Abstract
Background
Golgi phosphoprotein 3 (GOLPH3) has been reported to be involved in the development of several human cancers. Our previous study showed that GOLPH3 expression in glioma tissues was related to the severity of the malignancy of the cancer. However, the mechanism by which GOLPH3 affects cell apoptosis is largely unknown. The present study was designed to explore the possible mechanism of GOLPH3 in cell apoptosis.
Material/Methods
To analyze the biological role of GOLPH3 in glioma cells, we used GOLPH3 small interference RNA in apoptosis of glioma cells. The apoptosis of glioma cells was detected by flow cytometry. The expression level of GOLPH3 and NDRG1 protein was determined by Western blot analyses and immunohistochemical staining, respectively, to evaluate their association with glioma. Tumor tissues were collected from patients with glioma. Normal cerebral tissues were acquired from cerebral trauma patients undergoing internal decompression surgery.
Results
We confirm that the decrease of GOLPH3 that promotes the apoptosis of glioma cells may be regulated by the activation of NDRG1 and cleaved capcase 3. There was a inverse association between GOLPH3 and NDRG1 in glioma samples.
Conclusions
Our findings indicate that GOLPH3 and NDRG1 both play an important role in glioma etiology. Either GOLPH3 or NDRG1 might be a potential candidate for malignant glioma therapy.
MeSH Keywords: Apoptosis, Glioma, Golgi Apparatus
Background
Glioma is the common and fatal primary brain tumor in adults and accounts for 44.69% of intracranial tumors [1]. The current therapies for glioma, especially for high-grade glioma, are unsatisfactory because of its infiltrating growth. The prognosis of glioma is still poor even when glioma is completely resected, or treated in combination with radiation and chemotherapy after surgery [2]. Therefore, further understanding of the molecular pathological mechanism of glioma may provide new ideas to improve the prognosis and therapy of glioma [3].
Golgi phosphoprotein 3 (GOLPH3) was first identified in 2000 [4]. Located on the chromosomal region 5pl3.3, GOLPH3 encodes a protein of 34 kD named GPP34/GMx33/MlDAS [5]. GOLPH3 is a member of trans-Golgi network (TGN) protein family, and affects the secretion of Golgi apparatus [6,7]. Proteins synthesized in the endoplasmic reticulum enter the cis-Golgi network in the form of vesicles. After processing and modification, these proteins get out of trans-Golgi network through a budding process as secretory proteins. Through participating in protein processing, classification, packaging, and transportation in the Golgi apparatus, GOLPH3 may play a part in tumor occurrence and progression [8].
In addition, GOLPH3 is required for contractile ring formation and membrane trafficking during cytokinesis. Several studies have confirmed that GOLPH3 is overexpressed in breast cancer [9], ovarian cancer [10], renal cancer [11], non-small cell lung cancer [12], and gastric cancer [13]. Recent studies provided compelling evidence that GOLPH3 is a target for cancer therapy [14]. However, the biological role of GOLPH3 still remains unclear in glioma.
N-myc downstream regulated gene 1 (NDRG1) was first isolated as a gene upregulated in N-myc knockout mouse embryos, and its expression was repressed by N-myc and c-myc [15]. NDRG1 is widely expressed in normal tissues but was downregulated in primary and metastatic cancers, including colon, prostate, breast, esophageal squamous cancer, pancreatic cancer, and glioma [16]. In this context, NDRG1 has been suggested as a tumor suppressor. NDRG1 has been implicated in many cellular processes including cell cycle progression, apoptosis, differentiation, and vesicular transport. Nevertheless, the relationship between GOLPH3 and NDRG1 in tumor development is unclear. The present study aimed to explore the molecular mechanism by which GOLPH3 promotes glioma. We further analyzed the role of NDRG1 and GOLPH3 in the apoptosis of glioma cells, and examined the expression of GOLPH3 and NDRG1 in glioma samples.
Material and Methods
Cell lines
The human glioma cell lines U87 and U251 were purchased from Shanghai Cell Bank, Type Culture Collection Committee, Chinese Academy of Sciences. All of the cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Hyclone). All cells were cultured in a sterile incubator maintained at 37°C with 5% CO2.
siRNA transfection
siRNA oligos for GOLPH3 (Cat#: sc-91952, Santa Cruz Biotechnology) and NDRG1 (Cat#: sc-36021, Santa Cruz Biotechnology) or negative control scramble siRNAs were transfected into glioma cells using Lipofectamine™ 2000 (Invitrogen) according to the manufacturer’s instructions. One day prior to transfection, 2×105 cells were plated in 6-well plates and grown to 30–50% confluence. 100 pmol of siRNA duplex and 5 μL of Lipofectamine 2000 were mixed and incubated at room temperature for 20 min, and then added into the cells.
