TtuA and TtuB are the sulfur transferase and sulfur carriers for the biosynthesis of 2-thioribothymidine in some bacterial tRNAs. To elucidate their mechanism of interaction, the TtuA–TtuB complex from T. thermophilus was crystallized and a Zn-MAD data set was collected to a resolution of 2.5 Å.
Keywords: post-transcriptional modification, sulfur transfer, ubiquitin-like protein, Zn-MAD, TtuA, TtuB
Abstract
The ubiquitin-like protein TtuB is a sulfur carrier for the biosynthesis of 2-thioribothymidine (s2T) at position 54 in some thermophilic bacterial tRNAs. TtuB captures a S atom at its C-terminus as a thiocarboxylate and transfers it to tRNA by the transferase activity of TtuA. TtuB also functions to suppress s2T formation by forming a covalent bond with TtuA. To explore how TtuB interacts with TtuA and switches between these two different functions, high-resolution structure analysis of the TtuA–TtuB complex is required. In this study, the TtuA–TtuB complex from Thermus thermophilus was expressed, purified and crystallized. To mimic the thiocarboxylated TtuB, the C-terminal Gly residue was replaced with Cys (G65C) to obtain crystals of the TtuA–TtuB complex. A Zn-MAD data set was collected to a resolution of 2.5 Å. MAD analysis successfully determined eight Zn sites, and a partial structure model composed of four TtuA–TtuB complexes in the asymmetric unit was constructed.
1. Introduction
Thiolation is a general transfer RNA (tRNA) post-transcriptional modification that is widely conserved in all three kingdoms of life (Machnicka et al., 2013 ▸). Thiolation is related to various important cellular functions such as maintenance of translation (Krüger et al., 1998 ▸; Yarian et al., 2002 ▸), recognition of aminoacyl-tRNA synthases (Krüger & Sørensen, 1998 ▸), sensing ultraviolet (UV) radiation stress (Caldeira de Araujo & Favre, 1985 ▸) and stabilization of the ternary structure of tRNA (Watanabe et al., 1976 ▸; Shigi, Suzuki et al., 2006 ▸; Alings et al., 2015 ▸). A total of four tRNA thiolation modification groups have been found to date: 2-thiouridine (s2U), 4-thiouridine (s4U), 2-thiocytidine (s2C) and 2-methylthio-N 6-alkyladenodine (ms2x6A) (Boschi-Muller & Motorin, 2013 ▸). Each modification is introduced via distinct sulfur-transfer pathways using specific sulfur transferases (Shigi, 2014 ▸), although the S atoms for these thiolation modifications are commonly supplied by the cysteine desulfurase IcsS. Although the pathways for introducing these thiolation modifications have been fully described, mechanistic knowledge of the pathways remains limited.
TtuB and TtuA are the sulfur carrier and transferase, respectively, for the biosynthesis of 2-thioribothymidine (s2T) at position 54 in some thermophilic bacterial tRNAs (Shigi, Sakaguchi et al., 2006 ▸), which is important for survival in a high-temperature environment (Watanabe et al., 1976 ▸; Shigi, Suzuki et al., 2006 ▸). TtuB, composed of approximately 65 amino-acid residues, is a homologue of eukaryotic ubiquitin (sharing about 35% identity). TtuB contains a characteristic Gly-Gly sequence at the C-terminus, which is used for holding an S atom on the C-terminus as a thiocarboxylate group (Shigi et al., 2008 ▸). This S atom is transferred to tRNA by the enzymatic activity of a sulfur transferase, TtuA. It has been reported that TtuB and TtuA form a stable complex during sulfur transfer to tRNA (Shigi, Sakaguchi et al., 2006 ▸; Shigi et al., 2008 ▸). Similar sulfur carriers are also found in other biosynthetic pathways of sulfur-containing biomolecules, such as ThiS in thiamin biosynthesis and MoaD in molybdenum-cofactor biosynthesis (Begley et al., 2012 ▸; Iobbi-Nivol & Leimkühler, 2013 ▸).
