Abstract
Background:
Despite the important biological effects of jabuticaba, its actions on the cardiovascular system have not been clarified.
Objectives:
To determine the effects of jabuticaba hydroalcoholic extract (JHE) on vascular smooth muscle (VSM) of isolated arteries.
Methods:
Endothelium-denuded aortic rings of rats were mounted in isolated organ bath to record isometric tension. The relaxant effect of JHE and the influence of K+ channels and Ca2+ intra- and extracellular sources on JHE-stimulated response were assessed.
Results:
Arteries pre-contracted with phenylephrine showed concentration-dependent relaxation (0.380 to 1.92 mg/mL). Treatment with K+ channel blockers (tetraethyl-ammonium, glibenclamide, 4-aminopyridine) hindered relaxation due to JHE. In addition, phenylephrine-stimulated contraction was hindered by previous treatment with JHE. Inhibition of sarcoplasmic reticulum Ca2+ ATPase did not change relaxation due to JHE. In addition, JHE inhibited the contraction caused by Ca2+ influx stimulated by phenylephrine and KCl (75 mM).
Conclusion:
JHE induces endothelium-independent vasodilation. Activation of K+ channels and inhibition of Ca2+ influx through the membrane are involved in the JHE relaxant effect.
Keywords: Jabuticaba (Myrciaria Cauliflora); Trees; Vasodilatation; Calcium Channels; Muscle, Smooth Vascular
Introduction
Cardiovascular diseases are a major cause of death worldwide, among which hypertension accounts for 9.4 million deaths per year.1 Around 1 billion adults in the world have hypertension, and that figure will have increased by 25% in 10 years.2
Vascular tonus regulation is fundamental to appropriate blood pressure control. Blood vessel contraction and dilation in response to physiological demands are controlled by changes in the intracellular concentration of Ca2+ in vascular smooth muscle (VSM) cells. The increase in intracellular concentration of Ca2+ occurs via both Ca2+ influx through the plasma membrane and Ca2+ release from inner sources, such as the sarcoplasmic reticulum.3,4 Effective drugs to blood pressure control, such as nifedipine, verapamil and diltiazem, which act as Ca2+ channel blockers, induce vasodilation and reduce blood pressure.5
The use of natural products as an alternative treatment for hypertension has been extensively studied, being known to induce hypotension with minimum side effects.6,7 Jabuticaba (Myrciaria cauliflora), also known as Brazilian grape, is a hard-skinned berry of the Myrtaceae family, largely distributed in Brazil. It can be consumed fresh or in the form of liqueurs, wines, jams and sweets, and its consumption has increased in Brazil and worldwide.8,9 In addition to the use of jabuticaba as food and beverage, in folk medicine, that fruit is used to treat some diseases, such as asthma, inflammations, and gastrointestinal and cardiovascular disorders.10 Recent findings have shown that jabuticaba can decrease oxidative process,11 hyperglycemia associated with insulin resistance12 and dyslipidemia.13 In addition, that species has a proven endothelium-dependent hypotensive and vasodilating effect, mediated by nitric oxide pathway.14
Considering that jabuticaba has important biological effects and that its action on the cardiovascular system has been little studied, this study was aimed at assessing the possible effect of the jabuticaba extract directly on the VSM, mainly its effect on Ca2+ influx through the plasma membrane and activation of K+ channels.
Methods
Preparation of jabuticaba extract
For this study, the plant specimens were donated by the "Jabuticabal" wine house in the city of Hidrolândia, Goiás state, Brazil. A sample of the plant specimen was stored at the herbarium of the department of botany of the Federal University of Goiás (UFG), Goiânia, Goiás state, Brazil (n. 21140). Seedless fruits were dried in a greenhouse with air circulation, powdered in a pulverizer mill and passed through a 60-mesh sieve at the Laboratory of Research on Natural Products, Pharmacy School/UFG. The powder obtained was stored at -20°C. To prepare the extract, the dried material was exhaustively percolated into an ethanol:water solution (55:45 v/v), and the material obtained was filtered and submitted to rotary evaporation under reduced pressure at 40°C, resulting in the ethanol-free jabuticaba hydroalcoholic extract (JHE). After that process, the JHE was maintained in a freezer (-20°C) protected from light. On the days of experiment, the JHE was solubilized in distilled water at the concentration of 120 mg/mL.
