Abstract
Purpose
B-cell lymphoma 2 (BCL2) is an antiapoptosis protein and an important clinical breast cancer prognostic marker. As the role of BCL2 is dependent on the estrogen receptor (ER) status, this effect might differ according to molecular subtypes. The aim of this study was to evaluate the relationship between the prognostic outcomes and BCL2 expression among the molecular subtypes.
Methods
We retrieved the data of 1,356 patients who were newly diagnosed with malignant breast cancer between November 2006 and November 2011. Immunohistochemistry was used to measure ER, progesterone receptor, human epidermal growth factor receptor 2 (HER2), Ki-67, and BCL2 expression. We classified breast cancer into five molecular subtypes based on the 13th St. Gallen International Expert Consensus, including luminal A, luminal B (HER2-negative), luminal B (HER2-positive), HER2-overexpression, and triple-negative subtypes. We analyzed the clinicopathological features and assessed the correlation between BCL2 expression and clinical outcomes, such as relapse-free survival (RFS) and disease-specific survival (DSS) according to the five molecular subtypes.
Results
A total of 605 cases of breast cancer (53.8%) showed BCL2 expression. BCL2-positive expression was associated with young age (<50 years, p=0.036), lower histological grade (p<0.001), low Ki-67 level (<14%, p<0.001), hormone receptor positivity (p<0.001), HER2 negativity (p<0.001), luminal breast cancer (p<0.001), and low recurrence rate (p=0.016). BCL2-positive expression was also associated with favorable 5-year RFS (p=0.008, 91.4%) and DSS (p=0.036, 95.6%) in all the patients. BCL2-positive expression in luminal A breast cancer resulted in significantly favorable 5-year RFS and DSS (p=0.023 and p=0.041, respectively). However, BCL2 expression was not associated with the prognosis in the other subtypes.
Conclusion
The prognostic role of BCL2 expression in breast cancer is subtype-specific. BCL2 expression differs according to the molecular subtype and is a good prognostic marker for only luminal A breast cancer.
Keywords: B-cell lymphoma 2 protein, Breast neoplasms, Prognosis, Tumor biomarkers
INTRODUCTION
B-cell Lymphoma 2 (BCL2) is an antiapoptotic protein belong to the BCL2 family [1,2]. The role of BCL2 differs depending on its interaction with other members of the BCL2 family [1,2,3]. BCL2 is expressed in solid tumors, such as breast, prostate, colorectal, lung, stomach, and ovarian cancers [1,2]. BCL2 family proteins, including BCL2 and BAX, are expressed in normal mammary tissue [2,4]. Of the BCL2 family proteins, BCL2 is upregulated by estrogens in breast cancer, through a direct consequence of transcriptional induction [4]. Therefore, BCL2-positive expression in breast cancer may be considered a sign of estrogen receptor (ER) functional activity and is associated with the outcomes [2,4,5,6,7]. Recent studies have suggested that BCL2 is a reliable prognostic marker, particular for hormone receptor (HR)-positive breast cancer [6,7].
Patients with BCL2-positive expression in breast cancer have a better prognosis considering the overall survival and relapse-free survival (RFS) [8]. BCL2-positive expression is associated with better outcomes of metastatic and early breast cancer treated with either hormone therapy or chemotherapy [9,10]. Some studies have consistently reported an association between BCL2 expression and better survival in patients with triple-negative breast cancer (TNBC) [11,12,13].
The mechanism for the differences in prognosis remains unclear, but BCL2 expression is dependent on the ER status, so the different roles of BCL2 appear to be dependent on the molecular subtype. In this study, we investigated the role of BCL2 as a prognostic marker according to the 13th St. Gallen immunohistochemical classification [14].
METHODS
Patient selection
In this single-center trial, a cohort of patients with biopsy-proven invasive breast cancer who underwent surgical treatment and adjuvant treatment was reviewed retrospectively from the hospital's breast cancer enter database and the patient's medical records. A total of 1,356 patients were newly diagnosed with malignant breast cancer in the Department of Surgery, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine between November 2006 and November 2011. This study was approved by the Institutional Review Board of Seoul St. Mary's Hospital (KC 15RISI0804).
The exclusion criteria were the presence of noninvasive carcinoma (e.g., ductal carcinoma in situ), distant metastasis at diagnosis, and any other malignancy. Patients underwent surgical treatment received adjuvant chemotherapy, and/or endocrine therapy, and/or radiation therapy according to standard protocols.
RFS was defined as the time from the first diagnosis of breast cancer to first recurrence including locoregional recurrence and distant recurrence. Disease-specific survival (DSS) was defined as the time from the first diagnosis of breast cancer to death due to breast cancer.
Immunohistochemistry and assay methods
Immunohistochemistry (IHC) for ER (SP1, prediluted; Roche Science, Mannheim, Germany), progesterone receptor (PR; 1E2, prediluted; Roche), human epidermal growth factor receptor 2 (HER2; 4B5, prediluted; Roche), Ki-67 (MIB-1, prediluted; Roche), and BCL2 (124, 1:50; Dako, Carpinteria, USA) was performed on whole tissue section slides using BenchMark ULTRA (Ventana Medical Systems Inc., Tucson, USA). A positive ER and PR status was defined as an Allred score (AS) of ≥3. IHC or fluorescence in situ hybridization was performed to evaluate HER2 status. A positive HER2 status was defined as an IHC score of 3+. If the IHC score was 2+, the sample was retested with single-probe silver in situ hybridization. The amplification ratio was defined as the HER2 gene locus copy number relative to the chromosome 17 centromere copy number, and an amplification ratio of ≥2.0 was considered positive. The Ki-67 level was classified into three cutoff points according to the expressing cell ratio (<14%, 14%–19%, and ≥20%). BCL2 expression was assessed using the percentage of positively stained cells. BCL2 expression was positive when >10% of the cells stained positive for BCL2. This cutoff value was used considering the statistical guidelines for prognostic factors reported previously [15,16]. A single pathologist interpreted all the IHC results.
