of 952 cases identified hypotension and bradycardia rates of 33% and 13%, respectively, during spinal anesthesia, which were attributed to thoraco-lumbar sympathetic block and relative activation of parasympathetic tone, leading to diminished cardiac output [2]. Sedation during spinal anesthesia undertaken to improve patient satisfaction and acceptance of regional anesthesia could aggravate spinal anesthesia-induced hemodynamic instability caused by the anxiolytic properties and negative inotropic actions of sedatives.
Recently, dexmedetomidine, a selective α2-adrenoreceptor agonist, was a focus of interest for sedation during regional anesthesia due to its rapid offset, prolongation of spinal anesthesia, and excellent postoperative analgesia characteristics [3,4]. In addition, dexmedetomidine has a biphasic hemodynamic effect, whereby an initial increase in blood pressure by α2-receptor-mediated peripheral vasoconstriction is followed by a decrease due to norepinephrine release and sympathetic activity inhibition in central nervous system [5,6].
Bispectral index (BIS) monitors offer distinct advantages of objective, real-time assessment of sedated patients without the application of external stimuli [7]. In addition to monitoring hypnotic state, BIS monitoring might be helpful during the titration of sedatives so as to avoid adverse effects such as awareness due to inappropriate dosage and the adverse effects of over-dosage. Although the rate of hypotension during spinal anesthesia with proper sedation has been reported to be high, no previous report has compared the hemodynamic effects of BIS-guided sedation using dexmedetomidine or midazolam during spinal anesthesia. We hypothesized that transient hypertension induced by dexmedetomidine might attenuate spinal anesthesia-induced hypotension. Thus, the aim of this study was to compare the effects of BIS-guided intravenous sedation using midazolam or dexmedetomidine on hemodynamics and recovery profiles in patients undergoing spinal anesthesia.
Material and Methods
Participants and group assignment
After obtaining Institutional Review Board approval, 116 adult patients with American Society of Anesthesiologists (ASA) physical status 1 or 2, aged 20–65 years, and scheduled for elective surgery from January 2014 to December 2014 were enrolled in this prospective randomized study. The exclusion criteria applied were: a history of uncontrolled diabetes mellitus or uncontrolled hypertension, severe cardiovascular or respiratory disease, or any contraindication for spinal anesthesia. Patients were randomly assigned to receive either midazolam (the midazolam group; n=58) or dexmedetomidine (the dexmedetomidine group; n=58) using a randomized list generated using Excel 2007 (Microsoft Office, Redmond, WA, USA) without stratification.
Anesthesia and outcome assessment
No patient was premedicated. On arrival at the operating room, standard monitors were applied for non-invasive blood pressure, EKG, and pulse oximetry. All patients received a fluid preload of 300 ml of saline solution. Spinal anesthesia was performed in the lateral decubitus position using 0.5% hyperbaric bupivacaine by an anesthesiologist unaware of group identities. Study drugs were prepared by an anesthesia nurse blinded to group assignments (50 ml of midazolam 0.2 mg/ml in saline for the midazolam group and 50 ml of dexmedetomidine 4 μg/ml in saline for the dexmedetomidine group). Ten minutes after an intrathecal injection, 1.5 ml/kg/h of the study drug in a 50-ml syringe was infused over 10 min as a loading dose and then 0.125 ml/kg/h was infused as an initial maintenance dose. Infusion volumes were determined using midazolam or dexmedetomidine loading doses of 0.05 mg/kg or 1 μg/kg, respectively, for 10 min, and initial maintenance doses of midazolam or dexmedetomidine of 0.025 mg/kg/h or 0.5 μg/kg/h, respectively. Using a target bispectral index score (BIS) of 65–85, infused solutions were titrated until the end of surgery. Hypotension was defined as a fall in systolic blood pressure (SBP) to 80% below baseline or <90 mmHg; phenylephrine (50 μg) or ephedrine (5 mg) was administered when SBP fell to <90 mmHg. Bradycardia was defined as heart rate (HR) of <50 beats/min; atropine (0.5 mg) was administered at 2 min intervals when HR fell to under 45 beats/min. Intravenous fluid was infused at a constant rate of 6 ml/kg/h. Systolic, diastolic, and mean arterial pressures, heart rates, pulse oximeter oxygen saturations, and bispectral index scores were recorded before anesthetic induction (T0), at 5 min before anesthetic induction (T5), at 10 min (T10; start of midazolam or dexmedetomidine administration), and every 5 min (T15–T40) after the intrathecal injection. After arrival at the postanesthetic care unit (PACU), we monitored hemodynamic variables, cold- and pin prick-determined spinal block levels, and Ramsay sedation scores (1=anxious, agitated, or restless; 2=cooperative, oriented, and tranquil; 3=only responsive to verbal commands; 4=asleep, but brisk response to a light glabella tap or loud auditory stimulus; 5=asleep and sluggish response to a glabella tap or loud auditory stimulus; 6 asleep=no response to a light glabella tap or loud auditory stimulus). Patients were discharged from the PACU when vital signs were within 20% of preanesthetic values, the Ramsay sedation score was <3, and spinal blocked level was under T10, as determined by an independent anesthesiologist.
