Abstract
Objective
To evaluate the efficacy of vaginal progesterone administration for preventing preterm birth and perinatal morbidity and mortality in asymptomatic women with a singleton gestation and a mid‐trimester sonographic cervical length (CL) ≤ 25 mm.
Methods
This was an updated systematic review and meta‐analysis of randomized controlled trials comparing the use of vaginal progesterone to placebo/no treatment in women with a singleton gestation and a mid‐trimester sonographic CL ≤ 25 mm. Electronic databases, from their inception to May 2016, bibliographies and conference proceedings were searched. The primary outcome measure was preterm birth ≤ 34 weeks of gestation or fetal death. Two reviewers independently selected studies, assessed the risk of bias and extracted the data. Pooled relative risks (RRs) with 95% confidence intervals (CI) were calculated.
Results
Five trials involving 974 women were included. A meta‐analysis, including data from the OPPTIMUM study, showed that vaginal progesterone significantly decreased the risk of preterm birth ≤ 34 weeks of gestation or fetal death compared to placebo (18.1% vs 27.5%; RR, 0.66 (95% CI, 0.52–0.83); P = 0.0005; five studies; 974 women). Meta‐analyses of data from four trials (723 women) showed that vaginal progesterone administration was associated with a statistically significant reduction in the risk of preterm birth occurring at < 28 to < 36 gestational weeks (RRs from 0.51 to 0.79), respiratory distress syndrome (RR, 0.47 (95% CI, 0.27–0.81)), composite neonatal morbidity and mortality (RR, 0.59 (95% CI, 0.38–0.91)), birth weight < 1500 g (RR, 0.52 (95% CI, 0.34–0.81)) and admission to the neonatal intensive care unit (RR, 0.67 (95% CI, 0.50–0.91)). There were no significant differences in neurodevelopmental outcomes at 2 years of age between the vaginal progesterone and placebo groups.
Conclusion
This updated systematic review and meta‐analysis reaffirms that vaginal progesterone reduces the risk of preterm birth and neonatal morbidity and mortality in women with a singleton gestation and a mid‐trimester CL ≤ 25 mm, without any deleterious effects on neurodevelopmental outcome. Clinicians should continue to perform universal transvaginal CL screening at 18–24 weeks of gestation in women with a singleton gestation and to offer vaginal progesterone to those with a CL ≤ 25 mm. Published 2016. This article is a U.S. Government work and is in the public domain in the USA.
Keywords: cervical length, neonatal morbidity, neonatal mortality, prematurity, preterm delivery, progestins, progestogens, transvaginal ultrasound
Short abstract
 This article has been selected for Journal Club. Click here to view slides and discussion points.
This article has been selected for Journal Club. Click here to view slides and discussion points.
INTRODUCTION
In 2013, preterm birth was the leading cause of both neonatal mortality (35% of 2.8 million deaths) and child mortality (17% of 6.3 million deaths) worldwide1, 2. Neonates born preterm are at increased risk of both short‐term complications, attributed to immaturity of multiple organ systems3, 4, and long‐term adverse health outcomes, such as neurodevelopmental disabilities4, 5, behavioral problems3, 4, childhood asthma6, cardiovascular disease7, diabetes8 and depression9, in adult life. In addition, preterm birth is associated with a substantial economic cost and adverse psychosocial and emotional effects on families3, 4.
Preterm birth is a syndrome attributable to multiple pathological processes such as infection, vascular disorders, decidual senescence, uterine overdistension, a decline in progesterone action, cervical disease, breakdown of maternal‐fetal tolerance and stress, among others10, 11, 12. A short cervix, traditionally defined as a transvaginal sonographic cervical length (CL) ≤ 25 mm in the mid‐trimester of pregnancy, is an important risk factor for preterm birth and has emerged as one of the strongest and most consistent predictors of preterm birth in asymptomatic women with a singleton or twin gestation13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27.
In 2012, an individual patient data (IPD) meta‐analysis evaluated the efficacy and safety of vaginal progesterone administration for the prevention of preterm birth and neonatal morbidity and mortality in asymptomatic women with a sonographic short cervix (CL ≤ 25 mm) in the mid‐trimester28. A total of 723 women with a singleton gestation from four randomized controlled trials (RCTs) were included in the study. Overall, the administration of vaginal progesterone significantly reduced the risk of preterm birth occurring at < 28 to < 35 gestational weeks, as well as respiratory distress syndrome (RDS), composite neonatal morbidity and mortality, birth weight < 1500 g and admission to the neonatal intensive care unit (NICU). Since then, authors and professional organizations around the world have recommended the use of vaginal progesterone in patients with a singleton gestation and a short cervix in the mid‐trimester29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42. In addition, it has been suggested that the use of vaginal progesterone in pregnant women with a short cervix is one of the interventions that has contributed to the reduction in the rate of preterm birth in the USA in the last 7 years43.
Recently, the OPPTIMUM study44 tested the effect of vaginal progesterone in 1228 women at risk for preterm birth due to three major risk factors: (1) a history of spontaneous preterm birth; (2) a positive cervicovaginal fetal fibronectin test combined with other clinical risk factors for preterm birth; or (3) a sonographic short cervix (CL ≤ 25 mm). This double‐blind, placebo‐controlled trial reported that vaginal progesterone did not reduce the risk of preterm birth or neonatal morbidity and mortality in the entire population, or in the subgroup of women with a CL ≤ 25 mm, and has created confusion as to the efficacy of vaginal progesterone in reducing the rate of preterm birth in women with a short cervix45.
To address this issue, we updated the previous systematic review and meta‐analysis to quantify the efficacy of vaginal progesterone administration in preventing preterm birth and perinatal morbidity and mortality in asymptomatic women with a singleton gestation and a mid‐trimester sonographic CL ≤ 25 mm.
METHODS
This study followed a prospective protocol and is reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) statement46.
Data sources and searches
A literature search was carried out in MEDLINE, EMBASE, POPLINE, CINAHL and LILACS (all from inception to 31 May 2016), the Cochrane Central Register of Controlled Trials and Research Registers of ongoing trials, using a combination of keywords and text words related to progesterone (‘progesterone’, ‘progestins’, ‘progestogen’, ‘progestagen’, ‘progestational agent’), preterm birth (‘preterm’, ‘premature’) and randomized controlled trial (‘randomized controlled trial’, ‘controlled clinical trial’). Google Scholar, proceedings of congresses on obstetrics, maternal‐fetal medicine and ultrasound in obstetrics, reference lists of identified studies, previously published systematic reviews and review articles were also searched. In addition, we contacted investigators involved in the field to locate unpublished studies. No language restrictions were applied.
Study selection
We included RCTs in which asymptomatic women with a singleton gestation and a sonographic short cervix (CL ≤ 25 mm) in the mid‐trimester were allocated randomly to receive vaginal progesterone or placebo/no treatment for the prevention of preterm birth and/or adverse perinatal outcomes. Trials were included if the primary aim of the study was to prevent preterm birth in women with a short cervix, or to prevent preterm birth in women with risk factors other than a short cervix but for which outcomes were available for women with a pre‐randomization CL ≤ 25 mm. Exclusion criteria included quasirandomized trials, trials that evaluated vaginal progesterone in women with multiple gestation, preterm labor, arrested preterm labor (as maintenance tocolysis), premature rupture of membranes or second‐trimester bleeding, trials that assessed vaginal progesterone in the first trimester only to prevent miscarriage and studies that did not report clinical outcomes. Studies published as abstracts alone were excluded if additional information on methodological issues and results could not be obtained. When a study included women with singleton and multiple gestations it was not considered for inclusion in the review unless data for women with a singleton gestation were extractable separately.
