Abstract
Exposure to a patch of dots produces a repulsive shift in the perceived numerosity of subsequently viewed dot patches. Although a remarkably strong effect, in which the perceived numerosity can be shifted by up to 50% of the actual numerosity, very little is known about the temporal dynamics. Here we demonstrate a novel adaptation paradigm that allows numerosity adaptation to be rapidly induced at several distinct locations simultaneously. We show that not only is this adaptation to numerosity spatially specific, with different locations of the visual field able to be adapted to high, low, or neutral stimuli, but it can occur with only very brief periods of adaptation. Further investigation revealed that the adaptation effect was primarily driven by the number of unique adapting events that had occurred and not by either the duration of each event or the total duration of exposure to adapting stimuli. This event-based numerosity adaptation appears to fit well with statistical models of adaptation in which the dynamic adjustment of perceptual experiences, based on both the previous experience of the stimuli and the current percept, acts to optimize the limited working range of perception. These results implicate a highly plastic mechanism for numerosity perception, which is dependent on the number of discrete adaptation events, and also demonstrate a quick and efficient paradigm suitable for examining the temporal properties of adaptation.
Keywords: number perception, adaptation, event-based
Introduction
Numerosity perception has been extensively examined in the last decade with researchers placing a strong emphasis on learning how numerosity is encoded within the brain. This has led to detailed studies of numerosity discrimination in human adults (Burr & Ross, 2008a; Droit-Volet, Clément, & Fayol, 2008), infants (Halberda, Mazzocco, & Feigenson, 2008; Xu & Spelke, 2000) and various clinical groups (Aagten-Murphy et al., 2015; Piazza, Pinel, Le Bihan, & Dehaene, 2007; Turi et al., 2015). Additionally, the neural regions underlying these responses have been examined with single-unit recording (Nieder, 2005), EEG studies (Kong et al., 2005; Park, DeWind, Woldorff, & Brannon, 2016), and fMRI studies (Harvey, Klein, Petridou, & Dumoulin, 2013; Piazza et al., 2007). Many of these studies focused on disentangling whether our perception of numerosity is a directly sensed perceptual category (Anobile, Cicchini, & Burr, 2014; Arrighi, Togoli, & Burr, 2014; Burr & Ross, 2008a; Viswanathan & Nieder, 2013) or one that is indirectly derived from other perceptual attributes (Dakin, Tibber, Greenwood, Kingdom, & Morgan, 2011; Durgin, 2008; Durgin & Huk, 1997; Gebuis & Reynvoet, 2013; Tibber, Greenwood, & Dakin, 2012). In general, although numerosity perception appears to be influenced by nonnumerical covariates in many circumstances, recent studies have provided evidence for the existence of a direct and independent sense of numerosity (Anobile, Cicchini, & Burr, 2016). The early evidence for this dissociation came from studies of numerosity adaptation (Burr & Ross, 2008a) in which the sustained viewing of a large number of dots was found to alter the perceived quantity of subsequently viewed dot patches. The substantial adaptation effects for the perception of quantity, occurring in the absence of adaptation effects for other features, were interpreted as evidence that the perception of numerosity behaved similarly to other primary visual properties (such as color, orientation or motion). However, since these initial investigations few studies have reexamined numerosity adaptation, and the mechanisms and temporal dynamics underlying this effect remain largely unknown.
