Abstract
One important, yet relatively unexplored question, is whether blindsight, i.e. unconscious visually guided behaviour in hemianopic patients, is endowed with basic perceptual properties such as detecting stimulus numerosity and overall configuration. Rather than a forced-choice procedure in which patients are supposed to guess about stimuli presented to the blind hemifield, we used a Redundant Signal Effect (RSE) paradigm, i.e. the speeding of simple reaction time (RT) when presenting multiple versus single similar stimuli. The presence of an effect of numerosity for the (unseen) stimuli presented to the blind field was indirectly assessed by measuring RT to bilateral vs unilateral stimuli presented to the intact hemifield. Chronic hemianopic patients were tested with unilateral or bilateral black dots both of which could be either single or quadruple. The latter could either have a fixed spatial configuration representing a diamond or be randomly spatially assembled on every trial. Both configurations covered the same extent of visual field and had the overall same luminance. We found that a numerosity effect as a result of increasing the number of stimuli in the blind field was indeed present but only with the diamond configuration. This is convincing evidence that this form of blindsight does not depend upon stimulus numerosity per se but is likely to be related to the presence of structured and memorized rather than meaningless changing stimuli.
Keywords: Blindsight, Hemianopia, Redundancy Gain, Subcortical Visual Pathways, Reaction time
1. Introduction
The term blindsight refers to unconscious visually guided behaviour in response to stimuli presented to the blind hemifield of patients with complete damage to the primary visual cortex (V1) with or without additional damage to extrastriate visual areas. This phenomenon was originally described in the mid-seventies (Poeppel et al. 1973; Weiskrantz et al. 1974) and subsequently was thoroughly and ingeniously pursued by Weiskrantz and various collaborators (see Weiskrantz, 1990, 1996, 2004). The interest for this peculiar phenomenon concerns a challenging topic of cognitive neuroscience such as the neural correlate of perceptual awareness and also the function of visual pathways alternative to V1, not to mention the possibility that it might represent an initial step for regaining conscious vision (for recent reviews see Leopold 2012; Overgaard 2011; Perez and Chokron 2014; Silvanto 2014; Urbanski et al. 2014).
The presence of blindsight can be assessed either with direct methods, notably with a forced-choice response, or with indirect methods (see Danckert and Rossetti 2005 for review). In the former procedure patients are asked to guess about the location or other attributes of stimuli presented to the blind hemifield and the presence of unconscious above chance performance is taken as evidence of blindsight. In the latter, patients are asked to respond to stimuli presented to the intact hemifield during (or following) stimulus presentation to the blind hemifield and blindsight is inferred by the influence of blind field stimulation on response to stimuli presented to the intact field. A major advantage of the latter method is that the patient is not forced to guess and stimuli are visible on every trial in the good field.
One of the first examples of an indirect method for revealing blindsight has been provided by an interfield summation task originally described by Marzi et al. (1986) by using a modified Redundant Signal Effect (RSE) paradigm. Briefly, in this paradigm single or double visual stimuli are tachistoscopically presented in random sequence to one or both visual hemifields. Participants are to manually press a key as quickly as possible following detection of either kind of stimuli, that is, unilateral (left or right) or bilateral stimuli presented simultaneously across the vertical meridian, without having to make a choice (simple reaction time paradigm-RT). Marzi et al. (1986) found that there was a RSE, i.e. faster RT for double as compared to single stimuli, not only in control participants with an intact visual field but also in some hemianopic patients in whom bilateral stimuli were perceived as a single stimulus in the good field. The presence of this form of unconscious RSE has been confirmed several times (Corbetta et al. 1990; Leh et al. 2010; Marzi et al. 2009; Tamietto et al. 2010; Tomaiuolo et al. 1997) and there is functional magnetic resonance imaging (fMRI) evidence for an important role of the superior colliculus (SC) in mediating this form of blindsight (Leh et al. 2010; Tamietto et al. 2010).
