Abstract
Setting
Five primary health care clinics in Kinshasa, Democratic Republic of Congo.
Objective
To examine timing and predictors of delayed ART initiation during TB treatment.
Design
Prospective observational cohort of adult patients receiving integrated TB/HIV treatment, expected to initiate ART at 1 month if CD4 count <100 cells/mm3 or WHO clinical stage 4 for reason other than extrapulmonary TB, at 2 months if CD4 count 100–350 cells/mm3, or at completion of TB treatment if subsequently CD4 count ≤350 cells/mm3 or WHO clinical stage 4.
Results
Of 492 patients, 235 (47.8%) experienced delayed ART initiation: 171 (72.8%) initiated ART late, after a median delay of 12 days (IQR 4–27) and 64 (27.2%) never initiated ART. Contraindication to any ARV drug (adjOR 2.91, 95% CI 1.22–6.95), lower baseline CD4 count (adjOR 1.20, 95% CI 1.08–1.33 per 100 cells/mm3), TB drug intolerance (adjOR 1.93, 95% CI 1.23–3.02), and non-disclosure of HIV-infection (adjOR 1.50, 95% CI 1.03–2.18) predicted delayed ART initiation.
Conclusion
Despite fully-integrated treatment, half of all patients experienced delayed ART initiation. Pragmatic approaches to ensure timely ART initiation in those at-risk of delayed ART initiation are needed.
Keywords: predictors, integration, HIV, TB, delay
INTRODUCTION
The burden of tuberculosis disease (TB) and human immunodeficiency virus (HIV) is a major public health concern. In 2012, there were an estimated 8.6 million people worldwide and 201,000 people in the Democratic Republic of Congo (DRC) with incident TB.1,2 The estimated HIV prevalence among people with TB is disproportionately high worldwide (13%) and in the DRC (16%).1 Although all HIV-infected patients with TB are eligible, according to recent World Health Organization (WHO) guidelines, among those who knew their HIV status, only 57% worldwide and 40% in the DRC, initiated antiretroviral therapy (ART) in 2012.1,3
Based on findings from recent randomized controlled trials4–6, WHO recommends initiation of ART within 2 weeks of TB treatment in people with CD4 count <50 cells/mm3 and as soon as possible in those with less severe immunodeficiency.4–7 Despite this, many people diagnosed with TB experience delayed ART initiation.8–10 Two South African studies identified lack of integration of TB treatment and ART as a main cause of delay.11,12
In most TB clinics, patients are referred off-site for ART; resulting in loss to follow-up and delay.11,13,14 Treatment integration, where ART is initiated along with TB treatment, has been suggested as a solution.7 Two studies comparing outcomes pre- and post-integration found that integration significantly increased uptake of and reduced time to ART initiation, but did not assess patient-level predictors of delayed ART initiation.15,16
We examined timing and patient-level predictors of delayed ART during TB treatment among a prospective cohort receiving integrated TB/HIV treatment in Kinshasa, DRC.
METHODS
Setting, Study Population, and Study Procedures
We implemented the Integrating Tuberculosis and Anti-Retroviral Treatment (ITART) study among patients enrolled between August 2007 and November 2009 at five primary health centers. Full description and clinical outcomes of this study have been previously reported.17 The ITART study was approved by institutional review boards at the University of North Carolina at Chapel Hill and the University of Kinshasa. All patients provided written informed consent for participation.
Tuberculosis was diagnosed per national guidelines, using smear microscopy as the primary laboratory diagnostic. All patients were offered on-site HIV rapid testing as routine in TB diagnosis. All HIV-infected patients diagnosed with TB were offered study participation. At baseline, patients were administered a questionnaire to collect demographic and clinical data and CD4 count tested. Patients were followed-up weekly during the 2-month intensive phase, monthly during the subsequent 4-month continuation phase, and at completion of TB treatment. Patients were considered lost to follow-up from study if they were more than three days late for a scheduled clinic visit and could not be located by phone or home visit within one month.
