Abstract
Background
Acute kidney injury and hyperchloremia are commonly present in critically ill septic patients. Our study goal was to evaluate the association of hyperchloremia and acute kidney injury in severe sepsis and septic shock patients.
Methods
In this retrospective cohort study in a provincial tertiary care hospital, adult patients with severe sepsis or septic shock and serum chloride measurements were included. Serum chloride was measured on a daily basis for 48 hours. Primary outcome was development of acute kidney injury (AKI) and association of AKI and serum chloride parameters was analyzed.
Results
A total of 240 patients were included in the study, 98 patients (40.8 %) had hyperchloremia. The incidence of acute kidney injury (AKI) was significantly higher in the hyperchloremia group (85.7 % vs 47.9 %; p < 0.001). Maximal chloride concentration in the first 48 hours ([Cl-]max) was significantly associated with AKI. In multivariate analysis, [Cl-]max was independently associated with AKI [adjusted odds ratio (OR) for AKI = 1.28 (1.02–1.62); p = 0.037]. The increase in serum chloride (Δ[Cl-] = [Cl-]max – initial chloride concentration) demonstrated a dose-dependent relationship with severity of AKI. The mean Δ[Cl-] in patients without AKI was 2.1 mmol/L while in the patients with AKI stage 1, 2 and 3 the mean Δ[Cl-] was 5.1, 5.9 and 6.7 mmol/L, respectively. A moderate increase in serum chloride (Δ[Cl-] ≥ 5 mmol/L) was associated with AKI [OR = 5.70 (3.00–10.82); p < 0.001], even in patients without hyperchloremia [OR = 8.25 (3.44–19.78); p < 0.001].
Conclusions
Hyperchloremia is common in severe sepsis and septic shock and independently associated with AKI. A moderate increase in serum chloride (Δ[Cl-] ≥5 mmol/L) is associated with AKI even in patients without hyperchloremia.
Keywords: Chloride, Hyperchloremia, Acute kidney injury, Sepsis, Septic shock
Background
Acute kidney injury (AKI) frequently occurs in patients with severe sepsis and septic shock [1]. A small rise in serum creatinine (26.5 μmol/L) in critically ill patients is associated with higher mortality, longer length of stay, greater need of vasopressor and mechanical ventilator support and worse long-term outcomes [2–5]. Identifying risk factors for development of AKI in sepsis may therefore be helpful to understand and avoid this profound complication.
Intravenous fluid resuscitation to restore effective circulatory volume in severely ill septic patients is a mainstay of early therapy of severe sepsis and septic shock. The Surviving Sepsis Campaign International Guidelines recommends crystalloids as the initial fluid of choice for resuscitation of septic shock patients [6]. Normal 0.9 % saline solution (Saline), is the most common isotonic crystalloid solution used for resuscitation globally [7–9]. However, the electrolyte composition of Saline is quite different from serum electrolyte composition; Saline has 50 % greater chloride than serum (154 vs 100 mmol/L, respectively) [10]. Consequently, hyperchloremic metabolic acidosis is a common consequence of chloride-rich solution resuscitation [11, 12].
Animal and human studies demonstrate that infusion of Saline results in decreased renal blood flow, reduced glomerular filtration rate, and delayed time to micturition [13–15]. In an animal model of sepsis, resuscitation with Saline resulted in a higher incidence of AKI compared to a balanced crystalloid solution with a more physiologic chloride concentration [16]. A recent study conducted by Yunos and colleagues [17] in critically ill adults found that restriction of chloride-rich fluid was associated with a significant decrease in the incidence of AKI and need for renal replacement therapy (RRT). However, a subsequent randomized controlled trial of normal saline vs. a balanced solution (Plasma-Lyte) found no difference in development of AKI. However, patients were unselected ICU patients, the exposure was only 2 L over the ICU stay, no renal biomarkers were measured, and the serum chloride was not reported. Thus it remains controversial how to manage fluids and chloride in the critically ill. To date, the association of hyperchloremia and AKI has been observed in unselected critically ill patients [18] but not specifically in septic patients [19, 20].
Rapid volume resuscitation of septic patients with Saline rapidly changes serum chloride concentration. Despite all of the above work, it is unclear whether AKI associated with chloride-rich volume resuscitation is due to the absolute value of serum chloride rising above a threshold – hyperchloremia – or whether the change of serum chloride concentration is more important. For example, a rapid increase in serum sodium concentration (rather than the absolute serum sodium concentration) can lead to central pontine myelinolysis.
Accordingly we first tested the hypothesis that hyperchloremia after volume resuscitation was associated with AKI in severe sepsis and septic shock patients. Second, we determined whether the change of serum chloride concentration resulting from volume resuscitation was associated with AKI in severe sepsis and septic shock patients.
