Abstract
Tumor heterogeneity of triple-negative breast cancer (TNBC) has been the main barrier in conquering breast cancer. To dissect the molecular diversity of TNBC and discover therapeutic targets for TNBC, the molecular classification of TNBC is a prioritized issue in research area. Accordingly, recent studies have been successful in classifying TNBC into several distinct subtypes with specific biologic pathways. Despite the different methodologies used and varied number of final subtypes, these studies identically suggested that TNBC consists of four major subtypes: basal-like, mesenchymal, luminal androgen receptor, and immune-enriched. By reviewing these methods of classifications of TNBC, we highlight the unmet need to develop a molecular classifier suited for TNBC.
Keywords: Breast neoplasms, Gene expression, Triple negative breast neoplasms
INTRODUCTION
Breast cancer is the most frequently diagnosed cancer in women worldwide [1]. Even though it remains the second most common cause of cancer deaths [1], survival outcomes have improved in patients with breast cancer. The advances in molecular biology have contributed to the recent survival improvement, in addition to the widespread use of breast screening programs and new drugs such as anthracyclines and taxanes in chemotherapy [2,3].
The recent advances in cancer genomics have led to the elucidation of the intrinsic subtypes of breast cancer, thereby resulting in the delivery of target therapies including endocrine therapy and anti-human epidermal growth factor receptor 2 (HER2) therapy for appropriate patients with breast cancer who have hormone receptor (HR)-positive or HER2-positive tumors [2,4,5]. The success of targeted therapies has been integral to the improved treatment outcome in patients with breast cancer. Conversely, nonspecific chemotherapy remains the mainstay for the management of patients with triple-negative breast cancer (TNBC), which lacks the expression of three cellular receptors: estrogen receptor (ER), progesterone receptor, and HER2. TNBC, which accounts for 15% to 20% of the cases of invasive breast cancer, is usually aggressive, with higher grades or frequent nodal metastasis, and usually develops at a higher rate in young patients [6,7]. Patients with TNBC tend to experience an increased likelihood of distant metastasis and early recurrence in 2 or 3 years after treatment, compared with patients with other subtypes of breast cancer; patients with TNBC also tend to have lower survival [6,7].
By adopting unsupervised clustering analyses with genomic data of cases of TNBC, several subtypes of TNBC have been identified over the years [8,9,10,11]. These studies have shown that TNBC is remarkably heterogeneous at the transcriptional level. They further revealed that TNBC could be classified into several subtypes, with unique biological pathways for each subtype. This molecular heterogeneity of TNBC has been the main barrier in improving survival and in developing targeted therapy for patients with TNBC. Therefore, to deliver personalized therapy for patients with TNBC, researchers have prioritized the development of a standardized method of subtyping. Herein, by reviewing previous genomic studies about the classifications of TNBC, we highlighted the unmet need for the development of a molecular classifier for TNBC.
TNBC SUBTYPES BY GENE EXPRESSION ANALYSES
The Vanderbilt subtype
In the last decade, there has been intensive research to identify therapeutic targets for TNBC based on genomics. Since Perou et al. [12] published their landmark study categorizing breast cancer by gene expression profiling into intrinsic subtypes, gene expression profiling analyses have been widely adopted in classifying and discovering relevant therapeutic targets among the various methods using genomic data. An early study using gene expression profiles from TNBC reported that triple-negative tumors are synonymous with basal-like cancer, although five distinct subgroups are observed on hierarchical analysis [9].