Both TB treatment and ART were delivered by nurses in the TB clinic. The first-line ART regimen consisted of stavudine plus lamivudine plus efavirenz; nevirapine was used instead of efavirenz for pregnant women and zidovudine was used instead of stavudine in case of pre-existing peripheral neuropathy. Per national ART guidelines, ITART nurses followed a strategy to initiate ART earlier during TB treatment in patients with greater immunosuppression and/or disease progression at baseline, similar to 2006 and 2012 WHO guidelines.7,18 Patients were eligible to initiate ART at 1 month if baseline CD4 count <100 cells/mm3 or WHO stage 4 condition other than extrapulmonary TB and at 2 months if baseline CD4 count 100–350 cells/mm3. Patients with baseline CD4 count >350 cells/mm3 were scheduled for repeat CD4 testing at month 5 of TB treatment and eligible at end of TB treatment if CD4 count dropped below 350 cells/mm3 or at time of an incident WHO stage 4 condition. When CD4 counts were not available (due to reagent shortage), all patients were considered eligible at 1 month. Providers assessed whether the patient was or was not tolerating TB treatment at the time of ART eligibility.
Data Analysis and Statistical Methods
We limited our analysis to patients age ≥13 years who were enrolled within 1 month of TB treatment, were ART-naïve, and had a baseline CD4 count result. Baseline CD4 count was the first CD4 test ordered within 30 days of TB treatment. Patients with baseline CD4 count >350 cells/mm3 who experienced incident WHO stage 4 condition were categorized as eligible at completion of TB treatment. To define timing of ART eligibility, we compared the ART initiation date with TB treatment start date. To accommodate scheduling limitations, clinic closure on weekends and holidays, and on-site availability of consulting physician, patients eligible at 1 month of TB treatment who did not initiate ART within 1 month plus 5 days of TB treatment, were classified as experiencing delayed ART. Similarly, a 5-day window was applied to define delayed ART among patients eligible at 2 months and at completion of TB treatment. Patients who died or were lost-to-follow-up from study prior to the time they were eligible who did not initiate ART, were not categorized as experiencing delayed ART. Patients with baseline and follow-up CD4 count >350 cells/mm3 who did not experience a WHO stage 4 condition during TB treatment, were also categorized as not experiencing delayed ART, since they were not scheduled to initiate ART during TB treatment.
Low socio-economic status (SES) was defined as self-report of not having enough money to meet needs most of the time. Patients who were bedridden or in bed >50% of the time were categorized as having poor functional status. Disclosure of TB and HIV status was defined based on patient self-report of disclosure to person of significance e.g. family or household member, partner, pastor, etc.
We first ran a full logistic model containing all selected demographic, behavioral, and clinical covariates hypothesized to predict delayed ART initiation. Subsequently, we used backwards elimination stepwise to generate a final predictive model. Covariates were assessed in order from highest to lowest Wald chi-square and eliminated using the likelihood ratio test with alpha of 0.05. We conducted a sensitivity analysis to explore the impact of broadening the definition of delayed ART to include patients who were lost to follow-up from study prior to time of eligibility, a subset of patients who could have started timely ART had they been retained. Characteristics at baseline are presented as absolute (n) and relative frequencies (%) for categorical variables and as medians with interquartile ranges (IQR) for continuous variables. Crude odds ratios (OR) and adjusted odds ratios (adjOR) are presented with standard Wald 95% confidence intervals (95% CI). All analyses were conducted using SAS 9.3 (Cary, NC).
RESULTS
Characteristics at Baseline and Scheduled ART Initiation
In total, 599 patients diagnosed with TB and HIV were enrolled within the ITART study. Among them, 107 were sequentially excluded based on age<13 years (n=18), enrollment more than one month after TB treatment initiation (n=22), exposure to ART prior to enrollment (n=35), and lack of baseline CD4 count (n=32).
