Abstract
BACKGROUND
Metformin is the first‐line oral antihyperglycemic of choice for individuals with type 2 diabetes. Recent evidence supports a role for metformin in prostate cancer chemoprotection. However, whether metformin indeed influences prostate biology is unknown. We aimed to study the association between metformin and serum prostate‐specific antigen (PSA) levels—the primary prostate cancer biomarker.
METHODS
We conducted a cross‐sectional study of 326 prostate cancer‐free men with type 2 diabetes were recruited between 2004 and 2013 at St. Michael's Hospital. Men were excluded if they had a PSA ≥10‐ng/ml, or used >2,550‐mg/d metformin or supplemental androgens. Multivariate linear regressions quantified the association between metformin dose and log‐PSA. Secondary analyses quantified the association between other antihyperglycemics (sulfonylureas, thiazolidinediones) and PSA; sensitivity analyses tested covariate interactions.
RESULTS
Median PSA was 0.9‐ng/ml (IQR: 0.5–1.6‐ng/ml). Metformin dose associated positively with BMI, HbA1c, diabetes duration, and number of statin, acetylsalicylic acid, diuretic users, and number of antihyperglycemics used, and negatively with LDL‐C. In multivariate models, PSA changed by −8% (95%CI: −13 to −2%, P = 0.011) per 500‐mg/d increase in metformin. Men with diabetes for ≥6 years (n = 163) saw a greater difference in PSA per 500‐mg/d metformin (−12% [95% CI: −19 to −4%, P = 0.002], P‐interaction = 0.018). Serum PSA did not relate with sulfonylureas, thiazolidinediones, or total number of antihyperglycemic agents used. Our findings are limited by the cross‐sectional design of this study.
CONCLUSIONS
Metformin dose‐dependently inversely associated with serum PSA, independent of other antihyperglycemic medications. Whether metformin confers a dose‐dependent benefit on prostate tumorigenesis and progression warrants investigation. Prostate 76:1445–1453, 2016. © 2016 The Authors. The Prostate published by Wiley Periodicals, Inc.
Keywords: metformin, prostate cancer, PSA, antihyperglycemic, cross‐sectional, dose‐response
INTRODUCTION
Prostate cancer is the most commonly diagnosed malignancy among men in the Western world, carrying an estimated lifetime risk of diagnosis of 14–16% 1; 3–4% of diagnosed men die the disease 2. There remains a need to identify factors that prevent both prostate cancer development and progression. One solution may include the incorporation of chemoprotective agents, such as 5‐α‐reductase inhibitors (5‐ARIs), which have been reported to reduce both prostate cancer development and progression 3, 4, 5. More recently, the cholesterol‐lowering agents, statins, and the anti‐inflammatory agents, nonsteroidal anti‐inflammatory drugs (NSAIDs), have also shown promise in reducing prostate cancer risk 6, 7. Another emerging agent as a chemopreventative is the oral antihyperglycemic metformin (1,1‐dimethylbiguanide hydrochloride) 8. Metformin's excellent safety profile, affordability and efficacy in glycemic control and mortality not only make it the first‐line therapy of choice for diabetics, but also an ideal candidate for cancer chemoprevention.
Epidemiological studies support modest protective effects of metformin on prostate cancer outcomes 9, 10. Studying changes in serum prostate‐specific antigen (PSA) values—the most widely employed biomarker in monitoring prostate cancer risk and progression—may provide an important window into the pathobiology of the prostate in response to medications. Indeed improvements in prostate cancer outcomes associated with 5‐ARIs, statins, and NSAIDs all correspond with reductions in PSA values 11, 12, 13. However, whether metformin impacts PSA levels or whether metformin dose‐dependently influence prostate pathogenesis is unclear 14, 15, 16. Thus to further explore metformin's chemoprotective role while awaiting results from ongoing clinical trials, we investigated the role of different metformin doses on PSA levels in a cross‐section of men with type 2 diabetes.
METHODS
Participant Eligibility
Participants were identified from an internal database of individuals who enrolled in five independent randomized nutritional studies for patients with type 2 diabetes between 2004 and 2013 at St. Michael's Hospital (SMH) 17, 18, 19, 20, 21. PSA data were available at trial screening visits. Men were recruited from around the Toronto area using newspaper and transit advertisements. Research Ethics Board approval was obtained at SMH or the University of Toronto and written consent was obtained from all participants.