In 2011, the researchers of the Vanderbilt University reported a seminal study classifying TNBC into distinct subtypes [8]. Using gene expression analyses from 587 TNBC tumors, they illustrated that TNBC consists of six distinguished subtypes and displays a unique biology that responds differently to various therapies. By k-means and consensus clustering, they found the following six subtypes: two basal-like subtypes, one with increased cell cycle and DNA damage response gene signatures (BL1) and the other one with high expression growth factor pathway and myoepithelial markers (BL2); two mesenchymal subtypes with up-regulated gene signatures associated with cell differentiation and growth factor signaling (M and MSL); an immunomodulatory (IM) type with enriched immune cell processes; and a luminal androgen subtype characterized by androgen signaling (LAR). They found that distinct gene ontologies are involved with each TNBC subtype as briefly described above. Furthermore, they identified TNBC cell lines representing these subtypes by using gene expression analysis. They generated preclinical evidence for the clinical application of TNBC subtyping by correlating driver signaling pathways with the results of in vitro drug response assays using pharmacologically targeted treatment, offering distinct gene signatures that could forecast an effective tailored treatment. These experiments showed that DNA damaging agents such as cisplatin are effective for the basal-like subtype, NVP-BEZ335 as an mammalian target of rapamycin (mTOR)-phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) co-inhibitor for the mesenchymal subtype, and bicalutamide as an androgen receptor (AR) blockade for the LAR subtype.
Comparisons between the Vanderbilt subtype and the PAM50 subtype
Among 374 of the 587 cases of TNBC used for molecular subtyping in the Vanderbilt study, the PAM50 intrinsic subtypes were directly compared with the TNBC subtypes [13]. As expected, most TNBC samples were classified into the basal-like subtype by PAM50 (80.6%). The HER2-enriched intrinsic subtype was the second most common subtype (10.2%), followed by the normal-like (4.6%), luminal B (3.5%), and luminal A (1.1%) subtypes by PAM50. Considering the Vanderbilt subtypes, most subtypes are composed of the basal-like PAM50 subtype, except for the MSL and LAR subtypes. In the MSL subtype, half of the cases were basal-like, and the other half consisted of the normal-like (27.8%) and luminal B (13.9%) subtypes. In contrast, the LAR subtype mainly consists of the HER2 (74.3%) and luminal B (14.3%) subtype by PAM50 subtyping. This comparison suggests that PAM50-based subtyping alone has the potential to identify approximately 75% of the LAR subtype when PAM50 assay indicates the HER2-intrinsic subtype.
Validation of the Vanderbilt subtypes
To test the clinical usefulness of the Vanderbilt subtype, researchers developed an online tool (TNBCtype) to classify the molecular subtypes of TNBC using raw data of gene expression profiling regardless of array platforms [14]. In 2013, Masuda et al. [15] utilized the subtyping tool and validated the clinical correlation of the Vanderbilt subtype in patients with TNBC who underwent neoadjuvant anthracyclines-taxanes containing chemotherapy. In the study by Masuda et al. [15], the overall pathologic complete response (pCR) rate was 28%. However, pCR rates substantially differed according to the subtypes. The highest pCR rate (52%) was observed in the BL1 subtype. By contrast, the pCR rate was lower in patients with the BL2, MSL, and LAR subtypes (0%, 23%, and 10%, respectively). When a likelihood ratio test was applied, the Vanderbilt subtype was demonstrated to be a significant factor for pCR status. They also validated the TNBC subtyping tool in 163 TNBC cases from The Cancer Genome Atlas (TCGA) [16]. In accordance with the previous work by Masuda et al. [15], the study by Abramson et al. [16] showed a similar proportion of the Vanderbilt subtypes and different survival outcome by the subtypes.