All published studies deemed suitable were retrieved and reviewed independently by two authors to determine inclusion. Disagreements about inclusion were resolved through discussion.
Outcome measures
The primary outcome measure of interest was preterm birth ≤ 34 weeks of gestation or fetal death. Prespecified secondary outcome measures included preterm birth < 37, < 36, < 35, < 34, < 33, < 32, < 30 and < 28 weeks of gestation; spontaneous preterm birth < 34 weeks of gestation; RDS; necrotizing enterocolitis; intraventricular hemorrhage; proven neonatal sepsis; retinopathy of prematurity; fetal death; neonatal death; perinatal death; a composite outcome of neonatal morbidity and mortality (defined as the occurrence of any of the following events: RDS, intraventricular hemorrhage, necrotizing enterocolitis, proven neonatal sepsis or neonatal death); birth weight < 1500 g and < 2500 g; admission to NICU; use of mechanical ventilation; and long‐term neurodevelopmental outcomes.
Assessment of risk of bias
Two authors evaluated the risk of bias in each study included in the meta‐analysis using the Cochrane Collaboration tool for assessing risk of bias47. This tool assesses seven domains related to risk of bias (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias) and categorizes studies by low, unclear or high risk of bias in each domain. Discrepancies in the risk of bias assessment were resolved by consensus.
Data extraction
One investigator extracted the relevant data from eligible studies, which were then checked independently by another investigator. Information was extracted on study characteristics (randomization procedure, concealment allocation method, blinding of clinicians, women and outcome assessors, follow‐up period, completeness of outcome data for each outcome, including attrition and exclusions from the analysis, and intention‐to‐treat analysis), participants (inclusion and exclusion criteria, number of women in randomized groups, baseline characteristics and country and date of recruitment), details of intervention (aim, gestational age at trial entry, daily dose of vaginal progesterone, duration, compliance and use of co‐interventions) and outcomes (prespecified outcome measures, definition of outcome measures and the number of events and total number of participants in each group to calculate effect sizes).
We included additional data that had been obtained for four studies48, 49, 50, 51 included in our previous IPD meta‐analysis28. Corresponding authors of three RCTs identified in the new literature search were contacted by email to obtain additional data44, 52, 53. No author supplied additional data. Disagreements regarding data extraction were resolved by discussion among the authors.
Data synthesis
The data synthesis was performed according to the guidelines of the Cochrane Collaboration54. Outcomes were analyzed on an intention‐to‐treat basis. Results from different trials were combined to calculate pooled relative risk (RR) with 95% CIs for dichotomous outcomes. Heterogeneity of the results among studies was tested with the quantity I 2 statistic55. A substantial level of heterogeneity was defined as I 2 ≥ 50%54, 55. We pooled results from individual studies using a fixed‐effect model if substantial statistical heterogeneity was not present. If I 2 values were ≥ 50%, a random‐effects model was used to pool data across studies. The number needed to treat (NNT) for benefit or harm, with a 95% CI, was calculated for outcomes for which there was a statistically significant reduction or increase in risk difference based on control event rates in the trials56.
Subgroup analyses were planned to assess primary and secondary outcome measures according to several characteristics such as CL, obstetric history, maternal age, race/ethnicity, body mass index and daily dose of vaginal progesterone. However, the limited data reported in the OPPTIMUM study44 allowed only the performance of one subgroup analysis for the primary outcome measure according to the daily dose of vaginal progesterone. A test for interaction between the treatment and subgroups was calculated to examine whether treatment effects differ between subgroups57, 58. An interaction P‐value ≥ 0.05 was considered to indicate that the effect of treatment did not differ significantly between subgroups. We also planned to explore potential sources of heterogeneity and to assess publication and related biases if at least 10 studies were included in a meta‐analysis, but these analyses were not undertaken due to the small number of trials included in the review.
All statistical analyses were performed by using the Review Manager (RevMan; version 5.3.5; The Nordic Cochrane Centre, Copenhagen, Denmark) and StatsDirect (version 3.0.167; StatsDirect Ltd, Cheshire, UK) statistical packages.
This systematic review and meta‐analysis was exempted for review by the IRB Administration Office of Wayne State University because it involved the study of publicly available data sources and recorded data did not allow patient identification.
RESULTS
Selection, characteristics and risk of bias of studies
The searches produced 708 records, of which 11 were considered relevant. Figure 1 summarizes the process of identification and selection of studies. Six studies were excluded52, 53, 59, 60, 61, 62. Five of these studies assessed vaginal progesterone in women at high risk for preterm birth (previous preterm birth52, 59, 60, 61, uterine malformation52, 59, 61, cervical insufficiency or history of prophylactic cervical cerclage59, 61, uterine leiomyoma52, ‘short cervix’52 and pregnancies conceived by in‐vitro fertilization or intracytoplasmic sperm injection62), but none reported results according to CL at randomization. The remaining study evaluated vaginal progesterone in 80 Dutch women with a singleton pregnancy, no previous spontaneous preterm birth < 34 weeks of gestation and a CL ≤ 30 mm at 18–22 weeks, but did not report results for women with a CL ≤ 25 mm53. This study, which was stopped early due to low enrollment, reported that vaginal progesterone was associated with a non‐significant reduction in the risk of preterm birth < 34 weeks (RR, 0.81 (95% CI, 0.27–2.44)) and < 32 weeks (RR, 0.58 (95% CI, 0.14–2.30)), and composite neonatal morbidity and mortality (RR, 0.47 (95% CI, 0.09–2.40)). Five studies, including 974 women with a CL ≤ 25 mm, met the inclusion criteria44, 48, 49, 50, 51.
Figure 1.
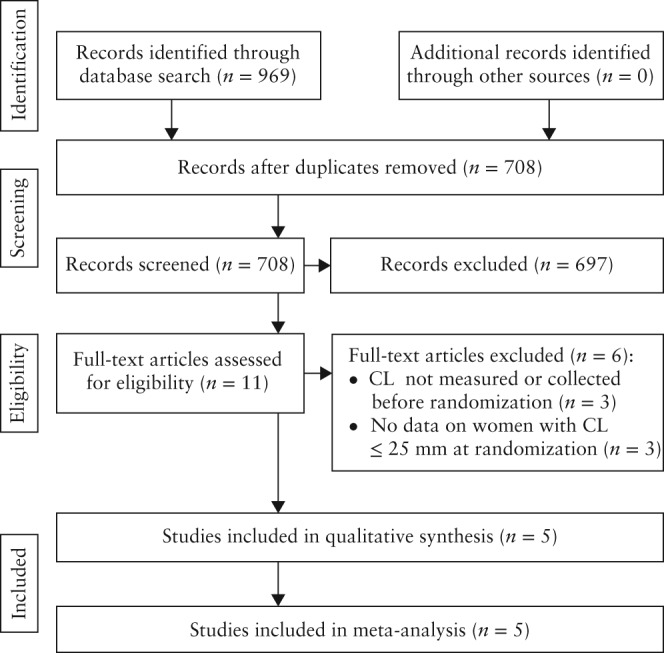
Study selection process.
