Summary
High‐dose chemotherapy (HDT) with autologous stem cell transplantation is the standard of care for relapsed/refractory (RR) Hodgkin lymphoma (HL). Given that HDT may cure a sizeable proportion of patients refractory to first salvage, development of newer conditioning regimens remains a priority. We present the results of a novel HDT regimen in which carmustine was substituted by a third‐generation chloroethylnitrosourea, fotemustine, with improved pharmacokinetics and safety (FEAM; fotemustine, etoposide, cytarabine, melphalan) in 122 patients with RR‐HL accrued into a prospective registry‐based study. Application of FEAM resulted in a 2‐year progression‐free survival (PFS) of 73·8% [95% confidence interval (CI), 0·64–0·81] with median PFS, overall survival and time to progression yet to be reached. The 2‐year risk of progression adjusted for the competitive risk of death was 19·4% (95% CI, 0·12–0·27) for the entire patient population. Most previously established independent risk factors, except for fluorodeoxyglucose (18 FFDG)‐uptake, were unable to predict for disease progression and survival after FEAM. Although 32% of patients had 18 FFDG‐positrin emission tomography‐positive lesions before HDT, the 2‐year risk of progression adjusted for competitive risk of death was 19·4% (95% CI; 0·12–0·27). No unusual acute toxicities or early/late pulmonary adverse events were registered. FEAM emerges as an ideal HDT regimen for RR‐HL patients typically pre‐exposed to lung‐damaging treatments.
Keywords: Hodgkin lymphoma, autologous stem cell transplantation, fotemustine, high‐dose chemotherapy
The standard treatment for patients with Hodgkin lymphoma (HL) who are unresponsive to upfront therapy or who relapse after primary treatment consists of salvage chemotherapy followed by high‐dose chemotherapy (HDT) and autologous stem cell transplantation (ASCT) (Linch et al, 1993; Sureda et al, 2012; Rancea et al, 2014). This strategy achieves long‐term progression‐free survival (PFS) in 50–60% of patients with chemosensitive relapse, but outcomes are poorer in those with primary chemorefractory disease; in these patients long‐term survival rarely exceeds 15–20% (Crump et al, 1993; Lavoie et al, 2005; Sirohi et al, 2008; Sureda et al, 2012).
Disease recurrence is the main cause of ASCT failure. Duration of response to upfront treatment, poor sensitivity to pre‐transplant salvage chemotherapy and early disease progression after ASCT were shown to be the most important predictors of unfavourable outcome in HL patients undergoing ASCT (Crump et al, 1993; Horning et al, 1997; Lazarus et al, 2001; Moskowitz et al, 2001; Josting et al, 2002, 2005; Sureda et al, 2005; Majhail et al, 2006; Smith et al, 2011; Martínez et al, 2013; Hertzberg, 2014). Accordingly, the persistence of metabolically active lymphoma lesions after salvage therapy and/or conditioning, as evidenced by 18Ffluorodeoxyglucose positron emission tomography (18FFDG‐PET), emerged as the strongest independent predictor for PFS and overall survival (OS) in patients with relapsed and refractory (RR) HL treated with ASCT (Hutchings, 2011; Smeltzer et al, 2011; Devillier et al, 2012; Moskowitz et al, 2012; von Tresckow & Engert, 2012; Akhtar et al, 2013; Hertzberg, 2014; Pinto et al, 2014). Patients who were still 18FFDG‐PET‐positive after salvage chemotherapy had a long‐term PFS (23–52%) that was significantly inferior (69–88%) to those who were 18FFDG‐PET negative (Hertzberg, 2014; Pinto et al, 2014). Taken together, these evidences indicate that suboptimal pre‐transplant cytoreduction and the inadequate efficacy of HDT conditioning to eradicate disease are the major determinants of ASCT failure in patients with RR‐HL. On the other hand, about 25–35% of HL patients whose disease appears resistant to salvage chemotherapy may nonetheless attain a long‐term survival after ASCT, emphasizing the potential of HDT to cure a sizeable fraction of patients who achieve an unsatisfactory cytoreduction before conditioning (Smith et al, 2011; Gerrie et al, 2014).
Few attempts have been made to develop more active HDT programmes for recurring HL and the vast majority of patients usually receive a combination of carmustine, etoposide, cytarabine and melphalan (BEAM). This regimen represented the experimental arm of randomized studies establishing the superiority of HDT over conventional salvage in HL and, also due its favourable efficacy‐toxicity trade‐off, has been adopted as the standard conditioning programme by most groups worldwide (Linch et al, 1993; Mills et al, 1995; Schmitz et al, 2002).
We have designed a novel HDT regimen in which carmustine was substituted by an equal dose of fotemustine, a third generation chloroethylnitrosourea with improved pharmacokinetics and safety profiles (Musso et al, 2010). The FEAM (fotemustine, etoposide, cytarabine and melphalan) conditioning, as applied to patients with RR‐lymphoma, compared favourably to BEAM in terms of engraftment times and toxicity, notably including the absence of pulmonary adverse events (AE) (Musso et al, 2010). Based on the favourable efficacy and safety profiles of FEAM, the Italian National Health Service (INHS) granted reimbursement for fotemustine in this new regimen (http://www.gazzettaufficiale.it/caricaHtml?nomeTiles=gazzettaUfficiale#parte1).
Due to its considerable anti‐tumour activity and given that a carmustine‐free conditioning may be particularly advantageous in RR‐HL patients, who carry an increased risk of pulmonary complications because of their exposure to bleomycin, mediastinal radiotherapy, gemcitabine and, more recently, to novel potential lung‐damaging agents, including brentuximab vedotin (BV) and nivolumab (Topalian et al, 2012; Younes et al, 2013; Hertzberg, 2014), we wished to prospectively evaluate safety and activity of FEAM in a substantial population of these patients.
Methods and patients
Following a specific assessment of safety and activity (23/648 procedures), the Italian regulatory agency for medical products allowed fotemustine to be reimbursed by the INHS as a component of the FEAM HDT regimen, which also included etoposide, cytarabine and melphalan. In April 2007, an Italian nationwide registry was established to prospectively collect safety and efficacy data of FEAM conditioning in patients with refractory and relapsed lymphoma undergoing ASCT. Fourteen transplant centres participated in the project and a total of 397 patients, 122 with HL and 275 with non‐Hodgkin lymphoma, were consecutively registered as to March 2012. Five years after registration of the first patient, the registry committee agreed to unlock the database and analyse the results for patients with HL. The study was conducted in accordance with the Declaration of Helsinki, good clinical practice guidelines and was approved by local Treatment Review Boards. All patients signed informed consent prior to FEAM conditioning.
Disease status and risk stratification
Depending on response to upfront chemotherapy, patients were categorized as having primary refractory disease [failure to achieve a complete response (CR), progression or transient response upon upfront treatment, i.e. CR/partial response (PR) lasting <3 months], early relapse (CR lasting <12 months) and relapse (CR lasting >12 months). Stage and performance status at relapse, were determined according to the Cotswold Modification of the Ann Arbor system (Lister et al, 1989) and Eastern Cooperative Oncology Group criteria, respectively. Prognostic variables analysed included age, gender, B symptoms, extranodal disease, elevated lactate dehydrogenase (LDH), bulky disease (presence of any mass >5 cm), response to upfront chemotherapy, number of previous chemotherapy lines (excluding conditioning), previous mediastinal radiotherapy, disease chemosensitivity to salvage treatments and 18FFDG‐PET status before HDT. Patients were also stratified according to two prognostic models, the International Prognostic Factors Project (IPFP) (anaemia, hypoalbuminaemia, lymphopenia, age) (Bierman et al, 2002) and the Memorial Sloan‐Kettering Cancer Center (MSKCC) model (B symptoms, presence of extra nodal disease and duration of first remission <12 months) (Moskowitz et al, 2001). For each model, a score was generated by totalling the variables present. 18FFDG‐PET status was scored as positive or negative according to published criteria (Cheson, 2007).
