ABSTRACT
Amnesia is associated with impairments in relational memory, which is critically supported by the hippocampus. By adapting the transitivity paradigm, we previously showed that age‐related impairments in inference were mitigated when judgments could be predicated on known pairwise relations, however, such advantages were not observed in the adult‐onset amnesic case D.A. Here, we replicate and extend this finding in a developmental amnesic case (N.C.), who also shows impaired relational learning and transitive expression. Unlike D.A., N.C.'s damage affected the extended hippocampal system and diencephalic structures, and does not extend to neocortical areas that are affected in D.A. Critically, despite their differences in etiology and affected structures, N.C. and D.A. perform similarly on the task. N.C. showed intact pairwise knowledge, suggesting that he is able to use existing semantic information, but this semantic knowledge was insufficient to support transitive expression. The present results suggest a critical role for regions connected to the hippocampus and/or medial prefrontal cortex in inference beyond learning of pairwise relations. © 2016 The Authors Hippocampus Published by Wiley Periodicals, Inc.
Keywords: memory, transitivity, developmental amnesia, semantic memory
INTRODUCTION
Relational memory is a critical component of higher‐order cognitive functioning (Konkel et al., 2008; Moscovitch, 2008; Addis et al., 2011; Duff and Brown‐Schmidt, 2012; Cohen, 2015) and is sensitive to neurological disturbances and aging (Ryan et al., 2013; D'Angelo et al., in press). Relational memory impairments can lead to impairments in the ability to infer novel information indirectly from prior learning (Smith and Squire, 2005; Ryan et al., 2016). Impairments in the ability to make inferential judgments in novel situations have implications for social learning, as inference is a key component in problem solving and for guiding behavior in social interactions (Holyoak, 2012; Koscik and Tranel, 2012a, 2012b). Although amnesic cases can demonstrate intact prior knowledge of relations, it remains unclear whether such prior knowledge can be used to support novel inferences in amnesic cases.
Amnesia is often characterized as a pervasive impairment in the ability to learn novel, arbitrary relations among individual stimuli due to damage to the hippocampus and the extended hippocampal system (EHS) (Cohen and Eichenbaum, 1993; Aggleton and Brown, 1999; Ryan et al., 2000; Moses and Ryan, 2006). To date, relational memory impairments in amnesic cases have most often been examined using tasks that test relations that had been directly studied. For example, in the transverse patterning (TP) task (Spence, 1952; Rickard and Grafman, 1998), individuals are typically required to learn novel relations among three stimuli (A, B, C), where each stimulus wins in the context of one of the other stimuli and loses in the context of the remaining stimulus. Amnesic cases show intact performance on TP when the relations were learned prior to their neurological insult (Moses et al., 2008a), and are impaired on TP when the relations are arbitrary and must be learned in the confines of the experiment (Rickard and Grafman, 1998; Moses et al., 2008a; Ryan et al., 2013; D'Angelo et al., 2015). However, these impairments can be mitigated when learning of arbitrary relations is supported by a unitization strategy (Ryan et al., 2013; D'Angelo et al., 2015).
Inference performance has often been studied using the transitive inference (TI) task (Dusek and Eichenbaum, 1997), in which individuals first learn a series of pairwise relations (e.g., A wins over B, B wins over C, C wins over D, D wins over E), after which they are tested on novel pairs (e.g., B vs. D), which require inference of a hierarchy within the stimuli (A > B > C > D > E). Prior work has demonstrated impaired TI performance in human amnesic cases (Smith and Squire, 2005; Smith et al., 2014) and in hippocampal lesion studies with non‐human animals (Dusek and Eichenbaum, 1997). However, in TI, some stimuli are always rewarded (e.g., A), and other stimuli are never rewarded (e.g., E). As a result, the intervening stimuli can be biased through the associative strength of the end stimuli (Frank et al., 2003), such that performance can be guided by associative strength of individual stimuli rather than by relational knowledge of the hierarchy (Moses et al., 2008b, 2010a).
To examine the extent to which prior knowledge can be used to support inferential judgements in amnesia, we used a version of the transitivity task (Bunsey and Eichenbaum, 1996) adapted for use with humans (Ryan et al., 2016). In the transitivity task, participants are shown a sample stimulus (e.g., A) and two choice stimuli (e.g., B, X) and are asked to select the choice stimulus that “belongs” with the sample stimulus (e.g., A → B). Through training, participants learn the relations among pairs of stimuli that comprise two sets (e.g., A → B, B → C, C → D; W → X, X → Y, Y → Z). Inference can then be tested using novel pairs containing indirect relations that can only be solved when knowledge from trained pairs is bridged (e.g., A → C?Y?, where C is the correct inference). Critically, unlike TI, the problems are setup such that associative strength cannot be used to guide performance, as the associative strength among the choice stimuli is equated (e.g., C and Y were rewarded an equal number of times during the training phase). Our adaptation of the transitivity task includes multiple conditions that vary in terms of whether the items and/or relations among items are known a priori from accumulated semantic knowledge. Critically, one condition (known items/pairwise relations) allowed us to test the expression of novel inference in the context of previously known relations. In this condition, the pairwise “premise” relations in each set (e.g., A → B, B → C, C → D) were comprised of relations known from prior knowledge [e.g., ball of yarn (A) → knitted scarf (B), knitted scarf (B) → ice skates (C), ice skates (C) → baseball, and glove (D)], but critically, the novel inference trials were not comprised of semantically rich relations [e.g., ball of yarn (A) → ice skates (C)]. This condition was contrasted with a condition requiring novel inference following learning of arbitrary relations (known items/arbitrary relations).
Using these conditions, we recently examined whether prior knowledge could support inferential judgments in older adults (Ryan et al., 2016), as older adults also show impairments on TP (Driscoll et al., 2003; Ostreicher et al., 2010) and TI (Ryan et al., 2009; Moses et al., 2010b). Relative to younger adults, older adults showed impaired relational learning of premise pairs in the known items/arbitrary relations condition, but older adults had intact knowledge of relations that could be based on prior semantic knowledge (known items/pairwise relations condition). Critically, although older adults had impaired transitive expression when novel inferences had to be made across pairs of relations that were learned within the confines of the experiment, transitive expression was intact when novel inferences were made across pairs of relations that were pre‐experimentally known (known items/pairwise relations condition). In sum, older adults showed intact inference for novel problems when there was intact knowledge of the premise relations.
Our prior study (Ryan et al., 2016) also examined whether prior knowledge could support inference in an amnesic case, D.A., who has bilateral damage to his medial temporal lobe (MTL), including the hippocampus, as well as damage to the right ventromedial prefrontal cortex (vmPFC) and right anterior temporal lobe (ATL) (Rosenbaum et al., 2008). Like the older adults, D.A. showed impaired relational learning of novel premise pairs, but intact relational knowledge based on semantic memory. However, unlike older adults, D.A.'s inference performance was impaired regardless if the transitive expression was based on arbitrary relations that had to be learned within the confines of the experiment or was based on pre‐experimentally known relations. We previously hypothesized that D.A.'s inability to use prior knowledge to mitigate his deficit may be the result of his damage to the right ATL, an area previously implicated in the ability to use prior knowledge to scaffold new learning (Kan et al., 2009) and as the hub of semantic memory processing (Patterson et al., 2007). It is therefore possible that intact transitive expression can be achieved in other amnesic cases through bridging in semantic memory if the semantic network (including ATL) is relatively intact.