The phytochemical characterization and pattern of the JHE showed 17.89% of phenolic compounds, quantified by using the Hagerman and Butler method, adapted by Mole and Watermen.15 The JHE showed ellagic acid (phytochemical marker, determined by HPLC-PDA) at 0.222% concentration.14 According to Abe et at.,9 the total ellagic acid content in M. cauliflora fruits ranges from 0.021% to 0.311%. Thus, that phytochemical marker concentration in JHE is in accordance with the fruit content.
Animals and preparation of isolated arteries
Wistar male rats (200-230 g) from the central vivarium of the UFG were used in this study. All experimental protocols abided by the UFG Animal Research Ethics Committee (protocol: 015/2014). This study is in accordance with the European Union Guide to the Care and Use of Experimental Animals (2010/63/UE).
The rats were sacrificed by use of cervical dislocation under inhalation anesthesia. Thoracic aorta was isolated, cleared of connective and adipose tissues, and sliced into rings (± 4 mm), which were mounted between two metal hooks, one of which was connected to a power transducer to record isometric tension (DATAQ Instruments, Akron, OH, USA) and the other was fixed to a cube for the isolated organ. The rings were placed into chambers for isolated organs, containing modified Krebs solution [composition in mM: NaCl, 130.0; KCl, 4.7; KH2PO4, 1.2; CaCl2,1.6; MgSO4, 1.2; NaHCO3, 14.9; glucose, 5.5], at pH of 7.4, under gasification with carbogen mixture (95% O2 + 5% CO2) at 37°C, and maintained at baseline tension of 1 g (ideal resting tension, previously standardized at our laboratory). To prevent the influence of vascular-endothelium-derived factors, endothelial cells were mechanically removed by rubbing the vessel lumen with a thin metal rod, the effectiveness of the removal being evidenced by lack of relaxation due to acetylcholine (1 µM) in aortic rings pre-contracted with EC50 of phenylephrine (0.1 µM).
Experimental protocols
After 60 minutes of stabilization at baseline tension (1 g), the arteries were pre-contracted with phenylephrine (0.1 µM), and cumulative relaxation-concentration-effect curves were built for JHE (0 to 1.92 mg/mL) and for verapamil, used as inner control (10 nM to 100 µM).
To assess the cellular pathways responsible for the relaxant effect of JHE, aortic rings were pre-contracted with phenylephrine (0.1 µM) for 20 minutes after incubation with the following agents: 1) Ca2+ ATPase of the sarcoplasmic reticulum, cyclopiazonic acid (CPA, 10 µM); 2) non-selective K+ channel blocker, tetraethyl-ammonium (TEA, 1 mM); 3) selective voltage-gated K+ channel (Kv) blocker, 4-aminopyridine (4-AP, 1 mM); 4) selective ATP-sensitive K+ channel (KATP) blocker, glibenclamide (3 µM); 5) Ca2+-dependent K+ channel (KCa) blocker, clotrimazole (5 µM).
To assess the influence of JHE on the contraction induced by adrenergic contractile agonist, concentration-effect curves were built for phenylephrine (selective α1-adrenergic agonist, 0.1 nM to 10 µM) in the presence (20 minutes) or absence of JHE at inhibitory concentration 50% (IC50, 0.5 mg/mL) or 100% (IC100, 1.92 mg/mL). In addition, the inhibitory effect of the Ca2+ channel blocker verapamil (IC50, 0.3 µM) was assessed as inner control.
In another series of experiments, JHE action on Ca2+ influx stimulated by two different agents was analyzed. The preparations were initially contracted with a KCl solution (75 mM) to cause maximum contraction of each preparation (100% contraction), being then rinsed with Ca2+-free Krebs solution until total relaxation. To exhaust the intracellular storage of Ca2+, the preparations were stimulated to contract with phenylephrine in Ca2+-free Krebs solution until any contractile response disappeared (approximately 5 or 6 times, for 30-50 minutes). Then the preparations were rinsed several times with Ca2+-free Krebs solution, and then incubated for 20 minutes with JHE at inhibitory concentration 50% (IC50, 0.51 mg/mL) or 100% (IC100, 1.92 mg/mL). In addition, the inhibitory effect of the Ca2+ channel blocker verapamil (IC50, 0.3 µM) was assessed as inner control. After incubation, the contractile stimulus was applied (phenylephrine, 0.1 µM, or KCL, 75 mM), and concentration-effect curves were built for CaCl2 (0 to 3.0 mM).