Conditioning regimen
The original FEAM regimen consisted of fotemustine 150 mg/m2 on days −7 and −6, etoposide 200 mg/m2 and cytarabine 400 mg/m2 on days −5, −4, −3 and −2, and melphalan 140 mg/m2 on day −1 (Musso et al, 2010). Fotemustine (Muphoran, Servier; Thissen Laboratoires, Braine L'alleud, Belgium) was dissolved in alcoholic solvent, diluted in polyvinyl chloride bags containing 5% dextrose solution, and administered intravenously over 1 h. Administration of fotemustine as a single dose of 300 mg/m2 on day −6, in analogy to carmustine in some BEAM schedules (Mills et al, 1995), was allowed according to the policy of participating centres. Autologous peripheral blood progenitor cells were infused on day 0, followed by lenograstim (5 μg/kg) from day 1 of ASCT until 2 consecutive days after the absolute neutrophil count (ANC) was ≥1000 × 109/l.
Supportive measures
Supportive measures were provided according to shared protocols and policies across the participating centres. Briefly, antimicrobial prophylaxis consisted of oral fluconazole, ciprofloxacin or levofloxacin and acyclovir, started on day 0 or on the first day of conditioning. In case of fever and ANC <0·5 × 109/l, empiric broad‐spectrum intravenous antibiotics were administered and antifungal treatment was initiated if the patient remained febrile at 96 h after first occurrence of fever in the absence of positive cultures. Packed red blood cells and platelet transfusions were administered in case of a haemoglobin level <80 g/l and platelet count <10 × 109/l. After ASCT, patients received prophylaxis with biweekly trimethoprim/sulfamethoxazole and acyclovir or valacyclovir for 3 months.
Study endpoints
The primary study endpoint was PFS, calculated from the start of FEAM conditioning until the date of progression, relapse or death from any cause. Secondary study endpoints were the proportion of patients entering CR after receiving FEAM, time to progression (TTP), OS and safety, in terms of acute and delayed AE. Responses after pre‐transplant salvage chemotherapy were assessed according to standard response criteria for lymphoma and all patients were evaluated with contrast‐enhanced computerized tomography (CT) and 18FFDG‐PET (Cheson, 2007). As to survival outcomes, TTP was calculated from the day of first fotemustine infusion until disease progression, relapse or recurrence and was adjusted for the competitive risk of death; OS was measured from fotemustine infusion to death from any cause or last follow‐up when the patient was known to be alive. Study follow‐up was stopped at 24 months for evaluation of the study endpoints and univariate/multivariate analyses.
Safety was evaluated by recording acute and delayed AEs and haemopoietic engraftment times. Acute AEs included mucositis, diarrhoea, chemotherapy‐induced nausea and vomiting, hepatotoxicity, nephrotoxicity and pulmonary toxicity. Delayed toxicity was defined as AEs that were possibly or probably related to the administration of FEAM, including pulmonary toxicity and secondary malignancies. All AEs were graded according to the common terminology criteria (CTCAE) v4.0. (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf). Transplant‐related mortality (TRM) was defined as any death related to a fatal complication in the absence of the underlying disease within 100 d from transplantation.
Statistical analysis
A total sample of 130 observations was computed to achieve 80% power at a 2‐sided 0·05 significance level to detect a hazard ratio (HR) ≥3·0 with a Cox regression of the log HR on a binary covariate with 50% prevalence. The sample size was adjusted for an anticipated event rate of 20%. Relationships of event incidence to covariates were investigated with univariate Cox regression (PFS from ASCT and time to death from ASCT) and univariate Fine & Gray regression for time to disease progression from ASCT corrected for the competing risk of death (Fine & Gray, 1999). Robustness and relative weight of univariate findings were quantified by the SAS macro %RELIMPCR (Heinze & Schemper, 2012). Multivariate Cox and Fine & Gray regression models were penalized by Firth's correction, while univariate and multivariable competitive risk analyses were generated through the sas macro %PSHREG (Heinze, 2012). All other analyses were generated through the sas software, version 9.2 (SAS Institute, Milan, Italy).
Results
Patient characteristics
After registry establishment up to March 2012, 122 consecutive patients with RR‐HL received the FEAM regimen as pre‐ASCT conditioning at 14 transplant centres. All patients were evaluable for analysis and their characteristics are summarized in Table 1. There were 69 males and 53 females with a median age at transplant of 35 years (range 12–69 years). Most patients (55·8%) had stage III–IV disease and all had received upfront chemotherapy with an anthracycline‐containing regimen, consisting of ABVD (adriamycin, bleomycin, vinblastine, dacarbazine) or escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) in 93·4% and 6·6% of cases, respectively. Twenty‐six patients (21·3%) received mediastinal radiotherapy as a part of their frontline treatment. Sixty‐four patients (52·5%) had primary refractory disease, 32 (26·2%) had an early relapse after upfront chemotherapy and 26 (21·3%) a late relapse. At relapse, extranodal disease, B symptoms and a bulky nodal mass (>5 cm) were present in 40·2%, 62·3% and 27·0% of patients, respectively. An abnormal LDH level was also present in 27·9% of cases. As to risk stratification, 36 patients (29·5%) had an IPFP score ≥3, and 78 patients (63·9%) had a MSKCC score ≥2 (Table 2).
Table 1.
Patient characteristics and previous treatments
| Characteristic | Number (n = 122) | % |
|---|---|---|
| Male/Female | 69/53 | 56·6/43·4 |
| Median age at transplant, years (range) | 35 (12–69) | |
| ECOG PS 0–1/2–3 | 90/24 | 73·8/19·7 |
| Stage I–II/III–IV | 54/68 | 44·2/55·8 |
| Histology | ||
| Nodular sclerosis | 102 | 83·6 |
| Frontline chemotherapy | ||
| ABVD | 114 | 93·4 |
| BEACOPP | 8 | 6·6 |
| Mediastinal radiotherapy | 26 | 21·3 |
| Response to frontline therapy | ||
| Primary refractory | 64 | 52·5 |
| Early relapse | 32 | 26·2 |
| Late relapse | 26 | 21·3 |
| Risk factors prior to salvage treatment | ||
| B symptoms | 76 | 62·3 |
| Extranodal disease | 49 | 40·2 |
| Abnormal LDH | 34 | 27·9 |
| Bulky disease (≥5 cm) | 33 | 27·0 |
| IPFP score | ||
| 0–2 | 86 | 70·5 |
| ≥3 | 36 | 29·5 |
| MSKCC score | ||
| 0 | 15 | 12·3 |
| 1 | 29 | 23·8 |
| 2 | 42 | 34·4 |
| 3 | 36 | 29·5 |
| Chemotherapy regimens before transplant | ||
| 2 | 92 | |
| >2 | 30 | |
| 18FDG‐PET after salvage chemotherapy | ||
| Negative | 83 | 68 |
| Positive | 39 | 32 |
ECOG PS, Eastern Cooperative Oncology Group performance score; IPFP, International Prognostic Factors Project; MSKCC, Memorial Sloan‐Kettering Cancer Center; ABVD, adriamycin, bleomycin, vinblastine, dacarbazine; BEACOPP, bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, prednisone; LDH, lactate dehydrogenase; 18FDG‐PET, 18Ffluorodeoxyglucose‐positron emission tomography.
Table 2.
Toxicity profile of the FEAM regimen
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | None | |
|---|---|---|---|---|---|
| Type of toxicity | |||||
| Mucositis | 27 | 34·4 | 11·5 | 3·3 | 23·8 |
| Diarrhoea | 33·6 | 32 | 5·7 | 0 | 28·7 |
| CINV | 30·3 | 34·4 | 6·6 | 0 | 28·7 |
| Liver | 1·6 | 2·5 | 0 | 0 | 95·9 |
| Renal | 0 | 0 | 0 | 0 | 100 |
| Pulmonary | 0 | 0 | 0 | 0 | 100 |
Data are reported as percent of patients with and without toxicity. Adverse events are graded according to the National Cancer Institute Common Terminology Criteria Version 4.0.
FEAM, fotemustine, etoposide, cytarabine, melphalan; CINV, chemotherapy‐induced nausea and vomiting.
Response to salvage treatment and 18FFDG‐PET status before FEAM conditioning
In 59% of patients the salvage regimen consisted of ifosfamide, gemcitabine and vinorelbine, while cytarabine/platinum and gemcitabine‐ifosfamide‐oxaliplatinum combinations were adopted in 14·7% and 15·6% of cases, respectively. After salvage, patients were restaged with CT and 18FFDG‐PET scanning. Fifty‐seven patients (46·7%) obtained a CR, 50 patients (41·0%) a PR and 15 patients (12·3%) did not qualify for CR or PR after salvage (Table 2). Before FEAM conditioning, 83 patients (68%) had a negative 18FFDG‐PET scan, including all CR patients and 20 patients in PR at CT evaluation. Overall, 39 patients (32%) displayed pathological 18FFDG uptakes before HDT.