Statistical analysis
The primary outcome variable was mean blood pressure (MBP) after spinal anesthesia. The sample size calculation was based on the findings of a previous study [8], which reported that the lowest mean blood pressure (MBP) was 82.7±7.4 mmHg in a midazolam group. Assuming a 5% difference in mean values of the lowest MBP, a power of 80%, and an α-error of 0.05, 49 patients were found to be required per group. Thus, 58 subjects were included per group to accommodate an expected loss of 20%.
The statistical analysis was performed using PASW Statistics 13® (SPSS Inc, Chicago, IL, USA). Results are expressed as means ±SDs or numbers of patients. Patient characteristics were compared using the t test or Fisher’s exact test, as appropriate. Hemodynamic variables and BIS values at each time point were compared using the t test. Changes in hemodynamic variables and BIS in the 2 groups were compared using repeated measures ANOVA. Post hoc comparisons within groups were performed using Bonferroni’s test. Statistical significance was accepted for P values <0.05.
Results
The data of 116 patients were analyzed (Figure 1). No significant intergroup differences were observed for patient characteristics, total operative time, or the type of surgery (Table 1).
Figure 1.
Patient assignment flow diagram.
Table 1.
Patient characteristics and perioperative data.
| Midazolam (n=58) | Dexmedetomidine (n=58) | P-value | |
|---|---|---|---|
| Age (yr) | 47.0±16.2 | 47.1±15.2 | 0.972 |
| Weight (kg) | 68.3±13.3 | 69.3±13.1 | 0.659 |
| Height (cm) | 168.4±7.9 | 167.8±8.3 | 0.698 |
| Gender (M/F) | 43/15 | 40/18 | 0.537 |
| Diabetes mellitus (n) | 9 | 8 | 0.793 |
| Hypertension (n) | 10 | 14 | 0.412 |
| Hypertensive medication (n) | 0.405 | ||
| β-blocker | 1 | 2 | |
| Calcium-channel blocker | 6 | 11 | |
| Others | 7 | 9 | |
| Anesthesia time (min) | 70.2±25.8 | 73.0±27.6 | 0.583 |
| Dose of bupivacaine (mg) | 13.0 [12–15] | 13.5 [12–16] | 0.358 |
| Infused fluid (ml) | 430±173 | 463±161 | 0.427 |
| Type of surgery (n) | 0.489 | ||
| Knee | 10 | 15 | |
| Tibiofibular/ankle/foot | 25 | 23 | |
| Urology | 21 | 16 | |
| Others | 2 | 4 |
Values are means ± standard deviations, medians [interquartile ranges], or numbers of patients.
During surgery, hypotension was more common in the midazolam group (P<0.001) and bradycardia was more common in the dexmedetomidine group (P<0.001). Phenylephrine and ephedrine requirements were non-significantly higher in the midazolam group. However, more atropine was required during surgery in the dexmedetomidine group (P=0.04) (Table 2). During sedation, no patient experienced arterial desaturation, defined as a SaO2 of <90% and no patients required transfusion of packed red blood cell due to blood loss.
Table 2.
