Abstract
Objectives
To evaluate the prevalence, impact and risk factors for pain among a cohort of HIV-infected adults treated with combination anti-retroviral therapy (cART) if indicated according to current guidelines.
Methods
This was a cross-sectional epidemiological observational study. All patients attending one HIV-outpatient centre in the UK in a 10-month period were eligible. Patients completed a validated questionnaire enquiring about demographics, HIV factors and symptoms of pain.
Results
Of 1050 eligible participants, 859 (82%) completed a questionnaire. The 1-month period prevalence of pain lasting > 1 day was 62.8% amongst whom 63% reported current pain. The prevalence of pain at most anatomical sites was broadly similar to that observed in population studies using the same questionnaires except that we found considerably higher rates of foot/ankle pain. The median duration of pain was 3 years (range 0-51 years) and the median pain score was 5.0 on an 11-point visual analogue score. Over 40% of people in pain had consulted their primary care physician and > 20% were taking analgesics daily. Independent risk factors for current pain were older age (p=0.001), time since diagnosis of HIV infection (p=0.001) and receipt of a protease inhibitor-based regimen (p=0.04).
Discussion
Pain, and notably foot/ankle pain, is common among adults living with prevalent HIV and is associated with substantial morbidity and healthcare utilisation.
Keywords: HIV, Pain, Impact, Combination anti-retroviral therapies (cART), Protease inhibitors
Introduction
Prior to effective combination anti-retroviral therapy (cART), the results of several studies suggested that severe, disabling pain affected 60-80% of people living with human immunodeficiency virus (HIV) [1–5]. However, since the advent of cART, the prognosis of HIV has been dramatically transformed with reductions in opportunistic infections and malignancies and life expectancy has normalised [6–7]. This transformed prognosis has resulted in a growing population of ageing individuals with prevalent HIV taking long-term cART who experience high levels of medical and psychiatric comorbidity [8]. Therefore, the emphasis of HIV management has changed to focus increasingly on symptoms, quality of life and prevention and management of comorbidities.
There is growing evidence that, despite cART, pain remains a common problem among HIV-infected patients [9–22]. Some, but not all, authors report prevalence rates very similar to those observed pre-cART [15–16,19]. Miaskowski and colleagues reported not only that pain was common but also that it was frequently ‘severe’ (59% of those reporting pain) [16] and Merlin et al showed that the pain was associated with significantly increased risk of impairment of physical function [19]. However, Cervia and colleagues reported lower pain intensity scores and more transient, rather than chronic, pain in 41 patients after treatment with cART [15]. There is controversy also about the role of immunological function and viral activity in the aetiology of pain. Pre-cART studies suggested that pain increased in prevalence and intensity with disease progression [5,23]. However, the findings of later studies suggested that effective cART attenuated the effects of disease stage or viral activity, as defined by CD4+ count or HIV viral load, on pain [15]. In some studies, risk factors for pain have included: female sex, lower socioeconomic status and educational attainment, depression and high rates of previous or recent use of illicit drugs [16,17]. There is also inconsistency in the literature as to whether pain is a side effect of some of the anti-retroviral therapies [10,13, 24–25]. Whilst a distal polyradiculopathy was closely linked with dideoxynucleosides in early cART regimens [26], these are generally avoidable with more modern treatment combinations. Therefore, our objective was to investigate the prevalence and distribution of pain among a UK cohort of HIV-infected adults treated according to best practice guidance with cART. We set out to quantify the prevalence of pain in the post-cART era, measure its impact in terms of intensity, effects on activities of daily living and healthcare utilisation and explore demographic, lifestyle and clinical risk factors for occurrence in order to elucidate possible strategies for prevention and treatment.
Methods
The sampling frame for this study included all HIV-infected adults who attended a routine outpatient appointment at a Teaching Hospital Centre for HIV Medicine in the UK January–October 2007. Patients were eligible if they were: aged ≥18 years and willing and able to provide written, informed consent. Eligible subjects were only approached once. Patients were offered the questionnaire to complete in a private space and a trained member of the research team was highlighted as available if any additional information or assistance was required. Permission was also sought to interrogate the confidential clinical database within the centre to collect HIV-related data (date of diagnosis, route of transmission, severity and course of disease, viral load, CD4 count, cART). The study protocol was approved by the Brighton Local Research and Ethics Committee (Ref: 06/Q1907/50).
The questionnaire enquired about demography (age, sex, ethnicity), lifestyle (smoking, alcohol), and employment status. The questions about pain were those used in a number of surveys of the prevalence of regional [Urwin] and widespread pain [27–30 . All participants were asked ‘during the past month, have you had any aches or pains which have lasted for one day or longer?’ The principal risk factor analyses were based upon those reporting current pain in response to the questions: ‘do you have any such aches or pains today?’ Pain intensity was self-rated on an 11-point visual analogue scale (VAS) for the intensity of pain during the past month. Functional impact of pain was measured on another 11-point VAS in response to the question ‘in the past month, how much has pain interfered with your daily activities, rated on a scale of 0-10, where 0 is “no interference” and 10 is “unable to carry out activities”? The site-specific prevalence rates presented here were obtained from a series of similarly-structured questions which asked: ‘During the past 1 month, have you had any pain from your (neck; shoulder; elbow; wrist/hand; hip; knee; ankle/foot) lasting for at least 1 week’? The questionnaire also enquired about healthcare use in relation to pain in the past month. Psychological health and wellbeing were measured using the vitality and mental health domains of the SF-36 instrument [31]. Each domain was scored according to the SF-36 algorithm for each individual and the scores were investigated in tertiles of the distribution in relation to the pain outcomes: lowest tertile ‘best’ mental health (referent) and highest tertile ‘worst’ mental health).