This kind of indirect method has proven to be a reliable indicator of blindsight but is quite conservative and usually only a relatively small fraction of patients has shown clear indications of this phenomenon. Here we report the results of a study using a paradigm which represents a modification of the original RSE wherein quadruple (rather than double) stimuli were displayed to each hemifield in alternative to single stimuli during either unilateral or bilateral simultaneous presentation. Perhaps more importantly, a novel addition to this paradigm is the use of two different configurations of quadruple stimuli, namely a structured and a randomized dot configuration. The former was constituted by four dots forming a diamond shape while the latter was a randomized configuration changing on every trial. Thus, the two configurations differed in familiarity as well as in overall gestaltic structure and either of them might be important factors for revealing blindsight. Previous evidence on the sensitivity of blindsight for overall stimulus configuration and other higher-order aspects is rather scanty and relies mainly on hemianopic completion across the vertical meridian (e.g. Torjussen 1978; Weil et al. 2009) or on implicit semantic priming (e.g. Marcel 1998). Moreover, it is worth mentioning that a recent single cell recording study in monkeys has demonstrated that SC neurons are sensitive to face and face-like configurations (Nguyen et al. 2014). All these studies encouraged us to try and test the possibility of finding gestalt-like as well as familiarity effects in blindsight.
2. Method
2.1. Participants
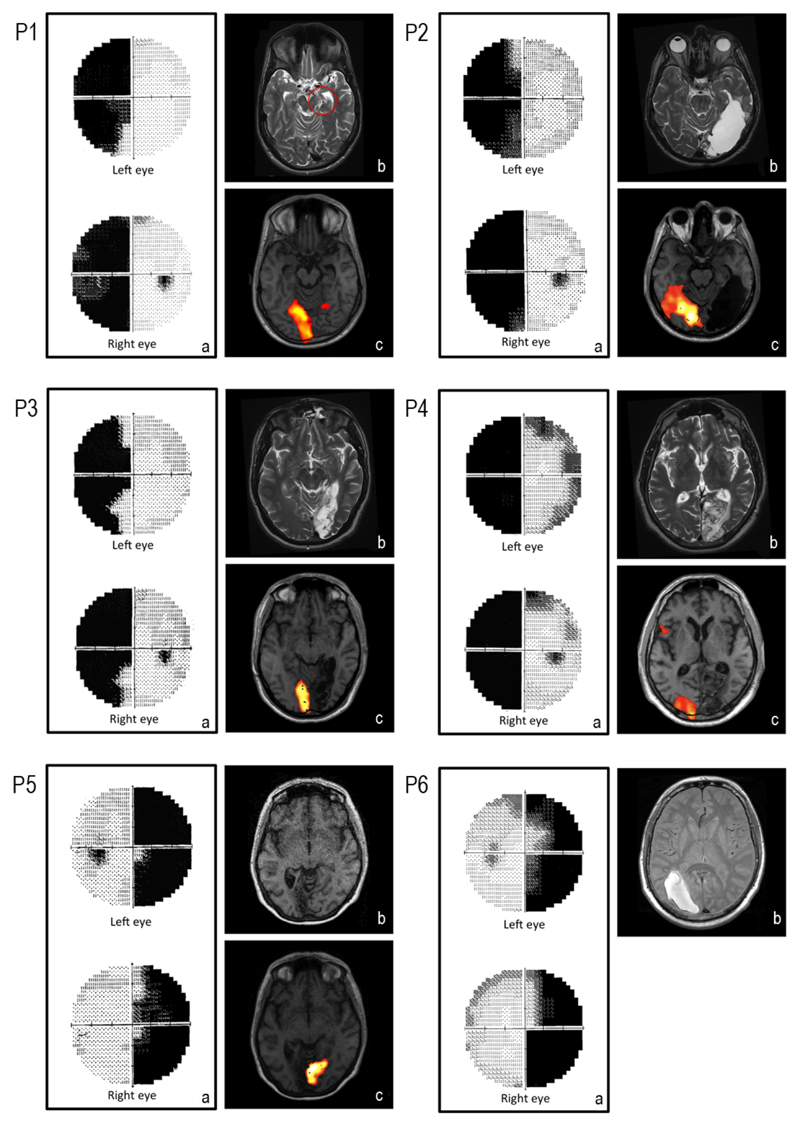
We tested six patients (mean age: 45.83 yrs.; SD:14.36) with hemianopia as a result of cortical or optic tract lesion, see details in Table 1 and Figure 1. All patients were right-handed and their acuity was normal or corrected-to-normal. They provided informed consent and the study was approved by our Departmental Ethics Committee and was carried out along the principles laid down by the Helsinki Declaration.