Baseline characteristics of the resultant 492 patients included in the analysis are shown in Table 1. Participants were 60.0% female, 44.5% married, and had a median age of 38 years (IQR 32–45). Most (92.3%) patients were of low SES, less than half (46.3%) were employed, and 61.6% completed secondary education. The majority (75.4%) of patients walked to the clinic, with about half (48.6%) traveling over 30 minutes. Of patients with pulmonary TB (79.7%), half were smear-negative. Overall 25.4% had prior history of TB treatment. Most (95.3%) patients were newly diagnosed with HIV.
TABLE 1.
Characteristics at baseline of 492 patients participating in the Integrated Tuberculosis and Anti-Retroviral Treatment (ITART) study at five primary care clinics in Kinshasa, Democratic Republic of Congo
| Characteristic | n | % |
|---|---|---|
| Female | 295 | 60.0 |
| Age <30 | 94 | 19.1 |
| 30–39 | 182 | 37.0 |
| 40–49 | 184 | 33.3 |
| ≥50 | 52 | 10.6 |
| Married | 219 | 44.5 |
| Completed secondary education | 303 | 61.6 |
| Employed | 228 | 46.3 |
| Low socio-economic status | 454 | 92.3 |
| Walk to clinic | 371 | 75.4 |
| Travel time to clinic (minutes) <10 | 18 | 3.7 |
| 10–19 | 141 | 28.7 |
| 20–29 | 94 | 19.1 |
| 30–39 | 136 | 27.6 |
| ≥40 | 103 | 20.9 |
| TB type Smear + Pulmonary | 193 | 39.2 |
| Smear − Pulmonary | 199 | 40.5 |
| Extrapulmonary | 100 | 20.3 |
| History of TB | 125 | 25.4 |
| HIV diagnosis at TB clinic | 469 | 95.3 |
| CD4 count (cells/mm3) <50 | 77 | 15.6 |
| 50–99 | 64 | 13.0 |
| 100–199 | 136 | 27.6 |
| 200–350 | 118 | 24.0 |
| >350 | 97 | 19.7 |
| Hospitalization in Prior Year | 77 | 15.7 |
| Poor Functional Status | 27 | 5.5 |
| Body mass index Underweight (<18.5) | 283 | 57.5 |
| Normal (18.5–24.9) | 194 | 39.4 |
| Overweight (25.0–29.9) | 12 | 2.4 |
| Obese (≥30) | 3 | 0.6 |
| Alcohol use (any) | 201 | 40.9 |
| Disclosure of tuberculosis status | 439 | 89.2 |
| Disclosure of HIV status | 197 | 40.0 |
At baseline, median CD4 count was 168 cells/mm3 (IQR 84–307) and 22.4% of patients were WHO clinical stage 4. Only 15.7% of patients had been hospitalized in the prior year, and few (5.5%) of patients had poor functional status. The median body mass index (BMI) was 17.9 (IQR 16.5–19.9) and over half (57.5%) of patients were underweight. At baseline, nearly all (89.2%) patients had disclosed their TB status, while less than half (40.0%) had disclosed their HIV status.
At their scheduled ART initiation visit, 145 (29.5%) of patients were assessed as not tolerating TB treatment. In total, 26 (5.3%) of patients had contraindication to one or more antiretroviral drug. Reasons for contraindication included: pre-existing peripheral neuropathy for stavudine (n=22), pregnancy for efavirenz (n=1), and anemia for zidovudine (n=2).
Timing of ART Initiation
A total of 143 (29.1%) patients were eligible for ART initiation at 1 month of TB treatment, nearly all (n=141) because of CD4<100 cells/mm3. Another 252 (51.2 %) patients were eligible at 2 months of TB treatment, based on CD4 100–350 cells/mm3. The majority (58.8%, n=57) of patients with baseline CD4 >350 (n=97) were eligible at completion of TB treatment based on CD4 ≤350 cells/mm3 at month 5 (n=18), lack of follow-up CD4 count (n=32), or incident WHO stage 4 condition (n=7).