Statistical analysis
The results of isometric tension were expressed as mean ± standard error of the mean (SEM) of at least five experiments (n = 5-8) obtained from different animals. The graphs were built and analyzed by use of the GraphPad Prism software (GraphPad Software Corporation, 5.0 version) with ANOVA and Bonferroni post-test. The 5% significance level (p < 0.05) was adopted for the differences.
Results
Relaxant effect of JHE on isolated arteries
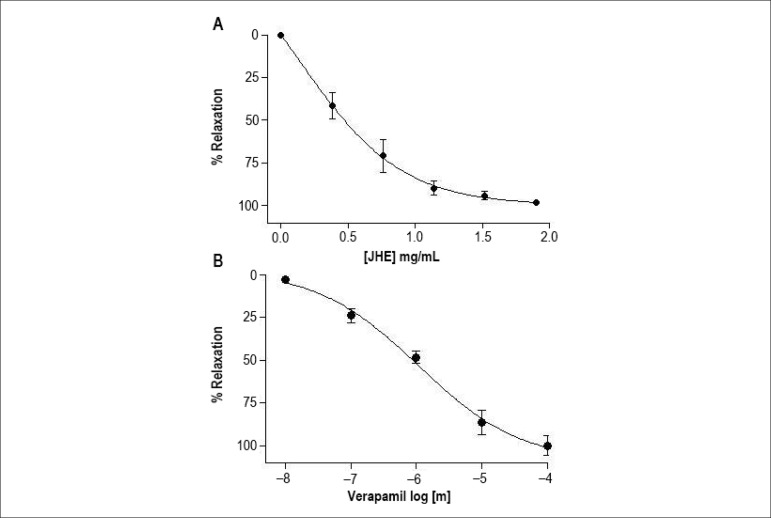
The JHE caused relaxation in preparations of endothelium-denuded arteries on a concentration-dependent way, relaxation initiating at the concentration of 0.38 mg/mL, and achieving the maximum effect (Emax) of 98.3% ± 0.4% (n = 6) at the concentration of 1.92 mg/mL (IC100) (Figure 1A). The JHE concentration that induced 50% relaxation (IC50) was 0.51 mg/mL. Similarly, verapamil (used as positive control) induced concentration-dependent relaxation with Emax of 99.8% ± 1.8% (n = 5) and IC50 of 0.3 µM.
Figure 1.
Cumulative concentration-response curves of jabuticaba hydroalcoholic extract (JHE) (A) and verapamil (B) in isolated endothelium-denuded arteries. The points represent mean ± SEM of the relaxant effect expressed as % relaxation.
Effect of JHE on the phenylephrine-induced contraction
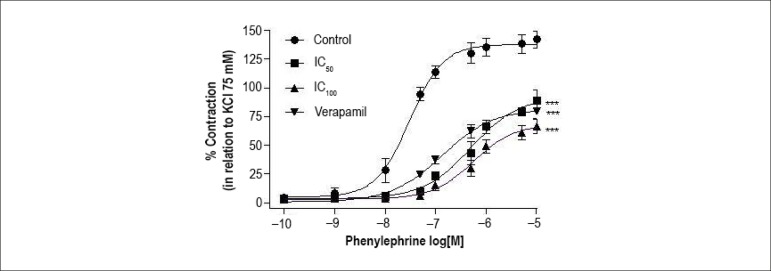
The Emax value for phenylephrine (142.1% ± 7.1%, n = 6) was significantly (p < 0.001) reduced to 88.7% ± 6.2% (n = 5), 66.1% ± 5.1% (n = 6) and 79.9% ± 5.5% (n = 5) after incubation with IC50 and IC100 of JHE or verapamil, respectively. The addition of IC50 and IC100 of JHE or verapamil significantly increased phenylephrine pD2 values (-log EC50) from 6.24 ± 0.09 to 5.35 ± 0.04, 5.14 ± 0.09 and 5.68 ± 0.07, respectively (Figure 2).
Figure 2.