The effect of numerosity adaptation is striking for both the strength and reliability of the changes in perception it induces. Indeed early numerosity adaptation studies found that the perceived numerosity of a patch of dots could be shifted by up to 50% of its unadapted value after adaptation (Burr, Anobile, & Turi, 2011; Burr & Ross, 2008a; Ross & Burr, 2010). Both under- and overestimation of the numerosity of the test patch could be induced, depending on the ratio between the adapter and test numerosity. As such it has been argued that numerosity adaptation, like other forms of adaptation, may represent the dynamic adjustment of perceptual responses to allow perception to operate within the limited working range available (Anobile et al., 2016; Gepshtein, Lesmes, & Albright, 2013). However, none of these studies have investigated how the effects of numerosity adaptation change across time. Instead researchers focused on examining the behavioral effects when numerosity adaptation was saturated. In these studies researchers utilized frequent and lengthy periods of adaptation to ensure that, regardless of how numerosity effects accumulate or decay over time, the observed effect would be maintained at ceiling. For example, Burr and Ross (2008a) used 30-s periods of initial adaptation followed by 5-s periods of top-up adaptation before each trial. While these paradigms produce robust adaptation effects, their design obscures the temporal development of the effect and they require a time-consuming experimental protocol that deters researchers from initiating detailed examinations of the effect. However, this avoidance is unfortunate as detailed studies of the dynamics of adaptation effects have proven to be important research tools as they not only demonstrate the dynamic nature of perception, but also provide a potent tool for probing the underlying sensory mechanisms (Clifford & Rhodes, 2005; Kohn, 2007; Solomon & Kohn, 2014).
Understanding the temporal dynamics of numerosity adaptation can also provide insight into the neural regions responsible for encoding numerosity. Whereas numerosity has been strongly linked with the intraparietal sulcus, many of the other visual attributes frequently found to covary with changes in numerosity (such as density and spatial frequency) are instead associated with early stages of the primary visual cortex. This is important when you consider that the behavioral effects of adaptation may stem from distinct plastic changes occurring at multiple different locations throughout the brain, each of which are induced and sustained over dramatically different timescales (Webster, 2011). While some adaptation effects are short-lived and associated with adapter exposure and decay rates within the millisecond range (Priebe, Churchland, & Lisberger, 2002; Priebe & Lisberger, 2002), other forms of adaptation are induced over several minutes and have been shown to persist for months or even years (Dodwell & Humphrey, 1990; McCollough, 1965; Robinson & MacLeod, 2011). However, even with the notoriously long-lasting McCollough effect, researchers have been able to demonstrate two distinct and separable adaptation processes (Vul, Krizay, & MacLeod, 2008). The first is a fast, transient form of adaptation (with similar dynamics to contrast adaptation) that rapidly saturates and decays, while the second is an essentially fixed bias that shows negligible decay if no additional de-adapting stimuli are presented. These experiments, among others, demonstrate how important aspects of the underlying mechanism can go unnoticed by only considering fully saturated adaptation effects.
Studies of adaptation have also suggested that the earlier in the visual hierarchy in which adaptation occurs, the longer the adaptation period required and the slower the adaptation effect decays (Kohn, 2007). Some evidence for this comes from fMRI studies which showed that while orientation-tuned adaptation signals in V1 require long-term (several seconds) adaptation (Fang, Murray, Kersten, & He, 2005), very short-term (millisecond) adaptation effects were seen in extrastriate areas (Henson, 2003). These findings suggest that the temporal dynamics of a specific adaptation effect may allow for some predictions to be made about the potential neural locus of the effect. However, it must be noted that this serves as a general principle and some exceptions exist. Indeed some complex face aftereffects, which almost certainly originate from higher visual areas, have also been found to persist for surprisingly long periods of time even after prolonged adaptation (Leopold, O’Toole, Vetter, & Blanz, 2001; Webster, Kaping, Mizokami, & Duhamel, 2004). These variations may at least partially arise from the prolonged exposure to a given stimulus inducing adaptation to all of its visual properties (i.e., also to orientation, contrast, spatial frequency), not just the target feature, or from similarity between nontarget visual properties in the test and adapter stimuli (Gepshtein et al., 2013; Webster, 2003). When it comes to the neural locus of the perception of numerosity, the majority of studies have provided substantial evidence for the critical involvement of higher regions of the cortex (Dehaene, Piazza, Pinel, & Cohen, 2003; Piazza & Izard, 2009; Pinel, Piazza, Le Bihan, & Dehaene, 2004). Given the link between visual hierarchy and the temporal dynamics of adaption effects, and that many of the covarying features in numerosity displays are low-level features, whether brief periods of adaptation would be sufficient for inducing substantial and long-lasting numerosity adaptation effects is an important unanswered question. If brief adaptation periods are sufficient to induce significant adaptation effects without inducing adaptation to other unwanted visual properties, then this may additionally allow numerosity adaptation effects to be studied independently from the influence of other stimulus dimensions.