Western blot assay
Cells were lysed by lysis buffer, and BCA Protein Assay Kit (Thermo Fisher Scientific) was used to measure protein concentrations. Equal amounts of proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE), transferred to polyvinylidene difluoride (PVDF) membrane, blocked with 3% bovine serum albumin (BSA), and incubated with specific antibodies against GOLPH3 (1: 1000, Abcam, UK), cleaved caspase 3 (1: 200, Santa Cruz, USA), β-actin (1: 3000, ZhongShan, China), and NDRG1 (1: 1000, Abcam, UK) at 4°C overnight. The blots were developed after incubation with peroxidase-conjugated secondary antibodies (1: 4000) at room temperature for 1 h and then were imaged with a chemiluminescence detection kit (Pierce, USA). The densitometry value of blots was normalized by the internal loading control to determine relative protein levels.
Apoptosis analysis
Apoptosis was detected using the FITC Annexin V Apoptosis Detection Kit (BD Biosciences) according to the manufacturer’s instructions. Briefly, U251 and U87 cells were washed twice with cold phosphate-buffered saline (PBS) and then resuspended in 1X Binding Buffer at a concentration of 1×106 cells/mL. Next, 100 μL of solution (1×105 cells) was incubated with 5 μL of FITC Annexin V and 5 μL of PI for 15 min at 25°C in the dark. The cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences). Cells that are considered viable are FITC Annexin V and PI negative; cells that are in early apoptosis are FITC Annexin V positive and PI negative; and cells that are in late apoptosis or already dead are both FITC Annexin V and PI positive.
Immunohistochemistry
Paraffin-embedded pathological sections including 6 non-tumor brain tissues from controls, 9 grade II glioma tissues, 10 grade III glioma tissues, and 15 grade IV glioma tissues. Normal cerebral tissues were acquired from cerebral trauma patients undergoing internal decompression surgery. For the use of these clinical materials for research purposes, prior consent of the patients and approval from the Institutional Research Ethics Committee were obtained.
The S-P immunohistochemistry kit (Bioss, China) was used for immunohistochemical staining. Tissue blocks were sectioned at a thickness of 4 μm, dewaxed in xylene, and then hydrated in 85% ethanol. Antigen retrieval was handled by heat treatment in a pressure cooker. Next, the tissues were incubated in 3% hydrogen peroxide for 5 minutes to block endogenous peroxidase and then blocked with 10% goat serum. The sections were then incubated with GOLPH3 (1: 200, Abcam, UK) or NDRG (1: 400, Abcam, UK) antibody overnight at 4°C. The tissue sections were then incubated with biotinylated secondary antibody (Bioss, China) at room temperature for 1 hour, followed by incubation with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Bioss, China) for 30 minutes. The sections were stained with diaminobenzidine and counterstained with hematoxylin. Appropriate positive and negative controls were also tested. All slides were interpreted by two experienced and independent pathologists who were unaware of the clinical data. For each slide, 5 visual fields were randomly selected and examined with a microscope to assess the staining intensity. The following scoring system was used for the staining results: negative (0); weak (1); moderate (2); and strong (3). The number of stained cells and the total number of cells in each visual field were also counted, and the ratio between the stained and total cells was calculated. The following scoring system was used for the stained cell ratio: <5% (0); 6–25% (1); 26–50% (2); and >50% (3). We multiplied the scores from the two systems on the same tissue sample, and the following definitions were used: negative (−) if the resulting score was 0; weak positive (+) if the resulting score was 1–3; strong positive (+ +) if the resulting score was 4–6; and very strong positive (+ + +) if the resulting score was 7–9. The numbers of positive cells were counted from 5 randomly selected fields, and the mean number of positive cells in each field was calculated.
The study protocol was approved by the Ethics Committee of Liaocheng People’s Hospital, and written informed consent was obtained from each patient. The experimental method also made reference to the article by Shang et al. [17].