Effect of jabuticaba hydroalcoholic extract (JHE) and verapamil on the phenylephrine-induced contraction in isolated endothelium-denuded arteries. Cumulative concentration-response curves were built in control conditions and after incubation (20 min) with JHE (IC50: 0.51 or IC100: 1.92 mg/mL) or verapamil (IC50: 0.3 µM). The points represent mean ± SEM of the contractile effect expressed as % contraction in relation to total KCl-induced contraction (75 mM). Significant difference: *** p<0.001 vs. Control
Effect of JHE on Ca2+-influx-induced contraction in preparations stimulated with phenylephrine or KCl
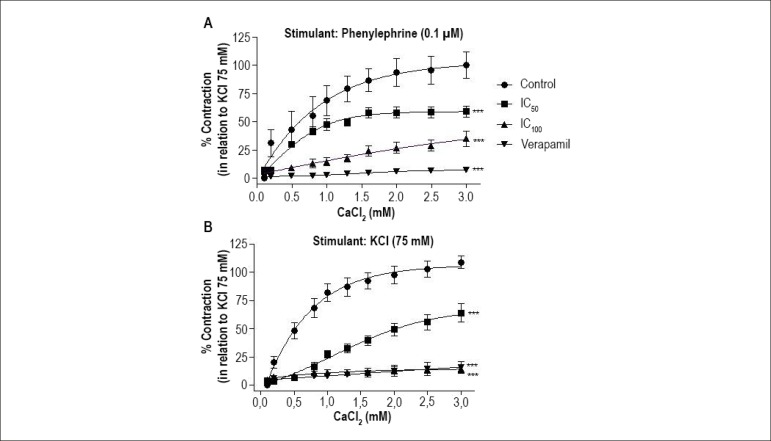
Regarding the Ca2+-influx-induced contraction stimulated by phenylephrine, pre-incubation with JHE (IC50 or IC100) significantly reduced (p < 0.001) the Emax values from 106.8% ± 7.5% (n = 5) to 58.8% ± 4.9% (n = 6) and 34.5% ± 3.2% (n = 6), respectively. In addition, treatment with verapamil significantly reduced (p < 0.001) the contraction to 7.1% ± 1.1% (n = 5) (Figure 3A).
Figure 3.
Effect of jabuticaba hydroalcoholic extract (JHE) and verapamil on the contractile response in isolated endothelium-denuded arteries. Cumulative concentration-response curves for CaCl2 were stimulated with phenylephrine (0.1µM) (A) or KCl 75 mM (B) in control conditions and after incubation (20 min) with JHE (IC50: 0.51 or IC100: 1.92 mg/mL) or verapamil (IC50: 0.3 µM). The points represent mean ± SEM of the contractile effect expressed as % contraction in relation to total KCl-induced contraction (75 mM). Significant difference: *** p<0.001 vs. Control.
Regarding the Ca2+-influx-induced contraction stimulated by KCl (75 mM), pre-incubation with JHE (IC50 or IC100) significantly reduced (p < 0.001) the Emax values from 108.8% ± 4.3% (n = 5) to 63.8% ± 6.1% (n = 6) and 14.6% ± 1.9% (n=6), respectively. In addition, treatment with verapamil significantly reduced (p < 0.001) the contraction to 15.5% ± 1.1% (n=6) (Figure 3B).
Effect of reticular Ca2+ ATPase inhibitor, CPA, and K+-channel blockers on JHE- induced relaxation
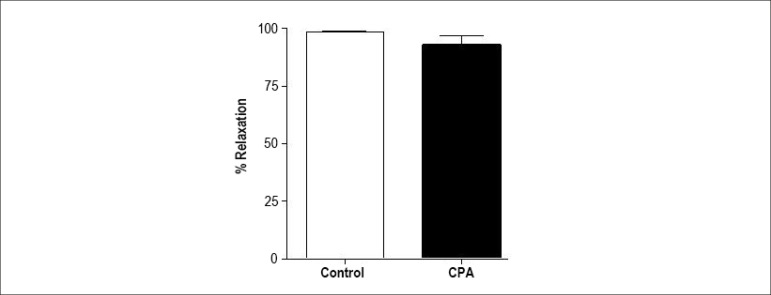
Treatment with CPA did not change the JHE-induced relaxation (93.8% ± 4.6%, n = 6) in isolated arteries (Figure 4). Thus, JHE did not change the inner Ca2+ uptake by the sarcoplasmic reticulum to induce vascular relaxation.
Figure 4.
Maximum relaxant effect induced by jabuticaba hydroalcoholic extract (JHE) in isolated arteries pre-contracted with phenylephrine (0.1 µM) in the absence or presence (20 min) of the reticular Ca2+ ATPase inhibitor, cyclopiazonic acid (CPA, 10 µM). The bars represent mean ± SEM of the maximum relaxant effect expressed as % relaxation.