Classification of intrinsic molecular subtypes using the 13th St. Gallen International Expert Consensus
IHC was used to classify the tumors into five different molecular subtypes [14]: (1) luminal A: all ER and/or PR positive, HER2-negative, Ki-67 low (<14%); (2) luminal B (HER2 negative): ER positive, HER2 negative, and at least one of: Ki-67 high (≥20%), PR negative or low (<20%); (3) luminal B (HER2 positive): ER positive, HER2 positive, any Ki-67, any PR; (4) HER2-overexpression: HER2 positive, ER and PR negative; and (5) TNBC: ER and PR negative, HER2 negative.
All cancers were staged according to the seventh edition of the American Joint Committee on Cancer staging manual.
Statistical analysis
Clinical and pathological features were assessed using the Student t-test, the chi-square test, and Fisher exact test. Cumulative survival probabilities were estimated using the Kaplan-Meier analysis. The log-rank test was used to compare differences between survival rates. A multivariate analysis was performed using the Cox proportional hazards model. All variables were described as the hazard ratio and 95% confidence intervals (CIs). A two-sided p-value <0.05 was considered significant. The statistical analyses were performed with IBM SPSS software version 18.0 for Windows (SPSS Inc., Chicago, USA).
RESULTS
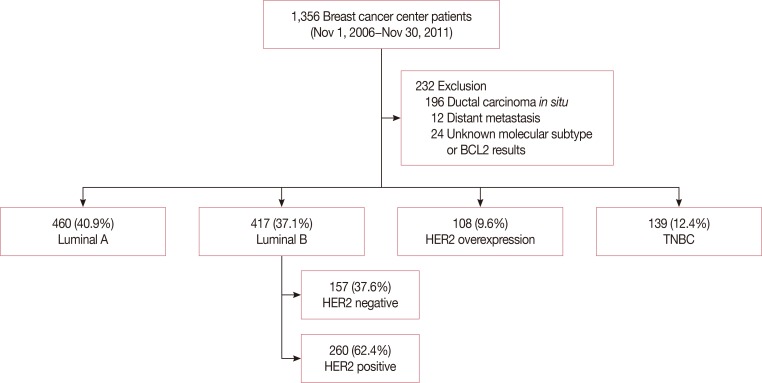
A total of 1,356 patients were enrolled in our study. After excluding 208 patients who satisfied the exclusion criteria, and 24 patients who had unknown molecular subtypes or unknown BCL2 results, 1,124 were finally included in the statistical analysis (Figure 1). The patients was classified into five groups based on the molecular subtype from the 13th St. Gallen International Expert consensus, including luminal A, luminal B (HER2-negative), luminal B (HER2-positive), HER2-overexpression, and TNBC (Figure 1). The mean patient age was 51.01±9.98 years, and the mean follow-up was 107.62±0.56 months. The clinicopathological characteristic and IHC results are summarized in Table 1.
Figure 1. Flowchart of the patient cohort included in this study. Exclusion criteria was noninvasive carcinoma (e.g., ductal carcinoma in situ), distant metastasis at diagnosis, and any other malignancy. Different frequency of BCL2 positive expression was observed depending on the molecular subtypes.
BCL2=B-cell lymphoma 2; HER2=human epidermal growth factor receptor 2; TNBC=triple-negative breast cancer.
Table 1. Clinicopathological characteristic according to B-cell lymphoma 2 expression.
| Characteristic | All patients (n = 1,124) | p-value | Characteristic | All patients (n = 1,124) | p-value | ||
|---|---|---|---|---|---|---|---|
| BCL2 negative (n = 519) No. (%) |
BCL2 positive (n = 605) No. (%) |
BCL2 negative (n = 519) No. (%) |
BCL2 positive (n = 605) No. (%) |
||||
| Mean age (yr)* | 51.46 ± 10.69 | 50.19 ± 10.33 | 0.045 | ER | < 0.001 | ||
| Age (yr) | 0.036 | Negative | 227 (43.7) | 40 (6.6) | |||
| < 50 | 222 (42.8) | 332 (54.9) | Positive | 292 (56.3) | 565 (93.4) | ||
| ≥ 50 | 297 (57.2) | 273 (45.1) | PR | < 0.001 | |||
| Breast operation | 0.821 | Negative | 294 (56.6) | 114 (18.8) | |||
| Breast conservation | 294 (56.6) | 339 (56.0) | Positive | 225 (43.4) | 491 (81.2) | ||
| Mastectomy | 225 (43.4) | 266 (44.0) | HER2 | < 0.001 | |||
| Axillary operation | 0.634 | Negative | 312 (60.1) | 444 (73.4) | |||
| No | 3 (0.6) | 5 (0.8) | Positive | 207 (39.9) | 161 (26.6) | ||
| SLNB | 307 (59.2) | 341 (56.4) | Ki-67 (%) | < 0.001 | |||
| ALND | 209 (40.3) | 259 (42.8) | <14 | 214 (41.4) | 383 (63.4) | ||
| T stage | 0.037 | ≥14 | 301 (58.2) | 218 (36.1) | |||
| T1 | 318 (61.3) | 415 (68.6) | Unknown | 4 (0.4) | 4 (0.5) | ||
| T2 | 179 (34.5) | 170 (28.1) | p53 | < 0.001 | |||