Analysis was carried out with the SAS v9.1 statistical package. The age-and sex-specific prevalence of pain lasting for more than one week out of the past month at different sites (with 95% confidence intervals generated using the exact binomial distribution where the group size was small, or the Normal approximation where group sizes were larger), the frequency of healthcare use and self-completed score for interference of the pain with daily activities were explored. The primary endpoint for the analysis was the reporting of current pain. Initial descriptive analyses illustrated the prevalence of current pain in each patient subgroup; univariate comparisons of the prevalence of pain were performed using Chi-squared tests. Potential risk factors considered are described in Table 1 and include: sex; age, BMI, smoking status, alcohol consumption, ethnicity, mode of HIV infection, time since HIV diagnosis, the patient’s current CD4 count, HIV viral load, Centers for Disease Control (CDC) classification, and cART status. Continuous covariates (e.g. age) were initially categorised as shown in Table 1, but were subsequently included in analyses in their continuous form if appropriate; the CDC stages B and C were combined as ‘symptomatic’ as the prevalence of pain appeared to be similar in these two groups. Subsequently, factors that were identified as being associated with current pain in these univariate analyses (P<0.10) were included in multivariable logistic regression analyses to identify factors that were independently associated with the outcome; factors were dropped from this model if non-significant until a parsimonious model was reached. Current working status and SF-36 scores were excluded from these models as factors which were likely to be consequent from the pain rather than causes of it.
Table 1. (i) Demographic and (ii) clinical characteristics of HIV-infected study participants, and the proportion of each group currently experiencing pain (n=859 unless otherwise stated).
| (i) Demographic characteristics | ||||
|---|---|---|---|---|
| Number (% of total sample) | Number with current pain (%) | p-value1 | ||
| n | 859 (100.0) | 341 (39.7) | ||
| Sex | Male | 775 (90.2) | 303 (39.1) | |
| Female | 84 (9.8) | 38 (45.2) | 0.33 | |
| Age (years) | Median (range) | 42 (19 - 77) | ||
| 19-34 | 171 (19.9) | 49 (28.7) | ||
| 35-39 | 164 (19.1) | 56 (34.2) | ||
| 40-44 | 186 (21.7) | 80 (43.0) | ||
| 45-49 | 143 (16.7) | 65 (45.5) | ||
| ≥50 | 195 (22.7) | 91 (46.7) | 0.002 | |
| Ethnicity | White | 631 (75.8) | 261 (41.4) | |
| (n=833) | Black African | 84 (10.1) | 27 (32.1) | |
| Other | 118 (14.2) | 43 (36.4) | 0.35 | |
| BMI (kg/m2) | Underweight (<18.5) | 40 (4.9) | 17 (42.5) | |
| (n=810) | Normal (≥18.5, <25) | 497 (61.4) | 197 (39.6) | |
| Overweight (≥25, <30) | 204 (25.2) | 81 (39.7) | ||
| Obese/morbidly obese (≥30) | 69 (8.5) | 26 (37.7) | 0.99 | |
| Smoking status | Current | 348 (40.9) | 138 (39.7) | |
| (n=850) | Ex-smoker | 266 (31.3) | 112 (42.1) | |
| Never smoked | 236 (27.8) | 88 (37.3) | 0.71 | |
| Consume alcohol | No | 198 (23.0) | 88 (44.4) | |
| Yes | 661 (77.0) | 253 (38.3) | 0.14 | |
| Hepatitis B +ve | No | 843 (98.1) | 334 (39.6) | |
| Yes | 16 (1.9) | 7 (43.8) | 0.94 | |
| Hepatitis C +ve | No | 848 (98.7) | 336 (39.6) | |
| Yes | 11 (1.3) | 5 (45.5) | 0.93 | |
| Working status | Working | 486 (57.0) | 145 (29.8) | |
| (n=852) | Not working | 366 (43.0) | 195 (53.5) | 0.0001 |
| SF-36 Mentality |
Median (IQR) | 14 (5, 30) | ||
| 5-11 | 264 (34.1) | 68 (25.8) | ||
| 12-16 | 254 (32.8) | 97 (38.2) | ||