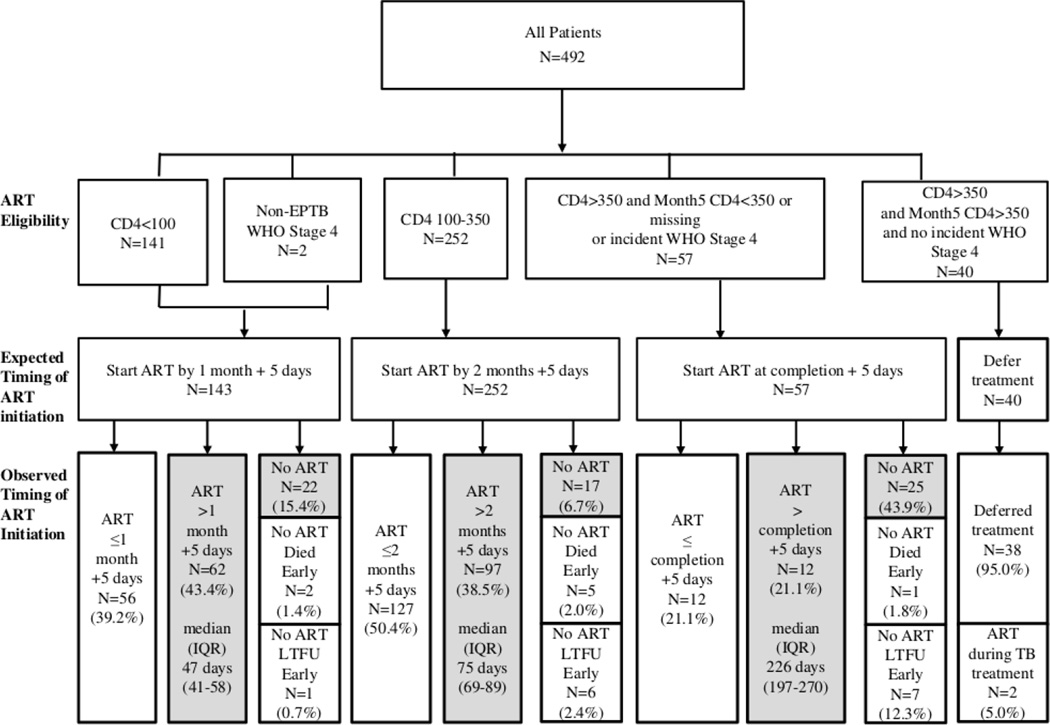
Overall, ART initiation was delayed in 235 (47.8%) patients (details in Figure 1). Patients scheduled to initiate ART at 2 months were less likely to experience delayed ART compared to patients scheduled to initiate ART at 1 month (45.2% v. 58.7%, p=0.010) and at TB treatment completion (45.2% v. 64.9%, p=0.007). Among the 235 (47.8%) who experienced delayed ART, 171 (72.8%) initiated ART late, after a median of 12 days (IQR 4–27) beyond time of eligibility and 64 (27.2%) never initiated ART. ART was delayed by the provider in five patients for a median of 13 days (IQR 13–19) due to untreated oral candidiasis (n=4) and short-term travel (n=1).
FIGURE 1.
Distribution of ART eligibility, expected timing of ART initiation, and observed timing of ART initiation in 492 patients receiving integrated TB and HIV treatment at five primary care clinics in Kinshasa, Democratic Republic of Congo.*
TB=tuberculosis; ART=antiretroviral therapy; CD4=CD4 count in cells/mm3; shading=delayed ART; median (IQR)=median and interquartile range of time to ART initiation from TB treatment start. *Expected timing of ART is at 1 month or 2 months from the start of TB treatment or at completion of TB treatment with a +5 day window around these timepoints to accommodate scheduling limitations, clinic closure on weekends and holidays, and on-site availability of consulting physician.