Engraftment and acute toxicity
All patients fully engrafted and achieved an ANC > 0·5 × 109/l at a median time of 10 d (range, 6–25 d) and >1·0 × 109/l after a median of 11 d (range, 8–27 d). Median times to platelet counts >20 × 109/l and >50 × 109/l were 12 d (range, 6–43 d) and 15 d (range, 9–51 d), respectively. Acute toxicity is reported in Table 2. The most frequent grade 3 AEs included mucositis (11·5%), diarrhoea (5·7%) and nausea/vomiting (6·6%). The only grade 4 toxicity recorded was mucositis, occurring in only 3·3% of patients. No severe hepatic or renal AEs were registered. No cases of interstitial pneumonitis or non‐infectious pneumonia were observed. TRM at 100 d occurred in three patients (2·5%) and was due in all cases to multi‐organ failure following a documented infection by Gram‐negative bacteria.
Outcomes
At 2 years from FEAM administration with a median follow‐up of 21 months, 104 of the 122 patients (85·2%) were alive, and median PFS, OS and TTP had not yet been reached. The 2‐year PFS, the primary study endpoint, was 73·8% (95% CI, 0·64–0·81) for the entire patient population (Fig 1A). The 2‐year risk of progression, adjusted for the competitive risk of death, was only 19·4% (95% CI, 0·12–0·27) for the entire patient population. This corresponded with the achievement of a CR rate of 82·8% after administration of FEAM, encompassing patients with primary refractory disease (73·5%) and early relapses (90·6%) (Table 3).
Figure 1.
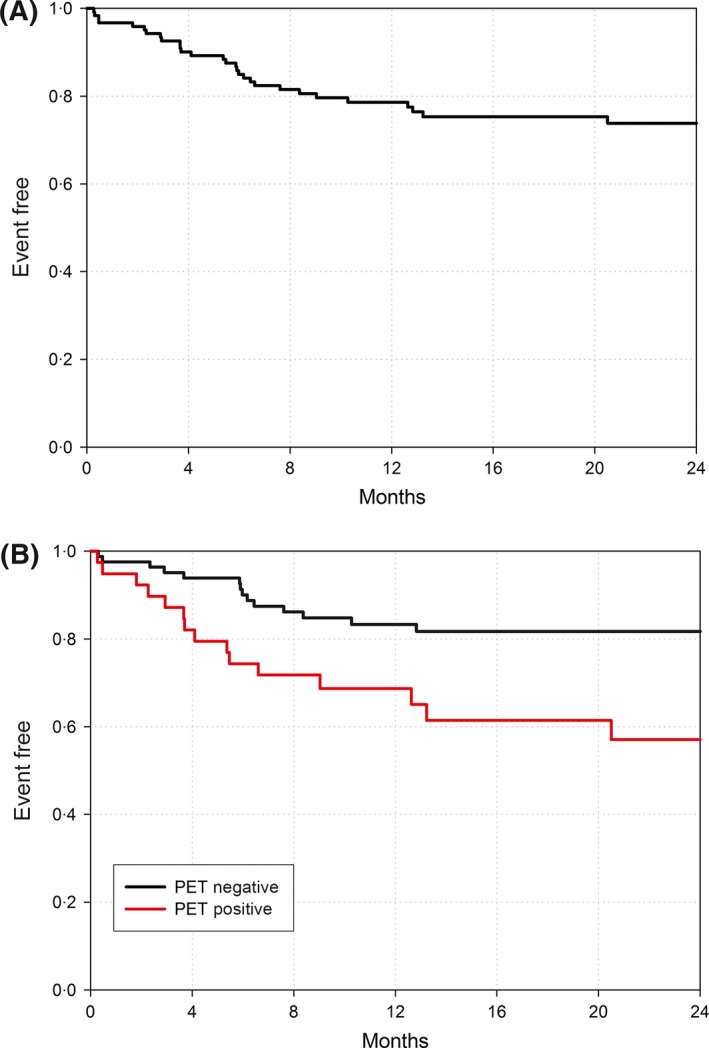
Kaplan–Meier curves for progression‐free survival (A) in the entire study population and (B) according to 18Ffluorodeoxyglucose‐positron emission tomography (PET) status before FEAM regimen (fotemustine, etoposide, cytarabine, melphalan).
Table 3.
Clinical response to FEAM conditioning regimen and patient status at last follow‐up
| Before FEAM | After FEAM | |||||
|---|---|---|---|---|---|---|
| Response | N (%) | Response | N (%) | Clinical status | N (%) | |
| All patients (n = 122) | CR | 57 (47·6) | CR | 101 (82·8) | CR | 87 (71·3) |
| PR | 50 (41) | PR | 9 (7·4) | PR | 5 (4·1) | |
| RD | 15 (12·3) | RD | 9 (7·4) | PD | 12 (9·8) | |
| DT | – | DT | 3 (2·4)a | DT | 18 (14·8) | |
| According to response to upfront chemotherapy | ||||||
| Primary refractory (n = 64) | CR | 21 (32·8) | CR | 47 (73·5) | CR | 39 (60·9) |
| PR | 31 (48·4) | PR | 7 (10·9) | PR | 4 (6·3) | |
| RD | 12 (18·8) | RD | 7 (10·9) | PD | 6 (9·4) | |
| DT | – | DT | 3 (4·7)a | DT | 15 (23·4) | |
| Early relapse (n = 32) | CR | 23 (71·9) | CR | 29 (90·6) | CR | 25 (78·1) |
| PR | 8 (25·0) | PR | 2 (6·3) | PR | 1 (3·1) | |
| RD | 1 (3·1) | RD | 1 (3·1) | PD | 5 (15·7) | |
| DT | – | DT | 0a | DT | 1 (3·1) | |
| Relapse (n = 26) | CR | 13 (5·0) | CR | 25 (96·2) | CR | 23 (88·5) |
| PR | 11 (42·3) | PR | 0 | PR | 0 | |
| RD | 2 (7·7) | RD | 1 (3·8) | PD | 1 (3·8) | |
| DT | – | DT | 0a | DT | 2 (7·7) | |
Primary refractory disease: failure to achieve CR, progression during first line chemotherapy or transient response (CR/PR lasting <3 months); early relapsed disease, CR lasting <12 months; relapsed disease, CR lasting >12 months).
FEAM, fotemustine, etoposide, cytarabine, melphalan; CR, complete response; PR, partial response; RD, resistant disease; PD, progression of disease; DT, death; ASCT, autologous stem cell transplantation.
Transplant‐related mortality.
In patients who received FEAM, most of previously established single prognostic factors, including those reflecting tumour extension and chemosensitivity at relapse, as well as different prognostic systems, i.e. the IPFPS and the MSKCC models, were unable to predict for PFS (Table 4). The only predictor statistically significant by univariate analysis (P = 0·0108) was the presence of 18FFDG‐avid tumour lesions before FEAM. As shown in Fig 1B, patients with a 18FFDG‐PET negative status displayed a 2‐year PFS of 81·7% (95% CI, 0·71–0·89) as opposed to 57% (95% CI, 0·38–0·72) for those who were 18FFDG‐PET positive.
Table 4.