| 17-30 | 256 (33.1) | 144 (56.3) | 0.0001 | |
| Physical functioning | Median (IQR) | 14 (4, 24) | ||
| 4-11 | 266 (34.6) | 59 (22.2) | ||
| 12-16 | 281 (36.5) | 121 (43.1) | ||
| 17-24 | 222 (28.9) | 126 (56.8) | 0.0001 | |
| (ii) Clinical characteristics | ||||
|---|---|---|---|---|
| Number (% of total sample) | Number with current pain (%) | p-value1 | ||
| n | 859 | 341 (39.7) | ||
| Mode of infection | MSM2 | 217 (25.3) | 84 (38.7) | |
| Other | 60 (7.0) | 21 (35.0) | ||
| Not known | 582 (67.8) | 236 (40.6) | 0.66 | |
| Latest CD4 (cells/mm3) | Median (range) | 475 (12, 1407) | ||
| (n=837) | <200 | 59 (7.1) | 24 (40.7) | |
| 200-349 | 164 (19.6) | 66 (40.2) | ||
| ≥350 | 614 (73.4) | 242 (39.4) | 0.99 | |
| Latest viral load (copies/ml) | Median (range) | 40 (40, 834687) | ||
| (n=842) | ≤40 | 569 (67.6) | 242(42.5) | |
| >40 | 273 (32.4) | 93 (34.1) | 0.02 | |
| Time since HIV diagnosis (years) | ≤1 | 88 (10.5) | 24 (27.3) | |
| (n=839) | >1, ≤3 | 132 (15.7) | 38 (28.8) | |
| >3, ≤5 | 133 (15.9) | 51 (38.4) | ||
| >5, ≤10 | 216 (25.7) | 98 (45.4) | ||
| >10, ≤20 | 220 (26.2) | 99 (45.0) | ||
| >20 | 50 (6.0) | 26 (52.0) | 0.0009 | |
| CDC status | A – asymptomatic | 489 (58.3) | 171 (35.0) | |
| (n=839) | B – symptomatic | 190 (22.7) | 89 (46.8) | |
| C – AIDS | 160 (19.1) | 71 (44.4) | 0.01 | |
| cART naive | Never had cART | 154 (17.9) | 46 (29.9) | |
| Have had cART | 705 (82.1) | 295 (41.8) | 0.008 | |
| Current ART status | Not on cART | 202 (23.5) | 66 (32.7) | |
| On cART | 657 (76.5) | 275 (41.9) | 0.02 | |
| PI based – No | 580 (67.5) | 209 (36.0) | ||
| PI-based - Yes | 279 (32.5) | 132 (47.3) | 0.002 | |
| NNRTI based - No | 503 (58.6) | 201 (40.0) | ||
| NNRTI based –Yes | 356 (41.4) | 140 (39.3) | 0.91 | |
| NRTI-based – No | 259 (30.1) | 90 (34.8) | ||
| NRTI-based - Yes | 600 (69.9) | 251 (41.8) | 0.06 | |
p-value obtained from a univariate comparison of the proportions currently experiencing pain in each group (Chi-squared test).
MSM: men having sex with men; CDC: Centers for Disease Control; cART: combination antiretroviral therapy; ART: antiretroviral therapy; PI: protease inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor; NRTI: nucleoside reverse transcriptase inhibitor
Results
In total, 1539 patients were registered with the Centre during the study period amongst whom 1050 patients attended ≥one outpatient clinic. Of these, 859 (81.8%) consented to participate. A comparison of the distributions of sex, age, years since HIV diagnosis, CDC stage and exposure to cART, showed no significant differences between those who did and did not complete a questionnaire (data not shown).
In total, 775 men and 84 women, median age 42 years, completed the questionnaire. Most were Caucasian and had prolonged duration since HIV diagnosis (Table 1). Most were currently receiving cART (76.5%) and 68% had undetectable viral loads.
Pain
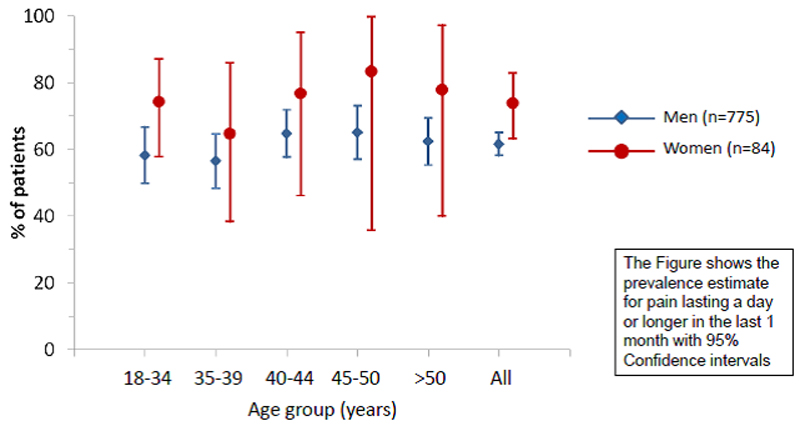
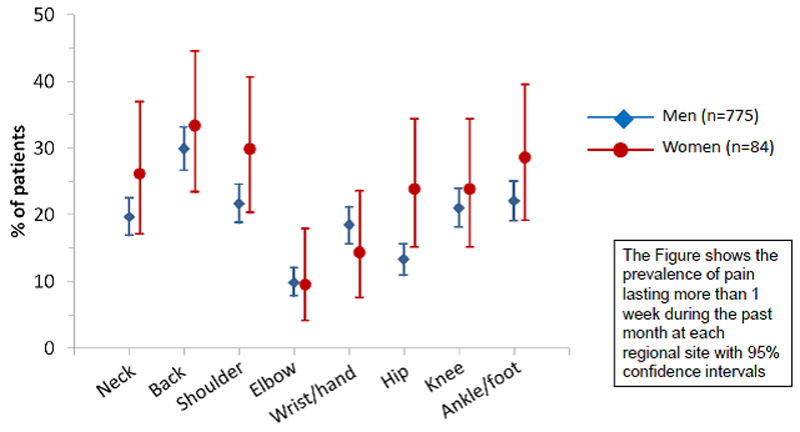
In total, 62.8% of respondents reported that they had experienced pain lasting >1 day in the past month (Table 2), of whom 63.3% reported pain on the day of the survey. Eighty percent indicated duration of pain >3 months and 23.6% that the pain was ‘all over the body’. The median number of sites affected was 2 but the range was 0-20 separate anatomical sites in men and 0-25 in women. The age- and sex-specific prevalence of pain is summarised in Figure 1. Pain frequency and location was similar among men and women and increased with age in both sexes. The most commonly affected sites were: axial (neck and back), shoulder, and foot/ankle (Figure 2).