Sensory block level and adverse events during spinal anesthesia.
| Midazolam (n=58) | Dexmedetomidine (n=58) | P-value | |
|---|---|---|---|
| Sensory block level at T10 (thoracic segments) | 6 [6–8] | 8 [6–8] | 0.259 |
| Time to reach to BIS 70 (min) | 14.3±6.2 | 12.6±3.8 | 0.498 |
| Time to recover to BIS 80 (min) | 11.5±6.6 | 13.5±7.1 | 0.546 |
| Incidence of hypotension (n) | 38 (66) | 18 (31) | <0.001 |
| Phenylephrine use (n) | 9 (16) | 4 (7) | 0.141 |
| Ephedrine use (n) | 17 (29) | 9 (16) | 0.075 |
| Incidence of bradycardia (n) | 11 (19) | 29 (50) | <0.001 |
| Atropine use (n) | 3 (5) | 14 (24) | 0.004 |
Values are means ±SDs, medians [interquartile ranges] or numbers of patients (%). T10 – 10 min after intrathecal injection (T10; start of midazolam or dexmedetomidine); BIS – bispectral index score.
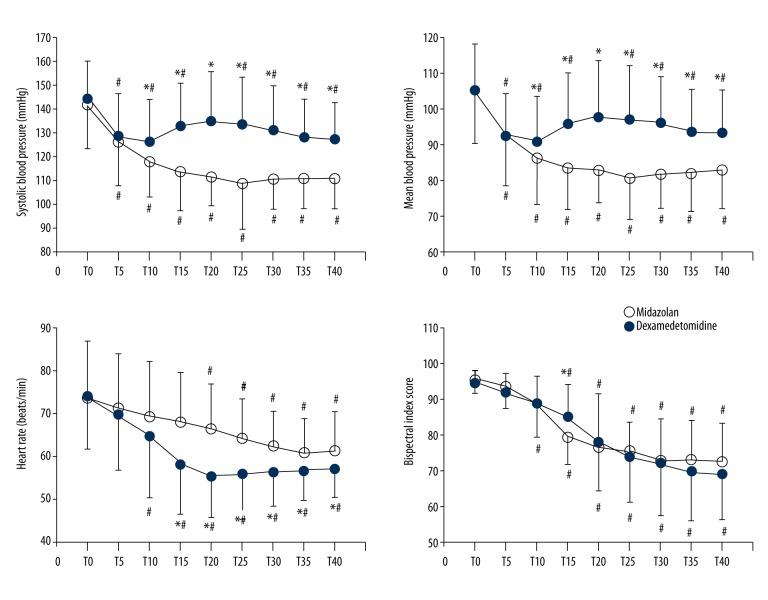
Intraoperative changes in systolic blood pressure (SBP), MBP, heart rate (HR), and BIS are shown in Figure 2. All variables were normally distributed (all P values >0.05). SBP and MBP were significantly higher in the dexmedetomidine group at T10–T40. SBP and MBP were significantly decreased after intrathecal injection in both groups versus baseline (P<0.001). Changes in SBP and MBP over time differed significantly in both groups (P<0.001). At T15–T40, HR was significantly lower in the dexmedetomidine group. HR decreased significantly from T10 in the dexmedetomidine group and from T20 in the midazolam group versus baseline. HR changes differed significantly in the 2 groups (P<0.001). In particular, BIS was significantly higher at T15 in the dexmedetomidine group. Over time, BIS changed significantly in both groups (P<0.001), but no intergroup difference was observed (P=0.677).
Figure 2.
Intraoperative hemodynamic changes in systolic blood pressure, mean blood pressure, heart rate, and bispectral index in patients that received midazolam (midazolam group, ?) or dexmedetomidine (dexmedetomidine group, during spinal anesthesia. Error bars represent standard deviations. Before anesthetic induction (T0), 5 min after intrathecal injection (T5), 10 min after intrathecal injection (T10; start of midazolam or dexmedetomidine), and every 5 min after sedative injection (T15–T40). * P<0.05, midazolam group vs. dexmedetomidine group, # P<0.05 vs. T0.
Lowest SBP and MBP were significantly higher (P values <0.001) and lowest HR and BIS were significantly lower (P<0.001 and 0.011, respectively) in the dexmedetomidine group. Changes in SBP, MBP, HR, and BIS ((baseline – lowest value)/baseline value) were significantly different in the 2 groups (P<0.001, P=0.001, P<0.001, P=0.030, respectively) (Table 3).
Table 3.