Furthermore, TtuB has been reported to be a bifunctional ubiquitin-like protein in eubacteria (Shigi, 2012 ▸). It forms a covalent linkage with Lys137, Lys226 and Lys229 of TtuA in a ubiquitin-like manner. These covalent linkages cause inhibition of the sulfur-transfer activity of TtuA; TtuB acts not only as a sulfur carrier, but also as a post-translational modifier. Interestingly, ubiquitin-related modifier-1 (Urm1), a similar bifunctional ubiquitin-like protein that acts both as a sulfur carrier and as a post-translational modifier, has similarly been found in eukaryotic cells (Leidel et al., 2009 ▸). These observations suggest that the eukaryotic ubiquitin system evolved from the prokaryotic sulfur-transfer system.
Although the structure of TtuA from Pyrococcus horikoshii has previously been reported (Nakagawa et al., 2013 ▸), the mechanism by which TtuB interacts with TtuA and switches between two distinct functions remains poorly understood. To address these questions, elucidation of the three-dimensional structure of the complex between TtuA and TtuB is required. In the present study, we expressed, purified and crystallized the TtuA–TtuB complex from Thermus thermophilus (hereafter referred to as TthTtuA–TtuB). Zn-MAD data were collected to a resolution of 2.5 Å, and multiple-wavelength anomalous diffraction (MAD) analysis successfully identified eight Zn sites.
2. Materials and methods
2.1. Cloning, expression and purification of TtuA–TtuB
A DNA fragment encoding TthTtuA was cloned into the NcoI and HindIII sites of the pCDFDuet-1 vector (Novagen). A DNA fragment encoding TthTtuB was cloned into the NdeI and XhoI sites of a modified pET-28a vector (Novagen), in which a Tobacco etch virus (TEV) protease recognition site was introduced between the His6 tag and the N-terminus of the target protein (Asano et al., 2015 ▸). TthTtuA and TthTtuB were co-expressed in Escherichia coli strain B834 (DE3). Macromolecule-production information is summarized in Table 1 ▸. E. coli strain B834 (DE3) cells harbouring expression vectors for both TthTtuA and TthTtuB were incubated on lysogeny broth (LB)–agar plates containing 50 mg l−1 streptomycin and 25 mg l−1 kanamycin overnight at 310 K. A single colony was inoculated into 100 ml LB preculture containing 50 mg l−1 streptomycin and 25 mg l−1 kanamycin and was incubated overnight at 310 K with shaking at 150 rev min−1. A 100 ml aliquot of the preculture was added to 900 ml LB culture containing 50 mg l−1 streptomycin and 25 mg l−1 kanamycin and incubated at 310 K. When the absorption at 600 nm reached 0.6, isopropyl β-d-1-thiogalactopyranoside (IPTG) was added to a final concentration of 0.1 mM. The culture was further incubated at 298 K for an additional 20 h. The E. coli cells were collected by centrifugation at 4000g for 10 min. Further procedures were conducted under anaerobic conditions consisting of 5% hydrogen and 95% nitrogen gas (Vinyl Anaerobic Chamber, Coy). The collected cells were sonicated for 20 min on ice in a sonication buffer consisting of 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) pH 7.6, 200 mM ammonium sulfate, 50 mM ammonium acetate, 1 mM magnesium acetate, 12% glycerol, 0.1% Triton X-100, followed by heat treatment at 343 K for 20 min. The precipitation was then removed by centrifugation at 7000g for 30 min. The supernatant was loaded onto a His-Trap HP column (GE Healthcare, Waukesha, Wisconsin, USA) pre-equilibrated with buffer A (25 mM HEPES pH 7.6, 200 mM ammonium sulfate, 50 mM ammonium acetate, 1 mM magnesium acetate, 12% glycerol). After washing with 15 ml buffer A, the adsorbed protein was eluted with a 0–0.5 M gradient of imidazole in buffer A. Fractions containing the TthTtuA–TtuB complex were desalted on a PD-10 desalting column (GE Healthcare) pre-equilibrated with buffer A and were then further purified on a HiLoad 16/60 Superdex 200 column (GE Healthcare). Purified TthTtuA–TtuB complex was concentrated to 10 mg ml−1 and used for crystallization. TthTtuA in complex with the TthTtuB (G65C) mutant was prepared by the same procedure as used for wild-type (WT) TthTtuA–TtuB.