In this study we investigated the spatial and temporal characteristics of numerosity adaptation. We developed a novel method for investigating adaptation in which multiple locations of visual space were differentially adapted within the same testing session. This allowed for the spatial specificity of numerosity adaptation to be examined, and also provided a technique with which different adaptation conditions (including neutral controls) could be tested simultaneously. In the second experiment, we introduced a simplified version of the paradigm that was optimized to allow an extensive parametric examination of which temporal properties of the adapter drive the numerosity adaptation effect. With this method we could examine the minimum conditions necessary to induce numerosity adaptation and examine both the magnitude and the rate of decay of the effect.
General methods
Participants
Eight participants (four males, four females; aged 21–30) participated in the first experiment, with a subset also performing the 5-second adapter control (three males, one female), while three participants (two males, one female; aged 25–28) participated in the longer second experiment. All participants had normal or corrected-to-normal vision, and all procedures involving human subjects were in accordance with the tenets of the Declaration of Helsinki.
Stimuli and apparatus
The experimental stimuli were presented in a dimly lit room and participants, at a distance of 57 cm, viewed binocularly a 21-in. Samsung LED monitor with 1920 × 1080 resolution, a refresh rate of 60 Hz and a mean luminance of 60 cd/m2. The stimuli used throughout the experiment were clouds of black and white (equal proportion) nonoverlapping dots, each 0.2° in diameter, subtending 12° of visual angle. Stimuli were displayed on a midgray background at 90% contrast. Each dot patch was procedurally generated, with the required number of elements assigned to random locations on each trial and presented under Matlab 7.6 using PsychToolbox routines (Brainard, 1997; Kleiner et al., 2007; Pelli, 1997).
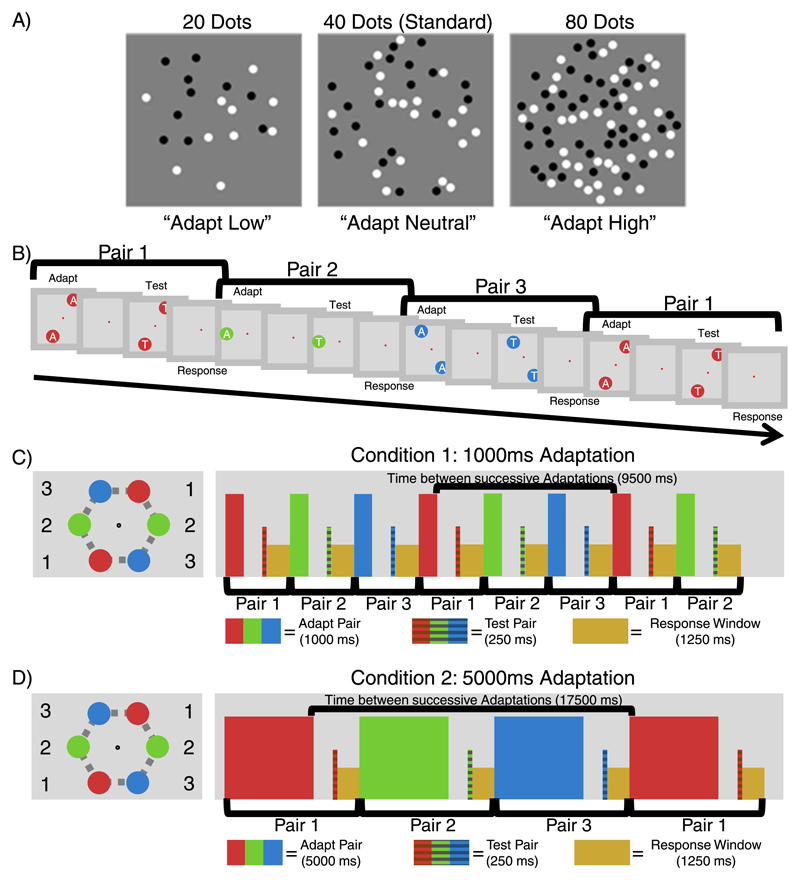
Adaptation effects were examined against a standard stimulus of 40 dots, with the adapters low (20), high (80), or neutral (40) in numerosity (see Figure 1A). Participants were required to indicate which of the two test stimuli appeared more numerous. The standard comprised 40 dots and, as adaptation to the same numerosity as the standard does not result in any change in perceived numerosity (Burr & Ross, 2008a), this meant a patch of 40 dots could also be used as a neutral adapter. All adaptation effects observed were repulsive effects with the perceived numerosity of subsequently viewed dot patches shifting away from the numerosity of the adapter. Effects in which the perceived numerosity was reduced (after adaptation to a high numerosity) are referred to as downward adaptation effects while those in which the perceived numerosity was increased (after adaptation to a low numerosity) are referred to as upward adaptation in order to avoid confusion with other terms (i.e., increasing/decreasing or positive/negative).