Intraoperative hemodynamic and bispectral index data.
| Midazolam (n=58) | Dexmedetomidine (n=58) | P-value | |
|---|---|---|---|
| Baseline | |||
| Systolic blood pressure (mmHg) | 141±18 | 144±16 | 0.377 |
| Mean blood pressure (mmHg) | 105±14 | 105±13 | 0.946 |
| Heart rate (beats/min) | 73±12 | 74±13 | 0.853 |
| Bispectral index | 95±4 | 95±4 | 0.249 |
| Lowest | |||
| Systolic blood pressure (mmHg) | 103±11 | 115±16 | <0.001 |
| Mean blood pressure (mmHg) | 76±10 | 84±11 | <0.001 |
| Heart rate (beats/min) | 58±9 | 52±7 | <0.001 |
| Bispectral index | 67±10 | 62±11 | 0.011 |
| Changes (%) | |||
| Systolic blood pressure | 27±10 | 20±11 | <0.001 |
| Mean blood pressure | 26±11 | 20±11 | 0.001 |
| Heart rate | 19±10 | 29±11 | <0.001 |
| Bispectral index | 29±11 | 34±12 | 0.030 |
Values are means ±SDs. Changes, (baseline-lowest)/baseline*100.
In the PACU, hemodynamic changes were similar in the 2 groups (Table 4). Ramsay sedation score was significantly lower in the dexmedetomidine group after arrival in the PACU (P=0.025), and PACU stay was significantly longer this group than in the midazolam group (P=0.003) (Table 5).
Table 4.
Postoperative hemodynamic data.
| P0 | P10 | P20 | P30 | ||
|---|---|---|---|---|---|
| SBP (mmHg) | Midazolam | 114±15 | 110±26 | 115±14 | 114±20 |
| Dexmedetomidine | 109±16 | 106±23 | 111±15 | 111±15 | |
| MAP (mmHg) | Midazolam | 84±15 | 85±11 | 84±10 | 84±12 |
| Dexmedetomidine | 81±12 | 81±12 | 82±11 | 82±11 | |
| HR (beats/min) | Midazolam | 61±9 | 58±11 | 59±9 | 59±8 |
| Dexmedetomidine | 58±11 | 56±10 | 57±11 | 57±9 |
Values are means ±SDs. SBP – systolic blood pressure; MBP – mean blood pressure; HR – heart rate; P 0–30 – 0–30 min after postanesthetic care unit admission.
Table 5.
Postoperative profiles.
| Midazolam (n=58) | Dexmedetomidine (n=58) | P-value | |
|---|---|---|---|
| Sensory block level at PACU arrival (thoracic segments) | 7 [6–8] | 8 [6–10] | 0.264 |
| PACU stay (min) | 42±17 | 55±27 | 0.003 |
| Ramsay sedation score at PACU arrival | 2.3 (2 [2–6]) | 2.7 (2 [2–6]) | 0.025 |
| Ramsay sedation score at 30 min after PACU arrival | 2.1 (2 [2–4]) | 2.3 (2 [2–5]) | 0.066 |
| Incidence of hypotension (n) | 4 (7) | 6 (10) | 0.508 |
| Phenylephrine use (n) | 0 (0) | 1 (2) | 0.315 |
| Ephedrine use (n) | 1 (2) | 1 (2) | 0.990 |
| Incidence of bradycardia (n) | 7 (12) | 13 (22) | 0.140 |
| Atropine use (n) | 1 (2) | 5 (9) | 0.098 |
Values are means ±SDs or means (medians [minimum-maximum]). PACU stay – time spent in the postanesthetic care unit (PACU).
Discussion
This prospective randomized study shows that BIS-guided dexmedetomidine sedation can decrease the incidence of hypotension as compared with midazolam sedation during spinal anesthesia. However, dexmedetomidine was found to provoke more episodes of bradycardia and to prolong PACU stay due to delayed recovery from its hypnotic effects.