Methods
Study design and participants
This study was a retrospective cohort study of patients with severe sepsis or septic shock admitted to St. Paul’s Hospital in Vancouver, a tertiary care referral hospital, from January 2011 to April 2015. We included all adults patients with the following criteria: (1) 18 years or older; (2) diagnosis of severe sepsis (defined by two of four Systemic Inflammatory Response Syndrome (SIRS) criteria plus a suspected or confirmed source of infection with at least one organ dysfunction or a lactate greater than 4 mmol/L) or septic shock (defined by sepsis-induced hypotension, tissue hypoperfusion or vasopressor requirement); and (3) initial serum chloride and daily serum chloride concentration for the first 48 hours were measured. We excluded patients with pre-existing chronic renal failure or chronic use of RRT. Written informed consent was obtained from all patients or their representative and the study protocol was approved by our institutional ethics board.
All of these patients were diagnosed via an institutional sepsis protocol in the Emergency Department. Study screening was triggered by this protocol. Most of the resuscitation was conducted by Emergency Department physicians. The primary resuscitation fluid was Saline. Thirty-seven percent of these patients were admitted to the ICU and managed by the attending intensivist. The treatment of these patients was evaluated and directed by attending physicians without a resuscitation guideline or protocol.
Study variables
Serum chloride concentration was measured by indirect potentiometry (ADVIA 1800 Chemistry System; Siemens Healthcare Diagnostic Inc., Oakville, ON, Canada). The initial chloride concentration, [Cl-]0 was the initial serum chloride concentration measured at the time that the patient fulfilled diagnostic criteria for severe sepsis or septic shock (above). Serum chloride concentration was measured at least daily for the first 48 hours and the maximal serum chloride concentration during this time period was designated as [Cl-]max. The increase in serum chloride, Δ[Cl-], was the difference between maximal serum chloride level and initial serum chloride level (Δ[Cl-] = [Cl-]max - [Cl-]0). Hyperchloremia was defined as [Cl-]max ≥ 110 mmol/L [20].
The Acute Physiology and Chronic Health Evaluation II (APACHE II) score was determined by using clinical and laboratory data on the first day of enrollment. AKI was diagnosed and classified by Kidney Disease Improving Global Outcomes (KDIGO) consensus criteria by an increase in serum creatinine concentration > 50 % from a baseline creatinine concentration measured within 3 months prior to enrollment [21]. If a previous serum creatinine was not available, it was calculated by assuming that the glomerular filtration rate (GFR) of the patient was 75 mL/min per 1.73 m2 and serum creatinine concentration was computed using the modified 4-variable Modification of Diet in Renal Disease (MDRD) formula [22].
Study outcomes
The primary outcome was development of AKI by KDIGO criteria. The secondary outcomes were requirement of RRT and 28-day mortality.
Statistical analysis
Demographic and clinical data were compared between patients with hyperchloremia and without hyperchloremia. Continuous data were reported by using mean ± standard deviation (SD) or median (interquartile range) and categorical data as percentage. We used t tests to compare normally distributed continuous data and Wilcoxon signed-rank tests for non-normally distributed data. For categorical variables, a chi-square test was used.
Logistic regression was used to test for association of chloride parameters ([Cl-]0, [Cl-]max and Δ[Cl-], hyperchloremia) with AKI, RRT, and 28-day mortality. Univariate logistic regression was used to test for unadjusted association between chloride parameters and AKI. Multivariate logistic regression was used to test for associations between chloride parameters and AKI after adjusting for potentially confounding covariates. Independent variables with p < 0.1 in univariate models were incorporated as covariates into the multivariate regression model. These variables consisted of demographic data (age, gender), underlying diseases (hypertension, congestive heart failure, chronic obstructive pulmonary disease) and clinical severity at presentation (APACHE II score, serum lactate, requirement of vasopressor, mechanical ventilator requirement).
All analyses were performed using SPSS, version 20 (SPSS, Chicago, IL, USA). A two-sided p value less than 0.05 was considered to be statistically significant.
Results
Patient characteristics
In this study, 275 patients with severe sepsis and septic shock were enrolled. Thirty-five patients were excluded because they had pre-existing chronic renal failure. Thus, 240 patients were eligible for further evaluation (Fig. 1). Of these, 98 patients (40.8 %) had hyperchloremia within the first 48 hours of resuscitation and 142 patients (59.2 %) did not have hyperchloremia. Demographics, baseline characteristics and clinical outcome of patients with hyperchloremia and without hyperchloremia are shown in Table 1. Patients with hyperchloremia had a higher heart rate and APACHE II score, vasopressor and ventilator requirement. Comorbidities, serum lactate and fluid intake/output were not different between the two groups. Baseline serum creatinine also was not significantly different in both groups. Notably, there was no difference in serum chloride before full resuscitation between those patients who developed hyperchloremia ([Cl-]0 104.3 ± 7.7 mmol/L) and those who did not ([Cl-]0 103.7 ± 4.6 mmol/L, p = 0.51).
Fig. 1.

Flow chart of the patients with severe sepsis and septic shock in the study. [Cl - ] max maximal chloride concentration in the first 48 hours, AKI acute kidney injury, CRF chronic renal failure
Table 1.