Univariate Cox regression analysis and penalized multivariate Cox regression analysis. Penalization was adopted to adjust for potential over‐fitting and was obtained using Firth's correction. Progression‐free survival after FEAM was the response variable used
| HC | 95% Lower confidence limit for HR | 95% Upper confidence limit for HR | Probability > χ2 | % of explained variation | |
|---|---|---|---|---|---|
| Univariate Cox regression analysis | |||||
| Variable(s) | |||||
| IPFP (≥3 vs. <3) | 1·990 | 0·949 | 4·172 | 0·0684 | 2·28 |
| MSKCC (≥2 vs. <2) | 1·707 | 0·756 | 3·857 | 0·1982 | 1·15 |
| Age (≥45 years vs. <45 years) | 2·099 | 0·975 | 4·517 | 0·0579 | 2·50 |
| Chemosensitive (no vs. yes) | 1·554 | 0·593 | 4·075 | 0·3701 | 0·62 |
| Response to first line chemotherapy (Rel + early rel vs. reference) | 2·798 | 0·847 | 9·251 | 0·0916 | 1·89 |
| Gender (male vs. female) | 0·541 | 0·258 | 1·132 | 0·1029 | 1·70 |
| Bulky disease >5 cm (yes vs. no) | 0·806 | 0·344 | 1·886 | 0·6185 | 0·01 |
| Number of previous chemotherapy lines (>2 vs. 2) | 0·969 | 0·414 | 2·268 | 0·9415 | 0·00 |
| B Symptoms (yes vs. no) | 1·069 | 0·504 | 2·263 | 0·8625 | 0·02 |
| 18FFDG‐PET status prior to ASCT (positive vs. negative) | 2·581 | 1·245 | 5·352 | 0·0108 | 4·07 |
| Serum LDH (abnormal vs. normal) | 1·527 | 0·721 | 3·234 | 0·2693 | 1·00 |
| Total | 14·63 | ||||
| Penalized multivariate Cox regression analysis | |||||
| Variable(s) | |||||
| IPFP (≥3 vs. <3) | 1·477 | 0·516 | 4·193 | 0·4768 | |
| MSKCC (≥2 vs. <2) | 1·396 | 0·387 | 6·417 | 0·6415 | |
| Age (≥45 years vs. <45 years) | 1·616 | 0·467 | 4·571 | 0·4107 | |
| Response to upfront chemotherapy (Rel + early rel vs. ref) | 2·480 | 0·452 | 25·737 | 0·3560 | |
| Gender (male vs. female) | 0·605 | 0·224 | 1·561 | 0·3166 | |
| 18FFDG‐PET status prior to ASCT (positive vs. negative) | 2·248 | 0·873 | 5·897 | 0·1009 | |
FEAM, fotemustine, etoposide, cytarabine, melphalan; IPFP, International Prognostic Factors Project score; MSKCC, Memorial Sloan‐Kettering Cancer Center score; ASCT, autologous stem cell transplantation; Rel, relapse; Ref, refractory; PET, positron emission tomography; LDH, lactate dehydrogenase; HR, hazard ratio; 18FDG‐PET, 18Ffluorodeoxyglucose‐positron emission tomography.
In multivariate analysis, after adjusting for the competitive risk of death, gender and 18FFDG PET results before ASCT were independent prognostic factors of disease progression after ASCT. All other risk factors were not statistically significant in our population and there were no prognostic predictors for either PFS or OS (data not shown). The results of univariate and multivariate analyses for PFS are listed in Tables 4 and 5 details the results of the analyses for TTP, adjusted for the competitive risk of death.
Table 5.
Univariate and penalized multivariate Fine & Gray regression analysis. Penalization was adopted to adjust for potential over‐fitting and was obtained using Firth's correction. Response variable was time to progression from ASCT and was adjusted for the competitive risk of death
| HR | 95% Lower confidence limit for HR | 95% Upper confidence limit for HR | Probability > χ2 | % Of explained variation | |
|---|---|---|---|---|---|
| Univariate Fine & Gray regression analysis | |||||
| Variable | |||||
| IPFP (≥3 vs. <3) | 1·656 | 0·655 | 3·931 | 0·2621 | 0·71 |
| MSKCC (≥2 vs. <2) | 1·229 | 0·511 | 3·244 | 0·6560 | 0·02 |
| Age (≥45 years vs. <45 years) | 1·850 | 0·701 | 4·448 | 0·1840 | 0·95 |
| Chemosensitive (no vs. yes) | 1·138 | 0·267 | 3·365 | 0·8353 | 0·03 |
| Response to first line chemotherapy (Rel + early rel vs. ref) | 1·833 | 0·620 | 7·827 | 0·3312 | 0·23 |
| Gender (male vs. female) | 0·378 | 0·143 | 0·908 | 0·0355 | 3·85 |
| Bulk disease >5 cm (yes vs. no) | 1·064 | 0·379 | 2·618 | 0·8980 | 0·01 |
| Number of previous chemotherapy lines (>2 vs. 2) | 0·939 | 0·307 | 2·398 | 0·9025 | 0·01 |
| B Symptoms (yes vs. no) | 0·850 | 0·360 | 2·084 | 0·7132 | 0·16 |
| 18FFDG‐PET status prior to ASCT (positive vs. negative) | 2·543 | 1·072 | 6·106 | 0·0327 | 2·90 |
| Serum LDH (abnormal vs. normal) | 1·858 | 0·758 | 4·389 | 0·1604 | 1·96 |
| Total | 13·97 | ||||
| Penalized multivariate Fine & Gray regression analysis | |||||
| Variable | |||||
| IPFP (≥3 vs. <3) | 2·047 | 0·746 | 5·683 | 0·1745 | |
| MSKCC (≥2 vs. <2) | 0·495 | 0·152 | 1·641 | 0·2530 | |
| Age (≥45 years vs. <45 years) | 2·203 | 0·816 | 5·474 | 0·1081 | |
| Response to first line chemotherapy (Rel + early rel vs. ref) | 1·885 | 0·516 | 8·363 | 0·3723 | |
| Gender (male vs. female) | 0·377 | 0·143 | 0·914 | 0·0413 | |
| 18FFDG‐PET status prior to ASCT (positive vs. negative) | 2·560 | 1·044 | 6·354 | 0·0443 | |
| Serum LDH (abnormal vs. normal) | 1·648 | 0·637 | 4·134 | 0·3023 | |
FEAM, fotemustine, etoposide, cytarabine, melphalan; IPFP, International Prognostic Factors Projectscore; MSKCC, Memorial Sloan‐Kettering Cancer Center score; ASCT, autologous stem cell transplantation; Rel, relapse; Ref, refractory; PET, positron emission tomography; LDH, lactate dehydrogenase; HR, hazard ratio; 18FDG‐PET, 18Ffluorodeoxyglucose‐positron emission tomography.
Late adverse events
After 58 months from inclusion of the first HL patient in the registry and 36 months from registration of the last patient, no pulmonary AEs have been recorded. Similarly, no delayed AEs or secondary malignancies had occurred at the time of the present analysis.
Discussion
Given that a sizeable proportion of patients who are chemorefractory to frontline and/or salvage treatments may achieve a long term survival after ASCT, the development of more effective conditioning regimens, in terms of antitumour activity and safety, represents a strategic priority in the setting of RR‐HL (Smith et al, 2011; Gerrie et al, 2014; Pinto et al, 2014).
This is the largest study conducted in patients with RR‐HL to assess the efficacy of a novel HDT programme based on the substitution of carmustine with the third‐generation chloroethylnitrosourea, fotemustine, within the BEAM platform. The patient population, accrued at nationwide transplant referral centres, included a substantial proportion of patients at a high risk for ASCT failure. Namely, 52% of patients were refractory to frontline chemotherapy, 26% had progressed shortly after primary treatment, 60% had a MSKCC score ≥2 and, most importantly, about one half of patients had an unsatisfactory response to pre‐conditioning salvage treatments, i.e. 41% did not achieve a CR and 12% were chemorefractory to salvage.
The results uncovered some key findings. First, the application of FEAM conditioning produced estimated post‐transplant 2‐year PFS and OS rates of 73·8% and 85·2%, respectively, with median PFS, OS and TTP yet to be reached. Second, most of the previously established prognostic factors lost their negative predictive value in patients conditioned with FEAM, except for pre‐transplant PET. Third, FEAM emerged as a very safe HDT regimen due to a highly favourable acute toxicity profile, the lack of any early or delayed episode of pulmonary toxicity and the absence of secondary malignancies after a substantial follow‐up period.