| T3–T4 | 22 (4.2) | 20 (3.3) | Negative | 177 (34.2) | 229 (37.9) | ||
| N stage | 0.211 | Positive | 130 (25.1) | 67 (11.1) | |||
| N0 | 331 (63.8) | 421 (69.6) | Unknown | 212 (40.6) | 309 (51.1) | ||
| N1 | 120 (23.1) | 123 (20.3) | EGFR | < 0.001 | |||
| N2 | 42 (8.1) | 32 (5.3) | Negative | 327 (63.0) | 537 (88.8) | ||
| N3 | 23 (4.4) | 24 (4.0) | Positive | 185 (35.6) | 49 (8.1) | ||
| Unknown | 3 (0.6) | 5 (0.8) | Unknown | 7 (1.4) | 19 (3.1) | ||
| Stage | 0.011 | CK5/6 | <0.001 | ||||
| I | 241 (46.4) | 340 (56.2) | Negative | 352 (67.8) | 367 (60.7) | ||
| II | 206 (39.7) | 199 (32.9) | Positive | 114 (22.0) | 50 (8.3) | ||
| III | 69 (13.3) | 61 (10.1) | Unknown | 53 (10.2) | 188 (31.0) | ||
| Unknown | 3 (0.6) | 5 (0.8) | Molecular subtypes | <0.001 | |||
| Multiplicity | 0.092 | Luminal A | 133 (25.6) | 327 (54.0) | |||
| One | 434 (83.6) | 506 (83.6) | Luminal B (HER2 negative) | 64 (12.3) | 93 (15.4) | ||
| Two | 41 (7.9) | 63 (10.4) | Luminal B (HER2 positive) | 107 (20.6) | 153 (25.3) | ||
| More than three | 44 (8.5) | 36 (6.0) | HER2 overexpression | 100 (19.3) | 8 (1.3) | ||
| Histologic grade (Nottingham Histologic Score system) | <0.001 | TNBC | 115 (22.2) | 24 (4.0) | |||
| Well | 114 (22.0) | 224 (37.0) | Neoadjuvant chemotherapy | 0.025 | |||
| Moderately | 245 (47.2) | 301 (49.8) | No | 455 (87.7) | 555 (91.7) | ||
| Poorly | 160 (30.8) | 80 (13.2) | Yes | 64 (12.3) | 50 (8.3) | ||
| LVI | 0.011 | Adjuvant chemotherapy | 0.028 | ||||
| No | 326 (62.8) | 422 (69.8) | No | 21 (4.0) | 11 (1.8) | ||
| Yes | 188 (36.2) | 182 (30.1) | Yes | 498 (96.0) | 592 (97.9) | ||
| Unknown | 5 (1.0) | 1 (0.2) | Unknown | 0 | 2 (0.3) | ||
| Perineural invasion | 0.126 | Recurrence | 0.008 | ||||
| No | 493 (95.0) | 573 (94.7) | No | 452 (87.1) | 554 (91.6) | ||
| Yes | 19 (3.7) | 30 (5.0) | Yes | 67 (12.9) | 51 (8.4) | ||
| Unknown | 7 (1.3) | 2 (0.3) | Death | 0.036 | |||
| No | 496 (95.6) | 590 (97.5) | |||||
| Yes | 23 (4.4) | 15 (2.5) | |||||
BCL2=B-cell lymphoma 2; SLNB=sentinel lymph node biopsy; ALND=axillary lymph node dissection; LVI=lymphovascular invasion; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; EGFR=epidermal growth factor receptor; CK5/6= cytokeratin 5/6; TNBC=triple-negative breast cancer.
*Mean±SD.
BCL2 expression as a prognostic factor
Of the 1,124 patients, 519 (46.2%) were BCL2-negative and 605 (53.8%) were BCL2-positive. The clinicopathological characteristic according to BCL2 expression are summarized in Table 1. BCL2-positive expression was associated with young age (<50 years, p=0.036), histological grade (p<0.001), low Ki-67 level (<14%, p<0.001), HR positivity (p<0.001), HER2 negativity (p<0.001), p53 negativity (p<0.001), epidermal growth factor receptor (EGFR) negativity (p<0.001), cytokeratin 5/6 (CK5/6) negativity (p<0.001), and the luminal type of breast cancer (p<0.001). Patients with ER-positive breast cancer were classified into two groups: low and high expression. Low ER-positive expression was defined as an AS between 3 and 5, and high ER-positive expression was defined as an AS between 6 and 8. High ER-positive expression was association with BCL2-positive expression (Table 2). However, there was no significant difference in the RFS and DSS according to the AS for ER.
Table 2. Correlation between B-cell lymphoma 2 expression and estrogen receptor positive expression.
| Characteristic | ER positive patient (n=857) | p-value | |
|---|---|---|---|
| BCL2 negative (n=292) No. (%) |
BCL2 positive (n=565) No. (%) |
||
| ER | <0.001 | ||
| Low expression (AS 3–5) | 42 (68.9) | 19 (31.1) | |
| High expression (AS 6–8) | 250 (31.4) | 546 (68.6) | |
ER=estrogen receptor; BCL2=B-cell lymphoma 2; AS=Allred score.