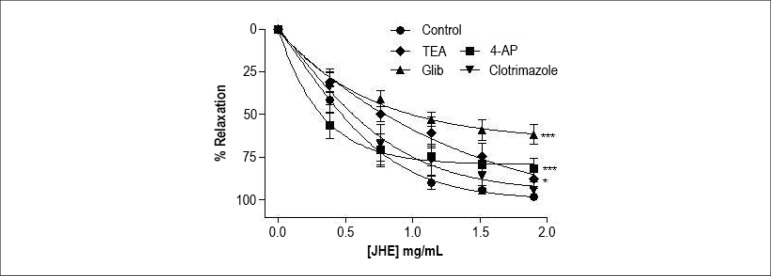
As shown in figure 5, except for clotrimazole (94.1% ± 4.5%, n = 5), K+-channel blockers changed the JHE-stimulated relaxation. The JHE-induced relaxation (Emax: 98.3% ± 0.4%, n = 6) was significantly (p < 0.05) reduced by TEA (Emax: 87.6% ± 5.7%, n=5), glibenclamide (Emax: 61.6% ± 5.8%, n = 6) and 4-AP (Emax: 81.6% ± 5.9%, n = 5). The results showed that JHE-induced relaxation depends on K+ efflux through the membrane.
Figure 5.
Effects of K+ channel blockers on the relaxation induced by jabuticaba hydroalcoholic extract (JHE) in isolated arteries pre-contracted with phenylephrine (0.1 µM) in the absence or presence (20 min) of the blockers tetraethyl-ammonium (TEA, 1 mM), glibenclamide (Glib, 3 µM), clotrimazole (5 µM) and 4-aminopyridine (4-AP, 1 mM). The points represent mean ± SEM of the relaxant effect expressed as % relaxation. Significant difference: *p < 0.05; *** p < 0.001 vs. Control
Discussion
The major finding of this study is that JHE, in addition to having a hypotensive effect and inducing vascular relaxation through endothelial nitric oxide pathway, as shown by our team,14 acts directly on VSM and leads to endothelium-independent relaxation. Therefore, jabuticaba clearly has cardiovascular effects through multiple endothelium-dependent and independent pathways. It is worth noting that the JHE concentration capable of inducing 100% vascular relaxation through the endothelial pathway is approximately 16 times lower (0.12 mg/mL)14 than the JHE concentration necessary to induce 100% relaxation acting directly on VSM (1.92 mg/mL).
Blood vessel contraction and relaxation in response to physiological demands are controlled by changes in intracellular Ca2+ concentration of VSM. The Ca2+ used for contraction includes intracellular or extracellular sources, or both. Sarcoplasmic reticulum is the major source of intracellular Ca2+.16 Our experiments showed that JHE does not change Ca2+ uptake by the sarcoplasmic reticulum, because its selective inhibitor, CPA, did not change the relaxation profile.
Voltage-gated Ca2+ channels (VGCC), also known as L-type Ca2+ channels, and receptor-operated Ca2+ channels (ROCC) located on the plasma membrane of VSM cells play a fundamental role in controlling Ca2+ influx.17,18 Phenylephrine-induced contraction is mediated by Ca2+ influx increase via VGCC and ROCC.19,20 However, contraction induced by membrane depolarization, such as in high KCl concentrations, activates preferentially VGCC.21 The results of the present study show that treating arteries with JHE inhibits the vascular contraction induced by the adrenergic stimulus with phenylephrine, suggesting that JHE blocks Ca2+ influx by interfering with VGCC and/or ROCC.
In an attempt to clarify the cell mechanism through which JHE induces vascular relaxation, experiments were performed in a Ca2+-free solution. Two different stimuli, phenylephrine and KCl (75 mM), were used to induce Ca2+ influx. The JHE, as well as verapamil, used as a positive control, inhibited the Ca2+-influx-induced contraction mediated by both stimuli. Because membrane depolarization with high concentrations of K+ activates specifically VGCC, we suggest that JHE acts directly or indirectly by blocking Ca2+ influx through the plasma membrane, acting preferentially on VGCC.