The main characteristics of the studies included in the systematic review are summarized in Table 1. All studies were double‐blind, placebo‐controlled trials, of which four were multicenter, conducted in hospitals from both developed and developing countries. Two trials were specifically designed to evaluate the use of vaginal progesterone in women with a sonographic short cervix48, 51, one evaluated the use of vaginal progesterone in women with a history of spontaneous preterm birth49, one examined the use of vaginal progesterone in women with a prior spontaneous preterm birth, uterine malformation or twin pregnancy50 and the remaining trial tested the effect of vaginal progesterone in women at risk of preterm birth because of previous spontaneous preterm birth ≤ 34 weeks of gestation, or a sonographic CL ≤ 25 mm at 18–24 weeks, or a positive cervicovaginal fetal fibronectin test combined with other clinical risk factors for preterm birth44. The two studies48, 51 specifically designed to assess the administration of vaginal progesterone in women with a short cervix provided 70% of the total sample size of the meta‐analysis.
Table 1.
Characteristics of studies included in the systematic review
| Study | Country | Primary target population | Inclusion and exclusion criteria | Women with CL ≤ 25 mm (n) | Intervention | Primary outcome measure |
|---|---|---|---|---|---|---|
| Fonseca (2007)48 | UK, Chile, Brazil, Greece | Women with short cervix |
Inclusion: women with singleton or twin gestation and transvaginal sonographic CL ≤ 15 mm Exclusion: major fetal abnormality, painful regular uterine contractions, history of ruptured membranes or cervical cerclage |
226 | Vaginal progesterone capsule (200 mg/day) or placebo from 24 to 33 + 6 weeks | Spontaneous PTB < 34 weeks |
| O'Brien (2007)49 | USA, South Africa, India, Czech Republic, Chile, El Salvador | Women with history of spontaneous PTB |
Inclusion: women with singleton gestation between 16 + 0 and 22 + 6 weeks and history of spontaneous singleton PTB at 20–35 weeks in immediately preceding pregnancy Exclusion: planned cervical cerclage, history of adverse reaction to progesterone, treatment with progesterone within 4 weeks before enrollment, treatment for seizure disorder, psychiatric illness or chronic hypertension at time of enrollment, history of acute or chronic congestive heart failure, renal failure, uncontrolled diabetes mellitus, active liver disorder, HIV infection with CD4 count < 350 cells/mm3 and requiring multiple antiviral agents, placenta previa, history or suspicion of breast or genital tract malignancy, history or suspicion of thromboembolic disease, Müllerian duct anomaly, major fetal anomaly or chromosomal disorder or multiple gestation |
31 | Vaginal progesterone gel (90 mg/day) or placebo from 18–22 to 37 + 0 weeks, rupture of membranes or preterm delivery, whichever occurred first | PTB ≤ 32 + 0 weeks |
| Cetingoz (2011)50 | Turkey | Women at high risk of PTB |
Inclusion: women with at least one previous spontaneous PTB, uterine malformation or twin gestation Exclusion: in‐place or planned cervical cerclage or serious fetal anomaly |
8 | Vaginal progesterone suppository (100 mg/day) or placebo from 24 to 34 weeks | PTB < 37 weeks |
| Hassan (2011)51 | USA, Republic of Belarus, Chile, Czech Republic, India, Israel, Italy, Russia, South Africa, Ukraine | Women with short cervix |
Inclusion: women with singleton gestation between 19 + 0 and 23 + 6 weeks, transvaginal sonographic CL of 10–20 mm and without signs or symptoms of preterm labor Exclusion: planned cerclage, acute cervical dilation, allergic reaction to progesterone, current or recent progestogen treatment within previous 4 weeks, chronic medical conditions that would interfere with study participation or evaluation of the treatment, major fetal anomaly or known chromosomal abnormality, uterine anatomic malformation, vaginal bleeding, known or suspected clinical chorioamnionitis |
458 | Vaginal progesterone gel (90 mg/day) or placebo from 20–23 + 6 to 36 + 6 weeks, rupture of membranes or preterm delivery, whichever occurred first | PTB < 33 weeks |
| OPPTIMUM (2016)44 | UK, Sweden | Women at high risk of PTB |
Inclusion: women with singleton gestation and (1) previous spontaneous PTB ≤ 34 + 0 weeks; or (2) transvaginal sonographic CL ≤ 25 mm at 18–24 weeks; or (3) positive cervicovaginal fetal fibronectin test at 22–24 weeks plus history of previous PTB or second‐trimester loss, PPROM or cervical procedure to treat abnormal smear Exclusion: congenital structural or chromosomal fetal anomaly, known sensitivity, contraindications or intolerance to progesterone or any excipient, rupture of fetal membranes at time of recruitment or prescription or ingestion of medication known to interact with progesterone |
251 | Vaginal progesterone capsule (200 mg/day) or placebo from 22–24 to 34 weeks, or delivery | PTB ≤ 34 + 0 weeks or fetal death, composite outcome of neonatal death, bronchopulmonary dysplasia or brain injury assessed by neurosonography, and Bayley‐III cognitive composite score at 2 years of age |
CL, cervical length; HIV, human immunodeficiency virus; PPROM, preterm prelabor rupture of the membranes; PTB, preterm birth.
Two studies used vaginal progesterone capsules 200 mg/day44, 48, two used vaginal progesterone gel 90 mg/day49, 51 and the other used vaginal progesterone suppositories 100 mg/day50. Treatment was started at 24 weeks of gestation in two trials48, 50, between 18 and 22 weeks of gestation in one trial49, between 20 and 23 weeks of gestation in another51 and between 22 and 24 weeks of gestation in the remaining trial44. Three studies reported that participants received study medication from the time of enrollment until 34 weeks of gestation44, 48, 50, and two administered treatment from enrollment until 37 weeks of gestation49, 51. The primary outcome measure differed among studies: spontaneous preterm birth < 34 weeks48, preterm birth ≤ 32 + 0 weeks49, preterm birth < 37 weeks50 and preterm birth < 33 weeks51. The remaining study44 had three primary outcome measures: preterm birth ≤ 34 + 0 weeks or fetal death; a composite outcome of neonatal death, bronchopulmonary dysplasia or brain injury assessed by neurosonography; and the Bayley‐III cognitive composite score at 2 years of age.