Of the 86 patients who never initiated ART, eight died a median of 20 days (IQR 8–30) before time of eligibility. Of these, two had baseline CD4 count <100 cells/mm3, five had baseline CD4 count 100–350 cells/mm3, and one had baseline CD4 count >350 cells/mm3. Fourteen patients were lost to follow-up from study prior to time of eligibility. One patient was considered ineligible for ART due to terminal illness at their scheduled ART initiation visit and died thereafter. Three patients refused ART as they did not feel ready to start long-term therapy. Two patients who never initiated ART self-reported suboptimal adherence to TB treatment (at least one missed dose within the prior four days) at the scheduled time of ART initiation.
Predictors of Delay in ART Initiation
Crude and fully adjusted model results are shown in Table 2. The final model indicated that lower CD4 count, lack of disclosure of HIV status, contraindication to one or more ARV drugs, and intolerance of TB drugs were predictive of delayed ART. Patients with contraindication to at least one antiretroviral drug were more likely to experience delayed ART (adjOR 2.91, 95% CI 1.22–6.95). Lower CD4 count at baseline was associated with 20% higher odds of delayed ART (adjOR 1.20, 95% CI 1.08–1.33 per 100 CD4 cell/mm3). Patients who did not tolerate their TB drugs were nearly 2 times as likely to experience delayed ART (adjOR 1.93, 95% CI 1.23–3.02). Failure to disclose HIV status, was associated with delayed ART (adjOR 1.50, 95% CI 1.03–2.18). These predictors were robust to sensitivity analysis when the definition of delayed ART was expanded to include patients who were lost to follow-up from study prior to the time eligibility. The full model results suggest that not being married (adjOR 1.46, 95% CI 0.99–2.16) and smear-negative pulmonary TB (v. smear-positive pulmonary TB) (adjOR 1.52, 95% CI 0.98–2.34) may predict delayed ART, although they did not meet a priori criteria for retention in the final model.
TABLE 2.
Predictors of Delayed ART Initiation in 492 patients participating in the Integrating Tuberculosis and Anti-Retroviral Treatment (ITART) study at five primary care clinics in Kinshasa, Democratic Republic of Congo
| Crude OR Univariate Model (95% CI) |
Adjusted OR Full Model (95% CI) |
Adjusted OR Reduced Model (95% CI) |
|
|---|---|---|---|
| Female | 1.00 (0.70–1.44) | 0.97 (0.65–1.45) | -- |
| Age >40 years | 1.06 (0.74–1.52) | 1.01 (0.68–1.51) | -- |
| Married | 0.73 (0.51–1.04) | 0.69 (0.46–1.01) | -- |
| Secondary Education | 0.88 (0.61–1.27) | 0.94 (0.64–1.40) | -- |
| Employed | 0.88 (0.62–1.26) | 1.04 (0.69–1.58) | -- |
| Low SES | 0.57 (0.29–1.12) | 0.59 (0.29–1.22) | -- |
| Walk to Clinic | 0.80 (0.53–1.20) | 0.83 (0.54–1.16) | -- |
| Travel Time >30 min | 0.85 (0.59–1.20) | 0.79 (0.54–1.16) | -- |
| TB Type Smear + Pulmonary | referent | referent | referent |
| Smear − Pulmonary | 1.42 (0.95–2.12) | 1.52 (0.98–2.34) | -- |
| Extrapulmonary | 1.15 (0.71–1.87) | 1.03 (0.61–1.72) | -- |
| History of TB | 0.85 (0.57–1.28) | 0.87 (0.56–1.35) | -- |
| HIV diagnosis at TB clinic | 1.20 (0.52–2.79) | 0.89 (0.36–2.20) | -- |
| CD4 count (per 100 cells/mm3 decrease) | 1.12 (1.02–1.23) | 1.18 (1.06–1.32) | 1.20 (1.08–1.33) |
| Prior Hospitalization | 1.01 (0.62–1.65) | 0.92 (0.54–1.54) | -- |
| Poor Functional Status | 1.19 (0.55–2.59) | 1.42 (0.60–3.33) | -- |
| Underweight (BMI<18.5) | 0.90 (0.63–1.29) | 0.89 (0.60–1.33) | -- |
| Alcohol use (any) | 0.97 (0.67–1.39) | 0.96 (0.65–1.43) | -- |
| Non-disclosure of TB status | 1.15 (0.65–2.04) | 0.87 (0.46–1.64) | -- |
| Non-disclosure HIV status | 1.46 (1.02–2.10) | 1.60 (1.05–2.44) | 1.50 (1.03–2.18) |