Demographic and clinical variables and outcome of patients classified by serum chloride status
| Variable | Hyperchloremia N = 98 |
No hyperchloremia N = 142 |
p |
|---|---|---|---|
| Demographics | |||
| Age, yr, mean ± SD | 57.5 ± 15.1 | 52.9 ± 18.4 | 0.05 |
| Male, % | 46.9 | 55.6 | 0.38 |
| Underlying diseases | |||
| Hypertension, % | 36.7 | 28.2 | 0.074 |
| Ischemic heart disease, % | 6.1 | 5.6 | 0.65 |
| CHF, % | 4.1 | 8.5 | 0.10 |
| DM, % | 21.4 | 21.1 | 0.71 |
| COPD, % | 21.4 | 18.3 | 0.086 |
| Cirrhosis, % | 7.1 | 4.9 | 0.082 |
| Malignancy, % | 4.1 | 5.6 | 0.20 |
| HIV, % | 11.2 | 9.2 | 0.56 |
| Chronic steroid treatment, % | 5.1 | 6.3 | 0.91 |
| Clinical parameters at presentation | |||
| MAP, mean ± SD | 85 ± 21 | 89 ± 17 | 0.12 |
| HR, mean ± SD | 104 ± 22 | 96 ± 21 | 0.003a |
| Lactate, mean ± SD | 2.9 ± 2.8 | 2.5 ± 2.4 | 0.29 |
| Creatinine(mg/dL), median (IQR) | 1.0 (0.7-1.4) | 0.9 (0.4-1.4) | 0.49 |
| APACHE II score, mean ± SD | 11.3 ± 5.0 | 7.8 ± 5.4 | <0.001a |
| Clinical parameters at 24 hr | |||
| Vasopressor, % | 31 | 11 | <0.001a |
| Mechanical ventilator, % | 64 | 32 | <0.001a |
| Fluid intake, mean ± SD | 4959 ± 3,417 | 4691 ± 2,341 | 0.71 |
| Urine output, mean ± SD | 2416 ± 1,146 | 2554 ± 1,463 | 0.644 |
| Chloride parameters | |||
| Initial chloride ([Cl-]0), mean ± SD | 104.3 ± 7.7 | 103.7 ± 4.6 | 0.51 |
| Maximal Cl in 48 hours ([Cl-]max) | 104.4 ± 3.7 | 114.4 ± 3.6 | <0.001a |
| Increase in serum Cl (Δ[Cl-]) | 3.2 ± 4.2 | 6.1 ± 5.0 | <0.001a |
| Clinical outcome | |||
| AKI, % | 85.7 | 47.9 | <0.001a |
| RRT, % | 7.1 | 3.5 | 0.206 |
| 28-day mortality, % | 6.1 | 1.4 | 0.066 |
SD standard deviation, CHF congestive heart failure, DM diabetes mellitus, COPD chronic obstructive pulmonary disease, HIV human immunodeficiency virus, MAP mean arterial pressure, HR heart rate, IQR interquartile rate, APACHE Acute Physiology and Chronic Health Evaluation, [Cl - ] 0 initial chloride concentration, [Cl - ] max maximal chloride concentration in the first 48 hours, Δ[Cl - ] increase in serum chloride; AKI acute kidney injury, RRT renal replacement therapy
aIndicates statistical significance, p < 0.05
Univariate analysis
Serum chloride and acute kidney injury
The initial serum chloride, [Cl-]0, before full resuscitation was not associated with development of AKI [odds ratio 1.01 (0.97–1.05); p = 0.51]. The incidence of AKI was significantly higher in the hyperchloremia group (84 of 98 = 85.7 % versus 68 of 142 = 47.9 %; p < 0.001). The maximum chloride concentration within the first 48 hours, [Cl-]max, was associated with AKI with an odds ratio 1.14 per mmol [Cl-] (95 % CI 1.08–1.20, p < 0.001) (Table 2). Non-significant trends for patients with hyperchloremia, compared to those without hyperchloremia, included a need of RRT (7.1 % versus 3.5 %; p = 0.206) and 28-day mortality (6.1 % versus 1.4 %; p = 0.066).
Table 2.