Figure 2 shows the risk of bias in each included study. All studies were judged to be at low risk for selection (random sequence generation and allocation concealment), performance (blinding of patients and clinical staff) and detection (blinding of outcome assessment) biases. All but the OPPTIMUM study44 had low risk of attrition (incomplete outcome data), reporting (selective reporting) and other biases. The OPPTIMUM study44 was considered to be at high risk of attrition bias because information on the Bayley‐III cognitive composite score at 2 years of age, one of the primary outcome measures, was available for only ∼70% of children (869/1228 in the entire population and 179/256 in the subgroup of women with a CL ≤ 25 mm). High attrition rates may bias an observed effect, mainly if the rate of the outcome measure is relatively low, as was ‘moderate‐to‐severe neurodevelopment impairment’ in the entire population (10.5%). Information on the two other primary outcome measures was available for > 95% of participants (97% for the obstetric outcome and 96% for the neonatal outcome); thus, there was no evidence of attrition bias for these outcome measures. Moreover, this study was judged to be at high risk of reporting bias because the publication did not include results for key outcomes such as preterm birth < 37, < 32, and < 28 weeks of gestation, RDS, retinopathy of prematurity and birth weight < 1500 g and < 2500 g, among others. In addition, most primary and secondary outcome measures were incompletely reported for the three subgroups of women at risk of preterm birth so they cannot be included in the meta‐analyses. Finally, the OPPTIMUM study44 was at high risk of compliance bias because only 68.6% of women (66.3% in the vaginal progesterone group) used at least 80% of study medication in comparison to 93.6% in the study by Fonseca et al. 48 and 88.5% in the study by Hassan et al. 51. In RCTs, non‐compliance or non‐adherence can be one of the major barriers to achieving statistical power to detect intervention effects63.
Figure 2.
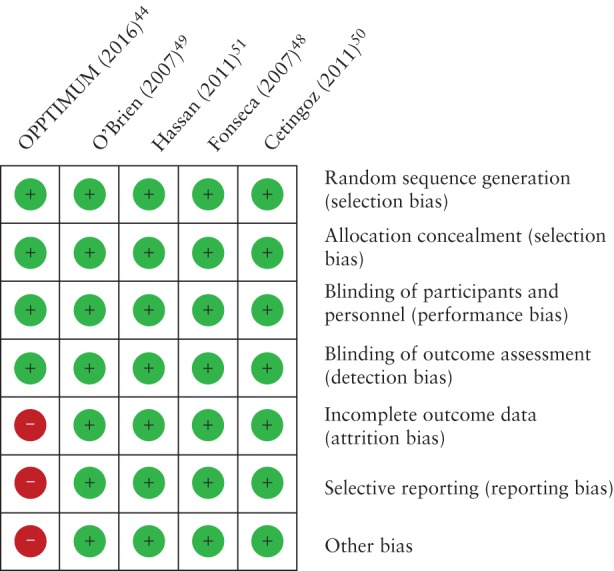
Risk of bias of studies included in the systematic review. Risk of bias:  , low;
, low;  , unclear;
, unclear;  , high.
, high.
Primary outcome
Vaginal progesterone administered to patients with a transvaginal sonographic short cervix was associated with a significant reduction in the risk of preterm birth ≤ 34 weeks of gestation or fetal death (18.1% vs 27.5%; RR, 0.66 (95% CI, 0.52–0.83); P = 0.0005; I 2 = 0%; five studies, 974 women; Table 2 and Figure 3). The number of patients needed to treat with vaginal progesterone to prevent one case of preterm birth ≤ 34 weeks of gestation or fetal death was 11 (95% CI, 8–21).
Table 2.
Effect of vaginal progesterone on the risk of preterm birth and adverse perinatal outcomes
| Outcome | Trials (n refs) | Events (n) Total(N) | Pooled RR (95% CI) | I 2 (%) | NNT (95% CI) | |
|---|---|---|---|---|---|---|
| Vaginal progesterone | Placebo | |||||
| Primary outcome | ||||||
| Preterm birth ≤ 34 weeks or fetal death | 544, 48, 49, 50, 51 | 90/498 | 131/476 | 0.66 (0.52–0.83) | 0 | 11 (8–21) |
| Secondary outcome | ||||||
| Preterm birth < 34 weeks | 448, 49, 50, 51 | 53/365 | 88/358 | 0.60 (0.44–0.82) | 0 | 10 (7–23) |
| Spontaneous preterm birth < 34 weeks | 448, 49, 50, 51 | 43/365 | 69/358 | 0.63 (0.44–0.88) | 0 | 14 (9–43) |
| Preterm birth < 37 weeks | 448, 49, 50, 51 | 127/365 | 141/358 | 0.89 (0.74–1.08) | 0 | — |
| Preterm birth < 36 weeks | 448, 49, 50, 51 | 93/365 | 117/358 | 0.79 (0.63–0.99) | 0 | 15 (8–306) |
| Preterm birth < 35 weeks | 448, 49, 50, 51 | 67/365 | 100/358 | 0.67 (0.51–0.87) | 0 | 11 (7–28) |
| Preterm birth < 33 weeks | 448, 49, 50, 51 | 41/365 | 72/358 | 0.56 (0.40–0.80) | 0 | 11 (8–25) |
| Preterm birth < 32 weeks | 448, 49, 50, 51 | 35/365 | 62/358 | 0.56 (0.38–0.82) | 0 | 13 (9–32) |
| Preterm birth < 30 weeks | 448, 49, 50, 51 | 27/365 | 46/358 | 0.59 (0.37–0.92) | 0 | 19 (12–97) |
| Preterm birth < 28 weeks | 448, 49, 50, 51 | 20/365 | 39/358 | 0.51 (0.31–0.85) | 0 | 19 (13–61) |
| Respiratory distress syndrome | 448, 49, 50, 51 | 17/365 | 37/358 | 0.47 (0.27–0.81) | 0 | 18 (13–51) |
| Necrotizing enterocolitis | 448, 49, 50, 51 | 5/365 | 6/358 | 0.88 (0.29–2.62) | 0 | — |
| Intraventricular hemorrhage | 448, 49, 50, 51 | 5/365 | 7/358 | 0.68 (0.22–2.13) | 0 | — |
| Proven neonatal sepsis | 448, 49, 50, 51 | 11/365 | 14/358 | 0.80 (0.37–1.74) | 0 | — |
| Retinopathy of prematurity | 448, 49, 50, 51 | 5/365 | 3/358 | 1.51 (0.40–5.69) | 0 | — |
| Fetal death | 448, 49, 50, 51 | 6/365 | 7/358 | 0.82 (0.28–2.40) | 0 | — |
| Neonatal death | 448, 49, 50, 51 | 6/365 | 11/358 | 0.53 (0.20–1.39) | 0 | — |
| Perinatal death | 448, 49, 50, 51 | 12/365 | 18/358 | 0.64 (0.31–1.31) | 0 | — |
| Composite neonatal morbidity/mortality* | 448, 49, 50, 51 | 29/365 | 49/358 | 0.59 (0.38–0.91) | 0 | 18 (12–81) |
| Birth weight < 1500 g | 448, 49, 50, 51 | 28/364 | 53/355 | 0.52 (0.34–0.81) | 0 | 14 (10–35) |
| Birth weight < 2500 g | 448, 49, 50, 51 | 102/364 | 117/355 | 0.86 (0.69–1.07) | 0 | — |
| Admission to NICU | 448, 49, 50, 51 | 59/365 | 87/358 | 0.67 (0.50–0.91) | 0 | 12 (8–46) |
| Mechanical ventilation | 448, 49, 50, 51 | 28/365 | 43/358 | 0.65 (0.41–1.01) | 0 | — |
Occurrence of any of the following events: respiratory distress syndrome; intraventricular hemorrhage; necrotizing enterocolitis; proven neonatal sepsis; neonatal death.
CI, confidence interval; NICU, neonatal intensive care unit; NNT, number needed to treat; RR, relative risk.
Figure 3.