Figure 1.
The multiple-location fast-adaptation paradigm. (A) Examples of the low, neutral, and high numerosity stimuli used in the experiments. Because the neutral stimulus was equal in numerosity to the standard (40 dots) there was no change in numerosity perception when it was used as an adapter. This meant adaptation to 40 dots could also be used as a neutral control. (B) Participants fixated on a central fixation point while the adaptation and test procedure occurred sequentially at each of the paired locations. This allowed multiple spatial locations to be tested sequentially within the same paradigm. (C) In the 1-second adapter condition each sequence comprised of a 1-s adaptation period, followed by a 1-s ISI pause, and then 0.25 s of the test stimuli. Afterwards participants were required to respond within 1.25 s which of the test stimuli (left or right) had been perceived as more numerous. The sequence then immediately repeated at the next paired location, regardless of whether participants had responded, to ensure regular timing across the task. This meant that there was a 9.5-s delay before adapter stimuli were shown again at any given spatial location. (D) the 5-second adapter condition was identical to the 1-second adapter condition, with the exception that the duration of the adapter increased to 5 s. As the other variables remained the same, this meant that there was now a 17.5-s delay before adapter stimuli were shown again at the same spatial location.
Experiment 1
We tested a novel, rapid, multiple-location adaptation paradigm for investigating numerosity adaptation in which the locations that were adapted and tested were alternated cyclically amongst different paired locations within the display. This format allowed adaptation effects at multiple spatial locations to be examined within the same experiment, although it resulted in an increase in the period of time between each test at any given location. As such it was important to first verify that the adaptation effects produced were comparable to previous experiments. Additionally, as several studies have suggested that numerosity adaptation may be spatially selective (Burr & Morrone, 2012; Burr & Ross, 2008a, 2008b), we wanted to directly investigate whether different adaptation effects could be observed at different locations.
We also examined the influence of the length of each individual adaptation period on the strength of the adaptation effect. Specifically, we contrasted our results with the study by Burr and Ross (2008a) in which participants were initially given a 30 s exposure to the adapter stimuli for 30 s and thereafter given 7 s periods of adaptation (with the interval between subsequent presentations separated by approximately 2 s) between every trial. In this experiment we tested multiple spatial locations (without an initial adaptation period) and had participants experience either 1 or 5 s of exposure to the adapter stimulus on each trial (with subsequent adaptation at the same location occurring either 9.5 or 17.5 s later).