Table 1. Macromolecule-production information.
| Macromolecule | TthTtuA | TthTtuB (G65C) |
|---|---|---|
| Source organism | T. thermophilus | |
| DNA source | UniProt Q72LF3 | UniProt Q72LF4 |
| Expression vector | pCDFDuet-1 | Modified pET-28a |
| Expression host | E. coli B834 (DE3), co-expression | |
| Complete amino-acid sequence of the construct produced† | MVCKVCGQKAQVEMRSRGLALCREHYLDWFVKETERAIRRHRMLLPGERVLVAVSGGKDSLALWDVLSRLGYQAVGLHIELGIGEYSKRSLEVTQAFARERGLELLVVDLKEAYGFGVPELARLSGRVACSACGLSKRYIINQVAVEEGFRVVATGHNLDDEAAVLFGNLLNPQEETLSRQGPVLPEKPGLAARVKPFYRFSEREVLSYTLLRGIRYLHEECPNAKGAKSLLYKEALNLVERSMPGAKLRFLDGFLEKIRPRLDVGEEVALRECERCGYPTTGAVCAFCRMWDAVYRRAKKRKLLPEEVSFRPRVKPLRAG | MGSSHHHHHHSSGLVPRGSHMRVVLRLPERKEVEVKGNRPLREVLEELGLNPETVVAVRGEELLTLEDEVREEDTLEVLSAISGC |
The artifactual sequences, i.e. His6 tag, TEV protease recognition site (LVPRGS) and point mutation G65C, are underlined.
2.2. Crystallization
The initial screening of crystallization conditions for each sample was performed with The JCSG I–IV, Classics, Classics II, MPD, PEGs, PEGs II, Protein Complex, Anions and Cations Suites (Qiagen) crystallization kits using the sitting-drop vapour-diffusion method at 293 K under anaerobic conditions. Although WT TthTtuA–TtuB did not crystallize, crystals of the TthTtuA–TtuB (G65C) mutant were successfully obtained. Crystals of the TthTtuA–TtuB (G65C) mutant suitable for further experiments grew from a buffer consisting of 0.1 M HEPES pH 7.6, 6%(w/v) PEG 8000, 4%(w/v) ethylene glycol. Crystallization information is provided in Table 2 ▸.
Table 2. Crystallization.
| Method | Sitting-drop vapour diffusion |
| Plate type | MRC 2 96-well crystallization plate |
| Temperature (K) | 293 |
| Protein concentration (mg ml−1) | 10 |
| Buffer composition of protein solution | 25 mM HEPES pH 7.6, 200 mM ammonium sulfate, 50 mM ammonium acetate, 1 mM magnesium acetate, 12% glycerol |
| Composition of reservoir solution | 0.1 M HEPES pH 7.6, 6%(w/v) PEG 8000, 4%(w/v) ethylene glycol |
| Volume and ratio of drop | 1 µl, 1:1 |
| Volume of reservoir (µl) | 70 |
2.3. Data collection and processing
An X-ray diffraction experiment was conducted at 100 K on the NE3A beamline at Photon Factory, Tsukuba, Japan. Crystals were mounted on the diffractometer after soaking in a buffer consisting of 0.1 M HEPES pH 7.6, 25%(w/v) PEG 6000, 4%(w/v) ethylene glycol. Three wavelengths were chosen for the MAD data on the basis of the fluorescence spectrum of the Zn K absorption edge: peak, 1.28251 Å; edge, 1.28311 Å; remote, 1.00000 Å. The MAD data set was collected to a resolution of 2.5 Å. The data were indexed, integrated and scaled with XDS (Kabsch, 2010 ▸).