Only men who reported metformin use or nonuse and dose, and had measured serum total PSA prior to starting an intervention were included. All participants had type 2 diabetes for ≥6 months, were on a stable dose of oral antihyperglycemic medications for ≥3 months, and free of any significant cardiovascular, renal (serum creatinine >150‐μmol/L), or liver (serum alanine aminotransferase >3× upper limit of normal) disease. Men were excluded if they had: a history of prostate cancer (n = 1); a metformin dose >2,550‐mg/d (maximum recommended dose) (n = 2) 22; a PSA ≥10‐ng/ml (n = 4); or a history of 5‐ARI or androgen therapy (n = 7). None of the men were receiving insulin therapy, or had a PSA <0.1‐ng/ml, or a history of prostate surgery.
Exposure Assessment
Serum PSA samples were collected and measured at SMH using the Access Hybertech PSA assay. Biochemical variables were measured following routine hospital laboratory protocol and are described in detail elsewhere 17, 18, 19, 20, 21. Registered dietitians collected medication histories during screening visits. All other demographic and categorical variables were collected through patient interviews prior to receiving an intervention. Serum testosterone was unavailable for analysis.
Primary and Secondary Analyses
The primary analysis continuously modeled metformin dose (at 500‐mg/d increments) as an independent variable with PSA as the outcome variable. Two secondary analyses were conducted: (i) the primary analysis was repeated with sulfonylurea and thiazolidinedione dose‐equivalents replacing metformin; and (ii) serum PSA was continuously modeled with total number of antihyperglycemic agents used. Sensitivity analyses explored any modifications to the metformin‐PSA relationship.
Statistical Analyses
We took the natural logarithm of PSA to normalize its distribution prior to performing analyses; our results are reported as geometric means of back‐transformed log‐PSAs. Log‐PSA satisfied normality criteria and showed no evidence of heteroscedasticity across metformin doses. Differences in PSA levels between low‐ (<1,000‐mg), intermediate‐ (1,000 to <2,000‐mg), and high‐ (≥2,000‐mg) dose metformin users, and between users versus nonusers of sulfonylureas, thiazolidinediones, and dipeptidyl peptidase‐4 (DPP‐4) inhibitors were evaluated as preliminary analyses. Linear regressions assessed the association between log‐PSA and metformin dose. The multivariate model was determined a priori to include: age, ethnicity (African, European, Far Eastern, Indian/South Asian, Other), BMI (continuous by category: <25, 25–29.9, 30–34.9, ≥35 in kg/m2), duration of type 2 diabetes (years), HbA1C (%), and LDL‐C, statins (atorvastatin dose‐equivalents: nonusers, ≤20, >20 in mg/d), ASA (nonusers, ≤81, >81 in mg/d), and thiazide diuretics (yes/no). Although non‐salicylate NSAIDs have been related to PSA levels 11, we did not study them specifically as 98% of our NSAID users used ASA. Secondary analyses linearly regressed dose‐equivalents of sulfonylureas (standardized as: nonusers, very low‐dose, low‐dose, intermediate‐dose, high‐dose, very high‐dose), thiazolidinediones (standardized as: low‐dose, intermediate‐dose, high‐dose), and number of total antihyperglycemics used (1, 2, or ≥3), as additional predictors in the primary multivariate model. DPP‐4 inhibitors were excluded from dose‐response analyses due to the limited number of users (n = 36). Use of meglitinides (n = 5), α‐glucosidase inhibitors (n = 6), and glucagon‐like peptide‐1 agonists (n = 1) were only considered for the number of total antihyperglycemic used.
Sensitivity analyses compared the metformin‐PSA association across subpopulations using interaction variables for: median age (<58 years vs. ≥58 years), median duration of diabetes (<6 years vs. ≥6 years), obesity (BMI <30‐kg/m2 vs. BMI ≥30‐kg/m2), concomitant sulfonylurea, thiazolidinedione, statin, ASA, or thiazide diuretic use (user vs. nonuser), and number of antihyperglycemics used (1 vs. ≥2).
Metformin dose did not show any evidence of multi‐collinearity with other predictors in our model. Univariate linear regressions evaluated covariate trends per 500‐mg/d metformin. Descriptive data are presented as medians with interquartile ranges (IQR). PSA differences between users versus nonusers, and results for primary and secondary analyses are presented as means and percent estimates with 95% confidence intervals (CIs), respectively. SAS 9.4 software (SAS Institute Inc., Cary, NC) computed all statistical analyses with significance set at two‐sided α < 0.05. The Tukey–Kramer method adjusted P‐values for multiple comparisons.