Methods
Each trial examined two paired locations and was comprised of an adaptation and a testing period. After completing the trial for a particularly pair, the next pair in the sequence was examined so that the locations tested alternated clockwise on every trial (see Figure 1A). During the adaptation period two stimuli were simultaneously shown at the paired location with the standard (40) at one location and a low (20), high (80), or neutral (40) adapter at the other location in the pair. Within a block this arrangement was fixed so that adaptation to a specific magnitude was able to accumulate at a specific location. On each trial the participant was required to maintain fixation at a red fixation dot presented at the screen center. Stimuli were then presented at one of the three paired locations, aligned on the edges of an (invisible) hexagon so that the center of each patch was 8° from the fixation point and the angle between each pair was 180° (see Figure 1B). Throughout the experiment the displayed pair was alternated in a clockwise fashion so that a different spatial location was tested on each trial (i.e., Pair 1, Pair 2, Pair 3, Pair 1, and so on). Each trial began with the presentation of either a 1-second adapter or a 5-second adapter, depending on the condition. The adapting pair comprised the two patches of dots with the adapting numerosity at one location (20, 40, or 80), and the standard (40) presented at the other. In this way only one of the locations in the pair was adapted (either to a high, low, or a neutral magnitude), with the neutral adapter always appearing at the other location. Following a 1-s pause, the test was presented for 0.25 s. During both adaptation and test periods, the patches of dots were presented at both of the paired locations simultaneously. Participants were then given 1.25 s to perform a 2-AFC task and indicate via the keyboard whether the patch of dots presented on the left or right was more numerous. Regardless of whether participants responded in time, the sequence would then continue for the next paired locations immediately to ensure that the timing was consistent throughout the entire experiment. Thus there was a consistent 9.5 s (or 17.5 s for the 5-second adapter control) delay from the end of the adaptation period for one pair until the next period of adaptation occurred at the same location (See Figure 1B and 1C). During this delay the adapter and test stimuli were presented at the other pair locations.
During the test period one of these locations was always the standard, comprising 40 dots, while the other was varied from trial to trial depending on participant response, with the numerosity determined by the QUEST algorithm (Watson & Pelli, 1983). To determine the numerosity of the next trial, the algorithm estimated the point of subjective equality (PSE) after each trial, and this estimation was then perturbed with a random number drawn from a Gaussian distribution of standard deviation 0.15 log-units in order to ensure a good sampling of most critical data across all participants even with variable effects of adaptation.
The proportion of trials in which the standard appeared more numerous than the probe was plotted against the probe numerosity and fitted with a cumulative Gaussian function to yield an estimate of the point of subjective equality (PSE). The PSE calculated in the adaptation condition was then divided by the PSE in the baseline condition so that the adaptation effect could be expressed as a percentage change from baseline performance.
Each session comprised two different conditions: an initial block of neutral baseline immediately followed by a block of adaptation. In order to control for attention effects during testing, where typically the patch to be adapted is presented on one side with nothing presented on the alternate side, we instead presented the neutral stimulus (with the same numerosity as the standard) at the other pair location during adaptation. All participants performed each session twice (on separate days), with the pair that was adapted to high and the pair that was adapted to low switched between sessions to ensure that verticality did not play a role in the effect. Additionally, participants who also completed the 5-second adapter sessions also did these on different days to minimize carryover effects. Thus, while in each session participants were simultaneously adapted at different spatial locations to high numerosity, low numerosity, and to neutral numerosity, on any single day each location in space was only adapted once (and in one consistent direction).