Table 1.
Demographic and Clinical data of the patients
| Sex | Age | Homonymous Hemianopia | Etiology | Lesions | |
|---|---|---|---|---|---|
| P1 | M | 23 | Left | Close Head trauma | Right optic tract |
| P2 | M | 55 | Left | Ischemic stroke with haemorrhagic evolution | Right parietal-occipital |
| P3 | M | 36 | Left | Ischemic Stroke | Right temporal-occipital |
| P4 | M | 60 | Left | Ischemic Stroke | Right mesial occipital |
| P5 | F | 44 | Right | Ischemic stroke with haemorrhagic evolution | Medial part of left occipital lobe |
| P6 | M | 57 | Right | Cerebral intraparenchimal haemorrhage | Left parietal-occipital |
Fig. 1.
Patients details a) Monocular visual fields showing a typical hemianopic loss with some spared areas b) Structural MRI showing the lesion c) Functional MRI with full field visual stimulation with checkerboards showing activation restricted to the contralesional visual cortex.
2.2. Stimuli and Apparatus
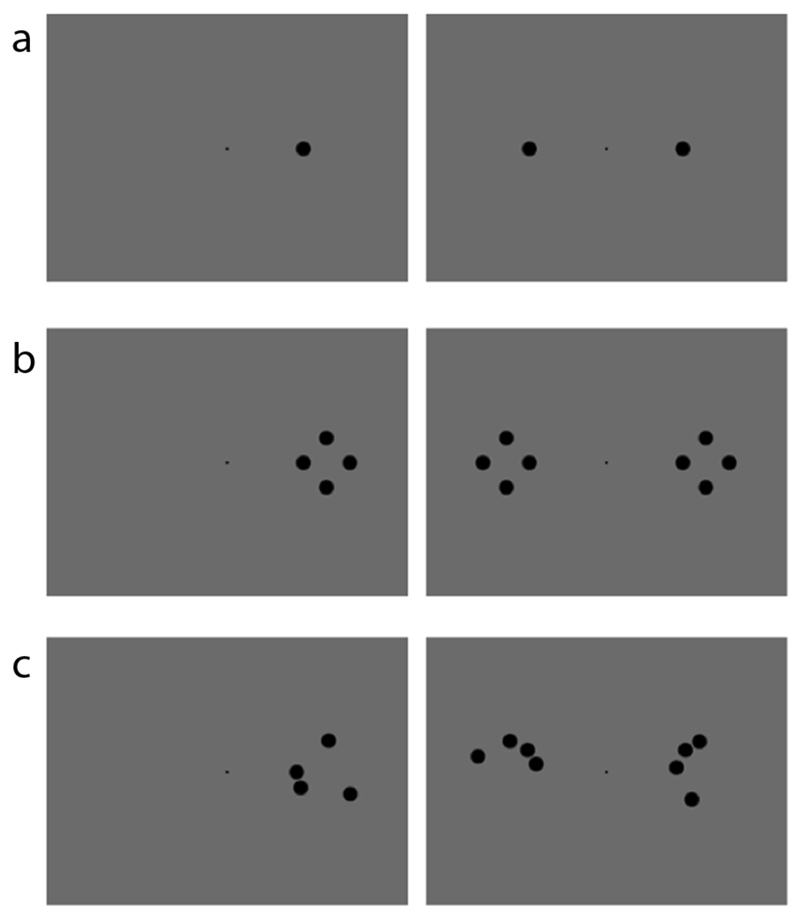
The stimuli were black dots on a grey background of 11.42 cd/m2 luminance presented with an exposure duration of 80 ms on a PC monitor. They could be either single or quadruple and could be presented either unilaterally or bilaterally. A unilateral single stimulus was presented along the horizontal meridian at an eccentricity of 6.5° from a central fixation point either in the left or the right hemifield. In some patients it has been necessary to present the stimuli a few degrees more laterally along the horizontal meridian to avoid encroaching upon partially spared portions of the visual field. Bilateral single stimuli were presented in the two hemifields simultaneously at the same eccentricity as unilateral single stimuli. The experiment was divided in two sessions. In the first the quadruple stimuli were four dots identical to the single stimuli and were presented in a diamond configuration. In the second we used the same four stimuli as in the first session but their position was randomized trial-by-trial so that they did not form a meaningful figure, see an example of the two configurations in Figure 2. The randomized stimuli were presented always in the second session but this did not yield any reliable practice effect, see Results and Discussion. For both configurations the centre was at 8.5° along the horizontal meridian and the innermost dot was at 6.5°.
Fig. 2.
Examples of stimuli and their spatial organization. a) Upper left=Unilateral Single; Upper right=Bilateral Single; b) Middle left=Unilateral Diamond Quadruple; Middle right=Bilateral Quadruple Diamond; c) Lower left=Unilateral Quadruple Random; Lower right= Bilateral Quadruple Random.
2.3. Procedure