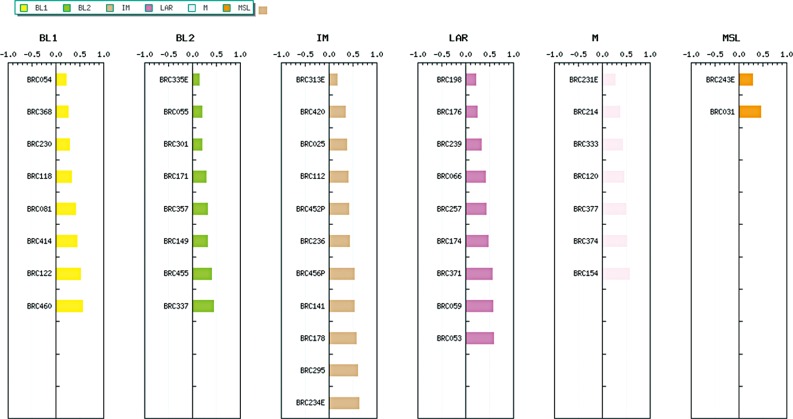
The working group of the Gangnam Severance Hospital also used the TNBCtype [14] and identified their own subtypes by uploading gene expression profiles of 62 Korean TNBC samples. They previously reported their analyses using gene expression profiling from 300 Korean breast cancer samples [17]. Among the 62 TNBC samples, except for 17 unspecified subtypes, the other cases were classified as eight BL1 (17.8%), eight BL2 (17.8%), 11 IM (24.4%), nine LAR (20.0%), seven M (15.5%), and two MSL subtypes (4.5%) (Figure 1). The distribution of the Vanderbilt subtypes in their data was similar to the results of the previous study [8], and indicates that this subtyping can be utilized for Korean patients with TNBC.
Figure 1. Distributions of the Vanderbilt subtypes using TNBCtype in Korean women with triple-negative breast cancer (n=45).
Even though there remains an unmet need for prospective validation of the Vanderbilt subtype in patients with TNBC, these findings showed that the Vanderbilt subtype guides the identification of the molecular subtype of TNBC, which may lead to subtype-driven chemotherapy or targeted therapy.
The Baylor subtype
In 2014, there was another classifier of TNBC proposed by the researchers of the Baylor University [10]. By integrating mRNA expression and DNA profiling for 198 TNBC tumor samples, they tried to classify the molecular subtypes of TNBC and discover therapeutic targets for each subtype. Using the non-negative matrix factorization method, they discovered classifier panels comprising 80 core genes. They classified TNBC tumors into the following four distinct subtypes: (1) LAR, (2) mesenchymal (MES), (3) basal-like immunosuppressed (BLIS), and (4) basal-like immune-activated (BLIA). Among all the subtypes, tumors with the BLIS subtype showed the worst prognosis, while tumors with the BLIA subtype showed the best prognosis.
The researchers performed a direct comparison between the Baylor subtype and the Vanderbilt subtype. They observed that the LAR subtype of the Baylor classifier was identical to the LAR subtype of the Vanderbilt classifier. In addition, most cases of the MES subtype contained the MSL and M subtypes according to the Vanderbilt classifier. However, there was discordance between the BL1, BL2, and IM subtypes by the Vanderbilt classifier with those of the Baylor subtype. Tumors with the BL1 and BL2 subtypes are distributed across BLIS and BLIA, while tumors with the IM subtype were classified as MES and BLIA. Their subtyping was validated in seven public datasets of gene expression profiles from TNBC.
When the Baylor subtype was compared with the PAM50 intrinsic subtype, BLIS and BLIA consisted of only the basal-like subtype. The MES subtype was separated into two PAM50 intrinsic subtypes: the basal-like and normal-like subtypes. Interestingly, the LAR subtype of the Baylor classifier was composed of the basal-like, luminal A, luminal B, HER2-enriched, and normal-like subtypes by PAM50, indicating the heterogeneity of the LAR subtype.
Furthermore, DNA copy number profiling separates the Baylor subtype into two major groups: LAR versus MES/BLIS/BLIA. The researchers suggested therapeutic candidates for specific subtypes: (1) targeting the AR and cell surface mucin (MUC1) for the LAR subtype; (2) inhibiting growth factor signaling such as platelet-derived growth factor (PDGF) receptor A and c-Kit, for the MES subtype; (3) inhibiting an immunosuppressing molecule such as V-set domain containing T cell activation inhibitor 1 (VTCN1) for the BLIS subtype; and (4) targeting stat signal transduction molecules and cytokines for the BLIA subtype.