Results and discussion
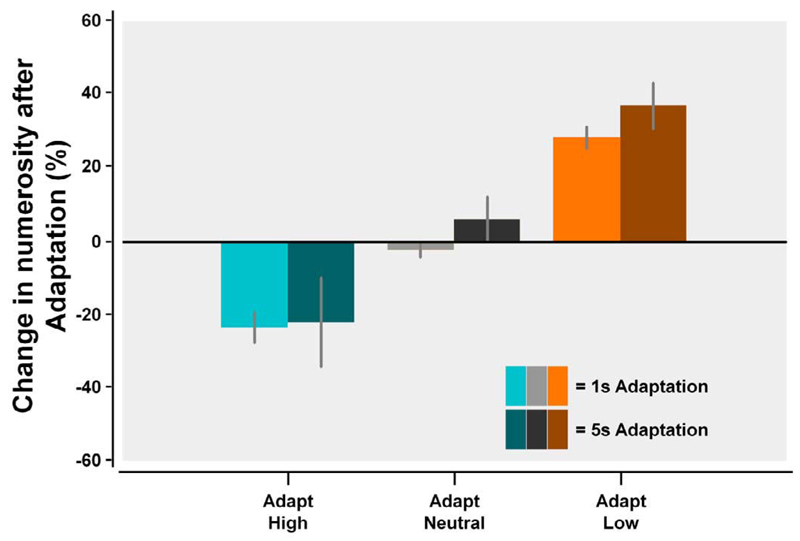
The amount of numerosity adaptation was expressed as the percentage change from the baseline condition to the adaptation (Figure 2). In the 1-second adapter condition there was a significant change in numerosity perception at both the adapt high, t(7) = −5.96, p < 0.001, and the adapt low, t(7) = 10.28, p < 0.001, locations with no significant changes occurring at the adapt neutral location, t(7) = −1.17, p = 0.28. Importantly, this result meant that the adaptation induced was spatially specific, as within the same testing block a downward, neutral, and upward adaptation effect was observed at different locations in the visual field. Although there was no initial adaptation period (the duration of each adapter was only 1 s, and subsequent adaptation at the same location was separated by 9.5 s), there was still a substantial numerosity adaptation effect (approximately a 25% change in PSE) for both upward and downward adaptation. Whereas we used significantly shorter periods of adaptation than Burr and Ross (2008a), the effects were nevertheless equivalent in magnitude to the 25%–30% adaptation effect they found when using the same ratio of adapter-to-standard (i.e., for 25 or 100 dot adapters with a 50 dot standard).
Figure 2.
The effect of adaptation at the high (cyan), neutral (gray), and low (orange) adapter locations for the 1-second adapter (lighter shades) and the 5-second adapter (darker shades) using the multiple locations short adaptation paradigm. As can be seen, both high and low adapters produced a substantial shift in the perception of the numerosity of the standard patch—eliciting approximately a 25%–30% change. There were no significant differences in numerosity adaptation between the 1-second adapter and 5-second adapter conditions.
As an additional control, half of the participants repeated the task with a 5-second adapter to examine whether the adaptation effects observed had saturated. The results showed that there was no change in the magnitude of adaptation with the longer adapter period (p > 0.05 for all comparisons). As such we can tentatively conclude that 1 s of exposure to the adapter stimulus every 9.5 s was sufficient to saturate the numerosity adaptation effect (or at least induced equivalent adaptation to 5-s of exposure every 17.5 s). This means that either an individual 1-second adapter trial was sufficient to reach adaptation ceiling, or that the adaptation was able to accumulate throughout the testing period. To better understand the dynamics of this short-interval numerosity adaption, Experiment 2 was devised to specifically look at the minimum exposure to the adapter required to elicit a numerosity adaptation effect and how this affects the rate at which the adaptation decays.
Experiment 2
The second experiment explored the influence of the quantity and duration of adapter trials on the temporal dynamics of adaptation to numerosity. We also aimed to understand whether the total length of exposure to adapter stimuli was crucial in determining the magnitude of the effect, and what were the minimal conditions necessary to yield numerosity adaptation. To do this we parametrically manipulated the length of the adapter, the number of adapter trials and the total amount of adaptation.