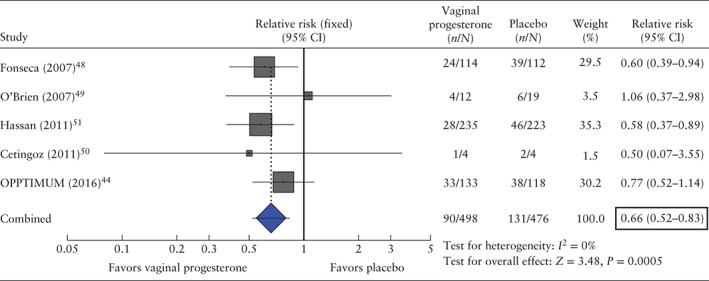
Forest plot of the effect of vaginal progesterone on the risk of preterm birth ≤ 34 weeks of gestation or fetal death.
A significant decrease in the risk of preterm birth ≤ 34 weeks of gestation or fetal death was found in women who received either 90–100 mg/day (RR, 0.62 (95% CI, 0.42–0.91); I 2 = 0%; three studies, 497 women) or 200 mg/day (RR, 0.69 (95% CI, 0.51–0.92); I 2 = 0%; two studies, 477 women) of vaginal progesterone. The interaction effect of vaginal progesterone based on daily dose was non‐significant (P = 0.65).
Secondary outcomes
All pooled estimates of the effects of vaginal progesterone on secondary outcome measures were obtained by the meta‐analysis of data from four trials48, 49, 50, 51 (Table 2). Treatment with vaginal progesterone was associated with a significantly lower risk of preterm birth < 36 weeks (RR, 0.79 (95% CI, 0.63–0.99)), < 35 weeks (RR, 0.67 (95% CI, 0.51–0.87)), < 34 weeks (RR, 0.60 (95% CI, 0.44–0.82)), < 33 weeks (RR, 0.56 (95% CI, 0.40–0.80)), < 32 weeks (RR, 0.56 (95% CI, 0.38–0.82)), < 30 weeks (RR, 0.59 (95% CI, 0.37–0.92)) and < 28 weeks (RR, 0.51 (95% CI, 0.31–0.85)), spontaneous preterm birth < 34 weeks (RR, 0.63 (95% CI, 0.44–0.88)); RDS (RR, 0.47 (95% CI, 0.27–0.81)); composite neonatal morbidity and mortality (RR, 0.59 (95% CI, 0.38–0.91)); birth weight < 1500 g (RR, 0.52 (95% CI, 0.34–0.81)) and admission to NICU (RR, 0.67 (95% CI, 0.50–0.91)). The NNT to prevent one case of preterm birth occurring at < 28 to < 36 gestational weeks or adverse neonatal outcomes varied from 10–19. There were no significant differences between the two groups for risk of preterm birth < 37 weeks of gestation, necrotizing enterocolitis, intraventricular hemorrhage, proven neonatal sepsis, retinopathy of prematurity, fetal death, neonatal death, perinatal death, birth weight < 2500 g and use of mechanical ventilation.
The OPPTIMUM study44 reported that infants whose mothers received vaginal progesterone had a non‐significant decreased risk of a composite outcome of neonatal death, bronchopulmonary dysplasia or brain injury (odds ratio, 0.54 (95% CI, 0.25–1.16); P = 0.113; 246 infants). The Bayley‐III cognitive composite scores at 2 years of age did not differ significantly between the vaginal progesterone and placebo groups (mean difference, –2.15 (95% CI, –7.23 to 2.93); P = 0.408; 179 children).
DISCUSSION
Principal findings
This updated systematic review and meta‐analysis, which includes data reported by the OPPTIMUM study44, shows that vaginal progesterone significantly decreases the risk of preterm birth ≤ 34 weeks of gestation or fetal death by 34%, among women with a singleton gestation and a mid‐trimester CL ≤ 25 mm. Clearly, the reduction in this composite outcome is attributable to a decrease in preterm birth ≤ 34 weeks of gestation rather than fetal death because vaginal progesterone had no effect on the risk of this adverse outcome in either the meta‐analysis of data from four studies (RR, 0.82 (95% CI, 0.28–2.40)) or in the OPPTIMUM study44 (RR, 1.14 (95% CI, 0.41–3.12) for the entire population). In addition, pooled estimates obtained by combining data from four trials indicate that vaginal progesterone administration was associated with a statistically significant reduction in the risk of preterm birth occurring at gestational ages of < 28 to < 36 weeks, RDS, composite neonatal morbidity and mortality, birth weight < 1500 g and admission to NICU.
Unfortunately, it was not possible to update most endpoints assessed in our previous meta‐analysis28 because the OPPTIMUM study44 did not report data for most adverse pregnancy and neonatal outcomes. It is noteworthy that the OPPTIMUM study44 was underpowered to detect a meaningful difference between vaginal progesterone and placebo in the subgroup of women with a CL ≤ 25 mm. Indeed, this study had a post‐hoc statistical power of only 26% to detect a 23% reduction in the risk of preterm birth ≤ 34 weeks of gestation or fetal death (from 32.2% in the placebo group to 24.8% in the vaginal progesterone group) and 33% to detect a 42% reduction in the risk of the composite outcome of neonatal morbidity and mortality (from ∼14% in the placebo group to ∼8% in the vaginal progesterone group) at an α‐level (two‐sided) of 0.05. Nonetheless, in this subpopulation, the OPPTIMUM study reported a non‐significant ∼42% reduction in the risk of neonatal death or serious neonatal morbidity, which is very similar to the 41% significant reduction in the risk of composite neonatal morbidity and mortality found in the meta‐analysis of data from the other four trials48, 49, 50, 51.
To explore the consequences of the lack of data in the OPPTIMUM study publication44, we performed several simulated meta‐analyses by using denominators of vaginal progesterone and placebo groups in the subgroup of women with a CL ≤ 25 mm reported in this study. In summary, we found that the statistically significant beneficial effects of vaginal progesterone administration on the risk of preterm birth < 35, < 33, < 32, < 30, and < 28 weeks of gestation, RDS, composite neonatal morbidity and mortality, birth weight < 1500 g and admission to NICU obtained in the meta‐analyses of data from four trials could only become non‐statistically significant if the rates of these adverse outcomes in the OPPTIMUM study44 were higher in the vaginal progesterone group than in the placebo group, and the RRs were > 1.12 for most of these outcomes. This hypothetical scenario is unlikely, given that the OPPTIMUM study44 showed a clear trend towards reduction in the risk of preterm birth ≤ 34 weeks of gestation (23%) and neonatal death or serious neonatal morbidity (∼42%) associated with the use of vaginal progesterone.
With regard to the effect of vaginal progesterone on the risk of adverse neurodevelopmental outcomes, the OPPTIMUM study44 found that there were no significant differences in the mean Bayley‐III cognitive composite scores or rates of neurodevelopmental impairment at 2 years of age between children exposed in utero to vaginal progesterone and those exposed to placebo. Similar findings were reported by O'Brien et al.64 who assessed neurodevelopmental outcomes among children born to women enrolled in their trial49 using the Denver II Developmental Screening Test at 6 months of age (445 children), 12 months of age (389 children) and 24 months of age (293 children). There was no significant difference in the rate of suspected developmental delay at any time during the 24‐month follow‐up between the vaginal progesterone and placebo groups. These findings are in accordance with those reported in children whose mothers participated in RCTs of vaginal progesterone vs placebo for the prevention of preterm birth in twin gestation65, 66. Rode et al.65 reported that the mean Ages and Stages Questionnaire scores (a tool that measures neurodevelopmental disability) at 6 months (1050 children) and 18 months (991 children) of age were not significantly different between the two groups, whereas McNamara et al.66 reported that there was no significant difference in neurodevelopmental outcomes (assessed using the Child Development Inventory tool) between twins in the vaginal progesterone and placebo groups at 3–6 years of age (759 children). In conclusion, the current available evidence suggests that in‐utero exposure to vaginal progesterone has no impact on neurodevelopmental outcomes at least until 2 years of age, and possibly until 6 years of age.