The researchers concluded that TNBC could be classified into four distinct subtypes with different prognoses. In agreement with the Vanderbilt study, they also concluded that targeted therapy for TNBC subtypes is possible in the future for more effective and tailored management.
The French subtype
The researchers of the Unicancer center in France reported another subtyping method for TNBC [11]. Similar to earlier studies on subtyping [8,10], they used gene expression profiling for 194 TNBC samples and adopted fuzzy clustering. They discovered three subtypes in the training set (n=107): C1, luminal AR, 22.4%; C2, basal-like with low immune response and high M2-like macrophages, 44.9%; C3, basal-enriched with high immune response and low M2-like macrophages, 32.7%; they validated these subtypes in another cohort (n=87). They found that the tumor grade and the Nottingham prognostic index were higher in C2 and C3 than in C1. On comparisons of event-free survival, patients with C3 tumors had a significantly better outcome compared to patients with C1 or C2 tumors. Their functional analyses showed that luminal androgen signaling was enriched in C1 tumors, similar to LAR in the Vanderbilt and the Baylor classifiers. The C2 type consisted of an almost pure basal-like cancer according to the PAM50 assay. The claudin-low subtype as well as the basal-like type was observed in 26% of C3 tumors. Furthermore, immune response signaling, which is associated with a high immune response and low M2-like macrophage was enriched in C3 tumors, which has similarities with the IM subtype of the Vanderbilt classifier or BLIA of the Baylor classifier. The findings highlight that targeting immune response genes and lowering macrophages would be an effective therapeutic strategy for TNBC.
Comparison across the three classifiers of TNBC
Despite the pervasive differences in the methodology and number of samples, all the three studies provided identical evidence that biological pathways predominantly exist for each subtype of TNBC. The similarities and differences across the three TNBC classifiers are shown in Table 1. All the studies performed unsupervised hierarchical clustering analysis using mRNA expression profiles as the initial step. Although core classifying gene panels and the number of final subtypes were different across the three studies, all the studies had four major subtypes: basal, mesenchymal, LAR, and IM. Owing to tumor heterogeneity, basal-like cancer comprised a large proportion of cases of TNBC. Tumors with enriched immune signaling or luminal androgen pathway were commonly noted across all the three classifiers. Even though the French study did not distinguish the mesenchymal subtype from the other TNBC subtypes, the Vanderbilt and the Baylor studies differentially identified subtypes with enriched mesenchymal features. We summarized emerging therapeutic strategies for each major molecular subtype in Table 2.
Table 1. Comparisons across three molecular classifications in triple-negative breast cancer.
| Author | Year of publication | Data set | No. of patients | Method | Subtype no. | Prognostic discrimination |
|---|---|---|---|---|---|---|
| Lehmann et al. [8] | 2011 | Public | 586 | K-means clustering | 6 | Poorly |
| Burstein et al. [10] | 2014 | Single institute | 198 | NMF | 4 | Well |
| Jézéquel et al. [11] | 2015 | Single institute | 194 | Fuzzy clustering | 3 | Well |
NMF=non-negative matrix factorization.
Table 2. Promising subtype-directed personalized therapy in triple-negative breast cancer.
| Basal-like | Mesenchymal | Immune | Luminal androgen | |
|---|---|---|---|---|
| Biologic pathway | DNA damage response | EMT signaling | Immune cell signaling | Luminal androgen signaling |
| Cell cycle pathway | Wnt signaling | |||
| Notch signaling | ||||
| Promising therapy | Platinum | MET inhibitor | Immune checkpoint inhibitor | Androgen blockade |
| PARP inhibitor | FGFR inhibitor | PIK3CA inhibitor | ||
| mTOR inhibitor |
EMT=epithelial-to-mesenchymal transition; MET=met tyrosine kinase; PARP=poly ADP-ribose polymerase; FGFR=fibroblast growth factor receptors; PIK3CA=phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; mTOR=mammalian target of rapamcyin.