3. Results and discussion
TthTtuA and TthTtuB are reported to form a stable complex in vivo (Shigi, Sakaguchi et al., 2006 ▸). Therefore, in the present study we attempted to express TthTtuA and TthTtuB together in E. coli cells and to purify the TthTtuA–TtuB complex spontaneously formed in the cell using a His6 tag attached to TthTtuB. SDS–PAGE of the fractions eluted from nickel-affinity chromatography clearly showed that TtuA is co-purified with TtuB, indicating that the TthTtuA–TtuB complex forms spontaneously in the E. coli cells. Although an excess of TtuB was present, further purification using size-exclusion chromatography yielded pure TthTtuA–TtuB complex (see Supporting Information). TthTtuA–TtuB had a strong tendency to aggregate, which hampered further experiments. Since we expected TtuA to be a sulfur transferase that transfers S atoms using Cys residue(s) and that the redox state of Cys residue may influence the protein stability, we tried to prepare the TthTtuA sample under anaerobic conditions. We finally determined that TthTtuA is unstable under aerobic conditions but stable under anaerobic conditions. Furthermore, a high concentration (200 mM) of ammonium sulfate is effective in enhancing the solubility of TtuA–TtuB. Finally, the TthTtuA–TtuB complex was successfully obtained with high yield and with a purity suitable for crystallization (Supplementary Fig. S1).
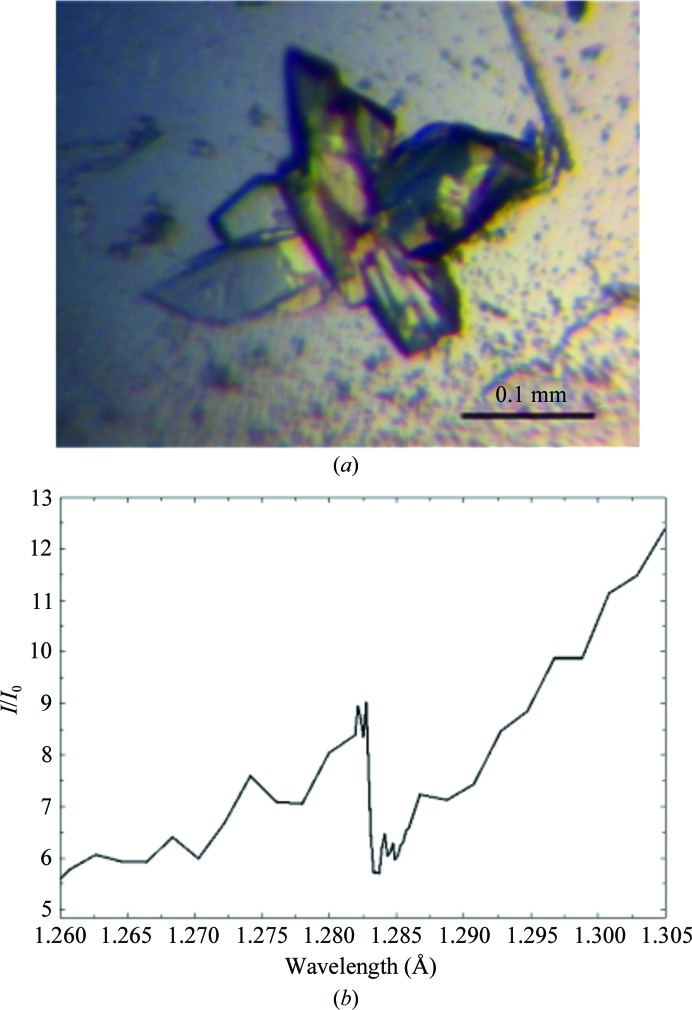
Next, we screened crystallization conditions for the purified TthTtuA–TtuB complex using commercially available crystallization kits. Unfortunately, no crystals of the WT TthTtuA–TtuB complex were obtained. Previous biochemical data indicated that TthTtuB carries an S atom using its thiocarboxylated C-terminus (Shigi et al., 2008 ▸), suggesting that the thiol group located at the C-terminus may play some significant role in the interaction with TthTtuA. We therefore substituted the C-terminal Gly residue with Cys (G65C; Supplementary Fig. S2c) to mimic the thiocarboxylated C-terminus (Supplementary Fig. S2b). It is expected that the chemical properties of Cys are more similar to those of the thiocarboxylated C-terminus than to Gly in terms of the presence of a free thiol group, and therefore the structure of the G65C mutant represents the binding manner and/or the reaction mechanism of the sulfur transfer more precisely. Using this mutant, we successfully obtained crystals of the TthTtuA–TtuB complex that diffracted well (Fig. 1 ▸ a).