RESULTS
Participant Characteristics
Participant characteristics for the 326 men included in analyses are presented in Table I. Median PSA was 0.9‐ng/ml (IQR: 0.5–1.6‐ng/ml). The median age was 58 years (IQR: 52–64 years); 85% of men were ≥50 years. Median BMI was 29‐kg/m2 (IQR: 26–33‐kg/m2): 40% and 41% of men were overweight and obese, respectively. Median duration of type 2 diabetes was 6 years (IQR: 3–10 years); 61% of men had diabetes for at least 5 years. Hyperglycemia was sufficiently controlled in most individuals (93% with HbA1c values between 6% and 8%) while dyslipidemia was inadequately controlled among most individuals (57% of individuals with LDL‐C >2.0‐mmol/L). Most men were of European (56%) or Indian/South Asian (28%) descent. Eighty‐nine percent (n = 289) used metformin alone or in combination with other antihyperglycemic medications; 50% used ≥2 oral antihyperglycemics. Forty, 21, and 11% of the cohort used sulfonylureas, thiazolidinediones, and DPP‐4 inhibitors, and 70, 54, and 15% of the cohort used statins, ASA, and thiazide diuretics, respectively (Table II ). The median dose among users was 1,500‐mg/d (IQR: 1,000–2,000‐mg/d) for metformin, intermediate‐dose (IQR: low‐dose to high‐dose) for sulfonylureas, intermediate‐dose (IQR: intermediate‐dose to high‐dose) for thiazolidinediones, 20‐mg/d (IQR: 10–40‐mg/d) for atorvastatin‐equivalents, and 81‐mg/d (IQR: 81–81 mg/d) for ASA. Men were using a median 1.5 (IQR: 1–2) antihyperglycemic agents, daily.
Table I.
Clinical and Demographic Characteristics of Included Participants
| Metformin Dose (mg) | 0–499 | 500–999 | 1,000–1,499 | 1,500–1,999 | 2,000–2,499 | ≥2,500 | P‐Trend | Overall |
|---|---|---|---|---|---|---|---|---|
| Participants (No. [%]) | 37 (11%) | 25 (8%) | 97 (30%) | 38 (12%) | 113 (35%) | 16 (5%) | – | 326 (100%) |
| Age (years) | 62 (58–71) | 57 (49–60) | 58 (51–64) | 60 (53–62) | 58 (53–64) | 60 (56–65) | 0.100 | 58 (52–64) |
| BMI (kg/m2) | 26.9 (24.4–31.6) | 27.8 (25.6–30.3) | 28.3 (25.7–33.4) | 27.8 (25.7–33.4) | 30.3 (27.2–33.9) | 30.3 (28.8–32.6) | 0.001 | 28.8 (25.7–33.1) |
| HbA1c (%) | 6.90 (6.60–7.60) | 6.90 (6.60–7.30) | 7.10 (6.70–7.50) | 7.05 (6.70–7.60) | 7.10 (6.80–7.60) | 7.50 (7.05–7.85) | 0.011 | 7.10 (6.70–7.60) |
| Duration of T2DM (years) | 7 (5.0–10.0) | 3.0 (2.0–6.0) | 4.5 (2.0–8.0) | 5.0 (2.0–10.0) | 7.0 (4.0–14.0) | 9.0 (5.0–11.5) | 0.015 | 6.0 (3.0–10.0) |
| LDL‐C (mmol/L) | 2.66 (1.99–3.12) | 2.28 (1.93–2.55) | 2.35 (1.57–2.92) | 2.15 (1.61–2.60) | 2.01 (1.41–2.42) | 2.20 (1.59–2.92) | <0.001 | 2.14 (1.58–2.76) |
| Ethnicity (No. [%]) | ||||||||
| African | 0 (0%) | 1 (4%) | 6 (6%) | 0 (0%) | 3 (3%) | 1 (6%) | 0.644 | 11 (3%) |
| European | 24 (65%) | 12 (48%) | 47 (48%) | 17 (45%) | 72 (64%) | 10 (63%) | 182 (56%) | |
| Far Eastern | 1 (3%) | 2 (8%) | 10 (10%) | 4 (11%) | 4 (4%) | 1 (6%) | 22 (7%) | |
| Indian/South Asian | 11 (29%) | 9 (36%) | 30 (31%) | 14 (37%) | 22 (19%) | 4 (25%) | 90 (28%) | |
| Other | 1 (3%) | 1 (4%) | 4 (4%) | 3 (8%) | 12 (11%) | 0 (0%) | 21 (6%) | |
| Antihyperglycemics (No. [%]) | ||||||||
| Metformin | 1 (3%) | 25 (100%) | 97 (100%) | 38 (100%) | 113 (100%) | 16 (100%) | – | 290 (89%) |
| Sulfonylureas | 28 (76%) | 3 (12%) | 20 (21%) | 14 (37%) | 56 (50%) | 8 (50%) | 0.596 | 129 (40%) |
| Thiazolidinediones | 10 (27%) | 3 (12%) | 16 (16%) | 4 (11%) | 27 (24%) | 8 (50%) | 0.167 | 68 (21%) |
| No. antihyperglycemics (No.) | 1 (1–1) | 1 (1–1) | 1 (1–2) | 2 (1–2) | 2 (1–2) | 2 (1.5–3) | <0.001 | 1.5 (1–2) |
| Other medications (No. [%]) | ||||||||
| Statins | 21 (57%) | 16 (64%) | 66 (68%) | 28 (74%) | 84 (74%) | 12 (75%) | 0.031 | 227 (70%) |
| ASA | 16 (43%) | 9 (36%) | 53 (55%) | 18 (47%) | 68 (60%) | 11 (69%) | 0.014 | 175 (54%) |
| Thiazide diuretics | 1 (3%) | 5 (20%) | 13 (13%) | 8 (21%) | 19 (17%) | 4 (22%) | 0.049 | 50 (15%) |
Table II.