Natural products have constantly shown the involvement of K+ channels in their vasodilating mechanism.22 Several types of K+ channels, such as ATP-sensitive K+ channels (KATP), Ca2+-dependent K+ channels (Kca) and voltage-gated K+ channels (Kv), are present in VSM.23,24 Those channels can be blocked by glibenclamide, clotrimazole and 4-AP, respectively.24,25 Tetraethyl-ammonium is a non-selective blocker of those channels. When activated, those channels allow K+ efflux, hyperpolarizing the VSM plasma membrane. This reduces Ca2+ influx through the VGCC and induces vasodilation.26,27 The present study shows that JHE-induced relaxation in endothelium-denuded arteries is hindered after K+ channel blockade. Except for clotrimazole, the other blockers hindered vascular relaxation, allowing relating its activation to the JHE effect.
Our results point to a new biological effect of jabuticaba, a Brazilian native specimen that has important biological effects on the cardiovascular system, such as glucose-lowering,12 lipid-lowering13 and hypotensive effects.14 Thus, the biological jabuticaba-induced effects demonstrated in this study will contribute to increase the knowledge about jabuticaba-derived compounds and their use as medicinal plant or functional food to prevent cardiovascular problems.
Conclusion
This study shows that JHE induces endothelium-independent vasodilation. The major cellular pathways used by JHE to cause vascular relaxation are inhibition of the Ca2+-influx through plasma membrane, in addition to K+ channel activation in VSM cells.
Acknowledgements
This research was funded by the Goiás State Research Support Foundation (FAPEG), process 201310267000013. The Brazilian National Council for Scientific and Technological Development (CNPq) provided funding to support the Master's program of Daniela L. M. de Andrade.
Footnotes
Author contributions
Conception and design of the research: Andrade DML, Borges LL, Torres IMS, Conceição EC, Rocha ML; Acquisition of data: Andrade DML, Borges LL, Conceição EC, Rocha ML; Analysis and interpretation of the data: Andrade DML, Torres IMS, Conceição EC, Rocha ML; Statistical analysis: Andrade DML, Rocha ML; Obtaining financing: Rocha ML; Writing of the manuscript: Andrade DML, Torres IMS, Rocha ML; Critical revision of the manuscript for intellectual content: Andrade DML, Borges LL, Conceição EC, Rocha ML.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
This study was partially funded by FAPEG/GO.
Study Association
This article is part of the thesis of master submitted by Daniela Medeiros Lobo Andrade, from Faculdade de Farmácia - Universidade Federal de Goiás.
References
- 1.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. Erratum in: Lancet. 2013;381(9867):628, Lancet. 2013;381(9874):1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 3.Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- 4.Somlyo AV, Bond M, Somlyo AP, Scarpa A. Inositol trisphosphate-induced calcium release and contraction in vascular smooth muscle. Proc Natl Acad Sci USA. 1985;82(15):5231–5235. doi: 10.1073/pnas.82.15.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker EH. Ion channels and the control of blood pressure. Br J Clin Pharmacol. 2000;49(3):185–198. doi: 10.1046/j.1365-2125.2000.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenway F, Liu Z, Yu Y, Gupta A. A clinical trial testing the safety and efficacy of a Standardized Eucommia ulmoides oliver bark extract to treat hypertension. Altern Med Rev. 2011;16(4):338–347. [PubMed] [Google Scholar]
- 7.Chan P, Tomlinson B, Chen YJ, Liu JC, Hsieh MH, Cheng JT. A double-blind placebo-controlled study of the effectiveness and tolerability of oral stevioside in human hypertension. Br J Clin Pharmacol. 2000;50(3):215–220. doi: 10.1046/j.1365-2125.2000.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortes GA, Naves SS, Godoi FF, Duarte AR, Ferri PH, Santos SC. Assessment of a maturity index in jabuticaba fruit by the evaluation of phenolic compounds, essential oil components, sugar content and total acidity. Am J Food Technol. 2011;6(11):974–984. [Google Scholar]