Figure 1.
(a) A crystal of the TthTtuA–TtuB complex obtained by the sitting-drop vapour-diffusion method. (b) X-ray absorption spectrum of the TthTtuA–TtuB complex crystal near the Zn K absorption edge.
TtuA contains five characteristic metal ion-binding motifs, CXXC/H, suggesting that TthTtuA may contain heavy atoms. We carried out an X-ray absorption spectrum experiment. The result clearly showed the presence of Zn atoms in the TthTtuA–TtuB complex crystal (Fig. 1 ▸ b). Therefore, we attempted to determine the structure using the Zn-MAD method.
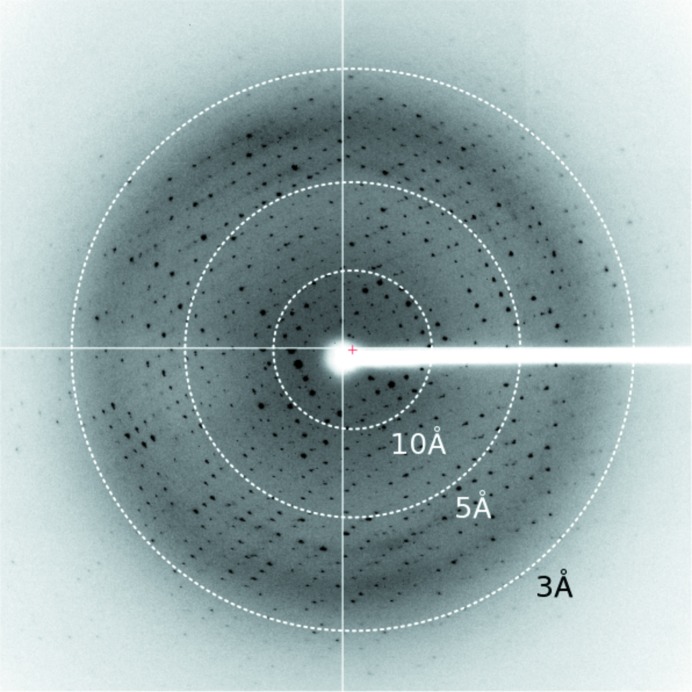
Zn-MAD data were collected to a resolution of 2.5 Å (Fig. 2 ▸). The crystal of the TthTtuA–TtuB (G65C) mutant complex belonged to space group P1, with unit-cell parameters a = 54.1, b = 93.9, c = 97.5 Å, α = 109.2, β = 104.6, γ = 106.9°. Data-collection statistics are summarized in Table 3 ▸. Four TthTtuA–TtuB complexes are expected to be located in the asymmetric unit, with a V M value of 2.26 Å3 Da−1 and a solvent content of 45.63%. SHELXD (Sheldrick, 2010 ▸) successfully determined eight Zn sites. SOLVE/RESOLVE (Terwilliger & Berendzen, 1999 ▸; Terwilliger, 2000 ▸, 2003 ▸) determined the initial phases with an overall figure of merit of 0.60 and automatically constructed an initial model containing 58% of the residues. Model building and refinement are in progress.