Table 2. Musculoskeletal pain and its impact among 859 HIV-infected adults.
| Total (n=859) | % of total | |
|---|---|---|
| Ever had pain lasting at least 1 day in the last month | 539 | 62.8 |
| Number of painful sites (range) | 6 (1-25) | - |
| Seen a Rheumatologist for pain | 110 | 12.8 |
| Seen an orthopaedic surgeon for pain | 102 | 11.9 |
| Attended Emergency Department for pain | 76 | 8.9 |
| Use analgesics most days for pain | 194 | 22.6 |
| Had an operation because of pain | 40 | 4.7 |
| Of those in pain: | ||
| Currently in pain | 341 | 39.7 (63.3 of those with pain) |
| Pain lasting >3 months (chronic pain) | 431 | 50.2 (80.0 of those with pain) |
| Time off work due to pain | 44 | 5.1 (16.2 of those with pain) |
| Seen a doctor because of the pain | 236 | 27.5 (43.8 of those with pain) |
| Median duration of pain (years)(range) | 3 (1-51) | - |
| Median pain score (range) | 5 (1-10) | - |
| Mean pain score (SD) | 5.2 (2.2) | - |
| Median disability score (range) | 4 (0-10) | - |
| Mean disability score (SD) | 4.4 (2.9) | - |
Figure 1. The cross-sectional age- and sex-specific prevalence of musculoskeletal pain among 859 adults infected with HIV.
Figure 2. The cross-sectional site-specific prevalence of musculoskeletal pain among 859 adults infected with HIV.
Impact of musculoskeletal pain
The median duration of pain was 3 years (range 1-51 years) (Table 2). The median pain intensity score recorded was 5.0 for men and 6.0 for women (mean scores 5.1 and 5.9 respectively). When asked to score the impact of pain on daily activities, the median score was 4.0 for men and 5.3 for women (mean scores 4.3 for men and 5.3 for women respectively). In total, 43.8% of those in pain reported that they had consulted in primary care because of their pain (41.7% of men and 59.7% of women). One hundred and ninety-four (22.6%) were taking analgesics most days; 70 (8.2%) had received injections of local corticosteroid; 110 (12.8%) had seen a rheumatologist, 102 (11.9%) had seen an orthopaedic surgeon and 76 (8.9%) had attended the Emergency Department to request treatment for the pain. Current pain was associated with not being in work (p=0.0001, 29.8% of those working vs. 53.5% of those not working reported current pain), and was strongly associated with psychological distress as measured by the SF-36 (mental health (p=0.0001) and physical functioning (p=0.0001)). Psychological wellbeing scores were significantly poorer among those with current pain (median (range) of 16 (6, 30) and 16 (5, 24) on the mental health and vitality scores respectively than among those without current pain (12 (5, 30) and 12 (4, 24) respectively)‥
Risk factors for pain
In descriptive analyses (Table 1), current pain was more prevalent in older individuals, in those with an undetectable (≤40 copies/ml) viral load, in those with a longer time since HIV diagnosis, in those with more advanced CDC status, in those who had ever received or were currently receiving cART and in those on a PI-based (vs. a non-PI-based) cART regimen and in those receiving NRTIs (vs. those not receiving NRTIs). Table 3 (left-hand side) reports the unadjusted odds ratios and 95% confidence intervals for these associations. Associations were also seen with working status and the SF-36 mentality and physical functioning scales in univariate analyses (Table 1) although these factors were not included in subsequent multivariable analysis as they are likely to be the result, rather than the cause, of the pain.
Table 3. Associations between demographic and clinical factors and current pain from univariate and multivariable logistic regression analyses.
| Characteristic | Unadjusted OR (95% CI)* | p-value | Adjusted OR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Age | /5 years older | 1.12 (1.05, 1.20) | 0.0008 | 1.10 (1.02, 1.19) | 0.02 |
| Time since HIV diagnosis | /5 years | 1.26 (1.13, 1.41) | 0.0001 | 1.17 (1.03, 1.32) | 0.02 |
| Viral load (copies/ml) | >40 | 1 | |||
| ≤40 | 1.43 (1.06-1.93) | 0.02 | - | ||
| HIV stage | Asymptomatic (CDC stage A) | 1 | |||
| Symptomatic (CDC stages B/C) | 1.57 (1.18, 2.07) | 0.002 | - | ||
| cART naive | No | 1 | |||
| Yes | 0.59 (0.41, 0.86) | 0.006 | - | ||
| Currently on cART | No | 1 | |||
| Yes | 1.48 (1.06, 2.07) | 0.02 | - | ||
| On NRTI | No | 1 | |||
| Yes | 1.35 (1.00, 1.83) | 0.05 | - | ||
| On PI | No | 1 | 1 | ||
| Yes | 1.59 (1.19, 2.13) | 0.002 | 1.39 (1.01, 1.91) | 0.04 |
OR: odds ratio; CI: confidence interval; CDC: Centers for Disease Control; cART: combination antiretroviral therapy; NRTI: nucleoside reverse transcriptase inhibitor; PI: protease inhibitor.