It is important to point out that unilateral stimuli presented to the blind field were not perceived by the patients who were requested to press the response key only when they were aware of the stimuli even if in a degraded and faint form. This did not occur in the above six patients with the exception of a few responses in patient SL. To further enquire about stimulus awareness, at the end of each session patients were asked if they had experienced or felt the occurrence of stimuli in the blind hemifield even when they did not respond. None of them reported having had such experience.
As mentioned above, the strategy followed to infer blindsight was to assess the effect of stimuli in the blind field on RT to stimuli perceived in the intact field. Unilateral stimuli presented to the intact hemifield served as control condition.
The initial event in a trial was a warning acoustic signal (duration: 150 ms; frequency: 1000 Hz) followed after a randomized temporal window (300 - 700 ms) by a single or a quadruple stimulus presented to the right or left hemifield or bilaterally. There were two sessions, one with diamonds as stimulus configuration and the other with randomized stimuli. Each session included 80 unilateral single stimuli and 80 unilateral quadruple stimuli, half to the left and half to the right hemifield (in a blocked alternation), 40 bilateral single stimuli and 40 bilateral quadruple stimuli in addition to 40 catch trials where the warning stimulus was not followed by a visual stimulus. The sequence of unilateral, bilateral, single or quadruple stimuli was randomized. Response was performed by pressing the spacing bar of a PC either with the left or the right hand according to a blocked ABBA sequence for each visual hemifield presentation. Participants were required to keep their fixation steady on a small black circle (diameter: 0.3°) in the centre of the visual field following onset of the warning signal and to refrain from moving the eyes until response had been completed. Fixation was remotely controlled by means of a closed TV system. RTs shorter than 140 ms (anticipations) and longer than 600 ms (retardations) were not included in the statistical analysis. Their overall percentage was less than 5 percent.
3. Results and Discussion
We carried out a repeated measures ANOVA on RT averaged across sessions with ‘Configuration’ (Diamond vs Random), ‘Numerosity in the Intact Field’ (1 vs 4) and ‘Numerosity in the Blind Field’ (0, 1, 4) as factors. The latter condition was represented by the following three levels: ‘0’, that is, unilateral stimuli (either single or quadruple) in the intact field and no stimuli in the blind field; ‘1’ bilateral single stimuli; ‘4’ bilateral quadruple stimuli. Therefore, we could compare the effect of 0, 1 or 4 stimuli presented to the blind field on RT to stimuli presented to the intact field. Obviously, the level ‘0’ stimuli was not present in the ‘Numerosity in the Intact Field’ condition because patients would not perceive anything and would not press the response key in this condition.