Four major classes of TNBC
Basal-like subtype
In basal-like subtype tumors, the biological pathways involving cell cycle and DNA damage response (e.g., ATR/BRCA) are highly activated, accelerating cell proliferation [8]. Therefore, targeting DNA damage response pathways could be an effective therapeutic approach. Two agents have been emerging as target drugs for tumors with DNA damage response pathways, such as platinum salt and poly ADP-ribose polymerase 1 (PARP) inhibitors [18]. In those tumor cells, there are defects in the homologous recombination repair system, which are vulnerable to platinum salts or PARP inhibitors that lead to DNA cross-link strand breaks.
Platinum salts have been mainly tested as neoadjuvant treatment for TNBC. Recent large phase II studies provided promising results regarding the activity of platinum salts for TNBC. The GeparSixto trial compared paclitaxel, doxorubicin, and bevacizumab with (n=159) or without (n=161) carboplatin as neoadjuvant treatment [19]. The pCR rate was significantly higher in patients treated with carboplatin (58.7%) than in patients treated without carboplatin (37.9%). Another study, CALGB40603, tested the addition of carboplatin or bevacizumab to backbone chemotherapy of weekly paclitaxel followed by dose-dense doxorubicin-cyclophosphamide as neoadjuvant treatment for patients with TNBC [20]. The pCR rate was also higher in women treated with carboplatin (54%) than in women treated without carboplatin (41%). Apart from the pCR rates, further results regarding survival outcomes are needed from both studies.
Platinum agents have also been tested for the treatment of metastatic TNBC. A phase III trial, the CBCSG006 study showed that cisplatin in addition to gemcitabine could be an alternative or the preferred first-line option for metastatic TNBC compared with paclitaxel plus gemcitabine [21]. The progression-free survival was 7.73 months in patients treated with cisplatin (n=120) and 6.47 months in patients treated with paclitaxel (n=120). A significant difference was found in both the noninferiority and superiority tests.
Another phase III trial, the TNT study compared carboplatin monotherapy with docetaxel monotherapy for patients with metastatic or recurrent locally advanced triple-negative or BRCA1/2 breast cancer [22]. The primary end-point was the objective response rate (ORR). The ORR was not significantly different between the two groups: 31.4% in patients treated with carboplatin (n=188) versus 35.6% in patients treated with docetaxel (n=188). However, the ORR of carboplatin was significantly higher than that of docetaxel in women with BRCA1/2 mutation (68.0% vs. 33.3%, respectively). The superiority of carboplatin to docetaxel considering the ORR was not found in patients without BRCA1/2 mutation. The observation of superior response to cisplatin in BRCA1/2 carriers implies that BRCA1/2 germline mutations can be predictive for platinum treatment.
Inconsistent results from those studies testing platinum agents in patients with TNBC may be associated with the fact that patients with non-basal-like tumors according to the Vanderbilt classifier may still be included in the study populations. Non-basal-like tumors occupying near half of TNBC have been a confounding component to identify the true benefit of platinum agents for patients with basal-like tumors. Further analyses excluding non-basal-like tumors by the Vanderbilt subtype in these studies will be needed to exactly evaluate the clinical benefit of platinum agents for patients with basal-like tumors.
PARP inhibitors—as a target agent for DNA repair systems—are also promising drugs for basal-like tumors. Despite the failure of iniparib in a phase III study [23], a new class of PARP inhibitors including olaparib and rucaparib has been tested in ongoing trials. Findings from earlier studies highlight the importance of predictive biomarkers for PARP inhibitors. In addition to germline BRCA mutations, biomarkers associated with BRCAness have been developed. For this purpose, the homologous recombination deficiency (HRD) score was also adopted to identify tumors with BRCAness, which has a homologous recombinant pathway deficiency [18]. In addition, the BRCA1 methylation status can be a potential marker associated with BRCAness [24,25]. To understand the clinical response rate of agents targeting the DNA repair system, predictive biomarkers are urgently needed.