Of the covariates considered, increasing age (adjusted odds ratio [aOR] per 5 years older 1.10 [95% confidence interval 1.02, 1.19], p=0.02), and longer time since diagnosis (aOR per 5 years longer 1.17 [1.03, 1.32], p=0.02) were independent predictors of current pain. Current use of a PI-based (1.39 [1.01, 1.91], p=0.04) regimen also remained significantly associated with current pain.
Finally, we considered whether the associations between current pain and sex and time since HIV diagnosis differed in men and women through the inclusion of interaction terms between sex and these covariates in the final model. Neither interaction term was significant (age: p=0.27; time since HIV diagnosis: p=0.46), suggesting that there was no evidence that these associations differed between sexes.
Discussion
This study confirms that the prevalence (in this case the one-month period prevalence) of pain is high in people living with HIV. The estimated prevalence rate of 63% is consistent with that reported in other studies in the cART era [14,16] and the findings of a systematic review of pain studies carried out before and after cART [22]. We found higher rates than the 39% reported by Cervia and colleagues in their US study of 41 subjects who completed pain scores before and after commencement of cART [15] but our study includes a larger population with a wide range of disease duration and longer-term exposure to cART. In keeping with the findings of some researchers [5,16], women in the current study reported higher rates of prevalence of pain than men, throughout the age range and at all anatomical sites. One study has however reported the opposite [18]. It is possible that the current study included a population that was more similar to those studied in the two (USA-based) studies than to the rural population studied by Mphalele and colleagues in South Africa and the differences may possibly be explained by cultural or ethnic differences in occurrence or reporting of pain. Clearly, this will require additional research in other ethnic groups and countries.
The median rating for pain severity in the current study was 5.0 (95% CI 2.0-9.0) (moderately severe), a rating consistent with the results of most studies which have included a measure of pain severity [22]. Moderate-to-severe intensity pain is recognised to have a significant impact on ability to function and quality of life. Our results bear out this association as respondents scored the interference of their pain in the past month with their daily activities a median of 4.0 (95% CI 0-9.0). This score is strikingly similar to that obtained by Breitbart and colleagues who surveyed ambulatory HIV patients in the pre-cART era and asked a similar question [5]. In 2012, Merlin and colleagues reported that pain in HIV patients was associated with a 10-fold greater risk of impaired physical function, even after adjusting for mood, age and substance abuse [19]. Our results further substantiate their conclusion that pain should be an important consideration in HIV primary care.
Overall, 35.5% of those with pain reported taking analgesics most days. Inadequacy of pharmacological pain management has been reported in previous studies among HIV-infected patients. Using pain management indexes (PMI), other investigators have reported sub-optimal effectiveness of pain management in the majority (66-100%) of respondents [22]. Although we did not include a PMI in this study, we were able to explore the percentage of people reporting pain receiving no treatment for pain, a measure used by other researchers as a marker of inadequate pain management. We found that 64.5% of our respondents in pain were not taking analgesics most days, a rate similar to that observed by others (40-73%) [22]. However, further exploration revealed that those taking analgesics most days rated their pain as more intense on a VAS and rated the interference of their pain with their daily activities as greater than those not taking analgesics most days. Our study design does not allow us to investigate whether patients have been prescribed analgesics and are choosing not to take them regularly perhaps because of toxicity or inefficacy, or because they believe their symptoms are insufficiently severe. Therefore, future research could usefully explore prescription and adherence with prescription of analgesia and the reasons for which patients do/do not take the medications regularly if we are to better understand how to manage pain in HIV.
In accord with the results of other studies [17,19,21], we found that pain in HIV was strongly associated with psychological ill-health (p<0.0001). Merlin and colleagues have demonstrated this in several studies and have also shown that there is a strong interaction between pain, mood, substance abuse and lower socioeconomic status [17,19,21]. They also showed that people in pain were more likely to miss clinical appointments but only if they were not substance abusers [17]. Failure to attend appointments has important implications for medication adherence and treatment success in HIV. Our survey did not allow characterisation of socioeconomic status in great detail but we found a much higher proportion of worklessness amongst those reporting pain than among those without pain (p<0.0001) and recognise the significance of worklessness as a factor importantly associated with poverty and socioeconomic status.