Statistical analysis
Comparisons between groups for statistical significance were carried out with a 2-tailed paired Student’s t test. The relationship between GOLPH3 and NDRG1 expression was analyzed by the χ2 test. P<0.05 indicated a statistically significant difference. SPSS software (SPSS version 17.0) was used for all statistical analyses.
Results
GOLPH3 knockdown promoted glioma cell apoptosis
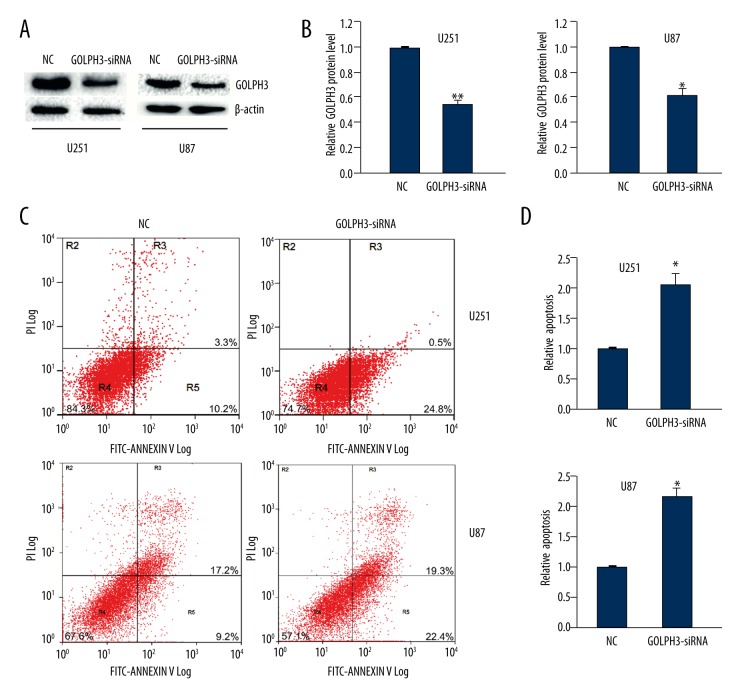
To explore the role of GOLPH3 in U251 and U87 cells apoptosis, we use siRNA to knock down GOLPH3 expression in glioma cells. Forty-eight hours after transfection, the knockdown efficacy of GOLPH3 siRNAs was examined by Western blot analysis. The results showed that GOLPH3 siRNAs effectively suppressed the expression of GOLPH3 by 50–60% in U251 cells and 40% in U87 cells (Figure 1A, 1B).
Figure 1.
GOLPH3 knockdown promoted the apoptosis of U251 and U87 cells. (A) The silencing efficacy of GOLPH3 siRNA was detected by Western blot analysis. β-actin was the loading control. (B) Quantitative analysis of GOLPH3 levels after GOLPH3 knockdown. (C, D) Flow cytometry assay showed the apoptosis rate of U251 and U87 cells at 48 h after transfection with GOLPH3 siRNA. R5 quadrant was calculated as the percentage of apoptosis rate. Data are shown as mean ±SEM (n=3). * P<0.05, ** P<0.01.
Next we surveyed the effect of GOLPH3 knockdown in cell apoptosis. The results showed that GOLPH3 siRNA-transfected U251 cells and U87 cells showed higher rate of apoptosis compared to the cells transfected with scrambled control (Figure 1C, 1D). These data demonstrated that GOLPH3 knockdown promoted glioma cell apoptosis.