Dexmedetomidine reduces the release of norepinephrine induced by presynaptic α2-receptor activation and inhibits sympathetic activity induced by postsynaptic receptors in the central nervous system, and these can decrease blood pressure and heart rate [6]. Some studies have reported blood pressure reductions after midazolam induction for ICU sedation or pre-hospital rapid sequence induction [9,10]. Given its anxiolytic and sedative properties, midazolam has negative inotropic activity in atrial tissues mediated by the inhibition of L-type calcium channels [11]. However, although dexmedetomidine and midazolam reduce blood pressure and heart rate, a previous comparative study demonstrated lower heart rate and blood pressure during third molar surgery for dexmedetomidine compared to midazolam during monitored anesthesia care [12]. However, in the present study, blood pressure was significantly higher in the dexmedetomidine group during the sedation period. Dexmedetomidine provokes an initial transient increase in blood pressure because of α2-adrenoceptor-mediated vasoconstriction in peripheral vessels, and a diminished heart rate could increase blood pressure mediated by the baroreceptor reflex [5]. A previous animal study demonstrated that pretreatment with oral dexmedetomidine before halothane administration can prevent the halothane-induced suppression of baroreceptor function, and produces a better hemodynamic profile than halothane alone [13]. However, midazolam causes a transient baroreflex depression and a sustained decrease of sympathetic tone in humans [14]. It has been reported that the preserved baroreflex and transient biphasic hemodynamic response observed during dexmedetomidine administration can attenuate hemodynamic changes induced by thoracolumbar sympathetic block and venous pooling during spinal anesthesia [15].
During the operations, patients in the dexmedetomidine group were administered atropine more frequently than patients in the midazolam group because of the higher incidence of bradycardia. Previous animal studies have demonstrated that atropine during dexmedetomidine administration can increase blood pressure [16,17]; therefore, the hemodynamic effect of atropine may have contributed to the relatively high blood pressures we observed in the dexmedetomidine group.
Sim et al. [18] reported that dexmedetomidine 1 μg/kg as a loading dose might lead to faster sedation without severe complications compared to a 0.5 μg/kg loading dose during spinal anesthesia; therefore, we used a loading dose of dexmedetomidine 1 μg/kg, which led to an onset similar to that observed for midazolam sedation.
In a previous comparative study, memory recalls at the end of 1-h infusions of 0.2 or 0.6 μg/kg dexmedetomidine were significantly worse than in a placebo control group, and BIS recovered to baseline 4 h after stopping dexmedetomidine [19]. We also found that PACU stays were longer with higher sedation score in the dexmedetomidine group than in the midazolam group. It has been previously suggested that proper control of infusion rate might help reduce delayed recovery from sedation [20].
The present study is limited because we could not measure the sensory regression time of spinal block because all patients were moderately sedated (BIS <85). A previously study reported that intravenous dexmedetomidine prolonged the duration of spinal anesthesia [21] and that sensory level could affect hemodynamic changes. However, despite the possibility of prolonged spinal block by dexmedetomidine, since blocked sensory levels were similar at the end of surgery, the hemodynamic effects induced by blocked levels might have been minimal in the present study.
Conclusions
BIS-guided dexmedetomidine sedation attenuated spinal anesthesia-induced hypotension more so than midazolam sedation, but dexmedetomidine induced bradycardia more frequently and was associated with greater atropine use. In addition, dexmedetomidine prolonged PACU stay and delayed sedation recovery after spinal anesthesia.
Footnotes
Conflict of Interest
The authors have no potential conflict of interest to declare.