Univariate logistic regression model to test association of AKI and initial serum chloride ([CL-]0), maximal serum chloride in first 48 hours ([Cl–]max), and increase in serum chloride (Δ[Cl-] = [Cl-]max–[CL-]0)
| Variable | AKI stage 1 to 3 | p | AKI stage 2 and 3 | p |
|---|---|---|---|---|
| Odds ratio (95 % CI) | Odds ratio (95 % CI) | |||
| [CL-]0 | 1.01 (0.97–1.05) | 0.478 | 0.99 (0.95–1.03) | 0.562 |
| [Cl-]max | 1.14 (1.08–1.20) | <0.001a | 1.07 (1.02–1.12) | 0.006a |
| Δ[CL-] | 1.25 (1.16–1.36) | <0.001a | 1.14 (1.07–1.21) | <0.001a |
AKI acute kidney injury, [Cl - ] 0 initial chloride concentration, [Cl - ] max maximal chloride concentration in the first 48 hours, Δ[Cl - ] increase in serum chloride, CI confidence interval
aIndicates statistical significance, p <0.05
Increase in serum chloride and severity of AKI
The increase in serum chloride, Δ[Cl-] (= [Cl-]max - [Cl-]0), was strongly associated with AKI. The odds ratio for development of AKI for Δ[Cl-] was 1.25 per mmol Δ[Cl-] (95 % CI 1.16–1.36; p < 0.001), which was a stronger effect than for [Cl-]max. Importantly, Δ[Cl-] remained significantly associated with development of AKI even in those patients who were never hyperchloremic with an odds ratio of 1.37 per mmol Δ[Cl-] (95 % CI 1.20–1.56). A dose-response relationship of Δ[Cl-] and severity of AKI was observed; the greater the Δ[Cl-], the more severe the AKI stage. The mean Δ[Cl-] in patients without AKI was 2.06 mmol/L, for AKI stage 1 Δ[Cl-] was 5.14 mmol/L, for AKI stage 2 Δ[Cl-] was 5.88 mmol/L, and for AKI stage 3 Δ[Cl-] was 6.70 mmol/L (Fig. 2).
Fig. 2.
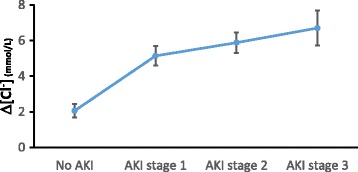
Increase in serum chloride and AKI severity. The mean increase in serum chloride (Δ[Cl-]) in AKI stage 1, 2 and 3 is significantly higher than in patients without AKI (p < 0.05) and these data suggest a dose-response relationship between Δ[Cl-] and AKI stage. Δ[Cl - ] increase in serum chloride, AKI acute kidney injury,
Moderate increase in serum chloride and AKI
Since AKI stage 1, 2, and 3 were all associated with a mean Δ[Cl-] ≥ 5 mmol/L we used this value as a threshold. A moderate increase in serum chloride (Δ[Cl-] ≥ 5 mmol/L) identified patients with an odds ratio of developing any AKI of 5.70 (3.00–10.82); p < 0.001 and an odds ratio of developing more severe AKI (AKI stage 2 and 3) of 3.40 (1.95–5.94); p < 0.001. Interestingly, in patients without hyperchloremia, Δ[Cl-] ≥ 5 mmol/L was also associated with AKI (OR = 8.25, 95 % CI 3.44–19.78; p < 0.001) and more severe AKI (AKI stage 2 and 3) (OR = 4.8, 95 % CI 2.1–10.7; p < 0.001) (Table 3).
Table 3.
Univariate logistic regression model to test association of AKI and increase in serum chloride (ΔCL = [Cl-]max - CL0) in all patients and patients without hyperchloremia
| Variable | AKI stage 1 to 3 | p | AKI stage 2 and 3 | p |
|---|---|---|---|---|
| Odds ratio (95 % CI) | Odds ratio (95 % CI) | |||
| All patients | ||||
| Δ[Cl-] | 1.25 (1.16–1.36) | <0.001a | 1.14 (1.07–1.20) | <0.001a |
| Δ[Cl-] ≥ 5 mmol/l | 5.70 (3.00–10.82) | <0.001a | 3.40 (1.95–5.94) | <0.001a |
| Patients without hyperchloremia | ||||
| Δ[Cl-] | 1.37 (1.20–1.56) | <0.001a | 1.25 (1.13–1.38) | <0.001a |
| Δ[Cl-] ≥ 5 mmol/l | 8.25 (3.44–19.78) | <0.001a | 4.77 (2.13–10.70) | <0.001a |
AKI acute kidney injury, Δ[Cl - ] increase in serum chloride, [Cl - ] max maximal chloride concentration in the first 48 hours, [Cl - ] 0 initial chloride concentration, CI confidence interval
aIndicates statistical significance, p < 0.05
Multivariate analysis
To adjust for baseline differences we incorporated age, gender and all potentially confounding variables [with p < 0.1 in univariate logistic regression analysis; hypertension, congestive heart failure, chronic obstructive pulmonary disease (COPD), APACHE II score, serum lactate, vasopressor and ventilator requirement] into the multivariate model. [Cl-]max remained significantly associated with AKI (OR = 1.35, 95 % CI 1.09–1.66, p = 0.006). Norepinephrine dosage was included quantitatively in the multivariable model and, while associated with AKI in the univariate analysis, it sufficiently covaried with other factors in the multivariate model (e.g., mean arterial pressure, APACHE II score) so that this association was not significant in the multivariate model. In contrast serum chloride remained an independent predictor of AKI (Table 4).
Table 4.