The present study is encouraged by recent neuroimaging studies that have investigated the role of the hippocampus in inferring new relations. During fMRI scanning, Schlichting et al. (2015) tested participants on an inference task similar to the transitivity paradigm used in the current study. The authors found a dissociation between anterior and posterior regions within the hippocampus, whereby anterior regions were associated with integration, while posterior regions were associated with maintaining distinct representations (see also Shohamy and Wagner, 2008; Collin et al., 2015). Using a similar task and MEG, Backus et al., (2016) found that hippocampal theta power at encoding predicted later inference, and found increased theta coherence between the hippocampus and medial PFC. These and other recent neuroimaging studies have highlighted a role for the hippocampus in inference, and in particular have highlighted how integration may occur at encoding and/or at test, depending on task demands (Zeithamova et al., 2012; see also Schlichting and Preston, 2015).
Although these neuroimaging studies provide insight into the regions and networks involved in relational learning and inference, they do not provide information regarding the critical role of these regions/networks. In particular, the findings from neuroimaging stand in stark contrast to a recent case study that reported impaired inference only in cases with vmPFC damage, but not in cases with unilateral hippocampal damage, when pairwise relations were known (Koscik and Tranel, 2012b). Therefore, to understand the critical role of the hippocampus and its extended network, case studies are required to test the predictions made by neuroimaging studies (see Rosenbaum et al., 2014). Given the predictions made by the fMRI work and our prior work with D.A. (Ryan et al., 2016), the present study examined whether D.A.'s impaired inference in the context of known pairwise relations would be replicated in a case of developmental amnesia, N.C. (D'Angelo et al., 2015). N.C. has damage to the EHS and diencephalic regions that does not include the hippocampus and vmPFC but that connects these regions. If, like D.A., N.C. shows impaired inference in the context of known pairwise relations, then it would suggest that deficits in binding and inference can occur when the hippocampal system is disconnected from the vmPFC, even in the absence of frank hippocampal damage. This finding would be consistent with the neuroimaging studies reviewed above (Schlichting et al., 2015). It would also suggest that D.A.'s impaired performance in this condition was not due to his additional right ATL damage. If it had been the case that N.C. were able to perform inference in the context of known pairwise relations, then this finding would be consistent with prior patient work showing intact inference in cases with MTL damage when the pairwise relations are known (Koscik and Tranel, 2012b).
This study was further motivated by the fact that N.C. is a developmental amnesic case, and thus there may be a greater chance of reorganization due to the early onset of the amnesia. Like other developmental amnesic cases, N.C. shows a dissociation of impaired episodic memory in the context of relatively spared semantic memory (Vargha‐Khadem et al., 1997; D'Angelo et al., 2015), which stands in contrast to adult‐acquired cases who typically show impaired semantic memory for learning that occurs after the onset of their amnesia (O'Kane et al., 2004; Bayley et al., 2008). One caveat is that the semantic learning observed in developmental amnesia occurs gradually over time and is typically not equivalent to the rapid learning observed in healthy adults (Gardiner et al., 2008) who can benefit from episodic encoding. Despite slowed semantic learning in developmental amnesia, there is some evidence that semantic knowledge can support other cognitive functions typically reliant on the hippocampal system, as the developmental amnesic case Jon was able to use semantic information to support “episodic” recall (Brandt et al., 2006). Jon's ability to use semantic learning to support other cognitive functions may be the result of plasticity and reorganization that can occur in developmental amnesia due to the early age of injury (Vargha‐Khadem et al., 2003).
In sum, the present study examined whether the findings of impaired inference observed in D.A. would also apply to a developmental amnesic person who has a greater chance for semantic reorganization. N.C.'s particular pattern of damage would also inform whether anteromedial thalamic damage is sufficient to lead to a similar pattern of impairment as observed in D.A., and whether the hippocampus and EHS are critical for inference. If N.C. shows impaired TI in the context of semantic pairwise knowledge, it would suggest that inference requires hippocampal binding even when pairwise knowledge is supported by existing semantic memory. The results of this study will further our understanding of the conditions under which strategies based on semantic knowledge can support other cognitive functions in amnesia. Moreover, the results of the present study would contribute to our understanding of the nature of inference itself.
METHODS
Amnesic Case
N.C. has previously been described in D'Angelo et al. (2015). Briefly, N.C. is a young, right‐handed man with 14 yr of education, including high school and 1 yr of technical college. He was aged 20 at the time of testing. N.C. experienced a thalamic stroke shortly after birth, which primarily affected the right mediodorsal nucleus, and partially affected the left mediodorsal nucleus and right anterior nucleus. N.C. also has reductions to his right fornix and his mammillary bodies, especially on the right. N.C.'s left lateral ventricle is larger than the right. He has white matter changes along the left lateral ventricle and also in the left temporal lobe, with the inferior longitudinal fasciculus most involved. See Figure 1 for MR scans highlighting N.C.'s damage.
Figure 1.
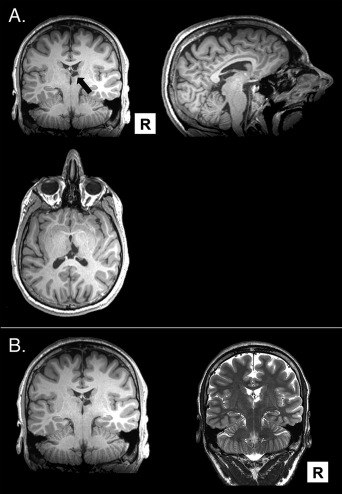
A. T1 weighted MRI scans of N.C., with arrow pointing to his right thalamic lesion. B. T1 weighted and T2 weighted MRI scans (left and right, respectively) showing that N.C.'s lesion shows slightly hypointense on the T1‐weighted image and hyperintense on the coronal T2‐weighted image. Figure from D'Angelo et al. (2015).
Neuropsychological Tests & Results
The results of an updated neuropsychological evaluation performed when NC was 20‐yr old are shown in Table 1. As described in D'Angelo et al. (2015), these results confirmed that in the context of average intelligence, N.C. was severely impaired on tests of delayed recall. N.C. also performed below expected levels on tests of working memory and more complex aspects of visuospatial processing (e.g., recognizing faces, integrating details into a complex figure). In contrast, his performance was largely intact on standard measures of semantic knowledge, language, processing speed, and executive function (see Table 1).
Table 1.