Strengths and limitations
The reliability and robustness of the results obtained in this updated review are supported by: (1) the use of the most rigorous methodology for performing a systematic review and meta‐analysis of RCTs; (2) the extensive literature searches without language restrictions; (3) the strict assessment of methodological quality of included trials based on widely recommended criteria; (4) the quantitative way of summarizing the evidence; (5) the evidence of clinical and statistical homogeneity in the meta‐analyses of all outcome measures evaluated; (6) the relatively narrow CIs obtained that made our estimates of effect size more precise; and (7) the subgroup analysis that did not show any significant influence of daily dose of vaginal progesterone on effect size. The main limitation of our study was the lack of data on several secondary outcome measures that were not reported in the OPPTIMUM study publication44. However, as mentioned previously, it is very unlikely that the significant beneficial effects of vaginal progesterone on the risk of preterm birth and neonatal morbidity and mortality become non‐significant after the inclusion of data from this study in the meta‐analyses.
Implications for practice and research
Evidence from this updated meta‐analysis reaffirms that vaginal progesterone reduces the risk of preterm birth ≤ 34 weeks of gestation in women with a singleton gestation and a mid‐trimester CL ≤ 25 mm. Therefore, clinicians should continue to perform universal transvaginal CL screening at 18–24 weeks of gestation in women with a singleton gestation, and to offer vaginal progesterone to those with a CL ≤ 25 mm, regardless of their history of spontaneous preterm birth, with the goal of preventing preterm birth and reducing neonatal morbidity and mortality. This recommendation is buttressed by the safety margin of vaginal progesterone44, 64, 65, 66 and the cost‐effectiveness of the intervention67, 68, 69, 70, 71, 72, 73, 74. We believe that an IPD meta‐analysis including data from the OPPTIMUM trial44 and the Dutch study53 is warranted to enable a more rigorous analysis and the performance of several subgroup analyses. We have invited the investigators of these trials to participate in such a study.
ACKNOWLEDGMENT
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services.
Disclosures
J.M.O'B. was involved in studies of progesterone gel treatment for preterm birth prevention sponsored by a maker of progesterone gel. He served on Advisory Boards and as Consultant for Watson Pharmaceuticals, a company with a financial interest in marketing vaginal progesterone gel for preterm birth prevention; he and others are listed in a patent on the use of progesterone compounds to prevent preterm birth (USA Patent Number 7884093: Progesterone for the Treatment and Prevention of Spontaneous Preterm Birth). G.W.C. was an employee of Columbia Laboratories, Inc. when the previous meta‐analysis was conducted.
 This article has been selected for Journal Club. Click here to view slides and discussion points.
This article has been selected for Journal Club. Click here to view slides and discussion points.
The copyright line for this article was changed on 5 September 2016 after original online publication.
REFERENCES
- 1. United Nations Children's Fund (UNICEF) . Committing to child survival: a promise renewed. Progress report 2014. New York, NY: UNICEF; 2014. [Google Scholar]
- 2. United Nations Inter‐agency Group for Child Mortality Estimation (UN IGME) . Levels & trends in child mortality. Report 2014. New York, NY: UNICEF; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008; 371: 261–269. [DOI] [PubMed] [Google Scholar]
- 4. Institute of Medicine US Committee on Understanding Premature Birth and Assuring Healthy Outcomes . Behrman RE, Butler AS, eds. Preterm birth: causes, consequences, and prevention. Washington, DC: National Academies Press; 2007. [PubMed] [Google Scholar]
- 5. Mwaniki MK, Atieno M, Lawn JE, Newton CR. Long‐term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet 2012; 379: 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Been JV, Lugtenberg MJ, Smets E, van Schayck CP, Kramer BW, Mommers M, Sheikh A. Preterm birth and childhood wheezing disorders: a systematic review and meta‐analysis. PLoS Med 2014; 11: e1001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parkinson JR, Hyde MJ, Gale C, Santhakumaran S, Modi N. Preterm birth and the metabolic syndrome in adult life: a systematic review and meta‐analysis. Pediatrics 2013; 131: e1240–1263. [DOI] [PubMed] [Google Scholar]
- 8. Li S, Zhang M, Tian H, Liu Z, Yin X, Xi B. Preterm birth and risk of type 1 and type 2 diabetes: systematic review and meta‐analysis. Obes Rev 2014; 15: 804–811. [DOI] [PubMed] [Google Scholar]
- 9. Loret de Mola C, de França GV, Quevedo L de A, Horta BL. Low birth weight, preterm birth and small for gestational age association with adult depression: systematic review and meta‐analysis. Br J Psychiatry 2014; 205: 340–347. [DOI] [PubMed] [Google Scholar]
- 10. Romero R. Prenatal medicine: the child is the father of the man. 1996. J Matern Fetal Neonatal Med 2009; 22: 636–639. [DOI] [PubMed] [Google Scholar]
- 11. Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG 2006; 113: 17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014; 345: 760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andersen HF, Nugent CE, Wanty SD, Hayashi RH. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol 1990; 163: 859–867. [DOI] [PubMed] [Google Scholar]
- 14. Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, Thom E, McNellis D, Copper RL, Johnson F, Roberts JM. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med 1996; 334: 567–572. [DOI] [PubMed] [Google Scholar]
- 15. Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol 1998; 12: 312–317. [DOI] [PubMed] [Google Scholar]
- 16. Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC, Wolfe HM. Patients with an ultrasonographic cervical length < or = 15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol 2000; 182: 1458–1467. [DOI] [PubMed] [Google Scholar]
- 17. Owen J, Yost N, Berghella V, Thom E, Swain M, Dildy GA 3rd, Miodovnik M, Langer O, Sibai B, McNellis D; National Institute of Child Health and Human Development , Maternal‐Fetal Medicine Units Network. Mid‐trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA 2001; 286: 1340–1348. [DOI] [PubMed] [Google Scholar]
- 18. To MS, Skentou C, Liao AW, Cacho A, Nicolaides KH. Cervical length and funneling at 23 weeks of gestation in the prediction of spontaneous early preterm delivery. Ultrasound Obstet Gynecol 2001; 18: 200–203. [DOI] [PubMed] [Google Scholar]