Medication Use Among Included Participants
| Medication | Users (No. [%]) |
|---|---|
| Metformin | |
| <1,000 mg | 26 (9) |
| 1,000–1,999 mg | 135 (47) |
| ≥2,000 mg | 129 (44) |
| Sulfonylureas | |
| Nonuser | 197 (60) |
| Low dose | 43 (13) |
| High dose | 57 (17) |
| Thiazolidinediones | |
| Nonuser | 258 (79) |
| Low dose | 38 (12) |
| High dose | 30 (9) |
| DPP‐4‐inhibitors | |
| No | 290 (89) |
| Yes | 36 (11) |
| Statins | |
| Nonusers | 99 (30) |
| 5–20 mg | 126 (39) |
| ≥20 mg | 101 (31) |
| ASA | |
| Nonusers | 151 (46) |
| ≤81 mg | 146 (45) |
| >81 mg | 29 (9) |
| Thiazide diuretics | |
| No | 276 (85) |
| Yes | 50 (15) |
Metformin dose positively related with BMI, HbA1c, duration of diabetes, and number of statin, ASA, diuretic users, and total number of antihyperglycemic agents; and negatively with LDL‐C (P for all <0.05).
Metformin Dose and PSA Levels
The relationship between metformin dose and serum PSA is presented in Figure 1. Mean PSA levels were 30% lower (95%CI: 47–13% [P = 0.012]) among users compared to nonusers. PSA levels of intermediate‐ and high‐dose metformin users were 32% (95%CI: 51–13% [adjusted‐P = 0.044]) and 37% (95%CI: 50–14% [adjusted‐P = 0.018]) lower, respectively, compared to the low‐dose group. PSA levels were not different between intermediate‐ and high‐dose users. Metformin dose was inversely related with PSA in univariate analyses (P = 0.005). In multivariate analyses, we identified a −7.6% (95%CI: −13.1 to −1.8% [P = 0.011]) relative difference in PSA for every 500‐mg/d increase in metformin dose up to a maximum dose of 2,550‐mg/d (Fig. 2). Accordingly, individuals using ≥2,500‐mg/d of metformin had a 33% lower PSA level relative to men taking <500‐mg/d.
Figure 1.
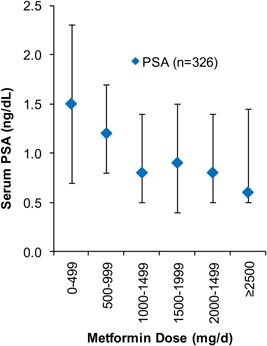
Distribution of PSA across metformin dose categories. The diamond represents median PSA at each dose threshold. The vertical bars represent interquartile ranges.
Figure 2.
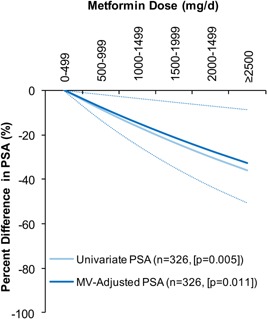
Regression‐predicted percent difference in serum PSA levels across continuously modeled metformin doses, compared to men using <500‐mg/d metformin. The light solid line presents the univariate relationship in all men (n = 326). The dark solid link presents the multivariate‐adjusted relationship in all men with the dotted line representing the corresponding 95%CIs (n = 326). The multivariate‐adjusted model adjusted for age, ethnicity, BMI, duration of diabetes, serum LDL‐C, glycated hemoglobin, NSAIDs, statins, and thiazide diuretics.
A sensitivity analysis using a mixed model, treating individuals as blocks for multiple PSA measures (30 men had three PSA measures, 126 had two, and 170 had one) did not modify our results (P = 0.020).