Figure 2.
X-ray diffraction image of a crystal of the TthTtuA–TtuB complex.
Table 3. Data-collection and processing statistics for the TthTtuA–TtuB (G65C) complex.
Values in parentheses are for the outer shell.
| Peak | Edge | Remote | |
|---|---|---|---|
| Diffraction source | NE3A, Photon Factory | ||
| Detector | Quantum 270, ADSC | ||
| Space group | P1 | ||
| a, b, c (Å) | 54.1, 93.9, 97.5 | ||
| α, β, γ (°) | 109.2, 104.6, 106.9 | ||
| Temperature (K) | 100 | ||
| Crystal-to-detector distance (mm) | 300 | ||
| Rotation range per image (°) | 1 | ||
| Total rotation range (°) | 360 | ||
| Exposure time per image (s) | 1 | ||
| Wavelength (Å) | 1.28251 | 1.28311 | 1.00000 |
| Resolution range (Å) | 47.8–2.50 (2.65–2.50) | 47.9–2.50 (2.65–2.50) | 47.9–2.50 (2.65–2.50) |
| Mosaicity (°) | 0.414 | 0.420 | 0.410 |
| Total No. of reflections | 207808 | 208416 | 210467 |
| No. of unique reflections | 105373 | 105675 | 106753 |
| Completeness (%) | 94.9 (93.2) | 94.8 (92.2) | 95.8 (94.3) |
| Multiplicity | 1.97 (1.87) | 1.97 (1.87) | 1.97 (1.87) |
| 〈I/σ(I)〉 | 16.24 (2.79) | 15.88 (2.39) | 14.90 (1.93) |
| R meas † (%) | 5.5 (39.6) | 5.9 (47.2) | 6.7 (59.1) |
| R merge ‡ (%) | 3.9 (28.0) | 4.2 (33.4) | 4.7 (41.2) |
| CC1/2 § | 99.8 (85.8) | 99.8 (80.8) | 99.8 (73.0) |
| Overall B factor from Wilson plot (Å2) | 51 | 52 | 53 |
R
meas = 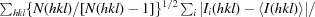
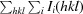 , where I
i(hkl) and N(hkl) are the mean intensity of a set of equivalent reflections and the multiplicity, respectively.
, where I
i(hkl) and N(hkl) are the mean intensity of a set of equivalent reflections and the multiplicity, respectively.
R
merge = 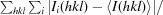
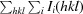 , where Ii(hkl) is the ith observation of reflection hkl and 〈I(hkl)〉 is the weighted average intensity for all i observations of reflection hkl.
, where Ii(hkl) is the ith observation of reflection hkl and 〈I(hkl)〉 is the weighted average intensity for all i observations of reflection hkl.
Percentage correlation between intensities from random half-data sets (Karplus & Diederichs, 2012 ▸).