BEAM is a worldwide standard HDT regimen for patients with recurring HL undergoing ASCT. While 50–60% of patients may achieve, overall, a long‐term PFS after BEAM, results are less satisfactory in those with chemorefractory disease and/or unfavourable prognostic features. In these patients, PFS rates varied between 15% and 40% across studies (Popat et al, 2004; Sureda et al, 2005, 2012; Hertzberg, 2014; Pinto et al, 2014; Rancea et al, 2014). To improve these outcomes, a few HDT regimens that target HL have been devised. A gemcitabine‐based conditioning, including busulfan and melphalan (Gem‐Bu‐Mel), achieved an event‐free survival rate at 36 months of 57%, as compared to 33% and 39% obtained for patients conditioned with busulfan‐melphalan and BEAM, respectively (Nieto et al, 2013). The inclusion of thiothepa (T) within the Bu‐Mel platform, yielded a 5‐year PFS of 66% in a group of 60 HL patients (Bains et al, 2014). The updated results of a BeEAM regimen, in which carmustine was replaced by bendamustine, indicated a 3‐year PFS of 72% in a mixed cohort of 43 lymphoma patients including, however, only 15 cases of HL (Visani et al, 2014).
Despite the limits of a prospective registry‐based study and a cross‐trial comparison, the efficacy outcomes of FEAM are remarkable and compare favourably with most HDT regimens, including BEAM and those more specifically designed for HL. Interestingly, most previously established independent risk factors, except for 18FFDG‐uptakes, lost their statistical significance in univariate and multivariate analyses and were unable to predict for disease progression and survival after ASCT in patients conditioned with FEAM. This was not always the case for other conditioning regimens (Nieto et al, 2013; Bains et al, 2014; Hertzberg, 2014; Visani et al, 2014). In our study, patients without 18FFDG uptakes before FEAM had a 2‐year PFS of 82% as opposed to 57% of those with residual 18FFDG‐avid tumour lesions at conditioning. It is of note, however, that this latter outcome favourably compares with the estimated PFS of PET‐positive patients conditioned with other regimens. This ranged from 18% to 33% at 3 years for patients given Gem‐Bu‐Mel and 23% to 41%, at 3 or 5 years, for patients conditioned with BEAM and similar regimens (Smeltzer et al, 2011; von Tresckow & Engert, 2012; Nieto et al, 2013; Hertzberg, 2014; Pinto et al, 2014). These results appear remarkable given that only 21% of patients accrued in our study had a favourable prognostic profile as opposed to about 50–80% of patients described in the Gem‐Bu‐Mel, Bu‐Mel‐T and BeEAM cohorts (Nieto et al, 2013; Bains et al, 2014; Visani et al, 2014).
While our results need reassessment at a later follow‐up, the low cumulative incidence of early progressions observed with FEAM appears very promising, in terms of long‐term outcomes, as most relapses usually occur within the first 2 years after ASCT (Majhail et al, 2009; William et al, 2013).
Efficacy outcomes need to be fully appreciated also in the context of the highly favourable toxicity profiles of the FEAM regimen. The present results fully confirm our early observation of a timely haemopoietic engraftment, with neutrophil and platelets recovery times, intensity of supportive measures, infectious rates and TRM comparable to those of most HDT regimens, including BEAM and those specifically devised for HL, such as Gem‐Bu‐Mel, Bu‐Mel‐T and BeEAM (Czyz et al, 2004; Nieto et al, 2013; Bains et al, 2014; Visani et al, 2014) Differently, the rate of severe mucositis was of only 15% as opposed to 41%, 26%, 28% and 98% for BEAM, BeEAM, Gem‐Bu‐Mel and Bu‐Mel‐T, respectively (Puig et al, 2006; Blijlevens et al, 2008; Nieto et al, 2013; Bains et al, 2014; Visani et al, 2014). A favourable trend for FEAM, as compared to other regimens, can be also envisaged by the very low occurrence of severe nausea and vomiting (6·6%) and diarrhoea (5·7%) along with the lack of any kidney and liver toxicity, including veno‐occlusive liver disease. This latter complication was reported to occur in up to 2–4% of patients receiving carmustine‐/melphalan‐based conditionings (Nademanee et al, 1995; Puig et al, 2006; Blijlevens et al, 2008).
More importantly, no episodes of pulmonary toxicity of any grade were recorded in our cohort of 122 patients. Non‐infectious pulmonary toxicity, such as interstitial pneumonitis or idiopathic pneumonia, represents an invalidating and sometimes fatal complication of the ASCT procedure, reported to occur in 11–26% of patients who received carmustine‐based conditioning regimens (Mills et al, 1995; Alessandrino et al, 2000; Stiff et al, 2003; Till & Madtes, 2012). Due to its strong inhibitory action on the glutathione reductase tissue detoxification system, carmustine has been implicated as one of the main causes of lung toxicity, particularly in association with previous radiation therapy (Kehrer, 1983; Alessandrino et al, 2000; Till & Madtes, 2012).
Application of a carmustine‐free HDT regimen, such as FEAM, may be of significant advantage in the setting of RR‐HL as these patients are typically exposed to a series of incoming lung injuries throughout their clinical history. First, bleomycin, an agent well known to cause lung damage in up to 27% of patients, is an integral component of frontline chemotherapy regimens for HL (Martin et al, 2005). Second, consistent proportions of patients receive mediastinal radiotherapy as a part of their upfront and/or salvage treatments (Moskowitz et al, 2001; Fox et al, 2012; Hertzberg, 2014). Third, several pre‐ and post‐transplant salvage regimens include the pneumotoxic drug gemcitabine (Hertzberg, 2014; Kharfan‐Dabaja et al, 2014; Reddy & Perales, 2014). Fourth, several novel agents active in RR‐HL, such mTOR and HDAC inhibitors, and, most importantly, BV and anti‐PD1/PD1‐ligand antibodies, such as nivolumab and pembrolizumab, may induce pulmonary complications (Topalian et al, 2012; Sasse et al, 2013; Younes et al, 2013; Provencio et al, 2014). Potential lung damage from these two latter agents may be of greater prospective relevance, as BV is being extensively tested in upfront and salvage regimens for HL and widely adopted across all transplantation phases, and previous use of elevated doses of carmustine may preclude patients from receiving nivolumab or pembrolizumab after ASCT failure (Ansell, 2014; ClinicalTrials.gov NCT02181738 and NCT02362997).
In fact, all of the patients in our study had received bleomycin within their upfront therapy, 79% were given a gemcitabine‐containing salvage regimen and 21% had mediastinal irradiation. The absence of any episode of non‐infectious lung toxicity in our patient cohort fully endorses the safety of the FEAM conditioning in terms of pulmonary complications.
While the registry consortium will continue to collect data to ensure a late time‐to‐event analysis, results from this large cohort of high‐risk patients support that FEAM is associated with a remarkable antitumour activity, a substantial 2‐year PFS rate and a highly advantageous safety profile. The absence of pulmonary toxicity also advocates FEAM as a valuable option for patients with RR‐HL given their frequent exposure, in both pre‐ and post‐transplantation phases, to lung‐damaging treatments.
Authorship contributions
MM and AP equally proposed, designed and supervised the study and wrote the manuscript. EB was responsible for statistical analysis. MM, AP, NDR, RS, GMe and GMa are members of the FEAM Registry Committee and all coauthors contributed to patient care, supported the study conduct, collected data, contributed to the final report and approved the manuscript.
Conflicts of interest
AP has received lecture fees from, Takeda, Roche, Celgene, Mundipharma and Italfarmaco S.p.A.; UV is a member of Roche advisory board and received lecture fees from Takeda, Roche and Celgene. All other authors have no financial or other conflicts of interest to declare. An unrestricted grant was provided by Italfarmaco S.p.A., Milan, Italy to establish and maintain the FEAM registry.
Acknowledgements
This work was supported in part by a grant to A. Pinto from Ministero della Salute, Ricerca Corrente, IRCCS, Rome, Italy.
Maurizio Musso and Antonello Pinto equally contributed to this work.
The copyright line for this article was changed on 26 September 2016 after original online publication.