Multivariate logistic regression of association of AKI with initial serum chloride ([Cl-]0), maximal serum chloride ([Cl-]max), initial serum sodium (Na0) and maximal serum sodium(Namax)
| OR | 95 % confidence interval | p | ||
|---|---|---|---|---|
| Age | 0.99 | 0.95 | 1.04 | 0.776 |
| Sex (male) | 0.40 | 0.09 | 1.77 | 0.228 |
| Hypertension | 0.68 | 0.13 | 3.70 | 0.656 |
| APACHE II | 1.55 | 1.19 | 2.03 | 0.001a |
| Serum lactate | 1.60 | 0.84 | 3.04 | 0.155 |
| Norepinephrine dosage | 1.06 | 0.82 | 1.36 | 0.680 |
| Ventilator requirement | 3.95 | 0.57 | 27.44 | 0.165 |
| [Cl-]0 | 0.87 | 0.70 | 1.10 | 0.240 |
| [Cl-]max | 1.28 | 1.02 | 1.62 | 0.037a |
| Δ[Cl-] | 1.32 | 1.07 | 1.61 | 0.008a |
| Na0 | 1.01 | 0.76 | 1.35 | 0.825 |
| Namax | 1.03 | 0.81 | 1.31 | 0.825 |
Multivariate logistic regression model was adjusted by incorporating all potentially confounding factors including age, gender, underlying diseases, initial serum lactate, APACHE II score and requirement of vasopressor and ventilator
AKI acute kidney injury, OR odds ratio, APACHE Acute Physiology and Chronic Health Evaluation
aIndicates statistical significance, p < 0.05
Serum sodium and bicarbonate and AKI
To test an alternative explanation that serum chloride was simply a marker of the degree of volume resuscitation, we repeated the multivariate analysis using alternative electrolytes that would similarly covary with Saline volume resuscitation. The multivariable analysis was repeated using initial and maximal serum sodium and initial and maximal serum bicarbonate. None of these variables were significant independent predictors of AKI (Table 4).
Discussion
The main result of this study is hyperchloremia is common and significantly associated with AKI in severe sepsis and septic shock patients. Although initial serum chloride concentration before volume resuscitation did not correlate with development of AKI, maximal serum chloride concentration in the first 48 hours ([Cl-]max) and the increase in serum chloride (Δ[Cl-]) were significantly associated with AKI. Because the patients in the hyperchloremia group had higher APACHE II score, vasopressor and ventilator requirement that might result in higher AKI than the patients in the normochloremia group rather than by hyperchloremia, we use a multivariate logistic regression model to minimize the effect of these confounders. After several potential confounders such as age, gender, hypertension, congestive heart failure, COPD, APACHE II score, serum lactate, vasopressor and ventilator requirement were adjusted for in the multivariate logistic regression model, [Cl-]max remained independently associated with AKI. Moreover, an increase in serum chloride was associated with AKI and more severe AKI (AKI stage 2 and 3). Importantly, Δ[Cl-] ≥ 5 mmol/L was associated with the development of AKI even in the patients who never developed hyperchloremia. Thus, these data suggest that a rapid change in serum chloride concentration may be more important than the absolute value of serum chloride in causing AKI.
To our knowledge this is the first report of the association of a moderate increase in serum chloride (Δ[Cl-] ≥ 5 mmol/L) and development of AKI in severe sepsis and septic shock, even in patients without hyperchloremia. Our results are consistent with a retrospective study of unselected critically ill patients in which there was an association of maximal chloride concentration and the development of AKI [18]. Our study used serum chloride concentration in the first 48 hours to define hyperchloremia and found the association of [Cl-]max with the development of AKI in severe sepsis and septic shock. We obtained chloride measurements prior to significant volume resuscitation and found that this initial serum chloride concentration ([Cl-]0) was not associated with development of AKI. Previous studies found a relationship between initial chloride concentration and development of AKI, although these initial measurements may have followed a substantial saline resuscitation [19, 20]. More recently, a double-blind randomized trial evaluating the effect of Saline versus Plasma-Lyte in the intensive care unit (the SPLIT trial) demonstrated no difference of AKI incidence and severity between the two groups [23]. However, most patients were postoperative (71 %) and only 4 % of patients were diagnosed as sepsis. Overall incidence of AKI by KDIGO criteria was lower (27 %) compared to what we observed (63 %) in our sepsis/septic shock population. The volume of crystalloid solution used in that trial (2000 mL) was much less than the average volume that was infused in our patients (4825 mL). This study did not report serum chloride concentrations so it is unknown whether this small volume of Saline resuscitation over an extended period of time was sufficient to cause either hyperchloremia or Δ[Cl-] ≥ 5 mmol/L.
We did not find a statistically significant association between hyperchloremia and need for RRT although we observed a trend in this direction. A previous study found a similar statistically significant trend in the association of chloride restrictive strategy and reduction of RRT requirement [17]. However, we excluded the patients with pre-existing chronic renal failure and need of RRT in our study was lower (5 % vs 10 %). Second, a smaller sample size in our study limited the statistical power of detection. Moreover, our populations were exclusively severe sepsis or septic shock while in the previous study only 7 % were severe sepsis or septic shock.