Results showed a significant main effect of ‘Numerosity in the Intact Field’ (F(1,5) = 37.384, p < .002) with Quadruple Stimuli (330.93 ms) faster than Single Stimuli (338.19 ms) and of ‘Numerosity in the Blind field’ (F(2,10) = 6.113, p < .018) with Quadruple Stimuli (332.40 ms) faster than Single (335.05 ms) and 0 Stimuli (336.23 ms) that did not differ one from the other. The main effect of ‘Configuration’ was not significant (F(1,5)= .331, p = .589) with the Random (332.35 ms) not reliably faster than the Diamond Configuration (336.77 ms). Importantly, we found a significant effect of the ‘Configuration by Numerosity in the Blind Field’ interaction (F(2,10) = 4.214, p < .05) while ‘Configuration by Numerosity in the Intact Field’ was not significant (F(1,5) = 2.332, p = 0.187). Finally, the three-ways interaction ‘Configuration by Numerosity in the Intact Field by Numerosity in the Blind Field’ was not significant (F(2,10) = .0137, p = .986).
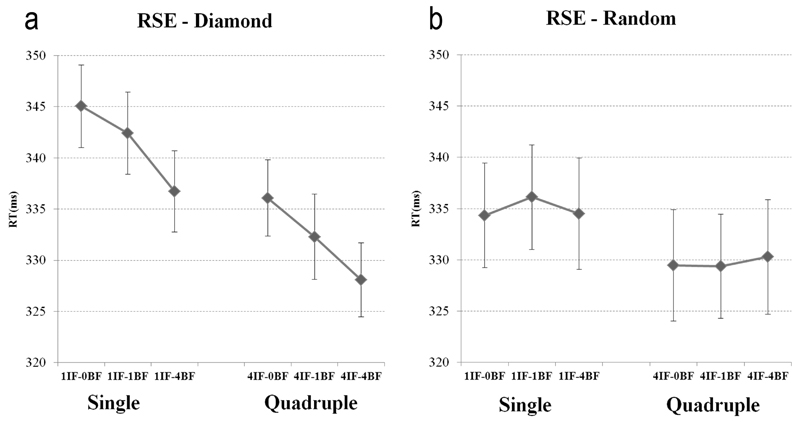
In order to analyze the ‘Configuration by Numerosity in the Blind Field’ interaction two ANOVAs were carried out separately for each ‘Configuration’ (Diamond and Random) with ‘Numerosity in the Intact Field’ (1 and 4 stimuli) and ‘Numerosity in the Blind Field’ (0, 1 and 4 stimuli) as factors. For the Diamond configuration there was a significant effect of ‘Numerosity in the Intact Field’ (F(1,5) = 28.852, p = .003) indicating that 4 stimuli were reacted faster than 1. Importantly, we also found a significant effect of ‘Numerosity in the Blind Field’ (F(2,10) = 7.477, p = .010) indicating that RT (to stimuli in the intact field – the only ones to which patients could respond) decreased by increasing the number of stimuli in the blind field, see Fig. 3 left panel. A polynomial analysis showed that the decrease of RT was significantly linearly related (p = .008) to the increase of the stimuli in the blind field. Moreover, no interaction was found (F(2,10) = .060, p = .942) indicating that the speeding up of RT induced by the number of stimuli presented in the blind field was unrelated to the number of stimuli simultaneously presented in the intact field.
Fig. 3.
RT to stimuli presented to the Intact Field (IF) as a function of stimulus numerosity (0,1,4) in the Blind Field (BF) for the Diamond (a) and Random configurations (b). On each panel: Left: Single stimuli; Right: Quadruple stimuli. Bars represent standard errors (SE).