Neuropsychological Profile of N.C
| Test | Normed score |
|---|---|
| General Intelligence | |
| WAIS‐IV: Full Scale IQ (standard score)a | 94 |
| Verbal Comprehension Index | 101 |
| Perceptual Reasoning Index | 106 |
| Working Memory Index | 76b |
| Processing Speed Index | 91 |
| Semantic Knowledge | |
| WAIS‐IV Vocabulary (scaled score)a | 10 |
| Language Production | |
| Boston Naming Test (percentile)c | 39th |
| Semantic Fluency (animals) (z‐score)d | 1.47 |
| Anterograde Memory | |
| WMS‐IV Logical Memory | |
| Logical Memory I: Immediate recall (scaled score) | 7 |
| Logical Memory II: Delayed recall (scaled score) | 2b |
| Logical Memory II: Recognition (percentile) | 3–9thb |
| California Verbal Learning Test‐II | |
| Total trials 1–5 (t score) | 29b |
| Short delay free recall (z‐score) | −2.5b |
| Short delay cued recall (z‐score) | −1.5b |
| Long delay free recall (z‐score) | −2.5b |
| Long delay cued recall (z‐score) | −3b |
| Learning (z‐score) | −1.5b |
| Total intrusions (z‐score)e | 5b |
| Total repetitions (z‐score)e | 1.5b |
| Recognition (Hits) (z‐score) | 0.5 |
| Recognition (False Positives) (z‐score) | 3b |
| Discrimination | −1.5b |
| Rey‐Osterrieth complex figure (t score) | |
| Immediate recall | < 20b |
| Delayed recall | < 20b |
| Test Normed Score | |
| Processing Speed | |
| WASI‐IV Codinga | 7 |
| WASI‐IV Symbol Searcha | 10 |
| Visuospatial Function | |
| WAIS‐IV Block Designa | 13 |
| Rey‐Osterrieth Complex Design—Copy (percentile) | 11–16th |
| Judgment of Line Orientation (percentile) | 72nd |
| Benton Facial Recognition Test | Borderline |
| Working Memory | |
| WAIS‐IV Letter‐Number Sequencinga | 6 |
| WAIS‐IV Digit Spana | 5b |
| Attention and Executive Function | |
| Trail Making Test (z‐score)f | |
| Part A (sec) | −0.74 |
| Part B (sec) | −0.95 |
| Phonemic Fluency (FAS) (z‐score)d | 0.31 |
| WAIS‐IV Similarities (scaled score)a | 10 |
| WAIS‐IV Matrix Reasoning (scaled score)a | 11 |
Apparatus and Stimuli
The apparatus and stimuli were identical to those used in our prior work (Ryan et al., 2016). The experiment was programmed and run on a desktop computer connected to a 19‐inch monitor using E‐prime 1.1. N.C. responded using the keys “Q” and “P” on a standard keyboard. Each of the four stimulus conditions consisted of eight colored stimuli divided into two sets (see Fig. 2). The four stimulus conditions differed based on whether the objects and their relations were known before the experimental session.
Figure 2.
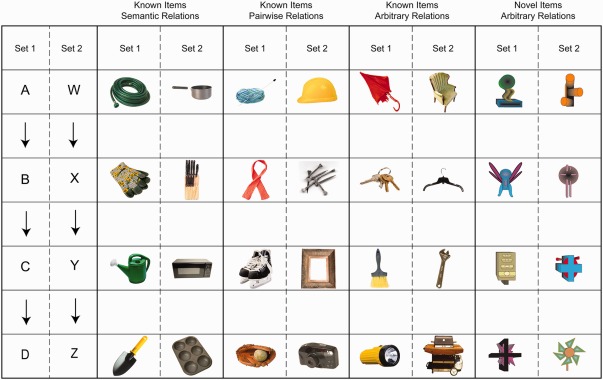
Stimuli used for the ABCD and WXYZ stimulus sets across the four experimental conditions. Figure taken from Ryan et al. (2016). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Known items/semantic relations (K‐S)
The K‐S condition consisted of two groups of known objects, whose relations within each group were also known before the experimental session. In this condition, one group of objects (A, B, C, and D) were all items typically found in a kitchen, while the other group of objects (W, X, Y, and Z) were all items used for gardening.
Known items/pairwise relations (K‐P)
The K‐P condition consisted of two groups of known objects where the relations among all of the items within each group were not known a priori, but in which consecutive pairs of items in each group had meaningful relations. For example in the first set of objects (A, B, C, and D), the yarn (object A) is meaningfully related to the scarf (object B), the scarf is related to the ice skates (object C), and the skates are related to the baseball glove (object D). However, there are no pre‐existing, commonly used, meaningful relations among the nonconsecutive items (A–C, A–D, B–D), such as between the yarn and the baseball glove, the yarn and the skates, or the scarf and the baseball glove. Similarly, in the second set (W, X, Y, Z), the construction hat (object W) is meaningfully related to the nails (object X), the nails are related to the picture frame (object Y), and the picture frame is related to the camera (object Z). Once again, although these pairwise relations exist among the consecutive items, there are no meaningful relations among nonconsecutive items (W–Y, W–Z, X–Z; the construction hat and the picture frame, the construction hat and the camera, or the nails and the camera).
Known items/arbitrary relations (K‐A)
The K‐A condition consisted of two sets of known objects, whose relations within each group were not known prior to the experimental session, and for which no overall or pairwise relations were known prior to training. For example, in one set the relations among an umbrella (object A), a set of keys (object B), a brush (object C), and a flashlight (object D) were learned and were separate from the relations among an armchair (object W), a hanger (object X), a wrench (object Y), and a barbeque (object Z). All objects in the known item conditions were common, nameable objects selected from the Hemera 3.01 database.
Novel items/arbitrary relations (N‐A)
The N‐A condition was identical to the known items/arbitrary relations condition, with the exception that eight novel, abstract objects were used as the stimuli. These objects were created using Corel Draw v.12.
Procedure
Each condition consisted of three training blocks followed by a no‐feedback test block, described below. N.C. was trained and tested on the four conditions over five separate sessions (see Table 2). The conditions were always administered in the following order: K‐S, K‐P, K‐A, and N‐A to ensure understanding of the task requirements. In Session 1, N.C. did not complete the test phase for the K‐A condition due to low performance in the training phase. Given his poor performance in the K‐A, the N‐A condition was not administered in Session 1. In Session 2, N.C. was trained and tested on all conditions. In Session 3, N.C. completed all conditions with the exception of N‐A, which was excluded due to time constraints. In Session 4, N.C. was trained and tested on all conditions, with the exception that he was not given the test phase for condition N‐A, due to low performance in the training phase. In Session 5, N.C. was trained and tested on all four conditions.
Table 2.
Overview of Conditions Completed by N.C. in Each Session
| Session | Known items/Semantic relations | Known items/Pairwise relations | Known items/Arbitrary relations | Novel items/Arbitrary relations |
|---|---|---|---|---|
| 1 | ✓ | ✓ | ✗ | – |
| 2 | ✓ | ✓ | ✓ | ✓ |
| 3 | ✓ | ✓ | ✓ | – |
| 4 | ✓ | ✓ | ✓ | ✗ |
| 5 | ✓ | ✓ | ✓ | ✓ |
✓= Training and test phases completed.
✗ = Failed training phase, test phase not administered.
– = Condition not administered.
Training phase
On every trial, N.C. was shown a problem set containing three items (see Fig. 3). A sample object was presented centrally on the upper half of the screen for 2 s on its own, after which two choice items appeared on the lower half of the screen, one on the left and one on the right. N.C. was instructed to pick the choice object that made a correct pairing with the sample object, using the left and right response keys to pick the left and right choice items, respectively. A happy cartoon face and the phrase “Correct!” was presented following correct responses, and an angry cartoon face and the phrase “Wrong!” was presented following incorrect responses. N.C. was required to learn the relations among the items through trial and error.
Figure 3.