Other Oral Antihyperglycemics and PSA
Mean PSA was similar between users and nonusers of sulfonylureas (difference: −1.3% [95%CI: −17.2 to 17.7%, P = 0.886]), thiazolidinediones (difference: 6.7% [95%CI: −13.1 to 31.1%, P = 0.553]), and DPP‐4 inhibitors (difference: −3.3% [95%CI: −26.2 to 26.7%, P = 0.805]). PSA was not associated with sulfonylurea dose‐equivalents (PSA difference per 1‐unit increase: 1.1% [95%CI: −4.7 to 7.2%, P = 0.727]) or thiazolidinediones dose‐equivalents (PSA difference per 1‐unit increase: 3.7% [95%CI: −6.4 to 14.9, P = 0.483]) in multivariate analyses. Mean PSA did not differ between men using 1 versus ≥2 antihyperglycemic medications (difference: −5.7% [95%CI: −25.5 to 19.4%, P = 0.831]). Serum PSA did not change with increasing number of antihyperglycemic agents used: −3.9% (95%CI: −20.5 to 16.2%, P = 0.682). Including sulfonylurea and thiazolidinedione dose‐equivalents, and number of total antihyperglycemics as covariates did not affect the metformin‐PSA association.
Interactions With Metformin and PSA
The metformin‐PSA association tended to be stronger in men who were older (≥58 years: −10% [95%CI: −18 to −1%], P = 0.028), not obese (−9% [95%CI: −16 to −1%], P = 0.025), had diabetes for ≥6 years (−12% [95%CI: −19 to −4%], P = 0.002), and among statins nonusers (−9% [95%CI: −17 to −1%], P = 0.041), thiazolidinedione users (−18% [95%CI: −27 to −9%], P = 0.001), and users of ≥2 antihyperglycemics (−12% [95%CI: −20 to −4%], P = 0.007) (Fig. 3). This interaction was only significant for duration of diabetes (P‐interaction = 0.018).
Figure 3.
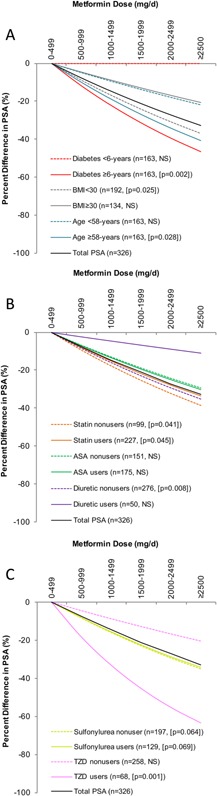
Regressionpredicted percent differences in serum PSA levels across metformin doses within covariate subpopulations. Each line represents the multivariate‐adjusted relationship at each of the following subpopulations: median diabetes duration, median age, obesity (A); statin, ASA, and diuretic use status (B); and sulfonylurea and thiazolidinedione (TZD) use status (C).
DISCUSSION
Metformin is the oral antihyperglycemic of choice for people with type 2 diabetes. A growing body of evidence supports a chemoprotective role for metformin in prostate cancer, although whether metformin impacts the intraprostatic environment remains unclear. Our study identified a significant non‐linear inverse relationship between metformin dose and serum PSA in prostate cancer‐free men with type 2 diabetes, independent of both other antihyperglycemics and number of total antihyperglycemics used.
Findings in the Context of the Literature
To the best of our knowledge, this is the first study to assess the relationship between metformin dose and serum PSA levels. In vitro experiments report reductions in PSA gene expression and dose‐dependent reductions in cancer cell viability with metformin treatment 23, 24, 25, 26. Two observational studies in European populations identified 11–15% lower PSA levels in metformin users compared to nonusers 14, 15. The implications of these results are complicated, however, as the studies did not adjust for prostate cancer status, or diabetes status and severity—both known modifiers of metformin dose, PSA levels and prostate cancer risk 27, 28. In a “window of opportunity” neoadjuvant trial, Joshua et al. found a 10% non‐significant PSA reduction in 22 men with stage T2a‐T3b disease when treated with 2,000‐mg/d of metformin for a median 41 days (P = 0.08) 29. Likewise, Rothermundt et al. treated 44 men with metastatic chemotherapy‐naïve castration‐resistant disease with 2,000‐mg/d of metformin for a median 85 days and found a prolongation of PSA doubling time in 23 patients, and >50% PSA reductions in two patients 16. The difference in PSA we observed between nonusers and high‐dose users (≥2,000‐mg/d) was substantially greater than the trial findings, potentially suggesting a diminished efficacy for metformin to lower PSA in men with more advanced cancer. Nevertheless, the available evidence is consistent with the current findings suggesting a lower PSA among metformin users.
The metformin‐PSA association was stronger among men with diabetes for ≥6 year, compared to <6 years. The implications of this interaction are unclear as some studies show a lower PSA among men with greater diabetes durations 28, while others do not 27. The existence of a temporal link between metformin use and PSA cannot be ruled out.