NDRG1 knockdown inhibited glioma cell apoptosis
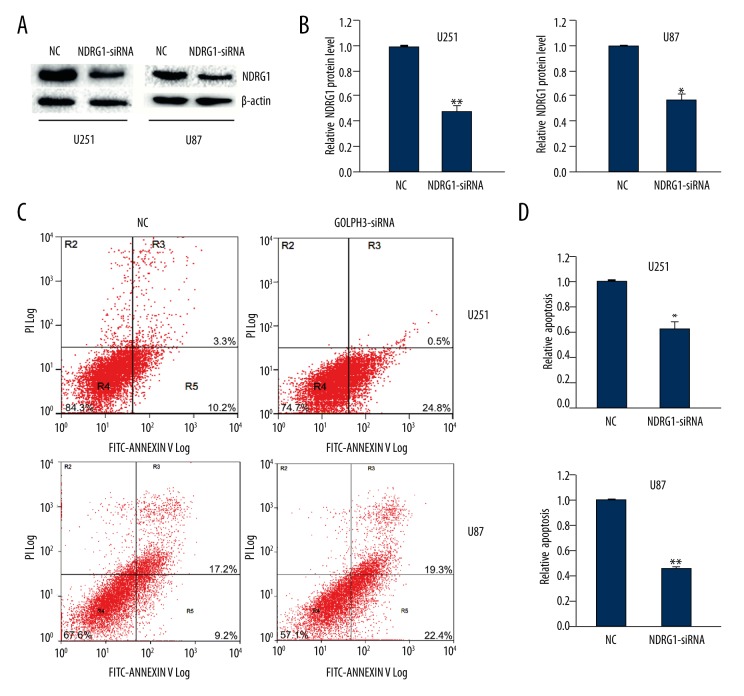
Furthermore, we examined the role of NDRG1 in cell apoptosis. We used siRNA to knock down NDRG1 expression in glioma cells. The knockdown efficacy of NDRG1 siRNAs was determined by Western blot analysis, and we found that NDRG1 siRNAs effectively suppressed the expression of NDRG1 by 55% in U251 cells and 45% in U87 cells (Figure 2A, 2B).
Figure 2.
NDRG1 knockdown inhibited the apoptosis of U251 and U87 cells. (A) The silencing efficacy of NDRG1 siRNA was detected by Western blot analysis. β-actin was the loading control. (B) Quantitative analysis of NDRG1 levels after NDRG1 knockdown. (C, D) Flow cytometry assay showed the apoptosis rate of U251 and U87 cells at 48 h after transfection with NDRG1 siRNA. R5 quadrant was calculated as the percentage of apoptosis rate. Data are shown as mean ±SEM (n=3). *P<0.05, ** P<0.01.
Moreover, we surveyed the effect of NDRG1 knockdown in cell apoptosis. The results showed that NDRG1 siRNA-transfected U251 cells and U87 cells showed a lower rate of apoptosis compared to the cells transfected with scrambled control (Figure 2C, 2D). These data demonstrated that NDRG1 knockdown inhibited glioma cell apoptosis, contrary to the effect of GOLPH3 knockdown.
They all had three replicates in Western blot and flow cytometry.
GOLPH3 increased the cleavage of caspase 3 partially via regulating NDRG1 in glioma cells
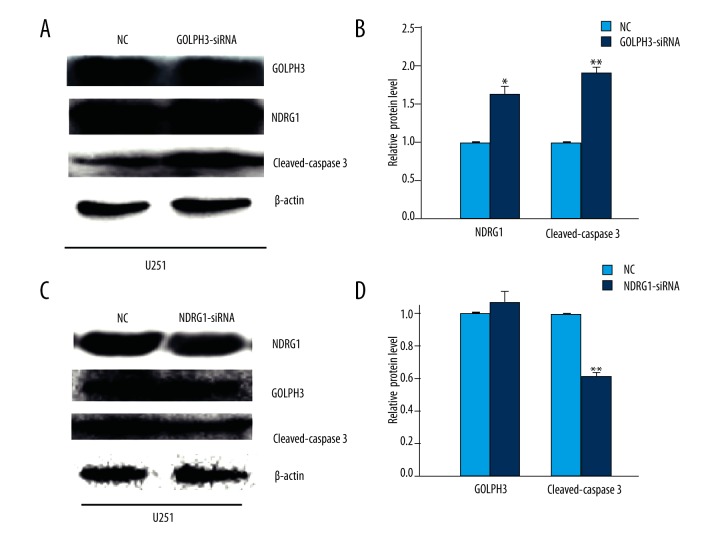
To further investigate the relationship between GOLPH3, NDRG1, and the apoptosis of glioma cells, we employed GOLPH3 siRNA to knock down GOLPH3 in U251 cells. Western blot analysis showed that the level of NDRG1 was increased by about 55% and the level of cleaved caspase 3 was increased by about 80% (Figure 3A, 3B). The results were consistent with the previous observation that GOLPH3 knockdown promoted glioma cell apoptosis.
Figure 3.
Knockdown of GOLPH3 increased the expression of NDRG1 and the cleavage of caspase 3. (A, B) Western blot analysis of protein levels of NDRG1 and cleaved caspase 3 at 48 h after transfection with GOLPH3 siRNA. (C, D) Western blot analysis of protein levels of GOLPH3 and cleaved caspase 3 at 48 h after transfection with NDRG1 siRNA. β-actin was the loading control. Data are shown as mean ±SEM (n=3). * P<0.05, ** P<0.01.