| Contraindication to ART* | 2.58 (1.10–6.05) | 2.90 (1.17–7.20) | 2.91 (1.22–6.95) |
| Intolerance of TB Drugs* | 1.36 (0.92–2.00) | 1.93 (1.21–3.07) | 1.93 (1.23–3.02) |
OR=odds ratio; CI=confidence interval; TB=tuberculosis; SES=socio-economic status; BMI=body mass index, ART=antiretroviral therapy; HIV=human immunodeficiency virus.
determined at scheduled ART initiation date (see text for details).
DISCUSSION
Despite access to fully integrated TB treatment and ART, delayed ART occurred in almost half (47.8%) of all patients. Delay was more likely among those with lower CD4 count, no HIV disclosure, intolerance of TB drugs, and contraindication to an ARV drug.
Our finding that half of patients experienced delayed ART despite full treatment integration confirms findings from two studies in South Africa. In a before-after study, Kershberger et al. found that integration reduced median time to ART from 147 to 75 days after TB treatment start; a timeframe that is still late, given a median CD4 count 84 cells/mm3 (IQR 32–158) in the study population.16 In a randomized controlled trial of ART timing during TB treatment by Abdool Karim et al, 16.7% of patients assigned to early ART (within 4 weeks) and 28.7% of patients assigned to late ART (within 3 months) did not initiate ART accordingly.6
Our study is the first to evaluate patient-level predictors of delayed ART in an integrated TB/HIV treatment setting. Two prior studies of delay in ART among patients diagnosed with TB were performed in a non-integrated setting.11,12 Similarly, these studies found that smear-negative pulmonary TB was associated with delay, which may be related to additional complexity and time required for a final diagnosis of this form of TB.19 In contrast, these studies found that lower CD4 count was associated with less delay. This contradictory finding may be because the outcome in these studies was absolute time to ART, without consideration of baseline status. The outcome in our study was delayed ART, defined by the study strategy, which recommended earlier ART for patients with lower CD4 count. Consequently, our finding, may in fact reflect the challenge of early ART due to factors including patient readiness to immediately start life-long ART; and potential for immune reconstitution inflammatory syndrome4,6,20, non-adherence due to increased pill burden, and overlapping drug toxicities.21 Additionally, we found that intolerance of TB treatment predicted delayed ART, suggesting that both providers and patients may be reluctant to initiate ART until TB treatment is tolerated. Our finding that non-disclosure of HIV status predicted delayed ART is consistent with other study findings of an association between non-disclosure of HIV status and non-readiness to initiate ART, which may be related to social support; a factor that may also explain being non-married as a potential predictor in our study22–24.
There are several study limitations that must be considered in the interpretation of our findings. First, transport for CD4 count specimens/results was provided to make CD4 count available for ART decision-making. Our findings may not be generalizable to settings in which CD4 count availability is limited. Second, our study provided additional training and support of nurses to provide integrated HIV/TB treatment. Consequently, our findings may not be fully generalizable to primary care clinics where nurses do not receive this level of support. Third, TB treatment intolerance was only qualitatively assessed by providers. Lack of information on the clinical conditions that contributed to this assessment limited our ability to consider the severity of TB treatment intolerance in our analysis.