Potential Mechanisms
Metformin has been shown to decrease PSA gene expression both through androgen receptor downregulation and androgen‐receptor independent mechanisms 23, 25, 26. Furthermore, metformin may indirectly lower PSA through its anti‐inflammatory 30 and hypolipidemic 31 capacity, as reductions in both cholesterol and systemic inflammation have independently been associated with lower PSA levels 11, 13. Lastly, since increasing doses of metformin correlated with markers of worsening diabetes, a worsening diabetes status may be responsible for lowering PSA 27, 32. However, the lack of a PSA association across increasing doses of either sulfonylureas or thiazolidinediones, or increasing number of antihyperglycemic agents used, lends support toward a link independent of other antihyperglycemics, or diabetes severity.
Implications for Practice and Research
While awaiting definitive answers regarding the causal relationship between metformin and PSA and prostate cancer, we take our observations of a metformin‐PSA dose‐response as objective evidence of metformin at least influencing prostate biology. As similar pathways are proposed for both metformin's PSA lowering and putative antitumor effects, it is possible that the lower PSA we observed may indicate metformin‐mediated changes to the intraprostatic micro environment, and consequently, reductions in the risk of developing prostate cancer or progression of undiagnosed indolent prostate cancer.
Several implications arise if these results are confirmed in larger cohorts. Most importantly, metformin's dose‐dependent relationship with PSA raises the key question of whether a corresponding relationship exists for prostate cancer outcomes. Indeed one study of patients with diabetes identified a significant 42% reduction in the risk of mortality from all cancers for every 1,000‐mg/d increase in metformin dose compared to nonusers 33. Likewise, Margel et al., found a 24% reduction in the risk of prostate cancer‐specific mortality for every 6‐months of cumulative metformin exposure after diagnosis 10. If a metformin dose‐response is confirmed for prostate cancer outcomes, classifying all doses as users—as the current epidemiological literature has done—may underestimate the true chemoprotective potential of metformin.
Lastly, some factors may modify PSA levels without corresponding changes in cancer risk 34, thus how metformin influences PSA‐screening warrants investigation. On one hand, a raised PSA among high‐dose metformin users may more accurately predict the presence of prostate cancer than a raised PSA among low‐dose users or nonusers, improving the PSA test's positive predictive value. On the other hand, low PSA levels among high‐dose users may lead clinicians to falsely perceive these men as low risk for prostate cancer and thus less likely to require biopsies, potentially leading to delayed diagnoses. Our results advocate for metformin dose‐adjusted interpretations of PSA levels, particularly at the screening stage.
Limitations
This study has several limitations. First, the cross‐sectional nature of this research prevents causal inferences or temporal assessments, limiting the direct clinical relevance of these findings. Second, we only included prostate cancer‐free men with diabetes and only 3% of the population was of African descent, limiting our external validity beyond this demographic. Lastly, we were unable to study the metformin dose‐response at different PSA thresholds (i.e., <2.0 vs. ≥2.0‐ng/ml, etc.), as the metformin dose range was insufficient and significantly differed between PSA subgroups.
CONCLUSIONS
This cross‐sectional analysis identified a significant inverse non‐linear association between metformin dose and serum PSA levels in 326 prostate‐cancer free men with type 2 diabetes. The association appears independent of other metabolic or clinical modifiers of serum PSA, and was not confounded by nor replicated with other antihyperglycemic agents. These results, and the impact of metformin dose on prostate cancer development and progression need to be confirmed in larger cohorts.
Conflicts of Interest/Financial Disclosures: VHJ, CI, NEF, RJH, and DJAJ have no reported conflicts of interest with this work.
Role of the Sponsors: None of the funding organizations or sponsors played any role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Aspects of this project were presented at the 79th Scientific Sessions and Annual Meeting of the American Society of Nutrition, in Boston, MA. March 28—April 1, 2015. Abstract available at: The FASEB Journal. 2015: 29(1):Supplement 406.8.
REFERENCES
- 1.SEER Stat Fact Sheets: Prostate Cancer. http://seercancergov/statfacts/html/prosthtml. 2014.