Normal saline solution was originally called “indifferent” saline because it was recognized that human erythrocytes did not lyse in the 0.9 % NaCl solution [24]. Despite being called “normal saline” solution, it has a near physiological concentration of sodium (154 mmol/L) but supraphysiologic concentration of chloride (154 mmol/L or 1.5 times of normal serum chloride concentration) [25]. Infusion of 0.9 % NaCl is significantly associated with hyperchloremic metabolic acidosis in both healthy volunteers and various types of patient [26–35]. The clinical significance of iatrogenic hyperchloremic metabolic acidosis remains uncertain. The hospital mortality is much higher in lactic acidosis than in hyperchloremic acidosis (56 % vs 29 %) compared with the hospital mortality of patients without acidosis (26 %) [36].
Supraphysiologic serum chloride concentration has detrimental effects on renal function. In animal models, infusion of 0.9 % NaCl resulted in renal vasoconstriction and decreased renal blood flow and glomerular filtration rate [13, 37–39] possibly due to chloride-induced thromboxane release [38] and augmented response to renal vasoconstrictors such as angiotensin II [40]. Furthermore, the tubuloglomerular feedback mechanism initiated by detection of chloride at the macula densa results in afferent arteriolar vasoconstriction, mesangial contraction and decreased glomerular filtration rate [41]. In healthy human volunteers, infusion of normal saline caused reductions in renal artery blood flow, renal cortical perfusion [14] and delayed first micturition [15, 27]. Saline infusion resulted in a higher incidence of AKI when compared with balanced crystalloids [17, 42, 43]. In an animal model of sepsis, resuscitation of septic animals with Saline resulted in not only higher incidence of AKI and greater severity of AKI but also higher inflammatory mediator concentrations (IL-6) when compared to Plasma-Lyte [16]. Interestingly, the study of effect of an acute Saline infusion on fluid and electrolyte metabolism revealed that the human body required 2 days to restore electrolyte and fluid balance to equilibrium [44].
There are several limitations to this study. First, it was a retrospective cohort study so the association of hyperchloremia with AKI does not necessarily imply causality. Second, we were not able to determine the amount of chloride administered from the medical records so the analysis is based on the consequence – the change in serum chloride concentration. Hyperchloremia may have negative effects on renal function and cause AKI. Alternatively, increased severity of shock would lead to AKI and increased administration of Saline without a causal relationship between the two. Second, these results do not address the mechanism or pathophysiology of hyperchloremia and AKI. Finally, the small sample size significantly limits power to detect an association of hyperchloremia or Δ[Cl-] with RRT and 28-day mortality.
Conclusions
Hyperchloremia is common in severe sepsis and septic shock patients and is independently associated with AKI. Initial serum chloride concentration is not associated with the development of AKI while hyperchloremia, defined as the maximum serum chloride concentrations measurement in the first 48 hours, is associated with development of AKI. Our most striking finding is the association of moderate increase in serum chloride (Δ[Cl-] ≥ 5 mmol/L) and AKI even in patients who had serum chloride within the normal range. Finally, there is a continuing need for RCTs of intravenous fluid in sepsis and septic shock that address the risks of saline vs. balanced solutions for development of AKI. Perhaps serum chloride could be a biomarker for response to various intravenous fluids.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Authors’ contributions
JB contributed to study conception and design, prepared and collected data and helped to draft the manuscript. JR participated in study conception and design, and helped to draft and revise the manuscript. CP participated in study conception and design, and performed statistical analysis. BS participated in study conception and design, collected data, performed statistical analysis, and drafted and revised the manuscript. KW participated in study conception and design, and helped to draft and revise the manuscript. All authors made substantial contribution. All authors read and approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved by the University of British Columbia, ethics approval H11-00505, and all patients gave written informed consent to the use of both their clinical and analytical data. In a blinded, observational, cohort study, patients with suspected sepsis were identified when the attending Emergency Department physician activated the Institutional Severe Sepsis Order Set.
Support
Canadian Institutes for Health Research.
Abbreviations
- [Cl-]0
initial chloride concentration
- [Cl-]max
maximal chloride concentration in the first 48 hours
- Δ[Cl-]
increase in serum chloride
- AKI
acute kidney injury
- APACHE
Acute Physiology and Chronic Health Evaluation
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CRF
chronic renal failure
- GFR
glomerular filtration rate
- KDIGO
Kidney Disease Improving Global Outcomes
- MDRD
Modification of Diet in Renal Disease
- Na0
initial serum sodium
- Namax
maximal serum sodium in first 48 hours
- OR
odds ratio
- RRT
renal replacement therapy
- SD
standard deviation
- SIRS
systemic inflammatory response syndrome
Contributor Information
Bandarn Suetrong, Email: bandarns@hotmail.com.
Chawika Pisitsak, Email: chawika_p@hotmail.com.