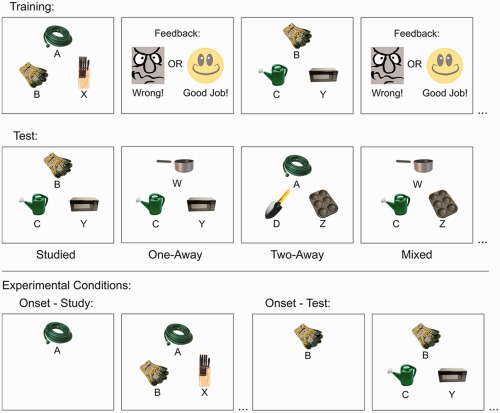
Depiction of the training and test sequences in the transitivity task. Trials were self‐paced and feedback was provided in the training phase only. The sample stimulus was presented at the top of the screen, and the two choice stimuli were presented along the bottom of the screen. Participants were required to select one of the two choice stimuli that ‘belonged’ with the sample stimulus. Test trials could depict previously studied relations (studied trials), or novel relations that had to be inferred across pairs of previously studied relations (novel probe pairs). Novel probe pairs include pairs in which the sample was separated from the choice items by one (1‐away), two (2‐away) or a mixture of one and two intervening items and their respective relations (mixed). Figure adapted from Ryan et al. (2016). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The training phase consisted of three blocks in which N.C. was trained on the relations among consecutive pairs of items in each set. In the first block, all of the samples were drawn from the first set of stimuli (A, B, C, D—see Fig. 1). Each problem set was presented consecutively and this sequence was repeated four times for a total of 12 trials (i.e., [A → B vs. X; B → C vs. Y; C → D vs. Z] x 4). The second block was identical to the first block with the exception that all of the samples were drawn from the second set of stimuli (W, X, Y, Z). In the third block, samples were drawn from both stimulus sets. Each problem set the two sets of stimuli were presented six times in the third block (i.e., six presentations of Set 1: [A → B vs. X; B → C vs. Y; C → D vs. Z] and Set 2: [W → X vs. A; X → Y vs. C; Y → Z vs. D]). Within each of the six presentations, the sequential order of the problem sets was maintained, but the order of Set 1 or Set 2 first was randomly selected. Therefore, learning always followed the same sequence, analogous to procedures used in animal work (Bunsey and Eichenbaum, 1996).
Training blocks advanced only if the criterion of 70% accuracy was achieved. If accuracy was less than 70%, the block repeated. Testing within a condition was terminated if a study block was repeated six times without criterion being reached.
Test phase
The test phase was identical to the training phase with the following exceptions. The test phase included four trial types: studied, one‐away, two‐away, and mixed. Studied trials were identical to those presented during training and tested the relations among consecutive items within each set. The remaining trial types tested N.C.'s ability to derive the relations among nonconsecutive items within each set. These novel probe trials included problem sets in which the sample was separated from the choice items by one (one‐away: A → C vs. Y, B → D vs. Z, W → C vs. Y, X → D vs. Z), two (two‐away: A → D vs. Z, W → D vs. Z), or a mixture of one and two intervening items and their respective relations (mixed: A → C vs. Z, A → D vs. Y, W → C vs. Z, W → D vs. Y). Each instance of each trial type presented six times (e.g., A → B vs. X was presented six times) for a total of 96 trials in the test phase. No feedback was given in the test phase.
In both the training and test phases the correct choice stimulus was counterbalanced such that it appeared an equal number of times on the left and right sides of the screen.
Healthy Controls
N.C.'s performance was contrasted with performance of the 36 healthy younger adults described in our prior work (Ryan et al., 2016). Briefly, 36 healthy younger adults (mean age = 23.1, SE = 0.5, range: 18–28) with no known pathology were recruited from the volunteer participant pool at the Rotman Research Institute at Baycrest. All participants gave informed written consent and participated in exchange for monetary compensation. This experiment received ethics approval from the Toronto Academic Health Science Council.
The procedure for the healthy controls was identical to the procedure for N.C. with the following exceptions. The first difference concerned whether the sample and choice stimuli were presented together during training and/or test. Twelve of the healthy controls completed the task with identical stimuli presentation as N.C.: the sample and choice stimuli were presented together both during training and test, as outlined above (sample alone for 2 s, after which the two choice stimuli were presented along with the sample until a response was made). This is the experiment version referred to as onset in our prior study. Twelve healthy controls saw the sample stimulus separately from the choice stimuli for both the training and test phases. In both phases, these participants were shown the sample stimulus presented alone for 2 s, followed by a blank screen for 3 s, and finally the two choice stimuli were presented until a response was made. This is the experiment version to as delay in our prior work. The remaining twelve healthy controls were shown the choice stimuli along with the sample stimulus during the training phase (as in the onset condition), but in the test phase the choice stimuli were presented separately from the sample stimulus (as in the delay condition). This is the experiment version referred to as onset + delay in our prior work. The sample and choice stimuli were presented together or separately during training and/or test to examine the role for the hippocampus in bridging information across time (Wallenstein et al., 1998; Bangasser et al., 2006). In our prior work, we found no consistent differences across the different experiment versions (Ryan et al., 2016), therefore N.C. was only trained on the onset condition.
Order of stimulus conditions and experiment version were counterbalanced across conditions in the healthy controls. N.C. was always given the task in the same order (K‐S, K‐P, K‐A, N‐A) in an effort to support his understanding of the task demands.
Analysis
Training accuracy was analyzed using a mixed‐effects repeated measures analysis of variance, where stimulus condition (K‐S, K‐P, K‐A, and N‐A) was included as a within‐subjects factor. Test accuracy was analyzed using a repeated measures analysis of variance, which included stimulus condition and trial type (studied, one‐away, two‐away, vs. mixed pairs) as within‐subject factors. To simplify the presentation of results, experiment version (onset, onset + delay, and delay) and stimulus condition presented first (K‐S, K‐P, K‐A, and N‐A) were not included in the present set of analyses. N.C. was not given either of these manipulations, and we found no consistent interactions of these variables with stimulus condition or trial type in our prior analyses (Ryan et al., 2016). The inclusion of these two variables in the present set of analyses showed the same pattern of results and the results of these analyses are available on request. N.C.'s performance was examined relative to healthy controls by contrasting his performance against the 95% confidence interval (CI) of healthy controls, as done in our prior work (Moses et al., 2008a; Ryan et al., 2013).
RESULTS
Training Phase—Healthy Controls
Accuracy in the training phase for the healthy controls and N.C. is plotted in Figure 4. The analysis of accuracy in the training phase revealed a main effect of stimulus condition (F(3,105) = 21.2, P < 0.001, ε = 0.70, = 0.38). As expected, accuracy was highest in the two conditions with semantic relations (M = 0.97, SE = 0.01 for both the K‐S and K‐P conditions), followed by the K‐A condition (M = 0.92, SE = 0.01) and was lowest in the N‐A condition (M = 0.89, SE = 0.02).
Figure 4.
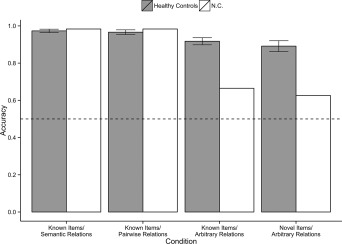
Mean accuracy in the training phase for healthy controls (from Ryan et al., 2016) and N.C. as a function of the four stimulus conditions. Error bars represent the 95% confidence interval (CI) of the mean accuracy in the healthy controls. The dotted line here and in all graphs represents chance performance (0.50).