Mesenchymal subtype
Diverse biological processes are enriched in tumors with the mesenchymal subtype. Genomic data suggested that gene clusters involving cell motility, extracellular matrix interaction, epithelial-to-mesenchymal transition (EMT), and growth factor signaling pathways contribute to the unique features of mesenchymal tumors. Interestingly, more than half of metaplastic carcinoma cases are classified into mesenchymal tumors (16 of 28; 57.1%) according to the histology [26].
To treat mesenchymal-like tumors, various therapeutic approaches have been evaluated owing to the heterogeneity of TNBC. In a previous study with the Vanderbilt subtype, Lehmann et al. [8] proposed that mesenchymal-like TNBC may be sensitive to mTOR inhibitors such as NVP-BEZ235 because these cancer cells have activated PI3K/AKT signaling owing to PIK3CA mutations or PTEN deficiency. In addition, eribulin mesylate, which significantly suppress the EMT pathway in breast cancer cells, may be another treatment option for mesenchymal-like tumors [27]. For targeting the EMT pathway, it is evident that inhibition of the fibroblast growth factor receptor pathway can be actionable in these tumors [28,29].
Immune-enriched subtype
The IM subtype is characterized as tumors that have enriched genes involving immune cell processes. Gene enrichment associated with immune cell signaling is a common characteristic in the IM subtype in the Vanderbilt classifier [8], BLIA in the Baylor classifier [10], and C3 in the French classifier [11]. Enriched gene clusters of the IM subtype include immune cell signaling associated with T cells, B cells, NK cells, and dendritic cells; antigen presentation signaling; cytokine signaling; and immune signal transduction such as NF-κB, JAK/STAT, and tumor necrotic factor (TNF) signaling. Considering distinct histologic phenotype such as lymphocytic infiltrations in stromal tissue, medullary carcinoma may be classified into this molecular subtype. Using the current treatments with cytotoxic chemotherapy, patients with the IM subtype showed a better treatment outcome compared with patients with other subtypes. However, it is unclear whether patients with the IM subtype may derive more benefits from immune checkpoint blockade, and ongoing studies with this type of immune drugs will answer this.
Luminal AR subtype
The LAR subtype is the most distinct subtype. In these tumors, hormone regulation pathways and estrogen/androgen metabolism pathways are expressed differentially compared to tumors with the other subtypes. In addition, DNA copy number analysis by Burstein et al. [10] revealed that LAR tumors are biologically distinguished from other subtypes. For tumors with the LAR subtype, an approach for luminal androgen blockade has a theoretical priority owing to its unique biological pathway, as this will aid in developing targeted therapy for LAR tumors.
Gucalp et al. [30] reported a phase II trial evaluating the clinical benefit of bicalutamide, an AR blocker, in patients with AR-positive TNBC. In 51 of 424 AR-positive patients (12%), the clinical benefit rate (CBR) was 19%, and supported the concept that androgen blockade is clinically actionable in AR-positive TNBC.
In the 2015 annual meeting of the American Society of Clinical Oncology, researchers presented the results of a phase II study of enzalutamide, another AR inhibitor, in advanced AR-positive TNBC [31]. In 75 patients with AR-positive TNBC (AR ≥10%), the CBR was 35%. In addition, the CBR was 39% for 56 patients with positive molecular AR-signatures, whereas it was 11% for 62 patients with negative signatures. These two studies provide clinical evidence that AR blockade may offer a clinical benefit for patients with AR-positive TNBC.
Furthermore, Lehmann et al. [32] suggested that PI3K inhibitors in addition to an AR antagonist would be more effective in treating AR-positive TNBC because PIK3CA mutations are frequently activated in these tumors. Further studies testing the clinical effect of concurrent treatment of PI3K inhibitors and AR blockades are warranted in future.