John H. Boyd, Email: John.Boyd@hli.ubc.ca
James A. Russell, Email: Jim.Russell@hli.ubc.ca
Keith R. Walley, Phone: (604) 806-8136, Email: Keith.Walley@hli.ubc.ca
References
- 1.Poukkanen M, Vaara ST, Pettilä V, et al. Acute kidney injury in patients with severe sepsis in Finnish Intensive Care Units. Acta Anaesthesiol Scand. 2013;57:863–72. doi: 10.1111/aas.12133. [DOI] [PubMed] [Google Scholar]
- 2.Lopes JA, Jorge S, Resina C, et al. Acute kidney injury in patients with sepsis: a contemporary analysis. Int J Infect Dis. 2009;13:176–81. doi: 10.1016/j.ijid.2008.05.1231. [DOI] [PubMed] [Google Scholar]
- 3.Vanmassenhove J, Lameire N, Dhondt A, Vanholder R, Van Biesen W. Prognostic robustness of serum creatinine based AKI definitions in patients with sepsis: a prospective cohort study. BMC Nephrol. 2015;16:112. doi: 10.1186/s12882-015-0107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopes JA, Fernandes P, Jorge S, et al. Long-term risk of mortality after acute kidney injury in patients with sepsis: a contemporary analysis. BMC Nephrol. 2010;11:9. doi: 10.1186/1471-2369-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linder A, Fjell C, Levin A, Walley KR, Russell JA, Boyd JH. Small acute increases in serum creatinine are associated with decreased long-term survival in the critically ill. Am J Respir Crit Care Med. 2014;189:1075–81. doi: 10.1164/rccm.201311-2097OC. [DOI] [PubMed] [Google Scholar]
- 6.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 7.Hahn RG. Should anaesthetists stop infusing isotonic saline? Br J Anaesth. 2014;112:4–6. doi: 10.1093/bja/aet292. [DOI] [PubMed] [Google Scholar]
- 8.Hammond NE, Taylor C, Saxena M, et al. Resuscitation fluid use in Australian and New Zealand Intensive Care Units between 2007 and 2013. Intensive Care Med. 2015;41:1611–9. doi: 10.1007/s00134-015-3878-y. [DOI] [PubMed] [Google Scholar]
- 9.Finfer S, Liu B, Taylor C, et al. Resuscitation fluid use in critically ill adults: an international cross-sectional study in 391 intensive care units. Crit Care. 2010;14:R185. doi: 10.1186/cc9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. 2013;369:1243–51. doi: 10.1056/NEJMra1208627. [DOI] [PubMed] [Google Scholar]
- 11.O'Dell E, Tibby SM, Durward A, Murdoch IA. Hyperchloremia is the dominant cause of metabolic acidosis in the postresuscitation phase of pediatric meningococcal sepsis. Crit Care Med. 2007;35:2390–4. doi: 10.1097/01.CCM.0000284588.17760.99. [DOI] [PubMed] [Google Scholar]
- 12.Noritomi DT, Soriano FG, Kellum JA, et al. Metabolic acidosis in patients with severe sepsis and septic shock: a longitudinal quantitative study. Crit Care Med. 2009;37:2733–9. doi: 10.1097/CCM.0b013e3181a59165. [DOI] [PubMed] [Google Scholar]
- 13.Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–35. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9 % saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24. doi: 10.1097/SLA.0b013e318256be72. [DOI] [PubMed] [Google Scholar]
- 15.Williams EL, Hildebrand KL, McCormick SA, Bedel MJ. The effect of intravenous lactated Ringer's solution versus 0.9 % sodium chloride solution on serum osmolality in human volunteers. Anesth Analg. 1999;88:999–1003. doi: 10.1097/00000539-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Zhou F, Peng ZY, Bishop JV, Cove ME, Singbartl K, Kellum JA. Effects of fluid resuscitation with 0.9% saline versus a balanced electrolyte solution on acute kidney injury in a rat model of sepsis. Crit Care Med. 2014;42:e270–8. doi: 10.1097/CCM.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566–72. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, Xu X, Fan H, Li D, Deng H. Higher serum chloride concentrations are associated with acute kidney injury in unselected critically ill patients. BMC Nephrol. 2013;14:235. doi: 10.1186/1471-2369-14-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan S, Maria H, Rahul N, Gagan K. Hyperchloremia after fluid resuscitation in severe sepsis - does it predict renal outcomes? Chest. 2014;146:232A. doi: 10.1378/chest.1994006. [DOI] [Google Scholar]
- 20.Neyra JA, Canepa-Escaro F, Li X, et al. Association of hyperchloremia with hospital mortality in critically ill septic patients. Crit Care Med. 2015;43:1938–44. doi: 10.1097/CCM.0000000000001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kellum JA, Lameire N. KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1) Crit Care. 2013;17:204–19. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 23.Young P, Bailey M, Beasley R, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA. 2015;314:1701–10. doi: 10.1001/jama.2015.12334. [DOI] [PubMed] [Google Scholar]
- 24.Awad S, Allison SP, Lobo DN. The history of 0.9 % saline. Clin Nutr. 2008;27:179–88. doi: 10.1016/j.clnu.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Kellum JA, Bellomo R, Kramer DJ, Pinsky MR. Etiology of metabolic acidosis during saline resuscitation in endotoxemia. Shock. 1998;9(5):364–8 [DOI] [PubMed]