References
- Akhtar, S. , Al‐Sugair, A.S. , Abouzied, M. , Alkadhi, Y. , Dingle, M. , Abdelsalam, M. , Soudy, H. , Darwish, A. , Eltigani, A. , Elhassan, T.A. , Nabil‐Ahmed, M. & Maghfoor, I. (2013) Pre‐transplant FDG‐PET‐based survival model in relapsed and refractory Hodgkin's lymphoma: outcome after high‐dose chemotherapy and auto‐SCT. Bone Marrow Transplantation, 48, 1530–1536. [DOI] [PubMed] [Google Scholar]
- Alessandrino, E.P. , Bernasconi, P. , Colombo, A. , Caldera, D. , Martinelli, G. , Vitulo, P. , Malcovati, L. , Nascimbene, C. , Varettoni, M. , Volpini, E. , Klersy, C. & Bernasconi, C. (2000) Pulmonary toxicity following carmustine‐based preparative regimens and autologous peripheral blood progenitor cell transplantation in hematological malignancies. Bone Marrow Transplantation, 25, 309–313. [DOI] [PubMed] [Google Scholar]
- Ansell, S.M. (2014) Brentuximab vedotin. Blood, 124, 3197–3200. [DOI] [PubMed] [Google Scholar]
- Bains, T. , Chen, A.I. , Lemieux, A. , Hayes‐Lattin, B.M. , Leis, J.F. , Dibb, W. & Maziarz, R.T. (2014) Improved outcome with busulfan, melphalan and thiotepa conditioning in autologous hematopoietic stem cell transplant for relapsed/refractory Hodgkin lymphoma. Leukemia & Lymphoma, 55, 583–587. [DOI] [PubMed] [Google Scholar]
- Bierman, P.J. , Lynch, J.C. , Bociek, R.G. , Whalen, V.L. , Kessinger, A. , Vose, J.M. & Armitage, J.O. (2002) The International Prognostic Factors Project score for advanced Hodgkin's disease is useful for predicting outcome of autologous hematopoietic stem cell transplantation. Annals of Oncology, 13, 1370–1377. [DOI] [PubMed] [Google Scholar]
- Blijlevens, N. , Schwenkglenks, M. , Bacon, P. , D'Addio, A. , Einsele, H. , Maertens, J. , Niederwieser, D. , Rabitsch, W. , Roosaar, A. , Ruutu, T. , Schouten, H. , Stone, R. , Vokurka, S. , Quinn, B. & McCann, S. ; European Blood and Marrow Transplantation Mucositis Advisory Group . (2008) Prospective oral mucositis audit: oral mucositis in patients receiving high‐dose melphalan or BEAM in patients receiving chemotherapy‐European blood and marrow transplantation mucositis advisory group. Journal of Clinical Oncology, 26, 1519–1525. [DOI] [PubMed] [Google Scholar]
- Cheson, B.D. (2007) The International harmonization project for response criteria in lymphoma clinical trials. Hematology/Oncology Clinics of North America, 21, 841–854. [DOI] [PubMed] [Google Scholar]
- Crump, M. , Smith, A.M. , Brandwein, J. , Brandwein, J. , Couture, F. , Sherret, H. , Sutton, D.M. , Scott, J.G. , McCrae, J. , Murray, C. & Pantalony, D. (1993) High‐dose etoposide and melphalan, and autologous bone marrow transplantation for patients with advanced Hodgkin's disease: importance of disease status at transplant. Journal of Clinical Oncology, 11, 704–711. [DOI] [PubMed] [Google Scholar]
- Czyz, J. , Dziadziuszko, R. , Knopinska‐Postuszuy, W. , Hellmann, A. , Kachel, L. , Holowiecki, J. , Gozdzik, J. , Hansz, J. , Avigdor, A. , Nagler, A. , Osowiecki, M. , Walewski, J. , Mensah, P. , Jurczak, W. , Skotnicki, A. , Sedzimirska, M. , Lange, A. , Sawicki, W. , Sulek, K. , Wach, M. , Dmoszynska, A. , Kus, A. , Robak, T. & Warzocha, K. (2004) Outcome and prognostic factors in advanced Hodgkin's disease treated with high‐dose chemotherapy and autologous stem cell transplantation: a study of 341 patients. Annals of Oncology, 15, 1222–1230. [DOI] [PubMed] [Google Scholar]
- Devillier, R. , Coso, D. , Castagna, L. , Brenot Rossi, I. , Anastasia, A. , Chiti, A. , Ivanov, V. , Schiano, J.M. , Santoro, A. , Chabannon, C. , Balzarotti, M. , Blaise, D. & Bouabdallah, R. (2012) Positron emission tomography response at the time of autologous stem cell transplantation predicts outcome of patients with relapsed and/or refractory Hodgkin's lymphoma responding to prior salvage therapy. Haematologica, 97, 1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine, J.P. & Gray, R.J. (1999) A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association, 94, 496–509. [Google Scholar]
- Fox, A.M. , Dosoretz, A.P. , Mauch, P.M. , Chen, Y.H. , Fisher, D.C. , LaCasce, A.S. , Freedman, A.S. , Silver, B. & Ng, A.K. (2012) Predictive factors for radiation pneumonitis in Hodgkin lymphoma patients receiving combined‐modality therapy. International Journal of Radiation Oncology, Biology, Physics, 83, 277–283. [DOI] [PubMed] [Google Scholar]
- Gerrie, A.S. , Power, M.M. , Shepherd, J.D. , Savage, K.J. , Sehn, L.H. & Connors, J.M. (2014) Chemoresistance can be overcome with high‐dose chemotherapy and autologous stem‐cell transplantation for relapsed and refractory Hodgkin lymphoma. Annals of Oncology, 25, 2218–2223. [DOI] [PubMed] [Google Scholar]
- Heinze, G. (2012) PSHREG – proportional and non‐proportional subdistribution hazards regression. Technical Report 3/2012. [DOI] [PMC free article] [PubMed]
- Heinze, G. & Schemper, M. (2012) RELIMPCR – SAS‐macro for the analysis of relative importance of prognostic factors in Cox regression. Technical Report 4/2012.
- Hertzberg, M. (2014) Relapsed/refractory Hodgkin lymphoma: what is the best salvage therapy and do we need RIC‐alloSCT? Hematology/Oncology Clinics of North America, 28, 123–147. [DOI] [PubMed] [Google Scholar]
- Horning, S.J. , Chao, N.J. , Negrin, R.S. , Hoppe, R.T. , Long, G.D. , Hu, W.W. , Wong, R.M. , Brown, B.W. & Blume, K.G. (1997) High‐dose therapy and autologous hematopoietic progenitor cell transplantation for recurrent or refractory Hodgkin's disease: analysis of the Stanford University results and prognostic indices. Blood, 89, 801–813. [PubMed] [Google Scholar]
- Hutchings, M. (2011) Pre‐transplant positron emission tomography/computed tomography (PET/CT) in relapsed Hodgkin lymphoma: time to shift gears for PET positive patients? Leukemia & Lymphoma, 52, 1615–1616. [DOI] [PubMed] [Google Scholar]
- Josting, A. , Franklin, J. , May, M. , Koch, P. , Beykirch, M.K. , Heinz, J. , Rudolph, C. , Diehl, V. & Engert, A. (2002) New prognostic score based on treatment outcome of patients with relapsed Hodgkin's lymphoma registered in the database of the German Hodgkin's lymphoma study group. Journal of Clinical Oncology, 20, 221–230. [DOI] [PubMed] [Google Scholar]
- Josting, A. , Rudolph, C. , Mapara, M. , Glossmann, J.P. , Sieniawski, M. , Sieber, M. , Kirchner, H.H. , Dörken, B. , Hossfeld, D.K. , Kisro, J. , Metzner, B. , Berdel, W.E. , Diehl, V. & Engert, A. (2005) Cologne high‐dose sequential chemotherapy in relapsed and refractory Hodgkin lymphoma: results of a large multicenter study of the German Hodgkin Lymphoma Study Group (GHSG). Annals of Oncology, 16, 116–123. [DOI] [PubMed] [Google Scholar]
- Kehrer, J.P. (1983) The effect of BCNU (carmustine) on tissue glutathione reductase activity. Toxicology Letters, 17, 63–68. [DOI] [PubMed] [Google Scholar]