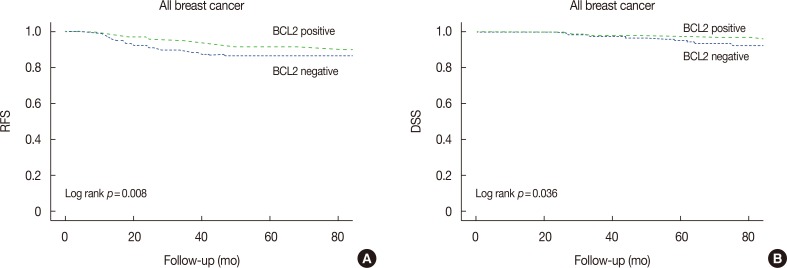
BCL2-positive breast cancer was significantly related with a favorable 5-year RFS (p=0.008; BCL2 negative vs. positive, 86.3% vs. 91.4%) and 5-year DSS (p=0.036; BCL2 negative vs. positive, 95.6% vs. 98.0%) (Figure 2).
Figure 2. Kaplan-Meier analysis of 5-year relapse-free survival (RFS) and disease-specific survival (DSS) for B-cell lymphoma 2 (BCL2) positive and BCL2-negative patients. Patients expressing BCL2 have longer 5-year RFS (A) (p=0.008) and 5-year DSS (B) (p=0.036) compared to patients with no BCL2 expression.
Different prognostic effect of BCL2 expression based on molecular subtypes
Different frequency and prognostic effects of BCL2-positive expression were observed depending on the 13th St. Gallen IHC classification. BCL2-positive expression was more common in luminal A and luminal B breast cancer than in other subtypes (Table 2).
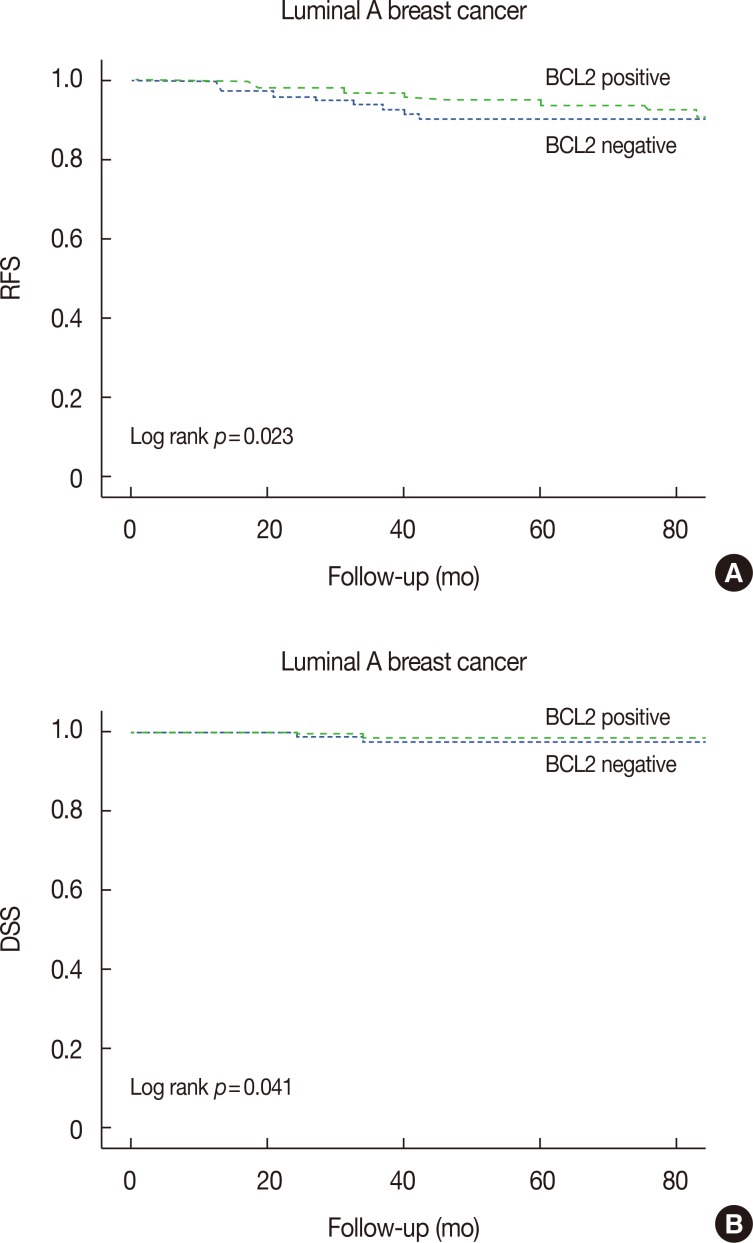
The prognostic significance of BCL2-positive expression for 5-year RFS and DSS was demonstrated in luminal A breast cancer (RFS, p=0.023; DSS, p=0.041) (Figure 3). BCL2-positive expression showed a more favorable prognostic effect than that of BCL2-negative expression in luminal A breast cancer (Figure 3).
Figure 3. Kaplan-Meier analysis of 5-year relapse-free survival (RFS) and 5-year disease-specific survival (DSS) for B-cell Lymphoma 2 (BCL2)-positive and BCL2-negative luminal A breast cancer patients. Patients expressing BCL2 have longer 5-year RFS (A) (p=0.023) and 5-year DSS (B) (p=0.041).