One of our aims was to explore the impact of parameters of HIV infection on pain. We found that duration of diagnosis of HIV was associated with pain and, in univariate analyses only, symptomatic stage of infection and cART exposure were also associated. Other studies have explored these parameters and produced inconclusive findings: for example, whilst the results of three studies suggested higher prevalence of pain with more advanced stage of infection [32–34], three others found no such association [5,35–36]. Moreover, two studies [14,37] found a higher prevalence of pain amongst those with lower CD+ counts and one showed more pain sites among those with lower CD+ counts [32], three others failed to see associations with CD+ counts [5,35,38]. We found no association with viral loads or CD4+ counts in this study but recognise that we were only able to explore the effects of the most recent results and that associations may have been present with nadir counts, which were not available for these participants. Some, but not all, studies have implicated cART in pain. Breitbart et al who studied patients commencing cART, reported beneficial effects of cART on pain [5], whilst Richardson and colleagues reported no difference in rates of pain [37]. In the current study, PI use was associated with pain (p=0.04) in univariate, but not multivariable models. In clinical trials of PIs amongst naïve HIV-infected patients and non-infected patients as post-exposure prophylaxis, symptoms of muscle pain and joint pain are relatively commonly (10-30% incidence) reported but usually described as ‘mild’ and ‘self-limiting’ [24,39]. Of interest, PIs have been implicated as a cause of pain in another study of female HIV patients [37]. Given that we had relatively numbers of female participants, this study was not powered to investigate a gender effect further and more research will be needed. Notably, use of PIs was recently shown to be associated with increased risk of peripheral neuropathy [40]. It is possible that PIs have some effect on peripheral or central pain pathways but more research into the long term impact of PIs will be required.
This study included specific questions about pain at different anatomical sites, which allowed comparison of pain at different regional sites with those obtained from UK and US general population surveys [27,41]. Broadly, the rates of prevalence and distribution were similar among those with HIV to those found in the general population with the exception of the results at the foot/ankle where we found much higher rates of pain prevalence. We hypothesise that this may reflect the common occurrence of peripheral neuropathy amongst HIV-infected adults. Some of the burden of neuropathy was caused by the neurotoxicity of some nucleoside analogue reverse transcriptase inhibitors (NRTI), particularly stavudine, didanosine and zalcitabine however other HIV factors have been implicated including older age, co-infection, co-existent diabetes mellitus and TB therapy with isoniazid. A recent study found that 32.1% of 2141 subjects starting cART had evidence of a peripheral neuropathy after 3 years of follow-up [40] despite excellent levels of viral and immunological control. It has been shown that the majority of patients (50-90%) with HIV-associated sensory neuropathy experience pain, proportions that are greater than in other common types of peripheral neuropathy such as diabetes and that recognition and treatment of painful sensory neuropathy in HIV is frequently sub-optimal [42]. Interestingly, people with more advanced HIV infection have been shown more likely to report pain with their neuropathy [43–44]. This epidemiologic study does not allow investigation of the aetiology of pain but we hypothesise that much of the excess reporting of foot/ankle pain in this study might be related to underlying sensory neuropathy.
The findings of this study must be taken alongside several limitations. This was a cross-sectional study so that the associations reported were cross-sectional and do not allow speculation about cause or effect or direction of association. Whilst the response rates were excellent (82%), and comparison of some of the key characteristics between those who did/did not complete the questionnaire revealed no significant differences, we cannot rule out the possibility that those who chose to complete the questionnaire were those who considered themselves affected by musculoskeletal symptoms and that the estimated prevalence rates reported are therefore relative over-estimates. These results were found from our survey among a well-characterised cohort of HIV-infected adults attending one UK centre for their HIV care. However, this cohort of patients may differ from those attending other HIV centres in the UK and elsewhere. For example, most of this cohort are male and Caucasian and most acquired HIV through sexual contact. We cannot exclude the possibility that there are factors peculiar to this cohort that also affect musculoskeletal pain that are not generaliseable to patients infected with the virus through different modes of transmission or from different ethnicities. For example, we showed no statistically significant association between mode of transmission of HIV and pain in the current study but other investigators found that mode of transmission was important and, in particular, that intravenous drug use was associated with higher pain levels and pain at a higher mean number of sites [32,35]. Our cohort only included 8 subjects known to have been infected by intravenous drug use so that this study was under-powered to detect this association. Similar studies will need to be carried out in different HIV cohorts to see if our findings are generaliseable.
Our results suggest that duration of HIV diagnosis is importantly associated with pain and this may reflect a number of factors: immunological function and viral activity; increasing burden of physical and psychological co-morbidities; increasing numbers of non-HIV medications; reduced resilience to side effects. Current immunological function and viral activity were investigated using the most recent CD4+ cell count and viral load for each subject and showed no important relationships with pain. However, it may well be that disease status might be more usefully represented by nadir CD4+ count, as a marker of disease state at its worst, rather than recent count and it is a limitation of this study that these data were not available for this cohort of patients. Further research will be needed to clarify the role of disease stage and viral activity on pain in cART treated patients. This study was also not designed to collect information about non-HIV morbidities or medications used for other diseases so we are also unable to investigate the role of those factors.
In summary, we have reported the results of a large-scale epidemiological survey of the occurrence of musculoskeletal pain in HIV-infected adults and found that it is a very common symptom associated with substantial morbidity and that feet/ankles are more commonly affected than in other populations. Cross-sectionally, the risk factors were age and time since diagnosis of HIV. Current use of protease inhibitors may be associated with pain but further research is warranted.
Acknowledgements
We would like to thank the patients and staff of the outpatient centre that took part in the study. This research was supported by a grant from the NHS R&D Department of the Brighton & Sussex University Hospitals NHS Trust.
Footnotes
Competing Interests
There are no conflicts of interest from any of the authors.