Training Phase—N.C
N.C. performed similarly to healthy controls on the conditions when prior knowledge supported performance (K‐S: M = 0.99; K‐P: M = 0.98). In contrast to his high performance in the two conditions supported by prior knowledge, N.C. was impaired on the conditions where arbitrary relations had to be learned (K‐A: M = 0.64; N‐A: M = 0.61). N.C.'s pattern of performance in the study phase replicates what was found with amnesic case D.A. (Ryan et al., 2016), as well as prior work showing impaired learning of arbitrary relations in amnesic cases, but intact processing of relations known prior to neurological insult (Moses et al., 2008a; Ostreicher et al., 2010; Ryan et al., 2013; D'Angelo et al., 2015). Importantly, N.C.'s intact performance on the conditions supported by prior knowledge is consistent with prior work showing evidence for the prior and ongoing acquisition of semantic knowledge in developmental amnesia (Vargha‐Khadem et al., 1997; Gardiner et al., 2008).
No‐Feedback Test Phase—Healthy Controls
Accuracy in the no‐feedback test phase for the healthy controls and N.C. is plotted in Figure 5. Similar to the analysis of the training phase, the analysis of the test phase revealed a significant main effect of stimulus condition (F(3,105) = 7.48, P < 0.001, ε = 0.87, = 0.18). As expected, accuracy was highest in the conditions with semantic (K‐S: M = 0.97, SE = 0.01), or pairwise relations (K‐P: M = 0.91, SE = 0.02), and was lowest in the two conditions that test pre‐experimentally arbitrary relations (K‐A: M = 0.86, SE = 0.03; N‐A: M = 0.86, SE = 0.03).
Figure 5.
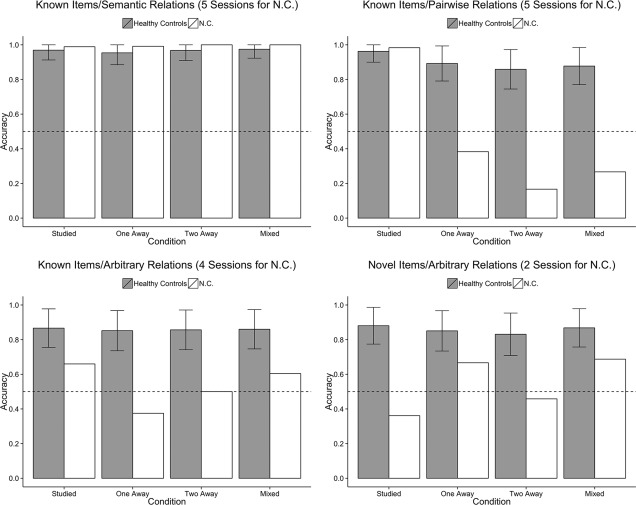
Mean accuracy in the test phase healthy controls (from Ryan et al., 2016) and N.C. as a function of the four stimulus conditions and four trial types. Error bars represent the 95% CI of the mean accuracy in the healthy controls.
The analysis revealed a significant main effect of trial type (F(3,105) = 7.47, P < 0.001, ε = 0.87, = 0.18). Participants had higher accuracy for the previously studied pairs (M = 0.92, SE = 0.02) than for the inference pairs (range = 0.88–0.89 for one‐away, two‐away, and mixed pairs).
Trial type also interacted with stimulus condition (F(9,315) = 3.02, P = 0.002, ε = 0.56, = 0.08). The general pattern of higher accuracy for studied pairs relative to inference pairs was found for all conditions; however, the range of accuracies across trial types was exaggerated in the K‐P condition (range = 0.86–0.96). As discussed in our prior work (Ryan et al., 2016), this exaggerated range of performance is not surprising, as the studied trials tested relations known prior to the experiment, while the inference trial types (one‐away, two‐away, and mixed) tested relations that had to be indirectly expressed based on knowledge of the premise pairs. In contrast, trial types in the other conditions either required the expression of pre‐experimentally known relations (K‐S condition) or novel relations that had to be acquired within the experimental session (K‐A and N‐A conditions).
No‐Feedback Test Phase—N.C
N.C.'s accuracy on the studied trials in the test phase mirrors his performance from the training phase. N.C.'s accuracy was similar to controls on the two conditions for which the relations were known pre‐experimentally (K‐S and K‐P). N.C. was markedly impaired on the conditions where novel relations had to be learned (K‐A and N‐A), falling outside the 95% CI of controls' performance on studied trials for the two conditions, and with mean performance below chance in the N‐A condition.
N.C. performed within the 95% CI of controls on the inference trials in the K‐S condition, where performance was still supported by pre‐experimentally known relations. Although N.C. demonstrated intact performance on the studied pairs for the K‐P condition, he was unable to express novel inference with respect to these semantically rich premise relations, as demonstrated by his poor performance on the inference pairs. Therefore, although prior knowledge supported performance for the studied pairs, this pre‐existing knowledge was unable to support the expression of inference across intervening levels of relational distance.
N.C. also showed impaired performance with accuracy outside the 95% CI of controls on the inference trials in the K‐A and N‐A conditions. Note that although N.C.'s accuracy on the inference trials appears to be higher in the N‐A relative to K‐A condition, he only completed two test phases in the N‐A condition and thus his performance may not be reliable.
GENERAL DISCUSSION
The present study examined whether prior knowledge can support inference in a developmental amnesic case, N.C., who has diencephalic damage and atrophy within the EHS and associated episodic memory impairment. Despite N.C.'s early amnesia, he shows evidence of semantic knowledge acquisition. We examined whether N.C. was able to use this knowledge to support inference, as there is some evidence that developmental amnesic cases can use semantic information to support other cognitive functions (Brandt et al., 2006), perhaps due to reorganization that occurs during development (Vargha‐Khadem et al., 2003). N.C. was unable to use his existing semantic knowledge to support novel inference, a finding that resembles that seen in the older, adult‐onset, amnesic case, D.A., who has damage to the MTL bilaterally and right vmPFC and ATL (Ryan et al., 2016). The results in N.C. extend this finding by indicating that anteromedial thalamic damage affecting hippocampal‐vmPFC connectivity is sufficient to produce general impairments in relational learning and inference, even in the context of intact ATL and semantic knowledge.
During training, N.C. showed impaired relational learning relative to healthy controls in conditions where arbitrary relations had to be learned in the absence of prior knowledge (known items/arbitrary relations and novel items/arbitrary relations conditions). This result is consistent with prior work examining impaired relational learning in amnesia (Ryan et al., 2000; Moses et al., 2008a; D'Angelo et al., 2015) and replicates what we previously observed in D.A. using this paradigm (Ryan et al., 2016). N.C. showed intact performance when the relations among premise pairs were known pre‐experimentally (known items/semantic relations and known items/pairwise relations conditions). N.C.'s intact expression of pre‐existing relational knowledge is consistent with previous work in adult‐acquired amnesia (Moses et al., 2008a; Ryan et al., 2013; D'Angelo et al., 2015) and developmental amnesia (Vargha‐Khadem et al., 1997), and is consistent with D.A.'s performance on this same task (Ryan et al., 2016).