Next we found that NDRG1 knockdown had no significant effect on GOLPH3 expression, but led to a decreased level of cleaved caspase 3 (Figure 3C, 3D). The results were consistent with the previous observation that NDRG1 knockdown inhibited the apoptosis of glioma cells. These data indicated that GOLPH3 increased the cleavage of caspase 3 and apoptosis of glioma cells partially via downregulating NDRG1.
Negative correlation of GOLPH3 and NDRG1 expression in glioma samples
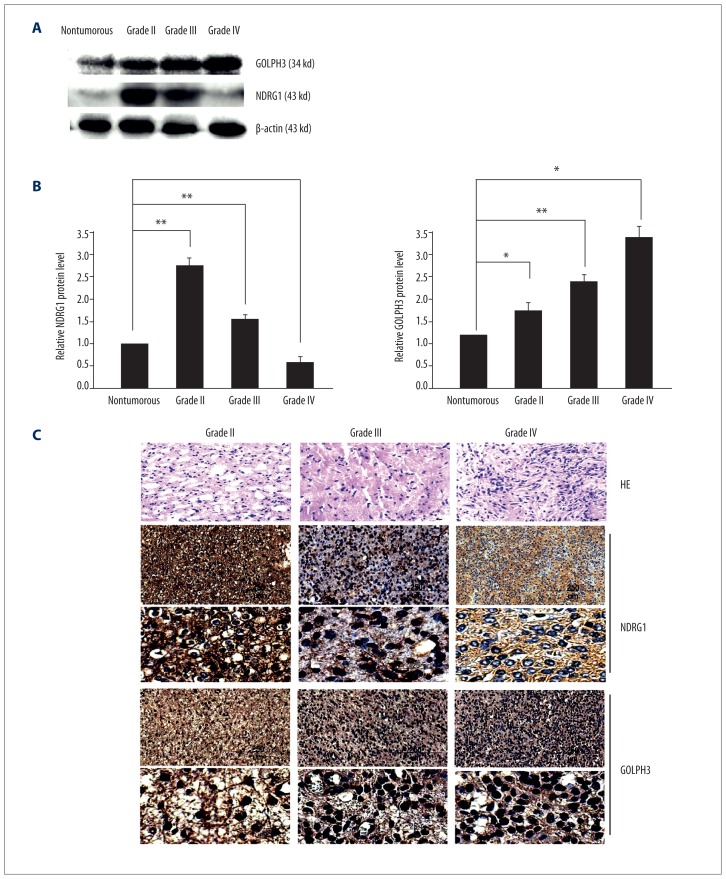
To confirm the opposite roles of GOLPH3 and NDRG1 in the progression of glioma, we detected GOLPH3 and NDRG1 expression in clinical glioma samples. Western blot analysis showed that the protein level of GOLPH3 increased with the advancement of glioma grade, while the protein level of NDRG1 decreased with the advancement of glioma grade (Figure 4A, 4B).
Figure 4.
The expression of GOLPH3 and NDRG1 in human glioma tissues. (A) GOLPH3 and NDRG1 expression in non-tumorous brain and human glioma tissues was determined by Western blot analysis. Non-tumorous brain samples were from controls. β-actin was the loading control. (B) Quantitative analysis of GOLPH3 and NDRG1 protein levels in non-tumorous brain and human glioma tissues. Data are shown as mean ±SEM (n=3). (C) Representative images of GOLPH3 and NDRG1 staining in human glioma tissues with different grades.
Furthermore, the distribution and expression of NDRG1 and GOLPH3 in glioma samples were assessed by immunohistochemistry (Figure 4C). We found that higher glioma grade was correlated with a higher percentage of GOLPH3-positive cells and a lower percentage of NDRG1-positive cells. The relative staining intensity was as follows: NDRG1 59±3.8% for grade II, 33±2.9% for grade III, and 11±2.9% for grade IV; GOLPH3 32±4.4% for grade II, 49±3.5% for grade III, and 79±4.8% for grade IV. Notably, GOLPH3 expression and NDRG1 expression in glioma samples were negatively correlated (r=−0.837; P<0.01). Collectively, these data suggested that GOLPH3 and NDRG1 play opposite roles in the growth of human glioma.