Patient-level factors can influence both patient and provider decision-making about ART initiation; therefore, interventions should be designed accordingly. Training of providers and health communication to patients should emphasize early ART initiation, particularly among patients presenting with low CD4 count. Availability of multiple first-line ARV regimens could expedite ART in patients with contraindications. Furthermore, severity of TB drug intolerability may vary substantially; therefore, guidance to providers on if and when it warrants delayed ART is needed. Finally, HIV testing in TB settings should emphasize and facilitate safe HIV disclosure by patients.
CONCLUSION
Despite fully integrated TB/HIV treatment, nearly half of all HIV-infected patients diagnosed with TB experienced delayed ART during TB treatment. Pragmatic approaches to ensure timely ART, particularly targeted to patients identified as at-risk of delay, are needed.
Acknowledgments
M.R.P. worked closely with A.VR. to design and conduct the data analyses and write the final paper. A.VR. conceptualized the ITART study in close collaboration with F.B. M.N., M.T., and M.Y. implemented the ITART study including patient enrollment, data collection, and clinical supervision and follow-up. All authors reviewed and contributed to the final paper.
The authors acknowledge the Centers of Disease Control and Prevention, President’s Emergency Plan for AIDS Relief, and The Global Fund for AIDS, TB and Malaria for funding this study. M.R.P. was partially supported by National Institutes of Health training grant 2T32AI070114. We thank Steve Mpuate and Elizabeth Cromwell for assistance in preparing the ITART dataset and Joseph Eron and Daniel Westreich for thoughtful comments on the paper.
MRP was a summer intern and research assistant at GlaxoSmithKline from 2010–2011.
Footnotes
Conflicts of interest: A.VR., M.N., M.Y., M.T., and F.B. have no conflicts of interest.
REFERENCES
- 1.World Health Organization. Global Tuberculosis Report 2013. 2013 WHO/HTM/TB/2013.11.
- 2.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global Report: UNAIDS Report on the Global AIDS Epidemic 2013. 2013 UNAIDS/JC2502/1/E. [Google Scholar]
- 3.World Health Organization. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach. 2010 [PubMed]
- 4.Havlir DV, Kendall MA, Ive P, Kumwenda J, Swindells S, Qasba SS, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011 Oct 20;365(16):1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanc FX, Sok T, Laureillard D, Borand L, Rekacewicz C, Nerrienet E, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011 Oct 20;365(16):1471–1481. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray AL, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011 Oct 20;365(16):1492–1501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. WHO Policy on collaborative TB/HIV activities: Guidelines for national programmes and other stakeholders. 2012 WHO/HTM/TB/2012.6. [PubMed]
- 8.Manosuthi W, Chottanapand S, Thongyen S, Chaovavanich A, Sungkanuparph S. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J Acquir Immune Defic Syndr. 2006 Sep;43(1):42–46. doi: 10.1097/01.qai.0000230521.86964.86. [DOI] [PubMed] [Google Scholar]
- 9.Varma JK, Nateniyom S, Akksilp S, Mankatittham W, Sirinak C, Sattayawuthipong W, et al. HIV care and treatment factors associated with improved survival during TB treatment in Thailand: an observational study. BMC Infect Dis. 2009 Apr 13;9:42. doi: 10.1186/1471-2334-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henegar C, Behets F, Vanden Driessche K, Tabala M, Bahati E, Bola V, et al. Mortality among tuberculosis patients in the Democratic Republic of Congo. The International Journal of Tuberculosis and Lung Disease. 2012;16(9):1199–1204. doi: 10.5588/ijtld.11.0613. [DOI] [PubMed] [Google Scholar]