N.C. showed impaired relational memory and transitive expression relative to controls in the test phase when performance could not be supported by prior knowledge (known items/arbitrary relations and novel items/arbitrary relations conditions). This finding is perhaps unsurprising given his impaired relational learning during training. When all possible relations among items in a set were supported by prior knowledge (known items/semantic relations), N.C.'s performance did not differ from that of controls: he was able to express his relational knowledge. Critically, when only pairwise relations among items in a set were supported by prior knowledge (known items/pairwise relations), N.C. showed intact knowledge of the premise pairs, but showed impaired transitive expression. In fact, N.C.'s performance was numerically below chance on all inference trials in this condition. N.C.'s below‐chance performance reflects an inability to overcome biases in responding based on weak relational information from prior knowledge (e.g., construction helmet going with ice skates, or construction helmet going with baseball and glove). Although N.C. made responses based on weak relational information during the test phase, his postexperiment explicit responses did not always map onto these task responses. For example, he only explicitly grouped the construction helmet with the ice skates in Session 4, and never explicitly grouped the construction helmet with the baseball and glove.
N.C.'s impaired inference in the context of known pairwise relations suggests that he is unable to flexibly bridge across existing relations in semantic knowledge. This inability to flexibly bridge across existing relations is likely due to deficits in relational binding. We hypothesize that bridging across relations is critically dependent on the hippocampus, but that deficits can also occur when the hippocampal system is disconnected from the vmPFC, as may be the case in N.C. Our interpretation is also consistent with prior work with two other developmental amnesic cases (Gardiner et al., 2008; Rosenbaum et al., 2015). For instance, although developmental amnesic case Jon has intact semantic memory for pre‐experimentally known facts, he was impaired and had slowed learning relative to controls when learning novel facts (Gardiner et al., 2008). Likewise, developmental amnesic case H.C. showed intact spatial knowledge of her neighborhood and downtown area of her hometown, but showed impaired performance on tasks where she was asked to describe alternate routes to avoid blocked roads or barriers (Rosenbaum et al., 2015). Her impairments suggested that she could not use her existing knowledge with the same flexibility expressed in typically developing controls. This prior work shows that the relatively intact semantic knowledge that is often observed in developmental amnesia is qualitatively different from semantic knowledge observed in controls. The results from this prior work highlight the role of the hippocampus and its extended system in the development of semantic knowledge, as has been previously described (Cohen and Eichenbaum, 1993; Squire, 2004).
Despite the many differences between D.A. and N.C. in terms of their pattern of damage, age, and the etiology of their amnesia, both cases were unable to perform inference in the context of premise pairs that contained relations known pre‐experimentally. Although N.C.'s hippocampi are volumetrically normal, the damage to his EHS (Aggleton and Brown, 1999), including bilateral anteromedial thalamic damage, appears to have disrupted his relational learning and transitive expression. Within the thalamus, N.C. has volume reduction primarily within the right mediodorsal nucleus of the thalamus, with an additional lesion but no volume reduction within the left mediodorsal nucleus (Rosenbaum et al., in prep). The mediodorsal nucleus forms part of a network between cortical MTL areas, including perirhinal and entorhinal cortices, and both medial and lateral PFC (Ketz et al., 2015). N.C. also has a lesion (but no significant volume reduction) within the right anterior nucleus as well as an atrophied right fornix and small mammillary bodies, which may be of relevance for the present findings. These structures form part of a circuit connecting the hippocampus to medial PFC (Ketz et al., 2015). It is possible that N.C.'s impaired ability to bridge across existing relations may reflect a critical disruption to this network. However, research involving additional cases is needed to test this hypothesis, in particular cases who have isolated deficits in one or another circuit.
The present set of findings add to prior work with non‐human animals with lesions to the hippocampus that has identified a role for the hippocampus in TI in the context of successful learning of premise pairs (Bunsey and Eichenbaum, 1996). N.C.'s impaired inference despite successful expression of relational knowledge of premise pairs is also consistent with prior work showing a role for the hippocampus in inference that extends beyond mere relational learning of the premise pairs (Myers et al., 2003; Preston et al., 2004; Shohamy and Wagner, 2008; Zeithamova and Preston, 2010; Collin et al., 2015; Schlichting et al., 2015; Backus et al., 2016). The present work critically extends this prior work by showing that even when pairwise relations are pre‐experimentally known, damage to the EHS nonetheless impairs inference, thus demonstrating a critical role for the hippocampal system in inference. The present work contributes to our understanding of what inference is by showing that inference involves the ability to flexibly bridge existing relations in memory, which likely entails the creation of new relations, a process that is critically dependent on the hippocampus. Based on prior neuroimaging work (Schlichting et al., 2015), we suggest that a level of stability of the premise relations may be required before bridging or integration can take place. This explanation is consistent with the results of our prior study (Ryan et al., 2016), where we found that older adults were impaired in establishing premise relations and performing inference in the arbitrary conditions, but were able to perform inference when the pairwise relations were pre‐experimentally known and stable (i.e., well‐established during encoding/training phases). Depending on task demands and/or the stability of the premise relations, bridging (or integration) can occur at encoding, particularly for already stable premise relations, and/or at test, when memory and inference are probed. Critically, the present work also shows that deficits in binding and inference can occur in the absence of hippocampal damage, when the hippocampal system is disconnected from the vmPFC. Deficits in inference can occur despite the existence of seemingly stable premise relations (i.e., intact performance on the premise relations during the training phase).
In prior work, we suggested the possibility that D.A.'s inability to use prior knowledge to mitigate his deficit was due to damage to neocortical areas including the right ATL, which has been implicated in the ability to use prior knowledge to scaffold new learning (Kan et al., 2009), and the right vmPFC, which has been implicated in inference (Koscik and Tranel, 2012b) and in rapid retrieval of relevant schemas to support flexible inference (Gilboa et al., 2009). While N.C. does not have damage to the ATL or vmPFC, suggesting that these regions may not be critical to supporting inference in the pairwise condition, N.C. does have a lesion to the anterior nucleus of the thalamus and degradation of the right fornix and mammillary bodies, which may impact how information is relayed to the vmPFC (Aggleton et al., 2011; Pergola and Suchan, 2013; Ketz et al., 2015). Therefore, it remains unknown whether N.C.'s performance is the result of damage to this pathway, which would be consistent with a prior study showing impaired inference despite intact pairwise learning in cases with vmPFC damage (Koscik and Tranel, 2012b). Although more work is needed to clarify the roles of the EHS and the vmPFC in inference, the present findings highlight a potential critical role for the EHS in inference.
The hippocampus and its extended system may support inference in two ways. First, the bridging and expression of known relations in the known relations/pairwise condition may require storage of new relational knowledge representing indirect relations, which may be mediated by the hippocampus (Kumaran and McClelland, 2012). In our prior work, we speculated that healthy older adults likely had sufficient residual hippocampal function to support this storage, but that D.A. did not (Ryan et al., 2016). Although N.C. does not have hippocampal damage, it is likely that the damage to his anteromedial thalamus and reductions in his mammillary bodies and right fornix were sufficient to impair either the storage or retrieval of new relational information (Aggleton and Brown, 1999), at either training or test.