- 19. Honest H, Bachmann LM, Coomarasamy A, Gupta JK, Kleijnen J, Khan KS. Accuracy of cervical transvaginal sonography in predicting preterm birth: a systematic review. Ultrasound Obstet Gynecol 2003; 22: 305–322. [DOI] [PubMed] [Google Scholar]
- 20. Crane JM, Hutchens D. Transvaginal sonographic measurement of cervical length to predict preterm birth in asymptomatic women at increased risk: a systematic review. Ultrasound Obstet Gynecol 2008; 31: 579–587. [DOI] [PubMed] [Google Scholar]
- 21. Conde‐Agudelo A, Romero R, Hassan SS, Yeo L. Transvaginal sonographic cervical length for the prediction of spontaneous preterm birth in twin pregnancies: a systematic review and metaanalysis. Am J Obstet Gynecol 2010; 203: 128.e1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lim AC, Hegeman MA, Huis In 'T Veld MA, Opmeer BC, Bruinse HW, Mol BW. Cervical length measurement for the prediction of preterm birth in multiple pregnancies: a systematic review and bivariate meta‐analysis. Ultrasound Obstet Gynecol 2011; 38: 10–17. [DOI] [PubMed] [Google Scholar]
- 23. Barros‐Silva J, Pedrosa AC, Matias A. Sonographic measurement of cervical length as a predictor of preterm delivery: a systematic review. J Perinat Med 2014; 42: 281–293. [DOI] [PubMed] [Google Scholar]
- 24. Conde‐Agudelo A, Romero R. Prediction of preterm birth in twin gestations using biophysical and biochemical tests. Am J Obstet Gynecol 2014; 211: 583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Q, Reeves M, Owen J, Keith LG. Precocious cervical ripening as a screening target to predict spontaneous preterm delivery among asymptomatic singleton pregnancies: a systematic review. Am J Obstet Gynecol 2015; 212: 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kindinger LM, Poon LC, Cacciatore S, MacIntyre DA, Fox NS, Schuit E, Mol BW, Liem S, Lim AC, Serra V, Perales A, Hermans F, Darzi A, Bennett P, Nicolaides KH, Teoh TG. The effect of gestational age at cervical length measurements in the prediction of spontaneous preterm birth in twin pregnancies: an individual patient level meta‐analysis. BJOG 2016; 123: 877–884. [DOI] [PubMed] [Google Scholar]
- 27. Conde‐Agudelo A, Romero R. Predictive accuracy of changes in transvaginal sonographic cervical length over time for preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol 2015; 213: 789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Romero R, Nicolaides K, Conde‐Agudelo A, Tabor A, O'Brien JM, Cetingoz E, Da Fonseca E, Creasy GW, Klein K, Rode L, Soma‐Pillay P, Fusey S, Cam C, Alfirevic Z, Hassan SS. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol 2012; 206: 124.e1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Committee on Obstetric Practice . The American College of Obstetricians and Gynecologists. Committee opinion no. 522: incidentally detected short cervical length. Obstet Gynecol 2012; 119: 879‐882. [DOI] [PubMed] [Google Scholar]
- 30. Society for Maternal‐Fetal Medicine Publications Committee, with assistance of Vincenzo Berghella . Progesterone and preterm birth prevention: translating clinical trials data into clinical practice. Am J Obstet Gynecol 2012; 206: 376–386. [DOI] [PubMed] [Google Scholar]
- 31. Committee on Practice Bulletins—Obstetrics . The American College of Obstetricians and Gynecologists. Practice bulletin no. 130: prediction and prevention of preterm birth. Obstet Gynecol 2012; 120: 964–973. [DOI] [PubMed] [Google Scholar]
- 32. Romero R, Yeo L, Miranda J, Hassan SS, Conde‐Agudelo A, Chaiworapongsa T. A blueprint for the prevention of preterm birth: vaginal progesterone in women with a short cervix. J Perinat Med 2013; 41: 27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iams JD. Clinical practice. Prevention of preterm parturition. N Engl J Med 2014; 370: 254–261. [DOI] [PubMed] [Google Scholar]
- 34. Romero R, Yeo L, Chaemsaithong P, Chaiworapongsa T, Hassan SS. Progesterone to prevent spontaneous preterm birth. Semin Fetal Neonatal Med 2014; 19: 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. FIGO Working Group On Best Practice In Maternal‐Fetal Medicine; International Federation of Gynecology and Obstetrics . Best practice in maternal‐fetal medicine. Int J Gynaecol Obstet 2015; 128: 80–82. [DOI] [PubMed] [Google Scholar]
- 36. Kuon RJ, Abele H, Berger R, Garnier Y, Maul H, Schleußner E, Rath W; Experts for the Prediction and Prevention of Preterm Birth (X4PB) – www.x4pb.de. Progesterone for Prevention of Preterm Birth‐‐Evidence‐based Indications [in German]. Z Geburtshilfe Neonatol 2015; 219: 125–135. [DOI] [PubMed] [Google Scholar]
- 37. Fuchs F, Senat MV. Progesterone and prevention of preterm birth [in French]. J Gynecol Obstet Biol Reprod (Paris) 2015; 44: 760–770. [DOI] [PubMed] [Google Scholar]
- 38. Khalifeh A, Berghella V. Universal cervical length screening in singleton gestations without a previous preterm birth: ten reasons why it should be implemented. Am J Obstet Gynecol 2016; 214: 603.e1–5. [DOI] [PubMed] [Google Scholar]
- 39. O'Brien JM, Lewis DF. Prevention of preterm birth with vaginal progesterone or 17‐alpha‐hydroxyprogesterone caproate: a critical examination of efficacy and safety. Am J Obstet Gynecol 2016; 214: 45–56. [DOI] [PubMed] [Google Scholar]
- 40. Conde‐Agudelo A, Romero R. Vaginal progesterone to prevent preterm birth in pregnant women with a sonographic short cervix: clinical and public health implications. Am J Obstet Gynecol 2016; 214: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goodnight W. Clinical application of progesterone for the prevention of preterm birth, 2016. Am J Perinatol 2016; 33: 253–257. [DOI] [PubMed] [Google Scholar]
- 42. McKay LA, Holford TR, Bracken MB. Re‐analysis of the PREGNANT trial confirms that vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix. Ultrasound Obstet Gynecol 2014; 43: 596–597. [DOI] [PubMed] [Google Scholar]
- 43. Schoen CN, Tabbah S, Iams JD, Caughey AB, Berghella V. Why the United States preterm birth rate is declining. Am J Obstet Gynecol 2015; 213: 175–180. [DOI] [PubMed] [Google Scholar]
- 44. Norman JE, Marlow N, Messow CM, Shennan A, Bennett PR, Thornton S, Robson SC, McConnachie A, Petrou S, Sebire NJ, Lavender T, Whyte S, Norrie J; OPPTIMUM study group . Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): a multicentre, randomised, double‐blind trial. Lancet 2016; 387: 2106–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vaginal Progesterone Does Not Prevent Preterm Birth . Medscape http://www.medscape.com/viewarticle/858675. [Accessed 9 February 2016].