- 26.Story DA, Lees L, Weinberg L, et al. Cognitive changes after saline or plasmalyte infusion in healthy volunteers: a multiple blinded, randomized, cross-over trial. Anesthesiology. 2013;119:569–75. doi: 10.1097/ALN.0b013e31829416ba. [DOI] [PubMed] [Google Scholar]
- 27.Reid F, Lobo DN, Williams RN, Rowlands BJ, Allison SP. (Ab) normal saline and physiological Hartmann's solution: a randomized double-blind crossover study. Clin Sci (Lond) 2003;104:17–24. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 28.Hadimioglu N, Saadawy I, Saglam T, et al. The effect of different crystalloid solutions on acid-base balance and early kidney function after kidney transplantation. Anesth Analg. 2008;107:264–9. doi: 10.1213/ane.0b013e3181732d64. [DOI] [PubMed] [Google Scholar]
- 29.Young JB, Utter GH, Schermer CR, et al. Saline versus Plasma-Lyte A in initial resuscitation of trauma patients: a randomized trial. Ann Surg. 2014;259:255–62. doi: 10.1097/SLA.0b013e318295feba. [DOI] [PubMed] [Google Scholar]
- 30.O’Malley F, Frumento RJ, Catherine MN, et al. A randomized, double-blind comparison of Lactated Ringer’s solution and 0.9 % NaCl during renal transplantation. Anesth Analg. 2005;100:1518–24. doi: 10.1213/01.ANE.0000150939.28904.81. [DOI] [PubMed] [Google Scholar]
- 31.Scheingraber S, Rehm M, Sehmisch C, et al. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology. 1999;90:1265–70. doi: 10.1097/00000542-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Waters JH, Gottlieb A, Schoenwald P, et al. Normal saline versus Lactated Ringer’s solution for intraoperative fluid management in patients undergoing abdominal aortic aneurysm repair: an outcome study. Anesth Analg. 2001;93:817–22. doi: 10.1097/00000539-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Hasman H, Cinar O, Uzun A, et al. A randomized clinical trial comparing the effect of rapidly infused crystalloids on acid-base status in dehydrated patients in the emergency department. Int J Med Sci. 2012;9:59–64. doi: 10.7150/ijms.9.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zunini GS, Rando KA, Cox RG. Fluid replacement in craniofacial pediatric surgery: normal saline or ringer’s lactate? J Craniofac Surg. 2011;22:1370–4. doi: 10.1097/SCS.0b013e31821c94db. [DOI] [PubMed] [Google Scholar]
- 35.Mahler SA, Conrad SA, Wang H, et al. Resuscitation with balanced electrolyte solution prevents hyperchloremic metabolic acidosis in patients with diabetic ketoacidosis. Am J Emerg Med. 2011;29:670–4. doi: 10.1016/j.ajem.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Gunnerson KJ, Saul M, He S, Kellum JA. Lactate versus non-lactate metabolic acidosis: a retrospective outcome evaluation of critically ill patients. Crit Care. 2006;10:R22. doi: 10.1186/cc3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quilley CP, Lin YS, McGiff JC. Chloride anion concentration as a determinant of renal vascular responsiveness to vasoconstrictor agents. Br J Pharmacol. 1993;108:106–10. doi: 10.1111/j.1476-5381.1993.tb13447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bullivant EM, Wilcox CS, Welch WJ. Intrarenal vasoconstriction during hyperchloremia: role of thromboxane. Am J Physiol. 1989;256:F152–7. doi: 10.1152/ajprenal.1989.256.1.F152. [DOI] [PubMed] [Google Scholar]
- 39.Aksu U, Bezemer R, Yavuz B, Kandil A, Demirci C, Ince C. Balanced vs unbalanced crystalloid resuscitation in a near-fatal model of hemorrhagic shock and the effects on renal oxygenation, oxidative stress, and inflammation. Resuscitation. 2012;83:767–73. doi: 10.1016/j.resuscitation.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka M, Schmidlin O, Olson JL, Yi SL, Morris RC. Chloride-sensitive renal microangiopathy in the stroke-prone spontaneously hypertensive rat. Kidney International. 2001;59(3):1066–76. [DOI] [PubMed]
- 41.Yunos NM, Bellomo R, Story D, Kellum J. Bench-to-bedside review: chloride in critical illness. Crit Care. 2010;14:226. doi: 10.1186/cc9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yunos NM, Bellomo R, Glassford N, Sutcliffe H, Lam Q, Bailey M. Chloride-liberal vs. chloride-restrictive intravenous fluid administration and acute kidney injury: an extended analysis. Intensive Care Med. 2015;41:257–64. doi: 10.1007/s00134-014-3593-0. [DOI] [PubMed] [Google Scholar]
- 43.Krajewski ML, Raghunathan K, Paluszkiewicz SM, Schermer CR, Shaw AD. Meta-analysis of high- versus low-chloride content in perioperative and critical care fluid resuscitation. Br J Surg. 2015;102:24–36. doi: 10.1002/bjs.9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drummer C, Gerzer R, Heer M, et al. Effects of an acute saline infusion on fluid and electrolyte metabolism in humans. Am J Physiol. 1992;262:F744–54. doi: 10.1152/ajprenal.1992.262.5.F744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