Second, evidence points to a role for hippocampal‐neocortical interactions in the assimilation of new knowledge into existing schemas (Tse et al., 2007, 2011). Tse and colleagues have demonstrated a critical role for the rat mPFC and hippocampus in the integration of new information into previously stored knowledge. Although the homolog to the rat mPFC is under debate (Kesner, 2000; Farovik et al., 2008), the vmPFC has been suggested to play a role in allowing rapid retrieval of relevant schemas to support flexible inference in humans (Gilboa et al., 2009; Kan et al., 2009). Recent work has also shown that when new information is learned, vmPFC activity increases with increasing congruency between the new information and existing knowledge, while hippocampal activity shows the opposite pattern, showing increasing activity with decreasing congruency (van Kesteren et al., 2013). These findings are consistent with a recent proposal that the vmPFC engages in evaluative processing, whereby the vmPFC drives the hippocampus to make new relations when needed, such as in the case of decreasing congruency (Liu et al., 2016). Therefore, although N.C. was able to express knowledge for existing semantic information, his impaired inference in the pairwise condition may thus reflect an inability to reorganize the schema structure to reflect the incorporation of new, indirect relations, potentially because of disrupted hippocampal‐neocortical interactions (Wang and Morris, 2010; McKenzie and Eichenbaum, 2011).
In conclusion, the results from the present study confirm that relational learning and transitive expression in the context of arbitrary relations are impaired in a case of developmental amnesia, who has damage limited to the EHS. Importantly, the present results reveal intact semantic knowledge but show impaired inference in the context of intact pairwise knowledge. This work outlines the intriguing finding that the (extended) hippocampal system critically supports inference behavior, either due to functions within the hippocampus itself, or through the connections with neocortical regions. Moreover, the present work also suggests that inference is not simply the product of a process by which existing memory space is probed through prefrontal circuits, at least not without involving the hippocampus.
Acknowledgments
We thank N.C. and his family for his continued involvement in memory research. The authors state that they do not have any conflicts of interest to declare.
REFERENCES
- Addis DR, Cheng T, Roberts RP, Schacter DL. 2011. Hippocampal contributions to the episodic simulation of specific and general future events. Hippocampus 21:1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. 1999. Episodic memory, amnesia, and the hippocampal‐anterior thalamic axis. Behav Brain Sci 22:425–444, discussion 444–489. [PubMed] [Google Scholar]
- Aggleton JP, Dumont JR, Warburton EC. 2011. Unraveling the contributions of the diencephalon to recognition memory: A review. Learn Mem 18:384–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus AR, Schoffelen J‐M, Szebényi S, Hanslmayr S, Doeller CF. 2016. Hippocampal‐prefrontal theta oscillations support memory integration. Curr Biol 26:450–457. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Waxler DE, Santollo J, Shors TJ. 2006. Trace conditioning and the hippocampus: The importance of contiguity. J Neurosci 26:8702–8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley PJ, O'Reilly RC, Curran T, Squire LR. 2008. New semantic learning in patients with large medial temporal lobe lesions. Hippocampus 18:575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt KR, Gardiner JM, Vargha‐Khadem F, Baddeley AD, Mishkin M. 2006. Using semantic memory to boost “episodic” recall in a case of developmental amnesia. NeuroReport 17:1057–1060. [DOI] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H. 1996. Conservation of hippocampal memory function in rats and humans. Nature 379:255–257. [DOI] [PubMed] [Google Scholar]
- Cohen NJ. 2015. Navigating life. Hippocampus 25:704–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. 1993. Memory, Amnesia, and the Hippocampal System. Cambridge: MIT Press. [Google Scholar]
- Collin SHP, Milivojevic B, Doeller CF. 2015. Memory hierarchies map onto the hippocampal long axis in humans. Nat Neurosci 18:1562–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo MC, Kacollja A, Rabin JS, Rosenbaum RS, Ryan JD. 2015. Unitization supports lasting performance and generalization on a relational memory task: Evidence from a previously undocumented developmental amnesic case. Neuropsychologia 77:185–200. [DOI] [PubMed] [Google Scholar]
- D'Angelo MC, Smith VM, Kacollja A, Zhang F, Binns MA, Barense MD, Ryan JD. 2016. The effectiveness of unitization in mitigating age‐related relational learning impairments depends on existing cognitive status Neuropsychol Dev Cogn B Aging Neuropsychol Cogn, doi: 10.1080/13825585.2016.1158235. [DOI] [PMC free article] [PubMed]
- Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, Sutherland RJ. 2003. The aging hippocampus: Cognitive, biochemical and structural findings. Cereb Cortex 13:1344–1351. [DOI] [PubMed] [Google Scholar]
- Duff MC, Brown‐Schmidt S. 2012. The hippocampus and the flexible use and processing of language. Front Hum Neurosci 6:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek JA, Eichenbaum H. 1997. The hippocampus and memory for orderly stimulus relations. Proc Natl Acad Sci USA 94:7109–7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farovik A, Dupont LM, Arce M, Eichenbaum H. 2008. Medial prefrontal cortex supports recollection, but not familiarity, in the rat. J Neurosci 28:13428–13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Rudy JW, O'Reilly RC. 2003. Transitivity, flexibility, conjunctive representations, and the hippocampus. II. A computational analysis. Hippocampus 13:341–354. [DOI] [PubMed] [Google Scholar]
- Gardiner JM, Brandt KR, Baddeley AD, Vargha‐Khadem F, Mishkin M. 2008. Charting the acquisition of semantic knowledge in a case of developmental amnesia. Neuropsychologia 46:2865–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A, Alain C, He Y, Stuss DT, Moscovitch M. 2009. Ventromedial prefrontal cortex lesions produce early functional alterations during remote memory retrieval. J Neurosci 29:4871–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holyoak KJ. 2012. Analogy and relational reasoning In: Holyoak KJ, editor. The Oxford Handbook of Thinking and Reasoning. New York: The Oxford handbook of thinking and reasoning; pp 234–259. [Google Scholar]
- Kan IP, Alexander MP, Verfaellie M. 2009. Contribution of prior semantic knowledge to new episodic learning in amnesia. J Cogn Neurosci 21:938–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP. 2000. Subregional analysis of mnemonic functions of the prefrontal cortex in the rat. Psychobiology 28:219–228. [Google Scholar]
- Ketz NA, Jensen O, O'Reilly RC. 2015. Thalamic pathways underlying prefrontal cortex‐medial temporal lobe oscillatory interactions. Trends Neurosci 38:3–12. [DOI] [PubMed] [Google Scholar]
- Konkel A, Warren DE, Duff MC, Tranel DN, Cohen NJ. 2008. Hippocampal amnesia impairs all manner of relational memory. Front Hum Neurosci 2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koscik TR, Tranel D. 2012a. Brain evolution and human neuropsychology: The inferential brain hypothesis. J Int Neuropsychol Soc 18:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koscik TR, Tranel D. 2012b. The human ventromedial prefrontal cortex is critical for transitive inference. J Cogn Neurosci 24:1191–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, McClelland JL. 2012. Generalization through the recurrent interaction of episodic memories: A model of the hippocampal system. Psychol Rev 119:573–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z‐X, Grady C, Moscovitch M. 2016. Effects of prior‐knowledge on brain activation and connectivity during associative memory encoding. Cereb Cortex, doi: 10.1093/cercor/bhw047. [DOI] [PubMed] [Google Scholar]