PROGNOSTIC DIFFERENTIATION
In terms of the prognosis, survival differences according to the molecular subtypes are pronounced in the Baylor and the French studies, but not in the Vanderbilt study. All the three studies agree that the patients with immune-enriched subtype have the best survival outcomes. Conversely, for patients with the LAR subtype, the survival outcome was the worst in the French study. In the last two studies, the mesenchymal type or BLIS showed the worst outcomes. Thus, there is a discrepancy in predicting the prognosis according to the molecular subtypes of TNBC.
PERSPECTIVES AND FUTURE DIRECTION
As these studies exemplified, there are efforts in classifying TNBC by gene expression profiling with biologic relevance. Despite the discrepancy in the number of subtypes or the classifying methods, all the studies suggested that TNBC consists of several subtypes and may require subtype-specific therapy based on their biological characteristics. All these studies with molecular classifications provide sufficient evidence that there are four major subtypes, indicating the need for subtype-targeted therapy for TNBC.
In addition, a single biomarker has inherit limitations; for instance, the HRD score only identifies tumor with homologous recombinant deficiency that may be treated with DNA damage response targeting drugs, while tumors with a low HRD score remain a group without targeted therapy. The molecular diversity of TNBC cannot be dissected by a single biomarker.
CONCLUSION
In conclusion, to deliver optimizing therapies for most patients with TNBC, a comprehensive classification is necessary based on genomic data. This type of classifier will offer opportunities for both subtyping and subtype-guided therapy in patients with TNBC. In the near future, the subtypes of TNBC would be easily identified that, in conjunction with clinically available classifiers, will help advance the management of women with TNBC.
Footnotes
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C3396).
CONFLICT OF INTEREST: The authors declare that they have no competing interests
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 3.Trudeau M, Charbonneau F, Gelmon K, Laing K, Latreille J, Mackey J, et al. Selection of adjuvant chemotherapy for treatment of node-positive breast cancer. Lancet Oncol. 2005;6:886–898. doi: 10.1016/S1470-2045(05)70424-1. [DOI] [PubMed] [Google Scholar]
- 4.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 5.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 6.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 7.Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H, et al. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9:R65. doi: 10.1186/bcr1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burstein MD, Tsimelzon A, Poage GM, Covington KR, Contreras A, Fuqua SA, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21:1688–1698. doi: 10.1158/1078-0432.CCR-14-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jézéquel P, Loussouarn D, Guérin-Charbonnel C, Campion L, Vanier A, Gouraud W, et al. Gene-expression molecular subtyping of triple-negative breast cancer tumours: importance of immune response. Breast Cancer Res. 2015;17:43. doi: 10.1186/s13058-015-0550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann BD, Pietenpol JA. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J Pathol. 2014;232:142–150. doi: 10.1002/path.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Li J, Gray WH, Lehmann BD, Bauer JA, Shyr Y, et al. TNBCtype: a subtyping tool for triple-negative breast cancer. Cancer Inform. 2012;11:147–156. doi: 10.4137/CIN.S9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez-Angulo AM, Meric-Bernstam F, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res. 2013;19:5533–5540. doi: 10.1158/1078-0432.CCR-13-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abramson VG, Lehmann BD, Ballinger TJ, Pietenpol JA. Subtyping of triple-negative breast cancer: implications for therapy. Cancer. 2015;121:8–16. doi: 10.1002/cncr.28914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahn SG, Lee HM, Lee HW, Lee SA, Lee SR, Leem SH, et al. Prognostic discrimination using a 70-gene signature among patients with estrogen receptor-positive breast cancer and an intermediate 21-gene recurrence score. Int J Mol Sci. 2013;14:23685–23699. doi: 10.3390/ijms141223685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watkins JA, Irshad S, Grigoriadis A, Tutt AN. Genomic scars as biomarkers of homologous recombination deficiency and drug response in breast and ovarian cancers. Breast Cancer Res. 2014;16:211. doi: 10.1186/bcr3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15:747–756. doi: 10.1016/S1470-2045(14)70160-3. [DOI] [PubMed] [Google Scholar]