Discussion
In this research, we demonstrated that knockdown of GOLPH3 promoted the apoptosis of glioma cells and increased the level of cleaved caspase 3. In contrast, knockdown of NDRG1 inhibited the apoptosis of glioma cells and reduced the level of cleaved caspase 3. We further revealed the relationship between NDRG1 and GOLPH3 and found that GOLPH3 knockdown increased the expression of NDRG1, but knockdown of NDRG1 had no obvious effect on GOLPH3 expression. Furthermore, we found a negative correlation of NDRG1 and GOLPH3 expression in human glioma tissues. Thus, GOLPH3 may inhibit the apoptosis of glioma cells and promote glioma growth by downregulating NDRG1.
Scott et al. first claimed that GOLPH3 was a novel cellular oncogene that was frequently amplified in multiple solid tumors [6]. Further studies confirmed that GOLPH3 plays an important role in the progression of various cancers. Our previous work confirmed that GOLPH3 expression was high in glioma and that the expression level increased with the increased degree of malignancy of the glioma [18]. An additional study reported that overexpression of GOLPH3 in glioblastoma was associated with worse prognosis [19]. However, the mechanism underlying the potential oncogenic role of GOLPH3 in glioma is not clear. It was reported that GOLPH3 could regulate glioma cell invasion and migration through the GOLPH3-mTOR-YB1 or GOLPH3-RhoA signaling pathway [20–22]. In addition, the overexpression of GOLPH3 promoted the proliferation of glioma cells through the PKD2-GOLPH3-AKT signaling pathway [23]. Thus, GOLH3 may become a novel therapeutic target of glioma. In this research we provided the first evidence that GOLPH3 inhibited the apoptosis of glioma cells, and further explored the possible mechanism.
NDRG1 is a differentiation related gene that has been proposed as a tumor suppressor in several cancers, including pancreatic cancer, prostate cancer, breast cancer, colorectal cancer, and glioma [24–26]. In prostate and breast cancer, loss of NDRG1 expression has been consistently linked to tumor progression and metastasis as well as poor survival of cancer patients [25,27]. Interestingly, it has been reported that NDRG1 is necessary for p53-dependent apoptosis in colorectal adenocarcinoma cells [28]. In addition, indolic diet derivative 3,3′-diindolylmethane induced apoptosis in human colon cancer cells through upregulating NDRG1 [29]. These results clearly demonstrate the role of NDRG1 in inducing the apoptosis of tumor cells. Consistent with these data, in this study we found that NDRG1 knockdown led to decreased cleavage of caspase 3 and decreased apoptosis of glioma cells.
Sun et al. found that the mRNA and protein expression of NDRG1 was decreased with the rise of tumor grade in 168 cases of glioma [30]. Thus, we further investigated the correlation of GOLPH3 and NDRG1 in glioma samples. With the increase of malignancy of glioma tissue, the expression of GOLPH3 was gradually increased while the expression of NDRG1 was reduced. In addition, we found that GOLPH3 expression and NDRG1 expression were negatively correlated in clinical human glioma tissues. In U251 cells, we found that GOLPH3 could regulate NDRG1 expression, but NDRG1 could not regulate GOLPH3 expression, indicating that GOLPH3 may act upstream of NDRG1. However, the exact mechanisms of how GOLPH3 regulates NDRG1 expression need further studies.
Both GOLPH3 and NDRG1 are involved in vesicle transport of the Golgi apparatus. The potential role of GOLPH3 in trafficking is further supported by its direct, tight, and specific interaction with phosphatidylinositol 4-phosphate (PI4P), which is a phosphatidylinositol lipid molecule found in most subcellular locations, predominantly at the Golgi [31]. NDRG1 also strongly interacts with PI4P and participates in vesicle formation, transportation, and sorting cargo proteins to endosomes [32]. NDRG1 is a Rab4a effector protein that is localized at perinuclear recycling/sorting vesicles in the trans-Golgi network by binding to PI4P [33]. Therefore, it is possible that GOLPH3 might regulate NDRG1 by competitively binding to PI4P, which promotes vesicles transport of the related receptors to the membrane.
Conclusions
In summary, we demonstrated that knockdown of GOLPH3 promoted glioma cell apoptosis and increased the cleavage of caspase 3. In addition, we found negative correlation of GOLPH3 and NDRG1 expression in glioma tissues and provided evidence that GOLPH3 is involved in the downregulation of NDRG1 expression in glioma cells. Further exploration the relationship of GOLPH3 and NDRG1 may provide a potential candidate for the therapy of glioma and guide its prognosis.