- 2. Moyer VA. Screening for prostate cancer: U.S. Preventive services task force recommendation statement. Ann Intern Med 2012; 157:120–134. [DOI] [PubMed] [Google Scholar]
- 3. Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, Pettaway CA, Tammela TL, Teloken C, Tindall DJ, Somerville MC, Wilson TH, Fowler IL, Rittmaster RS. Effect of dutasteride on the risk of prostate cancer. N Engl J Med 2010; 362:1192–1202. [DOI] [PubMed] [Google Scholar]
- 4. Finelli A, Trottier G, Lawrentschuk N, Sowerby R, Zlotta AR, Radomski L, Timilshina N, Evans A, van der Kwast TH, Toi A, Jewett MA, Trachtenberg J, Fleshner NE. Impact of 5alpha‐reductase inhibitors on men followed by active surveillance for prostate cancer. Eur Urol 2011; 59:509–514. [DOI] [PubMed] [Google Scholar]
- 5. Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA, Jr . The influence of finasteride on the development of prostate cancer. N Engl J Med 2003; 349:215–224. [DOI] [PubMed] [Google Scholar]
- 6. Bansal D, Undela K, D'Cruz S, Schifano F. Statin use and risk of prostate cancer: A meta‐analysis of observational studies. PLoS ONE 2012; 7:e46691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang X, Lin YW, Wu J, Zhu Y, Xu XL, Xu X, Liang Z, Hu ZH, Li SQ, Zheng XY, Xie LP. Meta‐analysis of nonsteroidal anti‐inflammatory drug intake and prostate cancer risk. World J Surg Oncol 2014; 12:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Margel D. Metformin to prevent prostate cancer: A call to unite. Eur Urol 2014; 66:1021–1022. [DOI] [PubMed] [Google Scholar]
- 9. Yu H, Yin L, Jiang X, Sun X, Wu J, Tian H, Gao X, He X. Effect of metformin on cancer risk and treatment outcome of prostate cancer: A meta‐analysis of epidemiological observational studies. PLoS ONE 2014; 9:e116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Margel D, Urbach DR, Lipscombe LL, Bell CM, Kulkarni G, Austin PC, Fleshner N. Metformin use and all‐cause and prostate cancer‐specific mortality among men with diabetes. J Clin Oncol 2013; 31:3069–3075. [DOI] [PubMed] [Google Scholar]
- 11. Chang SL, Harshman LC, Presti JC, Jr . Impact of common medications on serum total prostate‐specific antigen levels: Analysis of the National Health and Nutrition Examination Survey. J Clin Oncol 2010; 28:3951–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. D'Amico AV, Roehrborn CG. Effect of 1 mg/day finasteride on concentrations of serum prostate‐specific antigen in men with androgenic alopecia: A randomised controlled trial. Lancet Oncol 2007; 8:21–25. [DOI] [PubMed] [Google Scholar]
- 13. Hamilton RJ, Goldberg KC, Platz EA, Freedland SJ. The influence of statin medications on prostate‐specific antigen levels. J Natl Cancer Inst 2008; 100:1511–1518. [DOI] [PubMed] [Google Scholar]
- 14. Nordstrom T, Clements M, Karlsson R, Adolfsson J, Gronberg H. The risk of prostate cancer for men on aspirin, statin or antidiabetic medications. Eur J Cancer 2015; 51:725–733. [DOI] [PubMed] [Google Scholar]
- 15. Randazzo M, Beatrice J, Huber A, Grobholz R, Manka L, Wyler SF, Chun FF, Recker F, Kwiatkowski M. Influence of metformin use on PSA values, free‐to‐total PSA, prostate cancer incidence and grade and overall survival in a prospective screening trial (ERSPC Aarau). World J Urol 2015; 33(8):1189–1196. [DOI] [PubMed] [Google Scholar]
- 16. Rothermundt C, Hayoz S, Templeton AJ, Winterhalder R, Strebel RT, Bartschi D, Pollak M, Lui L, Endt K, Schiess R, Ruschoff JH, Cathomas R, Gillessen S. Metformin in chemotherapy‐naive castration‐resistant prostate cancer: A multicenter phase 2 trial (SAKK 08/09). Eur Urol 2014; 66:468–474. [DOI] [PubMed] [Google Scholar]
- 17. Jenkins DJ, Kendall CW, Banach MS, Srichaikul K, Vidgen E, Mitchell S, Parker T, Nishi S, Bashyam B, de Souza R, Ireland C, Josse RG. Nuts as a replacement for carbohydrates in the diabetic diet. Diabetes Care 2011; 34:1706–1711. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Jenkins DJ, Kendall CW, McKeown‐Eyssen G, Josse RG, Silverberg J, Booth GL, Vidgen E, Josse AR, Nguyen TH, Corrigan S, Banach MS, Ares S, Mitchell S, Emam A, Augustin LS, Parker TL, Leiter LA. Effect of a low‐glycemic index or a high‐cereal fiber diet on type 2 diabetes: A randomized trial. JAMA 2008; 300:2742–2753. [DOI] [PubMed] [Google Scholar]
- 19. Jenkins DJ, Kendall CW, Augustin LS, Mitchell S, Sahye‐Pudaruth S, Blance Mejia S, Chiavaroli L, Mirrahimi A, Ireland C, Bashyam B, Vidgen E, de Souza RJ, Sievenpiper JL, Coveney J, Leiter LA, Josse RG. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: A randomized controlled trial. Arch Intern Med 2012; 172:1653–1660. [DOI] [PubMed] [Google Scholar]
- 20. Jenkins DJ, Kendall CW, Vuksan V, Faulkner D, Augustin LS, Mitchell S, Ireland C, Srichaikul K, Mirrahimi A, Chiavaroli L, Blanco Mejia S, Nishi S, Sahye‐Pudaruth S, Patel D, Bashyam B, Vidgen E, de Souza RJ, Sievenpiper JL, Coveney J, Josse RG, Leiter LA. Effect of lowering the glycemic load with canola oil on glycemic control and cardiovascular risk factors: A randomized controlled trial. Diabetes Care 2014; 37:1806–1814. [DOI] [PubMed] [Google Scholar]
- 21. Chiavaroli L. Low glycemic index diet for type 2 diabetics. 2010;2015.