Reference List
- 1.Bouhassira D. Pain from AIDS (adult) Dev Sante. 1997;131:24–5. [PubMed] [Google Scholar]
- 2.Kelleher P, Cox S, McKeogh M. HIV infection: the spectrum of symptoms and disease in male and female patients attending a London hospice. Palliat Med. 1997;11:152–8. doi: 10.1177/026921639701100210. [DOI] [PubMed] [Google Scholar]
- 3.Norval DA. Symptoms and sites of pain experienced by AIDS patients. S Afr Med J. 2004;94:450–4. [PubMed] [Google Scholar]
- 4.Schofferman J. Care of the terminally ill person with AIDS. Int Ophthalmol Clin. 1989;29:127–30. doi: 10.1097/00004397-198902920-00011. [DOI] [PubMed] [Google Scholar]
- 5.Breitbart W, McDonald MV, Rosenfeld B, et al. Pain in ambulatory AIDS patients. I: Pain characteristics and medical correlates. Pain. 1996;68:315–21. doi: 10.1016/s0304-3959(96)03215-0. [DOI] [PubMed] [Google Scholar]
- 6.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293–9. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.May MT, Gompels M, Delpech V, et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy: UK cohort study. AIDS. 2014 Feb 19; doi: 10.1097/QAD.0000000000000243. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim DJ, Westfall AO, Chamot E, et al. Multimorbidity patterns in HIV-infected patients: the role of obesity in chronic disease clustering. J Acquir Immnue Defic Syndr. 2012;61:600–5. doi: 10.1097/QAI.0b013e31827303d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newshan G, Bennett J, Holman S. Pain and other symptoms in ambulatory HIV patients in the age of highly active antiretroviral therapy. J Assoc Nurses AIDS Care. 2002;13:78–83. doi: 10.1016/S1055-3290(06)60373-7. [DOI] [PubMed] [Google Scholar]
- 10.Silverberg MJ, Gore ME, French AL, et al. Prevalence of clinical symptoms associated with highly active antiretroviral therapy in the Women’s Interagency HIV Study. Clin Infect Dis. 2004;39:717–24. doi: 10.1086/423181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harding R, Molloy T, Easterbrook T, et al. Is antiretroviral therapy associated with symptom prevalence and burden? Int J STD AIDS. 2006;17:400–5. doi: 10.1258/095646206777323409. [DOI] [PubMed] [Google Scholar]
- 12.Lee KA, Gay C, Portillo CJ, et al. Symptom experience in HIV-infected adults: a function of demographic and clinical characteristics. J Pain Symptom Manage. 2009;38:882–93. doi: 10.1016/j.jpainsymman.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silverberg MJ, Jacobson LP, French A, et al. Age and racial/ethnic differences in the prevalence of reported symptoms in HIV-infected persons on antiretroviral therapy. J Pain Symptom Manage. 2009;38:197–207. doi: 10.1016/j.jpainsymman.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aouizerat BE, Miaskowski CA, Gay C, et al. Risk factors and symptoms associated with pain in HIV-infected adults. J Assoc Nurses AIDS Care. 2010;21:125–33. doi: 10.1016/j.jana.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cervia LD, McGowan JP, Weseley AJ. Clinical and demographic variables related to pain in HIV-infected individuals treated with effective, combination antiretroviral therapy (cART) Pain Med. 2010;11:498–503. doi: 10.1111/j.1526-4637.2010.00802.x. [DOI] [PubMed] [Google Scholar]
- 16.Miaskowski C, Penko JM, Guzman D, et al. Occurrence and characteristics of chronic pain in a community-based cohort of indigent adults living with HIV infection. J Pain. 2011;12:1004–16. doi: 10.1016/j.jpain.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merlin JS, Westfall AO, Raper JL, et al. Pain, mood, substance abuse in HIV: implications for clinic visit utilisation, ART adherence and virologic failure. J Acquir Immune Defic Syndr. 2012;61:164–70. doi: 10.1097/QAI.0b013e3182662215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mphalele NR, Mitchell D, Kamerman PR. Pain in ambulatory HIV-positive South Africans. Eur J Pain. 2012;16:447–58. doi: 10.1002/j.1532-2149.2011.00031.x. [DOI] [PubMed] [Google Scholar]
- 19.Merlin JS, Cen L, Praestgaard A, et al. Pain and physical and psychological symptoms in the ambulatory HIV patients in the current treatment era. J Pain Symptom Manage. 2012;43:638–45. doi: 10.1016/j.jpainsymman.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merlin JS, Westfall AO, Chamot E, et al. Pain is independently associated with impaired physical function in HIV-infected patients. Pain Med. 2013;14:1985–93. doi: 10.1111/pme.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merlin JS, Zinski A, Norton WE, et al. A conceptual framework for understanding chronic pain in patients with HIV. Pain Pract. 2013;14:207–16. doi: 10.1111/papr.12052. [DOI] [PubMed] [Google Scholar]
- 22.Parker R, Stein DJ, Jelsma J. Pain in people living with HIV/AIDS: a systematic review. JIAS. 2014;17:18719. doi: 10.7448/IAS.17.1.18719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hewitt DJ, McDonald M, Portenoy RK, et al. Pain syndromes and etiologies in ambulatory AIDS patients. Pain. 1997;70:117–23. doi: 10.1016/s0304-3959(96)03281-2. [DOI] [PubMed] [Google Scholar]