- Kharfan‐Dabaja, M.A. , Hamadani, M. , Sibai, H. & Savani, B.N. (2014) Managing Hodgkin lymphoma relapsing after autologous hematopoietic cell transplantation: a not‐so‐good cancer after all!. Bone Marrow Transplantation, 49, 599–606. [DOI] [PubMed] [Google Scholar]
- Lavoie, J.C. , Connors, J.M. , Phillips, G.L. , Reece, D.E. , Barnett, M.J. , Forrest, D.L. , Gascoyne, R.D. , Hogge, D.E. , Nantel, S.H. , Shepherd, J.D. , Smith, C.A. , Song, K.W. , Sutherland, H.J. , Toze, C.L. , Voss, N.J. & Nevill, T.J. (2005) High‐dose chemotherapy and autologous stem cell transplantation for primary refractory or relapsed Hodgkin lymphoma: long‐term outcome in the first 100 patients treated in Vancouver. Blood, 106, 1473–1478. [DOI] [PubMed] [Google Scholar]
- Lazarus, H.M. , Loberiza, Jr, F.R. , Zhang, M.J. , Armitage, J.O. , Ballen, K.K. , Bashey, A. , Bolwell, B.J. , Burns, L.J. , Freytes, C.O. , Gale, R.P. , Gibson, J. , Herzig, R.H. , LeMaistre, C.F. , Marks, D. , Mason, J. , Miller, A.M. , Milone, G.A. , Pavlovsky, S. , Reece, D.E. , Rizzo, J.D. , van Besien, K. , Vose, J.M. & Horowitz, M.M. (2001) Autotransplants for Hodgkin's disease in first relapse or second remission: a report from the autologous blood and marrow transplant registry (ABMTR). Bone Marrow Transplantation, 27, 387–396. [DOI] [PubMed] [Google Scholar]
- Linch, D.C. , Winfield, D. , Goldstone, A.H. , Moir, D. , Hancock, B. , McMillan, A. , Chopra, R. , Milligan, D. & Hudson, G.V. (1993) Dose intensification with autologous bone‐marrow transplantation in relapsed and resistant Hodgkin's disease: results of a BNLI randomized trial. The Lancet, 341, 1051–1054. [DOI] [PubMed] [Google Scholar]
- Lister, T.A. , Crowther, D. , Sutcliffe, S.B. , Glatstein, E. , Canellos, G.P. , Young, R.C. , Rosenberg, S.A. , Coltman, C.A. & Tubiana, M. (1989) Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. Journal of Clinical Oncology, 7, 1630–1636. [DOI] [PubMed] [Google Scholar]
- Majhail, N.S. , Weisdorf, D.J. , Defor, T.E. , Miller, J.S. , McGlave, P.B. , Slungaard, A. , Arora, M. , Ramsay, N.K. , Orchard, P.J. , MacMillan, M.L. & Burns, L.J. (2006) Long‐term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin's lymphoma. Biology of Blood and Marrow Transplantation, 12, 1065–1072. [DOI] [PubMed] [Google Scholar]
- Majhail, N.S. , Bajorunaite, R. , Lazarus, H.M. , Wang, Z. , Klein, J.P. , Zhang, M.J. & Rizzo, J.D. (2009) Long‐term survival and late relapse in 2‐year survivors of autologous haematopoietic cell transplantation for Hodgkin and non‐Hodgkin lymphoma. British Journal of Haematology, 147, 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, W.G. , Ristow, K.M. , Habermann, T.M. , Colgan, J.P. , Witzig, T.E. & Ansell, S.M. (2005) Bleomycin pulmonary toxicity has a negative impact on the outcome of patients with Hodgkin's lymphoma. Journal of Clinical Oncology, 23, 7614–7620. [DOI] [PubMed] [Google Scholar]
- Martínez, C. , Canals, C. , Sarina, B. , Alessandrino, E.P. , Karakasis, D. , Pulsoni, A. , Sica, S. , Trneny, M. , Snowden, J.A. , Kanfer, E. , Milpied, N. , Bosi, A. , Guidi, S. , de Souza, C.A. , Willemze, R. , Arranz, R. , Jebavy, L. , Hellmann, A. , Sibon, D. , Oneto, R. , Luan, J.J. , Dreger, P. , Castagna, L. & Sureda, A. ; Lymphoma Working Party of the European Group for Blood and Marrow Transplantation (EBMT) and the Gruppo Italiano Trapianto di Midollo Osseo (GITMO) . (2013) Identification of prognostic factors predicting outcome in Hodgkin's lymphoma patients relapsing after autologous stem cell transplantation. Annals of Oncology, 24, 2430–2434. [DOI] [PubMed] [Google Scholar]
- Mills, W. , Chopra, R. , McMillan, A. , Pearce, R. , Linch, D.C. & Goldstone, A.H. (1995) BEAM chemotherapy and autologous bone marrow transplantation for patients with relapsed or refractory non‐Hodgkin's lymphoma. Journal of Clinical Oncology, 13, 588–595. [DOI] [PubMed] [Google Scholar]
- Moskowitz, C.H. , Nimer, S.D. , Zelenetz, A.D. , Trippett, T. , Hedrick, E.E. , Filippa, D.A. , Louie, D. , Gonzales, M. , Walits, J. , Coady‐Lyons, N. , Qin, J. , Frank, R. , Bertino, J.R. , Goy, A. , Noy, A. , O'Brien, J.P. , Straus, D. , Portlock, C.S. & Yahalom, J. (2001) A 2‐step comprehensive high‐dose chemoradiotherapy second‐line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood, 97, 616–623. [DOI] [PubMed] [Google Scholar]
- Moskowitz, C.H. , Matasar, M.J. , Zelenetz, A.D. , Nimer, S.D. , Gerecitano, J. , Hamlin, P. , Horwitz, S. , Moskowitz, A.J. , Noy, A. , Palomba, L. , Perales, M.A. , Portlock, C. , Straus, D. , Maragulia, J.C. , Schoder, H. & Yahalom, J. (2012) Normalization of pre‐ASCT, FDG‐PET imaging with second‐line, non‐cross‐resistant, chemotherapy programs improves event‐free survival in patients with Hodgkin lymphoma. Blood, 119, 1665–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso, M. , Scalone, R. , Marcacci, G. , Lanza, F. , Di Renzo, N. , Cascavilla, N. , Di Bartolomeo, P. , Crescimanno, A. , Perrone, T. & Pinto, A. (2010) Fotemustine plus etoposide, cytarabine and melphalan (FEAM) as a new conditioning regimen for lymphoma patients undergoing auto‐SCT: a multicenter feasibility study. Bone Marrow Transplantation, 45, 1147–1153. [DOI] [PubMed] [Google Scholar]
- Nademanee, A. , O'Donnell, M.R. , Snyder, D.S. , Schmidt, G.M. , Parker, P.M. , Stein, A.S. , Smith, E.P. , Molina, A. , Stepan, D.E. & Somlo, G. (1995) High‐dose chemotherapy with or without total body irradiation followed by autologous bone marrow and/or peripheral blood stem cell transplantation for patients with relapsed and refractory Hodgkin's disease: results in 85 patients with analysis of prognostic factors. Blood, 85, 1381–1390. [PubMed] [Google Scholar]
- Nieto, Y. , Popat, U. , Anderlini, P. , Valdez, B. , Andersson, B. , Liu, P. , Hosing, C. , Shpall, E.J. , Alousi, A. , Kebriaei, P. , Qazilbash, M. , Parmar, S. , Bashir, Q. , Shah, N. , Khouri, I. , Rondon, G. , Champlin, R. & Jones, R.B. (2013) Autologous stem cell transplantation for refractory or poor‐risk relapsed Hodgkin's lymphoma: effect of the specific high‐dose chemotherapy regimen on outcome. Biology of Blood and Marrow Transplantation, 19, 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto, A. , Corradini, P. , Mussetti, A. & Zinzani, P.L. (2014) Recurrent Hodgkin Lymphoma: towards a new definition of candidates to autologous stem cell transplantation in the era of PET scan and novel agents. Leukemia & Lymphoma, 3, 1–19. [DOI] [PubMed] [Google Scholar]
- Popat, U. , Hosing, C. , Saliba, R.M. , Anderlini, P. , van Besien, K. , Przepiorka, D. , Khouri, I.F. , Gajewski, J. , Claxton, D. , Giralt, S. , Rodriguez, M. , Romaguera, J. , Hagemeister, F. , Ha, C. , Cox, J. , Cabanillas, F. , Andersson, B.S. & Champlin, R.E. (2004) Prognostic factors for disease progression after high‐dose chemotherapy and autologous hematopoietic stem cell transplantation for recurrent or refractory Hodgkin's lymphoma. Bone Marrow Transplantation, 33, 1015–1023. [DOI] [PubMed] [Google Scholar]