- 20.Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance) J Clin Oncol. 2015;33:13–21. doi: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu XC, Zhang J, Xu BH, Cai L, Ragaz J, Wang ZH, et al. Cisplatin plus gemcitabine versus paclitaxel plus gemcitabine as first-line therapy for metastatic triple-negative breast cancer (CBCSG006): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2015;16:436–446. doi: 10.1016/S1470-2045(15)70064-1. [DOI] [PubMed] [Google Scholar]
- 22.Tutt A, Ellis P, Kilburn L, Gilett C, Pinder S, Abraham J, et al. The TNT trial: a randomized phase III trial of carboplatin compared with docetaxel for patients with metastatic or recurrent locally advanced triple negative or BRCA1/2 breast cancer. Cancer Res. 2015;75(9 Suppl):S3–01. [Google Scholar]
- 23.O'Shaughnessy J, Schwartzberg L, Danso MA, Miller KD, Rugo HS, Neubauer M, et al. Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2014;32:3840–3847. doi: 10.1200/JCO.2014.55.2984. [DOI] [PubMed] [Google Scholar]
- 24.Murria Estal R, Palanca Suela S, de Juan Jiménez I, Alenda Gonzalez C, Egoavil Rojas C, García-Casado Z, et al. Relationship of immunohistochemistry, copy number aberrations and epigenetic disorders with BRCAness pattern in hereditary and sporadic breast cancer. Fam Cancer. 2016;15:193–200. doi: 10.1007/s10689-015-9864-2. [DOI] [PubMed] [Google Scholar]
- 25.Ruscito I, Dimitrova D, Vasconcelos I, Gellhaus K, Schwachula T, Bellati F, et al. BRCA1 gene promoter methylation status in high-grade serous ovarian cancer patients: a study of the tumour Bank ovarian cancer (TOC) and ovarian cancer diagnosis consortium (OVCAD) Eur J Cancer. 2014;50:2090–2098. doi: 10.1016/j.ejca.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Weigelt B, Ng CK, Shen R, Popova T, Schizas M, Natrajan R, et al. Metaplastic breast carcinomas display genomic and transcriptomic heterogeneity [corrected] Mod Pathol. 2015;28:340–351. doi: 10.1038/modpathol.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida T, Ozawa Y, Kimura T, Sato Y, Kuznetsov G, Xu S, et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states. Br J Cancer. 2014;110:1497–1505. doi: 10.1038/bjc.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen PT, Tsunematsu T, Yanagisawa S, Kudo Y, Miyauchi M, Kamata N, et al. The FGFR1 inhibitor PD173074 induces mesenchymal-epithelial transition through the transcription factor AP-1. Br J Cancer. 2013;109:2248–2258. doi: 10.1038/bjc.2013.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wendt MK, Taylor MA, Schiemann BJ, Sossey-Alaoui K, Schiemann WP. Fibroblast growth factor receptor splice variants are stable markers of oncogenic transforming growth factor beta1 signaling in metastatic breast cancers. Breast Cancer Res. 2014;16:R24. doi: 10.1186/bcr3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gucalp A, Tolaney S, Isakoff SJ, Ingle JN, Liu MC, Carey LA, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin Cancer Res. 2013;19:5505–5512. doi: 10.1158/1078-0432.CCR-12-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Traina TA, Miller K, Yardley DA, O'Shaughnessy J, Cortes J, Awada A, et al. Results from a phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) inhibitor, in advanced AR+ triple-negative breast cancer (TNBC) J Clin Oncol. 2015;33(15 Suppl):S1003. [Google Scholar]
- 32.Lehmann BD, Bauer JA, Schafer JM, Pendleton CS, Tang L, Johnson KC, et al. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res. 2014;16:406. doi: 10.1186/s13058-014-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]