- 24.Duran S, Spire B, Raffi F, et al. Self-reported symptoms after initiation of a protease inhibitor in HIV-infected patients and their impact on adherence to HAART. HIV Clin Trials. 2001;2:38–45. doi: 10.1310/R8M7-EQ0M-CNPW-39FC. [DOI] [PubMed] [Google Scholar]
- 25.Johnson MO, Stallworth T, Neilands TB. The drugs or the disease? Causal attributions of symptoms held by HIV-positive adults on HAART. AIDS Behav. 2003;7:109–17. doi: 10.1023/a:1023938023005. [DOI] [PubMed] [Google Scholar]
- 26.Bacellar H, Munoz A, Miller E, et al. Temporal trends in the incidence of HIV-1-related neurologic diseases: Multicenter AIDS Cohort Study, 1985-1992. Neurology. 1994;44:1892–1900. doi: 10.1212/wnl.44.10.1892. [DOI] [PubMed] [Google Scholar]
- 27.Urwin M, Symmons D, Allison T, et al. Estimating the burden of musculoskeletal disorders in the community: the comparative prevalence of symptoms at different anatomical sites. Ann Rheum Dis. 1998;57:649–55. doi: 10.1136/ard.57.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McBeth J, Silman AJ, Gupta A, et al. Moderation of psychosocial risk factors through dysfunction of the hypothalamic-pituitary-adrenal stress axis in the onset of chronic widespread musculoskeletal pain: findings of a population-based prospective cohort study. Arthritis Rheum. 2007;56:360–71. doi: 10.1002/art.22336. [DOI] [PubMed] [Google Scholar]
- 29.McBeth J, Symmons DP, Silman AJ, et al. Musculoskeletal pain is associated with a long-term increased risk of cancer and cardiovascular-related mortality. Rheumatology (Oxford) 2009;48:74–7. doi: 10.1093/rheumatology/ken424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulvey MR, Macfarlane DJ, Beasley M, et al. Modest association of joint hypermobility with disabling and limiting musculoskeletal pain: results from a large-scale general population-based survey. Arthritis Care Res. 2013;65:1325–33. doi: 10.1002/acr.21979. [DOI] [PubMed] [Google Scholar]
- 31.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 32.Martin C, Pehrsson P, Osterberg A, et al. Pain in ambulatory HIV-infected patients with and without intravenous drug use. Eur J Pain. 1999;3:157–64. doi: 10.1053/eujp.1999.0111. [DOI] [PubMed] [Google Scholar]
- 33.Dobalian A, Tsao J, Duncan RP. Pain and the use of outpatient services among persons with HIV – results from a nationally representative survey. Med Care. 2004;42:129–38. doi: 10.1097/01.mlr.0000108744.45327.d4. [DOI] [PubMed] [Google Scholar]
- 34.Nair S, Mary TR, Prarthana, et al. Prevalence of pain in patients with HIV/AIDS: a cross-sectional survey in a South Indian State. Indian J Palliat Care. 2009;15:67–70. doi: 10.4103/0973-1075.53550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Borgo C, Izzi I, Chiarotti F, et al. Multidimensional aspects of pain in HIV-infected individuals. AIDS Patient Care STDs. 2001;15:95–102. doi: 10.1089/108729101300003690. [DOI] [PubMed] [Google Scholar]
- 36.Wahab KW, Salami AK. Pain as a symptom in patients living with HIV/AIDS seen at the outpatient clinic of a Nigerian tertiary hospital. J Int Assoc Physicians AIDS Care. 2011;10:35–9. doi: 10.1177/1545109710368863. [DOI] [PubMed] [Google Scholar]
- 37.Richardson JL, Heikes B, Karim R, et al. Experience of pain among women with advanced HIV disease. AIDS Patient Care STDs. 2009;23:503–511. doi: 10.1089/apc.2008.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hitchcock SA, Meyer HP, Gwyther E. Neuropathic pain in AIDS patients prior to antiretroviral therapy. S Afr Med J. 2008;98:889–92. [PubMed] [Google Scholar]
- 39.Cahn P, Montaner J, Junod P, et al. Pilot, randomized study assessing safety, tolerability and efficacy of simplified LPV/r maintenance therapy in HIV patients on the 1st PI-based regimen. PLoS. 2011;6:e23726. doi: 10.1371/journal.pone.0023726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans SR, Ellis RJ, Chen H, et al. Peripheral neuropathy in HIV; prevalence and risk factors. AIDS. 2011;25:919–28. doi: 10.1097/QAD.0b013e328345889d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cunningham LS, Kelsey JL. Epidemiology of musculoskeletal impairments and their associated disability. Am J Public Health. 1984;74:574–9. doi: 10.2105/ajph.74.6.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamerman PR, Wadley AL, Cherry CL. HIV-associated sensory neuropathy: risk factors and genetics. Curr Pain Headache Rep. 2012;16:226–36. doi: 10.1007/s11916-012-0257-z. [DOI] [PubMed] [Google Scholar]
- 43.Attal N, Lanteri-Minet M, Laurent B, et al. The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain. 2011;152:2836–43. doi: 10.1016/j.pain.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 44.Simpson DM, Haidich AB, Schiffito G, et al. Severity of HIV-associated neuropathy is associated with plasma HIV-1 RNA levels. AIDS. 2002;16:407–12. doi: 10.1097/00002030-200202150-00012. [DOI] [PubMed] [Google Scholar]