- Provencio, M. , Sánchez, A. & Sánchez‐Beato, M. (2014) New drugs and targeted treatments in Hodgkin's lymphoma. Cancer Treatment Reviews, 40, 457–464. [DOI] [PubMed] [Google Scholar]
- Puig, N. , de la Rubia, J. , Remigia, M.J. , Jarque, I. , Martín, G. , Cupelli, L. , Sanz, G.F. , Lorenzo, I. , Sanz, J. , Martínez, J.A. , Jiménez, C. & Sanz, M.A. (2006) Morbidity and transplant‐related mortality of CBV and BEAM preparative regimens for patients with lymphoid malignancies undergoing autologous stem‐cell transplantation. Leukemia & Lymphoma, 47, 1488–1494. [DOI] [PubMed] [Google Scholar]
- Rancea, M. , von Tresckow, B. , Monsef, I. , Engert, A. & Skoetz, N. (2014) High‐dose chemotherapy followed by autologous stem cell transplantation for patients with relapsed or refractory Hodgkin lymphoma: a systematic review with meta‐analysis. Critical Reviews in Oncology and Hematology, 92, 1–10. [DOI] [PubMed] [Google Scholar]
- Reddy, N.M. & Perales, M.A. (2014) Stem cell transplantation in Hodgkin lymphoma. Hematology/Oncology Clinics of North America, 28, 1097–1112. [DOI] [PubMed] [Google Scholar]
- Sasse, S. , Rothe, A. , Goergen, H. , Eichenauer, D.A. , Lohri, A. , Kreher, S. , Jäger, U. , Bangard, C. , Kuhnert, G. , Böll, B. , von Tresckow, B. & Engert, A. (2013) Brentuximab vedotin (SGN‐35) in patients with transplant‐naive relapsed/refractory Hodgkin lymphoma. Leukemia & Lymphoma, 54, 2144–2148. [DOI] [PubMed] [Google Scholar]
- Schmitz, N. , Pfistner, B. , Sextro, M. , Sieber, M. , Carella, A.M. , Haenel, M. , Boissevain, F. , Zschaber, R. , Müller, P. , Kirchner, H. , Lohri, A. , Decker, S. , Koch, B. , Hasenclever, D. & Goldstone, A.H. (2002) Aggressive conventional chemotherapy compared with high‐dose chemotherapy with autologous haemopoietic stem‐cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. The Lancet, 359, 2065–2071. [DOI] [PubMed] [Google Scholar]
- Sirohi, B. , Cunningham, D. , Powles, R. , Murphy, F. , Arkenau, T. , Norman, A. , Oates, J. , Wotherspoon, A. & Horwich, A. (2008) Long‐term outcome of autologous stem‐cell transplantation in relapsed or refractory Hodgkin's lymphoma. Annals of Oncology, 19, 1312–1319. [DOI] [PubMed] [Google Scholar]
- Smeltzer, J.P. , Cashen, A.F. , Zhang, Q. , Homb, A. , Dehdashti, F. , Abboud, C.N. , Dipersio, J.F. , Stockerl‐Goldstein, K.E. , Uy, G.L. , Vij, R. , Westervelt, P. , Bartlett, N.L. & Fehniger, T.A. (2011) Prognostic significance of FDG‐PET in relapsed or refractory classical Hodgkin lymphoma treated with standard salvage chemotherapy and autologous stem cell transplantation. Biology of Blood and Marrow Transplantation, 17, 1646–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S.D. , Moskowitz, C.H. , Dean, R. , Pohlman, B. , Sobecks, R. , Copelan, E. , Andresen, S. , Bolwell, B. , Maragulia, J.C. , Vanak, J.M. , Sweetenham, J. & Moskowitz, A.J. (2011) Autologous stem cell transplant for early relapsed/refractory Hodgkin lymphoma: results from two transplant centres. British Journal of Haematology, 153, 358–363. [DOI] [PubMed] [Google Scholar]
- Stiff, P.J. , Unger, J.M. , Forman, S.J. , McCall, A.R. , LeBlanc, M. , Nademanee, A.P. , Bolwell, B.J. & Fisher, R.I. ; Southwest Oncology Group . (2003) The value of augmented preparative regimens combined with an autologous bone marrow transplant for the management of relapsed or refractory Hodgkin disease: a Southwest Oncology Group phase II trial. Biology of Blood and Marrow Transplantation, 9, 529–539. [DOI] [PubMed] [Google Scholar]
- Sureda, A. , Constans, M. , Iriondo, A. , Arranz, R. , Caballero, M.D. , Vidal, M.J. , Petit, J. , López, A. , Lahuerta, J.J. , Carreras, E. , García‐Conde, J. , García‐Laraña, J. , Cabrera, R. , Jarque, I. , Carrera, D. , García‐Ruiz, J.C. , Pascual, M.J. , Rifón, J. , Moraleda, J.M. , Pérez‐Equiza, K. , Albó, C. , Díaz‐Mediavilla, J. , Torres, A. , Torres, P. , Besalduch, J. , Marín, J. , Mateos, M.V. , Fernández‐Rañada, J.M. , Sierra, J. & Conde, E. ; Grupo Español de Linfomas/Trasplante Autólogo de Médula Osea Cooperative Group . (2005) Prognostic factors affecting long‐term outcome after stem cell transplantation in Hodgkin's lymphoma autografted after a first relapse. Annals of Oncology, 16, 625–633. [DOI] [PubMed] [Google Scholar]
- Sureda, A. , Pereira, M.I. & Dreger, P. (2012) The role of hematopoietic stem cell transplantation in the treatment of relapsed/refractory Hodgkin's lymphoma. Current Opinions in Oncology, 24, 727–732. [DOI] [PubMed] [Google Scholar]
- Till, B.G. & Madtes, D.K. (2012) BCNU‐associated pneumonitis: portrait of a toxicity. Leukemia & Lymphoma, 53, 1019–1020. [DOI] [PubMed] [Google Scholar]
- Topalian, S.L. , Hodi, F.S. , Brahmer, J.R. , Gettinger, S.N. , Smith, D.C. , McDermott, D.F. , Powderly, J.D. , Carvajal, R.D. , Sosman, J.A. , Atkins, M.B. , Leming, P.D. , Spigel, D.R. , Antonia, S.J. , Horn, L. , Drake, C.G. , Pardoll, D.M. , Chen, L. , Sharfman, W.H. , Anders, R.A. , Taube, J.M. , McMiller, T.L. , Xu, H. , Korman, A.J. , Jure‐Kunkel, M. , Agrawal, S. , McDonald, D. , Kollia, G.D. , Gupta, A. , Wigginton, J.M. & Sznol, M. (2012) Safety, activity, and immune correlates of anti–PD‐1 antibody in cancer. New England Journal of Medicine, 366, 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Tresckow, B. & Engert, A. (2012) The emerging role of PET in Hodgkin lymphoma patients receiving autologous stem cell transplant. Expert Review of Hematology, 5, 483–486. [DOI] [PubMed] [Google Scholar]
- Visani, G. , Stefani, P.M. , Capria, S. , Malerba, L. , Galieni, P. , Gaudio, F. , Specchia, G. , Meloni, G. , Gherlinzoni, F. , Gonella, R. , Gobbi, M. , Santoro, A. , Ferrara, F. , Rocchi, M. , Ocio, E.M. , Caballero, M.D. , Loscocco, F. & Isidori, A. (2014) Bendamustine, etoposide, cytarabine, melphalan, and autologous stem cell rescue produce a 72% 3‐year PFS in resistant lymphoma. Blood, 124, 3029–3031. [DOI] [PubMed] [Google Scholar]
- William, B.M. , Loberiza, Jr, F.R. , Whalen, V. , Bierman, P.J. , Bociek, R.G. , Vose, J.M. & Armitage, J.O. (2013) Impact of conditioning regimen on outcome of 2‐year disease‐free survivors of autologous stem cell transplantation for Hodgkin lymphoma. Clinical Lymphoma Myeloma & Leukemia, 13, 417–423. [DOI] [PubMed] [Google Scholar]
- Younes, A. , Connors, J.M. , Park, S.I. , Fanale, M. , O'Meara, M.M. , Hunder, N.N. , Huebner, D. & Ansell, S.M. (2013) Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin's lymphoma: a phase 1, open‐label, dose‐escalation study. The Lancet Oncology, 14, 1348–1356. [DOI] [PubMed] [Google Scholar]