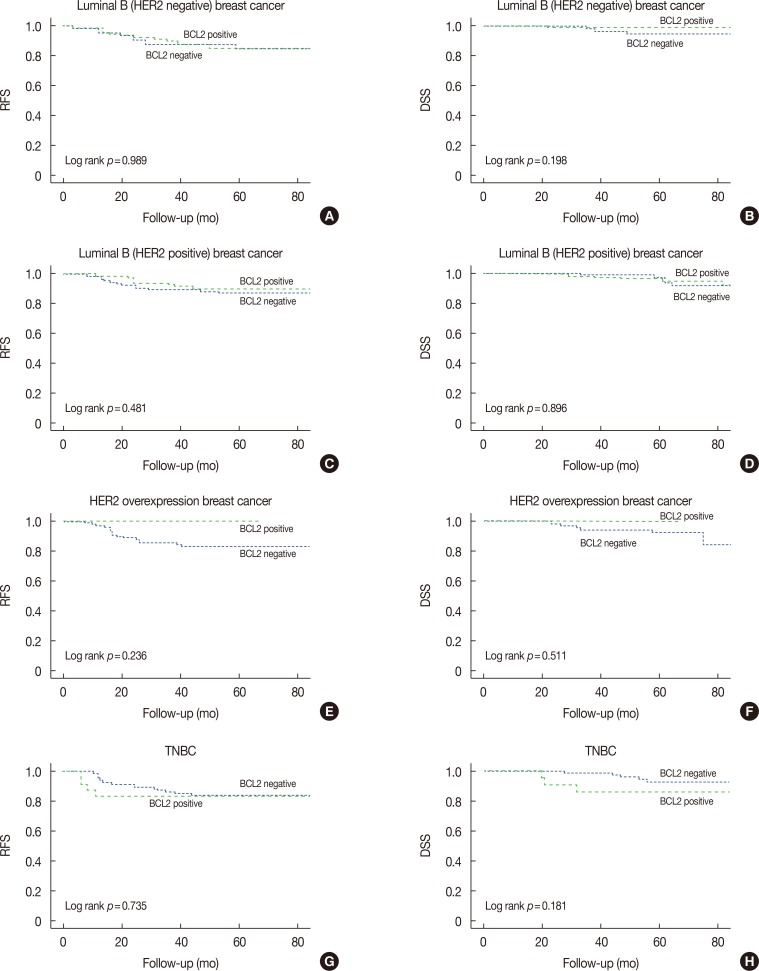
BCL2-positive expression tended to result in a longer 5-year RFS and DSS in luminal B (HER2-negative; RFS, p=0.989; DSS, p=0.198), luminal B (HER2-positive; RFS, p=0.481; DSS, p=0.896), and HER2-overexpression breast cancers (RFS, p=0.236; DSS, p=0.511) (Figure 4). However, BCL2-positive expression tended to result in a shorter 5-year RFS and DSS in TNBC (RFS, p=0.735; DSS, p=0.181) (Figure 4).
Figure 4. Kaplan-Meier analysis of 5-year relapse-free survival (RFS) and 5-year disease-specific survival (DSS) for B-cell lymphoma 2 (BCL2)-positive and BCL2-negative breast cancer patients. BCL2-positive luminal B, human epidermal growth factor receptor 2 (HER2) overexpression, and triple-negative breast cancer (TNBC) breast cancer patients have no longer 5-year RFS (A, C, E, and G) and 5-year DSS (B, D, F, and H) compared to patients with no BCL2 expression.
Multivariate analysis revealed that tumor size, lymph node metastasis, and BCL2 expression were independent prognostic factors for RFS (hazard ratio, 0.53; 95% CI, 0.29–0.97; p=0.015) (Table 3) and DSS (hazard ratio, 0.59; 95% CI, 0.08–0.73; p=0.021) in luminal A breast cancer (Table 4).
Table 3. Univariate and multivariate Cox regression analysis for relapse-free survival in luminal A breast cancer.
| Clinicopathological variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| Age (yr) | 0.002 | 0.033 | ||
| ≥50 | Ref | Ref | ||
| <50 | 2.63 (1.33–5.21) | 3.92 (1.12–13.77) | ||
| Tumor size (cm) | 0.004 | <0.001 | ||
| ≤2 | Ref | Ref | ||
| >2 | 2.81 (1.39–5.69) | 2.54 (1.21–5.29) | ||
| LN metastasis | 0.015 | <0.001 | ||
| No | Ref | Ref | ||
| Yes | 2.80 (1.40–5.62) | 5.22 (1.68–16.18) | ||
| LVI | 0.015 | 0.014 | ||
| No | Ref | Ref | ||
| Yes | 2.79 (1.39–5.60) | 1.30 (1.13–6.17) | ||
| Histologic grade | 0.029 | 0.018 | ||
| Well | Ref | Ref | ||
| Moderately to poorly | 2.73 (1.10–6.77) | 1.56 (1.38–7.81) | ||
| BCL2 expression | 0.013 | 0.025 | ||
| Negative | Ref | Ref | ||
| Positive | 0.20 (0.05–0.82) | 0.07 (0.01–0.72) | ||
CI=confidence interval; Ref=reference; LN=lymph node; LVI=lymphovascular invasion; BCL2=B-cell lymphoma 2.