Source of support: Departmental sources
References
- 1.Gonano C, Leitgeb U, Sitzwohl C, et al. Spinal versus general anesthesia for orthopedic surgery: anesthesia drug and supply costs. Anesth Analg. 2006;102:524–29. doi: 10.1213/01.ane.0000194292.81614.c6. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter RL, Caplan RA, Brown DL, et al. Incidence and risk factors for side effects of spinal anesthesia. Anesthesiology. 1992;76:906–16. doi: 10.1097/00000542-199206000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Kim J, Kim WO, Kim HB, Kil HK. Adequate sedation with single-dose dexmedetomidine in patients undergoing transurethral resection of the prostate with spinal anaesthesia: A dose-response study by age group. BMC Anesthesiol. 2015;15:17. doi: 10.1186/1471-2253-15-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niu XY, Ding XB, Guo T, et al. Effects of intravenous and intrathecal dexmedetomidine in spinal anesthesia: A meta-analysis. CNS Neurosci Ther. 2013;19:897–904. doi: 10.1111/cns.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–42. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: A novel sedative-analgesic agent. Proc (Bayl Univ Med Cent) 2001;14:13–21. doi: 10.1080/08998280.2001.11927725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Triltsch AE, Welte M, von Homeyer P, et al. Bispectral index-guided sedation with dexmedetomidine in intensive care: A prospective, randomized, double blind, placebo-controlled phase II study. Crit Care Med. 2002;30:1007–14. doi: 10.1097/00003246-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Kaya FN, Yavascaoglu B, Turker G, et al. Intravenous dexmedetomidine, but not midazolam, prolongs bupivacaine spinal anesthesia. Can J Anaesth. 2010;57:39–45. doi: 10.1007/s12630-009-9231-6. [DOI] [PubMed] [Google Scholar]
- 9.Davis DP, Kimbro TA, Vilke GM. The use of midazolam for prehospital rapid-sequence intubation may be associated with a dose-related increase in hypotension. Prehosp Emerg Care. 2001;5:163–68. doi: 10.1080/10903120190940065. [DOI] [PubMed] [Google Scholar]
- 10.Higgins TL, Yared JP, Estafanous FG, et al. Propofol versus midazolam for intensive care unit sedation after coronary artery bypass grafting. Crit Care Med. 1994;22:1415–23. doi: 10.1097/00003246-199409000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Nonaka A, Kashimoto S, Imamura M, et al. Mechanism of the negative inotropic effect of diazepam and midazolam in cultured foetal mouse cardiac myocytes. Eur J Anaesthesiol. 1997;14:481–87. doi: 10.1046/j.1365-2346.1997.00111.x. [DOI] [PubMed] [Google Scholar]
- 12.Cheung CW, Ying CL, Chiu WK, et al. A comparison of dexmedetomidine and midazolam for sedation in third molar surgery. Anaesthesia. 2007;62:1132–38. doi: 10.1111/j.1365-2044.2007.05230.x. [DOI] [PubMed] [Google Scholar]
- 13.Devcic A, Schmeling WT, Kampine JP, Warltier DC. Oral dexmedetomidine preserves baroreceptor function and decreases anesthetic requirements of halothane-anesthetized dogs. Anesthesiology. 1994;81:419–30. doi: 10.1097/00000542-199408000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Marty J, Gauzit R, Lefevre P, et al. Effects of diazepam and midazolam on baroreflex control of heart rate and on sympathetic activity in humans. Anesth Analg. 1986;65:113–19. [PubMed] [Google Scholar]
- 15.Stevens RA, Frey K, Liu SS, et al. Sympathetic block during spinal anesthesia in volunteers using lidocaine, tetracaine, and bupivacaine. Reg Anesth. 1997;22:325–31. doi: 10.1016/s1098-7339(97)80006-5. [DOI] [PubMed] [Google Scholar]
- 16.Congdon JM, Marquez M, Niyom S, Boscan P. Evaluation of the sedative and cardiovascular effects of intramuscular administration of dexmedetomidine with and without concurrent atropine administration in dogs. J Am Vet Med Assoc. 2011;239:81–89. doi: 10.2460/javma.239.1.81. [DOI] [PubMed] [Google Scholar]
- 17.Monteiro ER, Campagnol D, Parrilha LR, Furlan LZ. Evaluation of cardiorespiratory effects of combinations of dexmedetomidine and atropine in cats. J Feline Med Surg. 2009;11:783–92. doi: 10.1016/j.jfms.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sim JH, Yu HJ, Kim ST. The effects of different loading doses of dexmedetomidine on sedation. Korean J Anesthesiol. 2014;67:8–12. doi: 10.4097/kjae.2014.67.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall JE, Uhrich TD, Barney JA, et al. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 20.Snapir A, Posti J, Kentala E, et al. Effects of low and high plasma concentrations of dexmedetomidine on myocardial perfusion and cardiac function in healthy male subjects. Anesthesiology. 2006;105:902–10. doi: 10.1097/00000542-200611000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Hong JY, Kim WO, Yoon Y, et al. Effects of intravenous dexmedetomidine on low-dose bupivacaine spinal anaesthesia in elderly patients. Acta Anaesthesiol Scand. 2012;56:382–87. doi: 10.1111/j.1399-6576.2011.02614.x. [DOI] [PubMed] [Google Scholar]