- 11.Lawn SD, Campbell L, Kaplan R, Little F, Morrow C, Wood R, et al. Delays in starting antiretroviral therapy in patients with HIV-associated tuberculosis accessing non-integrated clinical services in a South African township. BMC Infect Dis. 2011 Sep 30;11:258. doi: 10.1186/1471-2334-11-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawn SD, Campbell L, Kaplan R, Boulle A, Cornell M, Kerschberger B, et al. Time to initiation of antiretroviral therapy among patients with HIV-associated tuberculosis in Cape Town, South Africa. J Acquir Immune Defic Syndr. 2011 Jun 1;57(2):136–140. doi: 10.1097/QAI.0b013e3182199ee9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zachariah R, Harries AD, Manzi M, Gomani P, Teck R, Phillips M, et al. Acceptance of anti-retroviral therapy among patients infected with HIV and tuberculosis in rural Malawi is low and associated with cost of transport. PloS one. 2006;1(1):e121. doi: 10.1371/journal.pone.0000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Legido-Quigley H, Montgomery CM, Khan P, Atun R, Fakoya A, Getahun H, et al. Integrating tuberculosis and HIV services in low- and middle-income countries: a systematic review. Trop Med Int Health. 2013 Feb;18(2):199–211. doi: 10.1111/tmi.12029. [DOI] [PubMed] [Google Scholar]
- 15.Huerga H, Spillane H, Guerrero W, Odongo A, Varaine F. Impact of introducing human immunodeficiency virus testing, treatment and care in a tuberculosis clinic in rural Kenya. Int J Tuberc Lung Dis. 2010 May;14(5):611–615. [PubMed] [Google Scholar]
- 16.Kerschberger B, Hilderbrand K, Boulle AM, Coetzee D, Goemaere E, De Azevedo V, et al. The effect of complete integration of HIV and TB services on time to initiation of antiretroviral therapy: a before-after study. PLoS One. 2012;7(10):e46988. doi: 10.1371/journal.pone.0046988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Rie A, Patel MR, Nana M, Vanden Driessche K, Tabala M, Yotebieng M, et al. Integration and task-shifting for TB/HIV care and treatment in highly resource-scarce settings: one size may not fit all. J Acquir Immune Defic Syndr. 2014 doi: 10.1097/01.qai.0000434954.65620.f3. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Antiretroviral Therapy of HIV Infection in Infants and Children: Towards Universal Access, Recommendations for a Public Health Approach. 2006 [PubMed] [Google Scholar]
- 19.Dimairo M, MacPherson P, Bandason T, Zezai A, Munyati SS, Butterworth AE, et al. The risk and timing of tuberculosis diagnosed in smear-negative TB suspects: a 12 month cohort study in Harare, Zimbabwe. PloS one. 2010;5(7):e11849. doi: 10.1371/journal.pone.0011849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007 Jan 30;21(3):335–341. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 21.McIlleron H, Meintjes G, Burman WJ, Maartens G. Complications of antiretroviral therapy in patients with tuberculosis: drug interactions, toxicity, and immune reconstitution inflammatory syndrome. J Infect Dis. 2007;196(Supplement 1):S63–S75. doi: 10.1086/518655. [DOI] [PubMed] [Google Scholar]
- 22.Wong LH, Van Rooyen H, Modiba P, Richter L, Gray G, McIntyre JA, et al. Test and tell: correlates and consequences of testing and disclosure of HIV status in South Africa (HPTN 043 Project Accept) J Acquir Immune Defic Syndr. 2009;50(2):215. doi: 10.1097/QAI.0b013e3181900172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abaynew Y, Deribew A, Deribe K. Factors associated with late presentation to HIV/AIDS care in South Wollo ZoneEthiopia: a case-control study. 2011 doi: 10.1186/1742-6405-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tessema B, Biadglegne F, Mulu A, Getachew A, Emmrich F, Sack U. Magnitude and determinants of nonadherence and nonreadiness to highly active antiretroviral therapy among people living with HIV/AIDS in Northwest Ethiopia: a cross-sectional study. AIDS Research and Therapy. 2010;7(1):2. doi: 10.1186/1742-6405-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]