Acknowledgments
The synchrotron-radiation experiments were performed at SPring-8 (proposal No. 2014B1033) and Photon Factory (proposal No. 2014G080). We thank the beamline staff of SPring-8 and Photon Factory for their assistance with data collection. This work was supported by the Platform Project for Supporting Drug Discovery and Life Science Research (Platform for Drug Discovery, Informatics and Structural Life Science; 11961) from Japan Agency for Medical Research and Development (AMED), JST PRESTO (JPMJPR1517) and Grants-in-Aid for Scientific Research (15J01961, 24000011, 26291008 and 16H00748) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Alings, F., Sarin, L. P., Fufezan, C., Drexler, H. C. A. & Leidel, S. A. (2015). RNA, 21, 202–212. [DOI] [PMC free article] [PubMed]
- Asano, N., Kato, K., Nakamura, A., Komoda, K., Tanaka, I. & Yao, M. (2015). Nucleic Acids Res. 43, 4746–4757. [DOI] [PMC free article] [PubMed]
- Begley, T. P., Ealick, S. E. & McLafferty, F. W. (2012). Biochem. Soc. Trans. 40, 555–560. [DOI] [PMC free article] [PubMed]
- Boschi-Muller, S. & Motorin, Y. (2013). Biochemistry (Mosc.), 78, 1392–1404. [DOI] [PubMed]
- Caldeira de Araujo, A. & Favre, A. (1985). Eur. J. Biochem. 146, 605–610. [DOI] [PubMed]
- Iobbi-Nivol, C. & Leimkühler, S. (2013). Biochim. Biophys. Acta, 1827, 1086–1101. [DOI] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 133–144. [DOI] [PMC free article] [PubMed]
- Karplus, P. A. & Diederichs, K. (2012). Science, 336, 1030–1033. [DOI] [PMC free article] [PubMed]
- Krüger, M. K., Pedersen, S., Hagervall, T. G. & Sørensen, M. A. (1998). J. Mol. Biol. 284, 621–631. [DOI] [PubMed]
- Krüger, M. K. & Sørensen, M. (1998). J. Mol. Biol. 284, 609–620. [DOI] [PubMed]
- Leidel, S., Pedrioli, P. G., Bucher, T., Brost, R., Costanzo, M., Schmidt, A., Aebersold, R., Boone, C., Hofmann, K. & Peter, M. (2009). Nature (London), 458, 228–232. [DOI] [PubMed]
- Machnicka, M. A., Milanowska, K., Osman Oglou, O., Purta, E., Kurkowska, M., Olchowik, A., Januszewski, W., Kalinowski, S., Dunin-Horkawicz, S., Rother, K. M., Helm, M., Bujnicki, J. M. & Grosjean, H. (2013). Nucleic Acids Res. 41, D262–D267. [DOI] [PMC free article] [PubMed]
- Nakagawa, H., Kuratani, M., Goto-Ito, S., Ito, T., Katsura, K., Terada, T., Shirouzu, M., Sekine, S.-I., Shigi, N. & Yokoyama, S. (2013). Proteins, 81, 1232–1244. [DOI] [PubMed]
- Sheldrick, G. M. (2010). Acta Cryst. D66, 479–485. [DOI] [PMC free article] [PubMed]
- Shigi, N. (2012). J. Biol. Chem. 287, 17568–17577. [DOI] [PMC free article] [PubMed]
- Shigi, N. (2014). Front. Genet. 5, 67. [DOI] [PMC free article] [PubMed]
- Shigi, N., Sakaguchi, Y., Asai, S.-I., Suzuki, T. & Watanabe, K. (2008). EMBO J. 27, 3267–3278. [DOI] [PMC free article] [PubMed]
- Shigi, N., Sakaguchi, Y., Suzuki, T. & Watanabe, K. (2006). J. Biol. Chem. 281, 14296–14306. [DOI] [PubMed]
- Shigi, N., Suzuki, T., Terada, T., Shirouzu, M., Yokoyama, S. & Watanabe, K. (2006). J. Biol. Chem. 281, 2104–2113. [DOI] [PubMed]
- Terwilliger, T. C. (2000). Acta Cryst. D56, 965–972. [DOI] [PMC free article] [PubMed]
- Terwilliger, T. C. (2003). Acta Cryst. D59, 38–44. [DOI] [PMC free article] [PubMed]
- Terwilliger, T. C. & Berendzen, J. (1999). Acta Cryst. D55, 849–861. [DOI] [PMC free article] [PubMed]
- Watanabe, K., Shinma, M., Oshima, T. & Nishimura, S. (1976). Biochem. Biophys. Res. Commun. 72, 1137–1144. [DOI] [PubMed]
- Yarian, C., Townsend, H., Czestkowski, W., Sochacka, E., Malkiewicz, A. J., Guenther, R., Miskiewicz, A. & Agris, P. F. (2002). J. Biol. Chem. 277, 16391–16395. [DOI] [PubMed]