Table 4. Univariate and multivariate Cox regression analysis for disease-specific survival in luminal A breast cancer.
| Clinicopathological variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| Age (yr) | 0.012 | 0.850 | ||
| ≥50 | Ref | Ref | ||
| <50 | 1.78 (1.37–3.53) | 1.09 (0.43–2.76) | ||
| Tumor size (cm) | 0.048 | <0.001 | ||
| ≤2 | Ref | Ref | ||
| >2 | 1.40 (1.29–3.57) | 2.46 (1.19–5.09) | ||
| LN metastasis | 0.016 | 0.027 | ||
| No | Ref | Ref | ||
| Yes | 2.50 (1.24–5.04) | 3.92 (1.12–13.77) | ||
| LVI | 0.007 | 0.191 | ||
| No | Ref | Ref | ||
| Yes | 1.23 (1.11–3.51) | 1.75 (1.43–4.05) | ||
| BCL2 expression | 0.037 | 0.021 | ||
| Negative | Ref | Ref | ||
| Positive | 0.77 (0.39–0.89) | 0.59 (0.21–0.73) | ||
CI=confidence interval; Ref=reference; LN=lymph node; LVI=lymphovascular invasion; BCL2=B-cell lymphoma 2.
DISCUSSION
According to the results of this study, the frequency of BCL2 expression differs among the molecular subtypes of breast cancer and it has a favorable prognostic effect. BCL2-positive expression results in a better prognosis considering the 5-year RFS and DSS, compared to BCL2-negative expression, and it is associated with a younger age, lower histological grade, low Ki-67 level, HR positivity, HER2 negativity, p53 negativity, EGFR negativity, CK5/6 negativity, and the luminal A and B breast cancer subtypes. The results also show that BCL2 expression has different prognostic roles according to the five molecular subtypes in the 13th St. Gallen International Expert Consensus. BCL2-positive expression is an independ-ent favorable prognostic marker for only luminal A breast cancer and is a significant factor in both univariate and multivariate analyses. On the other hand, BCL2 expression does not show a prognostic relationship for the other subtypes.
The prognostic role of BCL2 is first described in non-Hodgkin's lymphoma, and BCL2-positive expression is associated with a favorable prognosis in many solid tumors including breast cancer [17,18]. The mechanism of BCL2 as a prognostic factor in breast cancer remains unclear [2,19]. Many studies have suggested that interactions between BCL2 and ER modulate the prognostic effect [20,21,22]. Moreover, BCL2 expression is upregulated in response to estrogens, and so BCL2 is considered an estrogen-related protein [10,20,21,22].
This study focuses on the different interactions between BCL2 and ER according to the breast cancer molecular subtypes. In the present study, BCL2 was expressed in 52.8% of the patients, which is similar to the results of previous studies [17,23,24]. The frequency of BCL2-positive expression differed according to the molecular subtype. BCL2 was expressed in 54.0% of luminal A, 40.7% of luminal B, 1.3% of HER2-overexpression, and 4.0% of TNBC subtypes. BCL2-positive expression is increased in luminal breast cancer, and decreased in the HER2-overexpression, and TNBC subtypes. Seong et al. [17] reported a similar pattern of BCL2 expression, i.e., of 1,304 patients with breast cancer, 54.4% patients expressed BCL2. By subtypes, BCL2 expression was observed in 74% patients with HR-positive and HER2-negative, 10.7% patients with HR-positive and HER2-positive, 2.8% patients with HR-negative and HER2-positive, and 12.5% patients with HR-negative and HER2-negative breast cancer [17].
We also observed that BCL2 expression was significantly associated with low proliferative factors and ER and PR positivity; therefore, BCL2 expression can be used for predicting an overall good prognosis in patients with breast cancer. In particular, BCL2-positive expression has a positive correlation with the high expression of ER, as shown in the results of this study. Similarly, Dawson et al. [25] revealed that increased BCL2 staining intensity predicts a better prognosis in breast cancer. In contrast, Yang et al. [26] investigated patients with early breast cancer who underwent breast conservative surgery with radiotherapy and found that BCL2 expression correlates with ipsilateral breast recurrence. Yang et al. [26] speculated that BCL2 played an important role in the poor tumor response to radiotherapy and suggested that the conflicting result was possibly due to radiation, which affects the apoptotic function of BCL2.
In our study, BCL2 had different prognostic roles depending on the breast cancer molecular subtype. BCL2 expression was a favorable prognostic factor for 5-year RFS and DSS in luminal A breast cancer, which agrees with the results of previous studies [17,18]. Seong et al. [17] suggested that BCL2 expression is an independent, favorable prognostic factor in patients with HR-positive and HER2-negative breast cancer. In addition, Chen et al. [18] reported that patients with luminal breast cancer with high BCL2 expression have better outcomes compared to those with other breast cancer subtypes.
To the best of our knowledge, although there is no significant difference, patients with luminal B (HER2-negative and HER2-positive) and HER2-overexpression breast cancer with BCL2 expression tend to have good prognosis. This observation is supported by the results of previous studies that showed that BCL2 is inversely correlated with proliferative markers, such as Ki-67, and HER2-overexpression, and hence plays an antiproliferative role despite its antiapoptotic effect [27,28]. In luminal B (HER2-negative) breast cancer, which expresses a high Ki-67 level (threshold ≥20%), BCL2 expression is high, but BCL2 plays an antiproliferative role, resulting in a more favorable outcome compared to that of breast cancer with BCL2-negative expression. A low frequency of BCL2 expression (25.3% and 1.3%, respectively) was detected in luminal B (HER2-positive) and HER2-overexpression breast cancer, respectively, which was consistent with the finding in another study [28]. The strong inverse correlation between BCL2 and HER2 expression may be associated with non-significant prognostic value of BCL2 in luminal B (HER2-positive) and HER2-overexpression breast cancer.