In contrast, for the Random configuration the only significant effect was found for the ‘Numerosity in the Intact Field’ factor (F(1,5) = 8.651, p = .032) indicating that quadruple stimuli presented in the intact field were reacted faster than single stimuli. No effect was found for ‘Numerosity in the Blind Field’ (F(2,10) = .150, p = .863), see Fig. 3 right panel, or for the interaction ‘Numerosity in the Intact Field’ by ‘Numerosity in the Blind Field’ (F(2,10) = .245, p = .787).
The main thrust of these results is that increasing the number of stimuli in the blind field from 0 to one and to four, independently from the number of stimuli in the intact field, resulted in a progressive shortening of RT. This is clearly an implicit bilateral advantage in which unseen stimuli in the blind field are summated with normally perceived stimuli in the intact field. An intriguing finding supported by the significant interaction Configuration by Numerosity in the Blind Field is that the bilateral avantage was reliable only for the diamond rather than for the random configuration of quadruple stimuli. This suggests the fascinating possibility that unconscious vision might have a perceptual organization enhancing the processing of structured over meaningless stimuli. An important point is that a practice effect explaining the failure to find blindsight for the random stimuli session that was run always after the diamond stimuli session is an unlikely possibility given the lack of significance of the main effect of Configuration and of the three-ways interaction ‘Configuration by Numerosity in the Intact Field by Numerosity in the Blind Field’, see above.
As to the possible neural sites subserving the implicit effect described here, one should note that with the exception of patient P5 who has a lesion mainly restricted to V1 all other patients have either large lesions extending to extrastriate areas and often to the optic radiation and subcortical centers. Therefore, it is likely that the implicit RSE found might have a subcortical site, as argued by previous work with roughly similar paradigms (Tomaiuolo et al. 1997; Leh et al. 2010; Tamietto et al. 2010). This would suggest the presence of higher-order perceptual effects even when vision in the absence of the geniculate-striate pathway is subserved by subcortical centres and its extrastriate cortical projections.
An important question concerns the contribution of the intact hemisphere either at cortical or subcortical level. This is supported by a recent study (Celeghin et al. 2015) in which we used in hemianopic patients a behavioural paradigm originally developed by Poffenberger in 1912 for measuring interhemispheric transfer time (see Marzi, 1991, 1999). We found that unlike responses to stimuli presented to the intact hemifield of hemianopics, those to stimuli presented to the blind hemifield (patients were instructed to respond also to unseen stimuli) showed a paradoxically slower uncrossed than crossed visuomotor response. That is, responding with the hand ipsilateral to the stimulated hemifield (an uncrossed condition that does not require an interhemisphere transfer) was slower than the crossed condition in which responses are performed with the hand contralateral to the stimulated hemifield, i.e., a condition which does require an interhemispheric transfer (see Marzi et al, 1991, 1999). This indicates that responses to the blind hemifield were mediated by the intact hemisphere via a double crossing of the corpus callosum that is necessary first to access the visual information from the intact visual cortex and then to trigger the motor response from the ipsilesional side, see Fig. 5 in Celeghin et al. 2015. Also, diffusion tensor imaging (DTI) studies in blindsight patients provide converging evidence of post-lesion plasticity that involves subcortical visual structures such as the SC or the lateral geniculate nucleus (LGN) with aberrant fiber tracts reaching the intact hemisphere (e.g., Bridge et al., 2008; Leh et al., 2006; Tamietto et al., 2012).
Finally, of particular interest is patient P1 of the present study who sustained a closed head trauma and a unilateral lesion of the optic tract (OT) completely depriving the ipsilesional hemisphere of visual input. In this case blindsight could have been possible only through an aberrant (presumably post-lesional) misrouting of retinal fibers at the optic chiasm relying ipsilateral hemifield input to the intact hemisphere. Clearly, this input yielded blindsight but not conscious vision. The reason for that is an interesting issue to be further pursued.