Footnotes
Conflicts of interest
We have no financial disclosures to declare and no conflicts of interest.
Source of support: This study was sponsored by the Natural Science Foundation of China (No. 81372721) and the Natural Science Foundation of Shandong Province (No. ZR2013HM011)
References
- 1.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Gen Dev. 2007;21:2683–10. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 2.Van Meir EG, Hadjipanayis CG, Norden AD, et al. Exciting new advances in neuro-oncology: The avenue to a cure for malignant glioma. Cancer J Clin. 2010;60:166–93. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Rhun E, Taillibert S, Chamberlain MC. The future of high-grade glioma: Where we are and where are we going. Surg Neurol Intern. 2015;6:S9–44. doi: 10.4103/2152-7806.151331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu CC, Taylor RS, Lane DR, et al. GMx33: A novel family of trans-Golgi proteins identified by proteomics. Traffic. 2000;1:963–75. [PubMed] [Google Scholar]
- 5.Bell AW, Ward MA, Blackstock WP, et al. Proteomics characterization of abundant Golgi membrane proteins. J Biol Chem. 2001;276:5152–65. doi: 10.1074/jbc.M006143200. [DOI] [PubMed] [Google Scholar]
- 6.Scott KL, Kabbarah O, Liang MC, et al. GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature. 2009;459:1085–90. doi: 10.1038/nature08109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott KL, Chin L. Signaling from the Golgi: Mechanisms and models for Golgi phosphoprotein 3-mediated oncogenesis. Clin Cancer Res. 2010;16:2229–34. doi: 10.1158/1078-0432.CCR-09-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buschman MD, Rahajeng J, Field SJ. GOLPH3 links the Golgi, DNA damage, and cancer. Cancer Res. 2015;75:624–27. doi: 10.1158/0008-5472.CAN-14-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng Z, Lin H, Zhao X, et al. Overexpression of GOLPH3 promotes proliferation and tumorigenicity in breast cancer via suppression of the FOXO1 transcription factor. Clin Cancer Res. 2012;18:4059–69. doi: 10.1158/1078-0432.CCR-11-3156. [DOI] [PubMed] [Google Scholar]
- 10.Ma Y, Wang X, Wu Y, et al. Overexpression of GOLPH3 protein is associated with worse prognosis in patients with epithelial ovarian cancer. Tumour Biol. 2014;35:11845–49. doi: 10.1007/s13277-014-2411-1. [DOI] [PubMed] [Google Scholar]
- 11.Xue Y, Wu G, Liao Y, et al. GOLPH3 is a novel marker of poor prognosis and a potential therapeutic target in human renal cell carcinoma. Br J Cancer. 2014;110:2250–60. doi: 10.1038/bjc.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang R, Ke ZF, Wang F, et al. GOLPH3 overexpression is closely correlated with poor prognosis in human non-small cell lung cancer and mediates its metastasis through upregulating MMP-2 and MMP-9. Cell Physiol Biochem. 2015;35:969–82. doi: 10.1159/000369753. [DOI] [PubMed] [Google Scholar]
- 13.Peng J, Fang Y, Tao Y, et al. Mechanisms of GOLPH3 associated with the progression of gastric cancer: A preliminary study. PloS One. 2014;9:e107362. doi: 10.1371/journal.pone.0107362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sechi S, Frappaolo A, Belloni G, et al. The multiple cellular functions of the oncoprotein Golgi phosphoprotein 3. Oncotarget. 2015;6:3493–506. doi: 10.18632/oncotarget.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovacevic Z, Richardson DR. The metastasis suppressor, Ndrg-1: A new ally in the fight against cancer. Carcinogenesis. 2006;27:2355–66. doi: 10.1093/carcin/bgl146. [DOI] [PubMed] [Google Scholar]