Our result shows that BCL2 expression tends to be associated with a worse prognostic effect in patients with TNBC (5-year RFS, p=0.735; 5-year DSS, p=0.181). TNBC also has low BCL2 expression. This result agrees with those of previous studies reporting that low BCL2 expression is associated with good outcomes of TNBC [2,11,23,29]. Bouchalova et al. [29] investigated 187 patients with TNBC, 164 of who were treated with anthracycline-based adjuvant chemotherapy. They suggested that high BCL2 expression is a significant independent predictor of poor outcomes in patients with TNBC [29]. In contrast, Dawson et al. [25] reviewed 490 patients with stage I to III TNBC and concluded that BCL2 expression is related with better survival. Abdel-Fatah et al. [30] showed that low BCL2 expression resulted in a poor clinical outcome in 390 patients with TNBC. Although the role of BCL2 in TNBC remains controversial, most studies have concluded that BCL2 expression is lower and is associated with a poor prognosis. Eventually, there was no statistically significant relationship between BCL2 expression and TNBC and HER2-overexpression breast cancer in our study. Therefore, we can assume that BCL2 does not have a prognostic role in HR-negative breast cancer because this protein depends on estrogen and the ER pathway.
This study had some limitations. First, because our study was retrospective, selection bias may have been present. Second, as we used an IHC staining method, the results may be affected by intratumoral heterogeneity and interobserver heterogeneity. However, this is the first study to identify the different clinical effects based on BCL2 expression according to the molecular subtypes from the 13th St. Gallen International Expert Consensus. We used single-institutional data and obtained results based on coherent treatment protocols prescribed by two clinicians and standardized measurements of BCL2 performed by a single pathologist at a single institution. We also analyzed recurrence and survival to determine clinical significance of the breast cancer.
In summary, BCL2 expressed different frequency and played different prognostic roles according to breast cancer molecular subtype. BCL2 is an independent favorable prognostic marker for only luminal A breast cancer.
Footnotes
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.Basu A, Haldar S. The relationship between BcI2, Bax and p53: consequences for cell cycle progression and cell death. Mol Hum Reprod. 1998;4:1099–1109. doi: 10.1093/molehr/4.12.1099. [DOI] [PubMed] [Google Scholar]
- 2.Bouchalova K, Kharaishvili G, Bouchal J, Vrbkova J, Megova M, Hlobilkova A. Triple negative breast cancer: BCL2 in prognosis and prediction. Review. Curr Drug Targets. 2014;15:1166–1175. doi: 10.2174/1389450115666141106151143. [DOI] [PubMed] [Google Scholar]
- 3.Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci. 2009;122(Pt 4):437–441. doi: 10.1242/jcs.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung LK, Wang TT. Paradoxical regulation of Bcl-2 family proteins by 17beta-oestradiol in human breast cancer cells MCF-7. Br J Cancer. 1999;81:387–392. doi: 10.1038/sj.bjc.6690706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aleskandarany MA, Soria D, Green AR, Nolan C, Diez-Rodriguez M, Ellis IO, et al. Markers of progression in early-stage invasive breast cancer: a predictive immunohistochemical panel algorithm for distant recurrence risk stratification. Breast Cancer Res Treat. 2015;151:325–333. doi: 10.1007/s10549-015-3406-3. [DOI] [PubMed] [Google Scholar]
- 6.Choi JE, Kang SH, Lee SJ, Bae YK. Prognostic significance of Bcl-2 expression in non-basal triple-negative breast cancer patients treated with anthracycline-based chemotherapy. Tumour Biol. 2014;35:12255–12263. doi: 10.1007/s13277-014-2534-4. [DOI] [PubMed] [Google Scholar]
- 7.Samy N, Ragab HM, El Maksoud NA, Shaalan M. Prognostic significance of serum Her2/neu, BCL2, CA15-3 and CEA in breast cancer patients: a short follow-up. Cancer Biomark. 2010;6:63–72. doi: 10.3233/CBM-2009-0119. [DOI] [PubMed] [Google Scholar]
- 8.Kim HS, Moon HG, Han W, Yom CK, Kim WH, Kim JH, et al. COX2 overexpression is a prognostic marker for Stage III breast cancer. Breast Cancer Res Treat. 2012;132:51–59. doi: 10.1007/s10549-011-1521-3. [DOI] [PubMed] [Google Scholar]
- 9.Tsutsui S, Yasuda K, Suzuki K, Takeuchi H, Nishizaki T, Higashi H, et al. Bcl-2 protein expression is associated with p27 and p53 protein expressions and MIB-1 counts in breast cancer. BMC Cancer. 2006;6:187. doi: 10.1186/1471-2407-6-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gasparini G, Barbareschi M, Doglioni C, Palma PD, Mauri FA, Boracchi P, et al. Expression of bcl-2 protein predicts efficacy of adjuvant treatments in operable node-positive breast cancer. Clin Cancer Res. 1995;1:189–198. [PubMed] [Google Scholar]