4. Conclusions
The overall picture stemming from these results is that there was a clear cut speeding of RT with increasing numerosity of black dots. This effect occurred both in the intact hemifield and bilaterally despite the presence of a blind hemifield. This implicit RSE confirms earlier findings with bilateral single stimuli (Corbetta et al. 1990; Marzi et al. 1986, 2009; Tomaiuolo et al. 1997; Tamietto et al. 2010). The novel finding is that the present implicit RSE occurs only with quadruple stimuli with a structured rather than a random spatial configuration.
Clearly this result awaits confirmation by using various kinds of structured stimuli since in the present study the diamond configuration was the same in all trials while randomized stimuli varied from trial to trial. Thus, in principle, it could be argued that the observed implicit RSE was related to familiarity and/or to fixed versus variable stimuli rather than to the presence of a gestalt-like dot configuration. These possibilities need to be tested in further experiments and might provide further clues on the “cognitive” structure of blindsight.
Acknowledgments
The study was in part supported by ERC Advanced Grant “Perceptual Awareness” (C.A.M)
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Bridge H, Thomas O, Jbabdi S, Cowey A. Changes in connectivity after visual cortical brain damage underlie altered visual function. Brain. 2008;13:1433–1444. doi: 10.1093/brain/awn063. [DOI] [PubMed] [Google Scholar]
- Celeghin A, Barabas M, Mancini F, Bendini M, Pedrotti E, Prior M, Cantagallo A, Savazzi S, Marzi CA. Speeded manual responses to unseen visual stimuli in hemianopic patients: What kind of blindsight? Conscious Cogn. 2015;32:6–14. doi: 10.1016/j.concog.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Marzi C, Tassinari G, Aglioti S. Effectiveness of different task paradigms in revealing blindsight. Brain. 1990;113:603–616. doi: 10.1093/brain/113.3.603. [DOI] [PubMed] [Google Scholar]
- Danckert J, Rossetti Y. Blindsight in action: what can the different sub-types of blindsight tell us about the control of visually guided actions? Neurosci Biobehav Rev. 2005;29:1035–1046. doi: 10.1016/j.neubiorev.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Leh SE, Johansen-Berg H, Ptito A. Unconscious vision: new insights into the neuronal correlate of blindsight using diffusion tractography. Brain. 2006;129:1822–1832. doi: 10.1093/brain/awl111. [DOI] [PubMed] [Google Scholar]
- Leh SE, Ptito A, Schönwiesner M, Chakravarty MM, Mullen KT. Blindsight mediated by an S-cone-independent collicular pathway: an fMRI study in hemispherectomized subjects. J Cogn Neurosci. 2010;22:670–82. doi: 10.1162/jocn.2009.21217. [DOI] [PubMed] [Google Scholar]
- Leopold DA. Primary visual cortex: awareness and blindsight. Annu Rev Neurosci. 2012;35:91–109. doi: 10.1146/annurev-neuro-062111-150356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcel AJ. Blindsight and shape perception: deficit of visual consciousness or of visual function? Brain. 1998;121:1565–1588. doi: 10.1093/brain/121.8.1565. [DOI] [PubMed] [Google Scholar]
- Marzi CA, Bisiacchi P, Nicoletti R. Is interhemispheric transfer of visuomotor information asymmetric? Evidence froma a meta-analysis. Neuropsychologia. 1991;29:1163–1177. doi: 10.1016/0028-3932(91)90031-3. [DOI] [PubMed] [Google Scholar]
- Marzi CA. The Poffenberger paradigm: a first, simple, behavioral tool to study interhemispheric transmission in humans. Brain Res Bull. 1999;50:421–422. doi: 10.1016/s0361-9230(99)00174-4. [DOI] [PubMed] [Google Scholar]