- 22. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid = bbaad5e9‐38dd‐4c4a‐b278‐8d1fb01200cb. METFORMIN HYDROCHLORIDE—metformin hydrochloride tablet
- 23. Besla R, Venier N, Coloquhoun A, Fleshner NE, Klotz LH, Venkateswaran V. Dutasteride and metformin reduce the growth of LNCaP cells and alter the SREBP‐1 pathway. Open Prostate Cancer J 2013; 6:10–15. [Google Scholar]
- 24. Colquhoun AJ, Venier NA, Vandersluis AD, Besla R, Sugar LM, Kiss A, Fleshner NE, Pollak M, Klotz LH, Venkateswaran V. Metformin enhances the antiproliferative and apoptotic effect of bicalutamide in prostate cancer. Prostate Cancer Prostatic Dis 2012; 15:346–352. [DOI] [PubMed] [Google Scholar]
- 25. Lee SY, Song CH, Xie YB, Jung C, Choi HS, Lee K. SMILE upregulated by metformin inhibits the function of androgen receptor in prostate cancer cells. Cancer Lett 2014; 354:390–397. [DOI] [PubMed] [Google Scholar]
- 26. Wang Y, Liu G, Tong D, Parmar H, Hasenmayer D, Yuan W, Zhang D, Jiang J. Metformin represses androgen‐dependent and androgen‐independent prostate cancers by targeting androgen receptor. Prostate 2015; 75:1187–1196. [DOI] [PubMed] [Google Scholar]
- 27. Muller H, Raum E, Rothenbacher D, Stegmaier C, Brenner H. Association of diabetes and body mass index with levels of prostate‐specific antigen: Implications for correction of prostate‐specific antigen cutoff values? Cancer Epidemiol Biomarkers Prev 2009; 18:1350–1356. [DOI] [PubMed] [Google Scholar]
- 28. Werny DM, Saraiya M, Gregg EW. Prostate‐specific antigen values in diabetic and nondiabetic US men, 2001–2002. Am J Epidemiol 2006; 164:978–983. [DOI] [PubMed] [Google Scholar]
- 29. Joshua AM, Zannella VE, Downes MR, Bowes B, Hersey K, Koritzinsky M, Schwab M, Hofmann U, Evans A, van der Kwast T, Trachtenberg J, Finelli A, Fleshner N, Sweet J, Pollak M. A pilot 'window of opportunity' neoadjuvant study of metformin in localised prostate cancer. Prostate Cancer Prostatic Dise 2014; 17:252–258. [DOI] [PubMed] [Google Scholar]
- 30. Saisho Y. Metformin and inflammation: Its potential beyond glucose‐lowering effect. Endocr Metab Immune Disord Drug Targets 2015; 15(3):196–205. [DOI] [PubMed] [Google Scholar]
- 31. Wulffele MG, Kooy A, de Zeeuw D, Stehouwer CD, Gansevoort RT. The effect of metformin on blood pressure, plasma cholesterol and triglycerides in type 2 diabetes mellitus: A systematic review. J Intern Med 2004; 256:1–14. [DOI] [PubMed] [Google Scholar]
- 32. Fowke JH, Matthews CM, Buchowski MS, Signorello LB, Chang SS, Cookson MS, Blot WJ. Association between prostate‐specific antigen and leptin, adiponectin, HbA1c or C‐peptide among African‐American and Caucasian men. Prostate Cancer Prostatic Dis 2008; 11:264–269. [DOI] [PubMed] [Google Scholar]
- 33. Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC‐16. Diabetes Care 2010; 33:322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thompson IM, Tangen CM, Kristal AR. Prostate‐specific antigen: A misused and maligned prostate cancer biomarker. J Natl Cancer Inst 2008; 100:1487–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]