- 9.Abe LT, Lajolo FM, Genovese MI. Potential dietary sources of ellagic acid and other antioxidants among fruits consumed in Brazil jabuticaba (Myrciaria jaboticaba (Vell.) Berg) J Sci Food Agric. 2012;92(8):1679–1687. doi: 10.1002/jsfa.5531. [DOI] [PubMed] [Google Scholar]
- 10.Giraldi M, Hanazaki N. Use and traditional knowledge of medicinal plants at Sertão do Ribeirão, Florianópolis, Santa Catarina State, Brazil. Acta Bot Bras. 2010;24(2):395–406. [Google Scholar]
- 11.Reynertson KA, Yang H, Jiang B, Basile MJ, Kennelly EJ. Quantitative analysis of antiradical phenolic constituents from fourteen edible Myrtaceae fruits. Food Chem. 2008;109(4):883–908. doi: 10.1016/j.foodchem.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dragano NR, Marques AY, Cintra DE, Solon C, Morari J, Leite-Legatti AV, et al. Freeze-dried jaboticaba peel powder improves insulin sensitivity in high-fat-fed mice. Br J Nutr. 2013;110(3):447–455. doi: 10.1017/S0007114512005090. [DOI] [PubMed] [Google Scholar]
- 13.Araújo CR, Esteves EA, Dessimoni-Pinto NA, Batista AG. Myrciaria cauliflora peel flour had a hypolipidemic effect in rats fed a moderately high-fat diet. J Med Food. 2014;17(2):262–267. doi: 10.1089/jmf.2012.0256. [DOI] [PubMed] [Google Scholar]
- 14.Lobo de Andrade DM, Reis CF, Castro PF, Borges LL, Amaral NO, Torres IM, et al. Vasorelaxant and Hypotensive Effects of Jaboticaba Fruit (Myrciaria cauliflora) Extract in Rats. Evid Based Complement Alternat Med. 2015;2015:696135–696135. doi: 10.1155/2015/696135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mole S, Waterman PG. A critical analysis of techniques for measuring tannins in ecological studies-II Techniques for biochemically defining tannins. Oecologia. 1987;72(1):148–156. doi: 10.1007/BF00385059. [DOI] [PubMed] [Google Scholar]
- 16.Thomas AP, Bird GS, Hajoczky G, Robb-Gaspers LD, Putney JW., Jr Spatial and temporal aspects of cellular calcium signaling. FASEB J. 1996;10(13):1505–1517. [PubMed] [Google Scholar]
- 17.Berridge MJ. Cell signalling a tale of two messengers. Nature. 1993;365(6445):388–389. doi: 10.1038/365388a0. [DOI] [PubMed] [Google Scholar]
- 18.Hess P. Calcium channels in vertebrate cells. Annu Rev Neurosci. 1990;13:337–356. doi: 10.1146/annurev.ne.13.030190.002005. [DOI] [PubMed] [Google Scholar]
- 19.Wesselman JP, VanBavel E, Pfaffendorf M, Spaan JA. Voltage-operated calcium channels are essential for the myogenic responsiveness of cannulated rat mesenteric small arteries. J Vasc Res. 1996;33:32–41. doi: 10.1159/000159129. [DOI] [PubMed] [Google Scholar]
- 20.Lee CH, Poburko D, Sahota P, Sandhu J, Ruehlmann DO, van Breemen C. The mechanism of phenylephrine-mediated [Ca(2+)](i) oscillations underlying tonic contraction in the rabbit inferior vena cava. J Physiol. 2001;534(3):641–650. doi: 10.1111/j.1469-7793.2001.t01-1-00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochegarov AA. Pharmacological modulators of voltage-gated calcium channels and their therapeutic application. Cell Calcium. 2003;33(3):145–162. doi: 10.1016/s0143-4160(02)00239-7. [DOI] [PubMed] [Google Scholar]
- 22.McNeill JR, Jurgens TM. A systematic review of mechanisms by which natural products of plant origin evoke vasodilatation. Can J Physiol Pharmacol. 2006;84(8-9):803–821. doi: 10.1139/y06-028. [DOI] [PubMed] [Google Scholar]
- 23.Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res. 2008;44(2):65–81. doi: 10.1540/jsmr.44.65. [DOI] [PubMed] [Google Scholar]
- 24.Ferrer M, Marin J, Encabo A, Alonso MJ, Balfagon G. Role of K+ channels and sodium pump in the vasodilation induced by acetylcholine, nitric oxide, and cyclic GMP in the rabbit aorta. Gen Pharmacol. 1999;33(1):35–41. doi: 10.1016/s0306-3623(98)00259-6. [DOI] [PubMed] [Google Scholar]
- 25.Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1 a potential immunosuppressant. Proc Natl Acad Sci USA. 2000;97(14):8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Pt 1Am J Physiol. 1995;268(4):C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 27.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]