- 11.Abdel-Fatah TM, Perry C, Dickinson P, Ball G, Moseley P, Madhusudan S, et al. Bcl2 is an independent prognostic marker of triple negative breast cancer (TNBC) and predicts response to anthracycline combination (ATC) chemotherapy (CT) in adjuvant and neoadjuvant settings. Ann Oncol. 2013;24:2801–2807. doi: 10.1093/annonc/mdt277. [DOI] [PubMed] [Google Scholar]
- 12.Hwang KT, Woo JW, Shin HC, Kim HS, Ahn SK, Moon HG, et al. Prognostic influence of BCL2 expression in breast cancer. Int J Cancer. 2012;131:E1109–E1119. doi: 10.1002/ijc.27539. [DOI] [PubMed] [Google Scholar]
- 13.Tawfik K, Kimler BF, Davis MK, Fan F, Tawfik O. Prognostic significance of Bcl-2 in invasive mammary carcinomas: a comparative clinicopathologic study between "triple-negative" and non-"triple-negative" tumors. Hum Pathol. 2012;43:23–30. doi: 10.1016/j.humpath.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellemans P, van Dam PA, Weyler J, van Oosterom AT, Buytaert P, Van Marck E. Prognostic value of bcl-2 expression in invasive breast cancer. Br J Cancer. 1995;72:354–360. doi: 10.1038/bjc.1995.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callagy GM, Pharoah PD, Pinder SE, Hsu FD, Nielsen TO, Ragaz J, et al. Bcl-2 is a prognostic marker in breast cancer independently of the Nottingham Prognostic Index. Clin Cancer Res. 2006;12:2468–2475. doi: 10.1158/1078-0432.CCR-05-2719. [DOI] [PubMed] [Google Scholar]
- 17.Seong MK, Lee JY, Byeon J, Sohn YJ, Seol H, Lee JK, et al. Bcl-2 is a highly significant prognostic marker of hormone-receptor-positive, human epidermal growth factor receptor-2-negative breast cancer. Breast Cancer Res Treat. 2015;150:141–148. doi: 10.1007/s10549-015-3305-7. [DOI] [PubMed] [Google Scholar]
- 18.Chen LY, Tsang JY, Ni YB, Chan SK, Chan KF, Zhang S, et al. Bcl2 and Ki67 refine prognostication in luminal breast cancers. Breast Cancer Res Treat. 2015;149:631–643. doi: 10.1007/s10549-015-3288-4. [DOI] [PubMed] [Google Scholar]
- 19.Daidone MG, Silvestrini R, Luisi A, Mastore M, Benini E, Veneroni S, et al. Changes in biological markers after primary chemotherapy for breast cancers. Int J Cancer. 1995;61:301–305. doi: 10.1002/ijc.2910610304. [DOI] [PubMed] [Google Scholar]
- 20.Martin LA, Dowsett M. BCL-2: a new therapeutic target in estrogen receptor-positive breast cancer? Cancer Cell. 2013;24:7–9. doi: 10.1016/j.ccr.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Park SH, Kim H, Song BJ. Down regulation of bcl2 expression in invasive ductal carcinomas is both estrogen- and progesterone-receptor dependent and associated with poor prognostic factors. Pathol Oncol Res. 2002;8:26–30. doi: 10.1007/BF03033697. [DOI] [PubMed] [Google Scholar]
- 22.Teixeira C, Reed JC, Pratt MA. Estrogen promotes chemotherapeutic drug resistance by a mechanism involving Bcl-2 proto-oncogene expression in human breast cancer cells. Cancer Res. 1995;55:3902–3907. [PubMed] [Google Scholar]
- 23.Kallel-Bayoudh I, Hassen HB, Khabir A, Boujelbene N, Daoud J, Frikha M, et al. Bcl-2 expression and triple negative profile in breast carcinoma. Med Oncol. 2011;28(Suppl 1):S55–S61. doi: 10.1007/s12032-010-9694-x. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Yang D, Lippman ME. Targeting Bcl-2 and Bcl-XL with non-peptidic small-molecule antagonists. Semin Oncol. 2003;30(5 Suppl 16):133–142. doi: 10.1053/j.seminoncol.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Dawson SJ, Makretsov N, Blows FM, Driver KE, Provenzano E, Le Quesne J, et al. BCL2 in breast cancer: a favourable prognostic marker across molecular subtypes and independent of adjuvant therapy received. Br J Cancer. 2010;103:668–675. doi: 10.1038/sj.bjc.6605736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Q, Moran MS, Haffty BG. Bcl-2 expression predicts local relapse for early-stage breast cancer receiving conserving surgery and radiotherapy. Breast Cancer Res Treat. 2009;115:343–348. doi: 10.1007/s10549-008-0068-4. [DOI] [PubMed] [Google Scholar]
- 27.Knowlton K, Mancini M, Creason S, Morales C, Hockenbery D, Anderson BO. Bcl-2 slows in vitro breast cancer growth despite its antiapoptotic effect. J Surg Res. 1998;76:22–26. doi: 10.1006/jsre.1998.5277. [DOI] [PubMed] [Google Scholar]
- 28.Mitrović O, Čokić V, Ðikić D, Budeč M, Vignjević S, Subotički T, et al. Correlation between ER, PR, HER-2, Bcl-2, p53, proliferative and apoptotic indexes with HER-2 gene amplification and TOP2A gene amplification and deletion in four molecular subtypes of breast cancer. Target Oncol. 2014;9:367–379. doi: 10.1007/s11523-013-0297-2. [DOI] [PubMed] [Google Scholar]
- 29.Bouchalova K, Svoboda M, Kharaishvili G, Vrbkova J, Bouchal J, Trojanec R, et al. BCL2 is an independent predictor of outcome in basal-like triple-negative breast cancers treated with adjuvant anthracycline-based chemotherapy. Tumour Biol. 2015;36:4243–4252. doi: 10.1007/s13277-015-3061-7. [DOI] [PubMed] [Google Scholar]
- 30.Abdel-Fatah TM, Dickinson PD, Moseley P, Reis-Filho JS, Green AR, Ellis IO, et al. Bcl2 as a surrogate prognostic and predictive marker in triple-negative breast cancer. J Clin Oncol. 2011;29(15 Suppl):abstr 1024. [Google Scholar]