- McKenzie S, Eichenbaum H. 2011. Consolidation and reconsolidation: Two lives of memories? Neuron 71:224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M. 2008. The hippocampus as a “stupid,” domain‐specific module: Implications for theories of recent and remote memory, and of imagination. Can J Exp Psychol 62:62–79. [DOI] [PubMed] [Google Scholar]
- Moses SN, Ryan JD. 2006. A comparison and evaluation of the predictions of relational and conjunctive accounts of hippocampal function. Hippocampus 16:43–65. [DOI] [PubMed] [Google Scholar]
- Moses SN, Ostreicher ML, Rosenbaum RS, Ryan JD. 2008a. Successful transverse patterning in amnesia using semantic knowledge. Hippocampus 18:121–124. [Internet] Available at: http://onlinelibrary.wiley.com/doi/10.1002/hipo.20378/full [DOI] [PubMed] [Google Scholar]
- Moses SN, Villate C, Binns MA, Davidson PSR, Ryan JD. 2008b. Cognitive integrity predicts transitive inference performance bias and success. Neuropsychologia 46:1314–1325. [DOI] [PubMed] [Google Scholar]
- Moses SN, Brown TM, Ryan JD, McIntosh AR. 2010a. Neural system interactions underlying human transitive inference. Hippocampus 20:894–901. [DOI] [PubMed] [Google Scholar]
- Moses SN, Ostreicher ML, Ryan JD. 2010b. Relational framework improves transitive inference across age groups. Psychol Res 74:207–218. [DOI] [PubMed] [Google Scholar]
- Myers CE, Shohamy D, Gluck MA, Grossman S, Kluger A, Ferris S, Golomb J, Schnirman G, Schwartz R. 2003. Dissociating hippocampal versus basal ganglia contributions to learning and transfer. J Cogn Neurosci 15:185–193. [DOI] [PubMed] [Google Scholar]
- O'Kane G, Kensinger EA, Corkin S. 2004. Evidence for semantic learning in profound amnesia: An investigation with patient H.M. Hippocampus 14:417–425. [DOI] [PubMed] [Google Scholar]
- Ostreicher ML, Moses SN, Rosenbaum RS, Ryan JD. 2010. Prior experience supports new learning of relations in aging. J Gerontol Psychol Sci 65B:32–41. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. 2007. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci 8:976–987. [DOI] [PubMed] [Google Scholar]
- Pergola G, Suchan B. 2013. Associative learning beyond the medial temporal lobe: Many actors on the memory stage. Front Behav Neurosci 7:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, Shrager Y, Dudukovic NM, Gabrieli JDE. 2004. Hippocampal contribution to the novel use of relational information in declarative memory. Hippocampus 14:148–152. [DOI] [PubMed] [Google Scholar]
- Rickard TC, Grafman J. 1998. Losing their configural mind. Amnesic patients fail on transverse patterning. J Cogn Neurosci 10:509–524. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Moscovitch M, Foster JK, Schnyer DM, Gao F, Kovacevic N, Verfaellie M, Black SE, Levine B. 2008. Patterns of autobiographical memory loss in medial‐temporal lobe amnesic patients. J Cogn Neurosci 20:1490–1506. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Gilboa A, Moscovitch M. 2014. Case studies continue to illuminate the cognitive neuroscience of memory. Ann N Y Acad Sci 1316:105–133. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Cassidy BN, Herdman KA. 2015. Patterns of preserved and impaired spatial memory in a case of developmental amnesia. Front Hum Neurosci 9:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JD, Althoff RR, Whitlow S, Cohen NJ. 2000. Amnesia is a deficit in relational memory. Psychol Sci 11:454–461. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Moses SN, Villate C. 2009. Impaired relational organization of propositions, but intact transitive inference, in aging: Implications for understanding underlying neural integrity. Neuropsychologia 47:338–353. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Moses SN, Barense M, Rosenbaum RS. 2013. Intact learning of new relations in amnesia as achieved through unitization. J Neurosci 33:9601–9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JD, D'Angelo MC, Kamino D, Ostreicher M, Moses SN, Rosenbaum RS. 2016. Relational learning and transitive expression in aging and amnesia. Hippocampus 26:170–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting ML, Preston AR. 2015. Memory integration: Neural mechanisms and implications for behavior. Curr Opin Behav Sci 1:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting ML, Mumford JA, Preston AR. 2015. Learning‐related representational changes reveal dissociable integration and separation signatures in the hippocampus and prefrontal cortex. Nat Commun 6:8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Wagner AD. 2008. Integrating memories in the human brain: Hippocampal‐midbrain encoding of overlapping events. Neuron 60:378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Squire LR. 2005. Declarative memory, awareness, and transitive inference. J Neurosci 25:10138–10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CN, Urgolites ZJ, Hopkins RO, Squire LR. 2014. Comparison of explicit and incidental learning strategies in memory‐impaired patients. Proc Natl Acad Sci USA 111:475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence KW. 1952. The nature of the response in discrimination learning. Psychol Rev 59:89–93. [DOI] [PubMed] [Google Scholar]
- Squire LR. 2004. Memory systems of the brain: A brief history and current perspective. Neurobiol Learn Mem 82:171–177. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN. 2004. Trail making test A and B: Normative data stratified by age and education. Arch Clin Neuropsychol 19:203–214. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, Kozak J, Rees L. 1999. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 14:167–177. [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RGM. 2007. Schemas and memory consolidation. Science 316:76–82. [DOI] [PubMed] [Google Scholar]
- Tse D, Takeuchi T, Kakeyama M, Kajii Y, Okuno H, Tohyama C, Bito H, Morris RGM. 2011. Schema‐dependent gene activation and memory encoding in neocortex. Science 333:891–895. [DOI] [PubMed] [Google Scholar]
- van Kesteren MTR, Beul SF, Takashima A, Henson RN, Ruiter DJ, Fernández G. 2013. Differential roles for medial prefrontal and medial temporal cortices in schema‐dependent encoding: From congruent to incongruent. Neuropsychologia 51:2352–2359. [DOI] [PubMed] [Google Scholar]
- Vargha‐Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. 1997. Differential effects of early hippocampal pathology on episodic and semantic memory. Science 277:376–380. [DOI] [PubMed] [Google Scholar]
- Vargha‐Khadem F, Salmond CH, Watkins KE, Friston KJ, Gadian DG, Mishkin M. 2003. Developmental amnesia: Effect of age at injury. Proc Natl Acad Sci 100:10055–10060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein GV, Eichenbaum H, Hasselmo ME. 1998. The hippocampus as an associator of discontiguous events. Trends in Neurosci 21:317–323. [DOI] [PubMed] [Google Scholar]
- Wang S‐H, Morris RGM. 2010. Hippocampal‐neocortical interactions in memory formation, consolidation, and reconsolidation. Annu Rev Psychol 61:49–79. C1–4. [DOI] [PubMed] [Google Scholar]
- Zeithamova D, Preston AR. 2010. Flexible memories: Differential roles for medial temporal lobe and prefrontal cortex in cross‐episode binding. J Neurosci 30:14676–14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Schlichting ML, Preston AR. 2012. The hippocampus and inferential reasoning: Building memories to navigate future decisions. Front Hum Neurosci 6:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