- Marzi CA, Tassinari G, Aglioti S, Lutzemberger L. Spatial summation across the vertical meridian in hemianopics: A test of blindsight. Neuropsychologia. 1986;24:749–758. doi: 10.1016/0028-3932(86)90074-6. [DOI] [PubMed] [Google Scholar]
- Marzi CA, Mancini F, Metitieri T, Savazzi S. Blindsight following visual cortex deafferentation disappears with purple and red stimuli: a case study. Neuropsychologia. 2009;47:1382–1385. doi: 10.1016/j.neuropsychologia.2009.01.023. Epub 2009 Jan 22. [DOI] [PubMed] [Google Scholar]
- Nguyen MN, Mtsumoto J, Hori E, Maior RS, Tomaz C, Tran AH, Ono T, Nishijo H. Neuronal responses to face-like and facial stimuli in the monkey superior colliculus. Front Behav Neurosci. 2014;8:85. doi: 10.3389/fnbeh.2014.00085. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard M. Visual experience and blindsight: a methodological review. Exp Brain Res. 2011;209:473–479. doi: 10.1007/s00221-011-2578-2. Epub 2011 Feb 15. [DOI] [PubMed] [Google Scholar]
- Perez C, Chokron S. Rehabilitation of homonymous hemianopia: insight into blindsight. Front Integr Neurosci. 2014;8:82. doi: 10.3389/fnint.2014.00082. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppel E, Held R, Frost D. Residual visual function after brain wounds involving the central visual pathways in man. Nature. 1973;243:295–296. doi: 10.1038/243295a0. [DOI] [PubMed] [Google Scholar]
- Silvanto J. Why is “blindsight” blind? A new perspective on primary visual cortex, recurrent activity and visual awareness. Conscious Cogn. 2014;32:15–32. doi: 10.1016/j.concog.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Tamietto M, Pullens P, de Gelder B, Weiskrantz L, Goebel R. Subcortical connections to human amygdala and changes following destruction of the visual cortex. Curr Biol. 2012;22:1449–1445. doi: 10.1016/j.cub.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Tamietto M, Cauda F, Corazzini LL, Savazzi S, Marzi CA, Goebel R, Weiskrantz L, de Gelder B. Collicular vision guides nonconscious behavior. J Cogn Neurosci. 2010;22:888–902. doi: 10.1162/jocn.2009.21225. [DOI] [PubMed] [Google Scholar]
- Tomaiuolo F, Ptito M, Marzi CA, Paus T, Ptito A. Blindsight in hemispherectomized patients as revealed by spatial summation across the vertical meridian. Brain. 1997;120:795–803. doi: 10.1093/brain/120.5.795. [DOI] [PubMed] [Google Scholar]
- Torjussen T. Visual processing in cortically blind hemifields. Neuropsychologia. 1978;16:15–21. doi: 10.1016/0028-3932(78)90038-6. [DOI] [PubMed] [Google Scholar]
- Urbanski M, Coubard OA, Bourlon C. Visualizing the blind brain: brain imaging of visual field defects from early recovery to rehabilitation techniques. Front Integr Neurosci. 2014;8:74. doi: 10.3389/fnint.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil RS, Plant GT, James-Galton M, Rees G. Neural correlates of hemianopic completion across the vertical meridian. Neuropsychologia. 2009;47:457–464. doi: 10.1016/j.neuropsychologia.2008.09.020. Epub 2008 Oct 7. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L, Warrington EK, Sanders MD, Marshall J. Visual capacity in the hemianopic field following a restricted occipital ablation. Brain. 1974;97:709–728. doi: 10.1093/brain/97.1.709. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L. The Ferrier lecture, 1989. Outlooks for blindsight: explicit methodologies for implicit processes. Proc R Soc Lond B Biol Sci. 1990;239:247–278. doi: 10.1098/rspb.1990.0016. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L. Blindsight revisited. Curr Opin Neurobiol. 1996;6:215–220. doi: 10.1016/s0959-4388(96)80075-4. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L. Roots of blindsight. Prog Brain Res. 2004;144:229–241. doi: 10.1016/s0079-6123(03)14416-0. [DOI] [PubMed] [Google Scholar]