- 16.Broggini T, Wustner M, Harms C, et al. NDRG1 overexpressing gliomas are characterized by reduced tumor vascularization and resistance to antiangiogenic treatment. Cancer Lett. 2016;380(2):568–76. doi: 10.1016/j.canlet.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Shang C, Hong Y, Guo Y, et al. Mir-210 up-regulation inhibits proliferation and induces apoptosis in glioma cells by targeting sin3a. Med Sci Monit. 2014;20:2571–77. doi: 10.12659/MSM.892994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li XY, Liu W, Chen SF, et al. Expression of the Golgi phosphoprotein-3 gene in human gliomas: A pilot study. J Neurooncol. 2011;105:159–63. doi: 10.1007/s11060-011-0573-x. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J, Xu T, Qin R, et al. Overexpression of Golgi phosphoprotein-3 (GOLPH3) in glioblastoma multiforme is associated with worse prognosis. J Neurooncol. 2012;110:195–203. doi: 10.1007/s11060-012-0970-9. [DOI] [PubMed] [Google Scholar]
- 20.Abraham RT. GOLPH3 links the Golgi network to mTOR signaling and human cancer. Pigment Cell Melanoma Res. 2009;22:378–79. doi: 10.1111/j.1755-148X.2009.00596.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Ding Z, Mo J, et al. GOLPH3 promotes glioblastoma cell migration and invasion via the mTOR-YB1 pathway in vitro. Mol Carcinog. 2015;54:1252–63. doi: 10.1002/mc.22197. [DOI] [PubMed] [Google Scholar]
- 22.Zhou X, Zhan W, Bian W, et al. GOLPH3 regulates the migration and invasion of glioma cells though RhoA. Biochem Biophys Res Ccommun. 2013;433:338–44. doi: 10.1016/j.bbrc.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Zhou X, Xue P, Yang M, et al. Protein kinase D2 promotes the proliferation of glioma cells by regulating Golgi phosphoprotein 3. Cancer Lett. 2014;355:121–29. doi: 10.1016/j.canlet.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Angst E, Dawson DW, Stroka D, et al. N-myc downstream regulated gene-1 expression correlates with reduced pancreatic cancer growth and increased apoptosis in vitro and in vivo. Surgery. 2011;149:614–24. doi: 10.1016/j.surg.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Bandyopadhyay S, Pai SK, Hirota S, et al. Role of the putative tumor metastasis suppressor gene Drg-1 in breast cancer progression. Oncogene. 2004;23:5675–81. doi: 10.1038/sj.onc.1207734. [DOI] [PubMed] [Google Scholar]
- 26.Ma W, Na M, Tang C, et al. Overexpression of N-myc downstream-regulated gene 1 inhibits human glioma proliferation and invasion via phosphoinositide 3-kinase/AKT pathways. Mol Med Rep. 2015;12:1050–58. doi: 10.3892/mmr.2015.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bandyopadhyay S, Pai SK, Gross SC, et al. The Drg-1 gene suppresses tumor metastasis in prostate cancer. Cancer Res. 2003;63:1731–36. [PubMed] [Google Scholar]
- 28.Stein S, Thomas EK, Herzog B, et al. NDRG1 is necessary for p53-dependent apoptosis. J Biol Chem. 2004;279:48930–40. doi: 10.1074/jbc.M400386200. [DOI] [PubMed] [Google Scholar]
- 29.Lerner A, Grafi-Cohen M, Napso T, et al. The indolic diet-derivative, 3,3′-diindolylmethane, induced apoptosis in human colon cancer cells through upregulation of NDRG1. J Biomed Biotechnol. 2012;2012:256178. doi: 10.1155/2012/256178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun B, Chu D, Li W, et al. Decreased expression of NDRG1 in glioma is related to tumor progression and survival of patients. J Neurooncol. 2009;94:213–19. doi: 10.1007/s11060-009-9859-7. [DOI] [PubMed] [Google Scholar]
- 31.Bishe B, Syed GH, Field SJ, Siddiqui A. Role of phosphatidylinositol 4-phosphate (PI4P) and its binding protein GOLPH3 in hepatitis C virus secretion. J Biol Chem. 2012;287:27637–47. doi: 10.1074/jbc.M112.346569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choudhury RR, Hyvola N, Lowe M. Phosphoinositides and membrane traffic at the trans-Golgi network. Biochem Soc Symp. 2005;(72):31–38. doi: 10.1042/bss0720031. [DOI] [PubMed] [Google Scholar]
- 33.Kachhap SK, Faith D, Qian DZ, et al. The N-Myc down regulated Gene1 (NDRG1) Is a Rab4a effector involved in vesicular recycling of E-cadherin. PloS One. 2007;2:e844. doi: 10.1371/journal.pone.0000844. [DOI] [PMC free article] [PubMed] [Google Scholar]