- 46. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62: e1–34. [DOI] [PubMed] [Google Scholar]
- 47. Higgins JPT, Altman DG, Sterne JAC. Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. [Google Scholar]
- 48. Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH; Fetal Medicine Foundation Second Trimester Screening Group . Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med 2007; 357: 462–469. [DOI] [PubMed] [Google Scholar]
- 49. O'Brien JM, Adair CD, Lewis DF, Hall DR, Defranco EA, Fusey S, Soma‐Pillay P, Porter K, How H, Schackis R, Eller D, Trivedi Y, Vanburen G, Khandelwal M, Trofatter K, Vidyadhari D, Vijayaraghavan J, Weeks J, Dattel B, Newton E, Chazotte C, Valenzuela G, Calda P, Bsharat M, Creasy GW. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double‐blind, placebo‐controlled trial. Ultrasound Obstet Gynecol 2007; 30: 687–696. [DOI] [PubMed] [Google Scholar]
- 50. Cetingoz E, Cam C, Sakallı M, Karateke A, Celik C, Sancak A. Progesterone effects on preterm birth in high‐risk pregnancies: a randomized placebo‐controlled trial. Arch Gynecol Obstet 2011; 283: 423–429. [DOI] [PubMed] [Google Scholar]
- 51. Hassan SS, Romero R, Vidyadhari D, Fusey S, Baxter JK, Khandelwal M, Vijayaraghavan J, Trivedi Y, Soma‐Pillay P, Sambarey P, Dayal A, Potapov V, O'Brien J, Astakhov V, Yuzko O, Kinzler W, Dattel B, Sehdev H, Mazheika L, Manchulenko D, Gervasi MT, Sullivan L, Conde‐Agudelo A, Phillips JA, Creasy GW; PREGNANT Trial . Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double‐blind, placebo‐controlled trial. Ultrasound Obstet Gynecol 2011; 38: 18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Azargoon A, Ghorbani R, Aslebahar F. Vaginal progesterone effects for the prevention of preterm birth and neonatal complications in women at increased risk: A randomized placebo‐controlled double‐blind study. Int J Fertil Steril 2014; 8: 104. [PMC free article] [PubMed] [Google Scholar]
- 53. van Os MA, van der Ven AJ, Kleinrouweler CE, Schuit E, Kazemier BM, Verhoeven CJ, de Miranda E, van Wassenaer‐Leemhuis AG, Sikkema JM, Woiski MD, Bossuyt PM, Pajkrt E, de Groot CJ, Mol BW, Haak MC. Preventing preterm birth with progesterone in women with a short cervical length from a low‐risk population: a multicenter double‐blind placebo‐controlled randomized trial. Am J Perinatol 2015; 32: 993–1000. [DOI] [PubMed] [Google Scholar]
- 54. Deeks JJ, Higgins JPT, Altman DG. Analyzing data and undertaking meta‐analyses. In: Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. [Google Scholar]
- 55. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Altman DG. Confidence intervals for the number needed to treat. BMJ 1998; 317: 1309–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sun X, Ioannidis JP, Agoritsas T, Alba AC, Guyatt G. How to use a subgroup analysis: users' guide to the medical literature. JAMA 2014; 311: 405–411. [DOI] [PubMed] [Google Scholar]
- 58. Klebanoff MA. 17 Alpha‐hydroxyprogesterone caproate for preterm prevention: issues in subgroup analysis. Am J Obstet Gynecol 2016; 214: 306‐307. [DOI] [PubMed] [Google Scholar]
- 59. da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo‐controlled double‐blind study. Am J Obstet Gynecol 2003; 188: 419–424. [DOI] [PubMed] [Google Scholar]
- 60. Majhi P, Bagga R, Kalra J, Sharma M. Intravaginal use of natural micronised progesterone to prevent pre‐term birth: a randomised trial in India. J Obstet Gynaecol 2009; 29: 493–498. [DOI] [PubMed] [Google Scholar]
- 61. Akbari S, Birjandi M, Mohtasham N. Evaluation of the effect of progesterone on prevention of preterm delivery and its complications. Sci J Kurdistan Univ Med Sci 2009; 14:11–19. [Google Scholar]
- 62. Aboulghar MM, Aboulghar MA, Amin YM, Al‐Inany HG, Mansour RT, Serour GI. The use of vaginal natural progesterone for prevention of preterm birth in IVF/ICSI pregnancies. Reprod Biomed Online 2012; 25: 133–138. [DOI] [PubMed] [Google Scholar]
- 63. Jo B. Statistical power in randomized intervention studies with noncompliance. Psychol Methods 2002; 7: 178–193. [DOI] [PubMed] [Google Scholar]
- 64. O'Brien JM, Steichen JJ, Phillips JA, Creasy GW. Two year infant outcomes for children exposed to supplemental intravaginal progesterone gel in utero: secondary analysis of a multicenter, randomized, double‐blind, placebo‐controlled trial. Am J Obstet Gynecol 2012; 206: S223. [Google Scholar]
- 65. Rode L, Klein K, Nicolaides KH, Krampl‐Bettelheim E, Tabor A; Group PREDICT. Prevention of preterm delivery in twin gestations (PREDICT): a multicenter, randomized, placebo‐controlled trial on the effect of vaginal micronized progesterone. Ultrasound Obstet Gynecol 2011; 38: 272–280. [DOI] [PubMed] [Google Scholar]
- 66. McNamara HC, Wood R, Chalmers J, Marlow N, Norrie J, MacLennan G, McPherson G, Boachie C, Norman JE. STOPPIT Baby Follow‐up Study: the effect of prophylactic progesterone in twin pregnancy on childhood outcome. PLoS One 2015; 10: e0122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cahill AG, Odibo AO, Caughey AB, Stamilio DM, Hassan SS, Macones GA, Romero R. Universal cervical length screening and treatment with vaginal progesterone to prevent preterm birth: a decision and economic analysis. Am J Obstet Gynecol 2010; 202: 548.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Werner EF, Han CS, Pettker CM, Buhimschi CS, Copel JA, Funai EF, Thung SF. Universal cervical‐length screening to prevent preterm birth: a cost‐effectiveness analysis. Ultrasound Obstet Gynecol 2011; 38: 32–37. [DOI] [PubMed] [Google Scholar]
- 69. Brown S, Mozurkewich E. Cost analysis of universal cervical length screening and progesterone therapy in remote populations. Am J Obstet Gynecol 2014; 210: S201. [Google Scholar]
- 70. Fonseca EB, Nishikawa AM, Paladini L, Clark OAC. Cervical assessment with progesterone in the prevention of Preterm Birth: A strategy based on cost‐effectiveness. Value Health 2014; 17: A510. [DOI] [PubMed] [Google Scholar]
- 71. Pizzi LT, Seligman NS, Baxter JK, Jutkowitz E, Berghella V. Cost and cost effectiveness of vaginal progesterone gel in reducing preterm birth: an economic analysis of the PREGNANT trial. Pharmacoeconomics 2014; 32: 467–478. [DOI] [PubMed] [Google Scholar]
- 72. Eke A, Buras A, Drnec S, Woo J. Vaginal progesterone versus cervical cerclage for the prevention of preterm births in women with a sonographically short cervix ‐ a cost effectiveness and decision analysis. Am J Obstet Gynecol 2015; 212(Suppl): S367–S368. [Google Scholar]
- 73. Werner EF, Hamel MS, Orzechowski K, Berghella V, Thung SE. Cost‐effectiveness of transvaginal ultrasound cervical length screening in singletons without a prior preterm birth: an update. Am J Obstet Gynecol 2015; 213: 554.e1–6. [DOI] [PubMed] [Google Scholar]
- 74. Einerson BD, Grobman WA, Miller ES. Cost‐effectiveness of risk‐based screening for cervical length to prevent preterm birth. Am J Obstet Gynecol 2016. Feb 12. pii: S0002‐9378(16)00304‐5. doi: 10.1016/j.ajog.2016.01.192. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