Methods
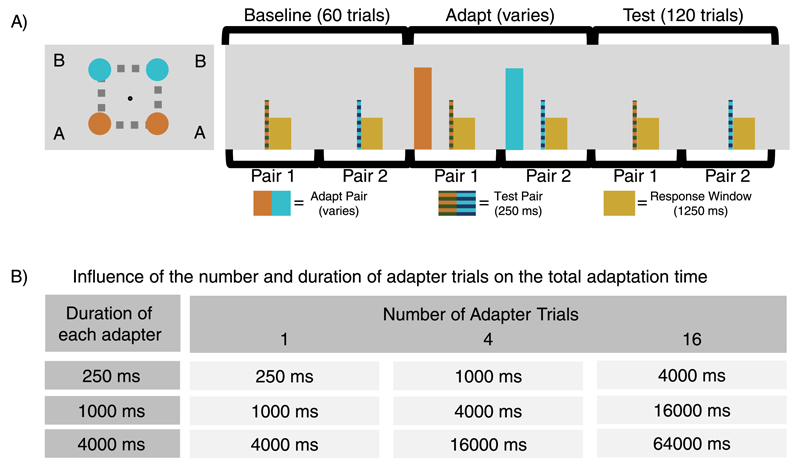
In order to better probe the temporal effects, Experiment 2 was simplified to contain just two pairs as shown in Figure 3. A small pause was introduced after the response window to ensure that the timing between subsequent adaptations at the same location remained the same (9.5 s) as in the first experiment. Additionally, during the adaptation period while one side of the pair was adapted to high (80) numerosity, the other side was adapted to low (20) numerosity. This manipulation effectively doubled the adaptation effect, and thus allowed for more a precise measurement of the changes in adaptation strength that occurred across time. Other than these small changes, the stimuli were identical to those of the first experiment.
Figure 3.
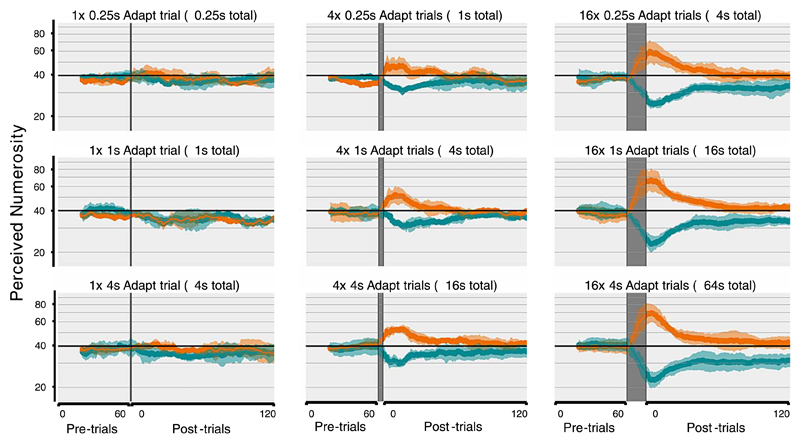
The paradigm and conditions for Experiment 2. (A) A reduced version of the brief multiple spatial location paradigm was used to examine the longevity of adaptation effects. The procedure was identical to Experiment 1, except it was reduced to two pairs, and following a variable period of adaptation, there was again a 1-s pause, followed by the 0.25-s test stimuli and 1.25-s response window. To account for the reduction in the number of pairs, a delay was introduced after the response (and before the next pair was shown) to ensure that the overall timing remained identical to Experiment 1. Furthermore, the adapter was only present in a small subset of trials (depending on the condition) while during the pre- and post-adapt trials the adapter period was blank (although the pause was maintained to ensure consistent timing throughout the paradigm). (B). The 3 × 3 design for examining the longevity of numerosity adaptation. In this experiment three different adapter lengths (0.25-second adapter, 1-second adapter, or 4-second adapter) were examined and presented a variable number of times (one, four, or 16 times) to examine the strength of the adaptation effect and its rate of decay. The conditions were selected to ensure the total exposure to adapting stimuli was equated along the diagonals (i.e., one trial of the 4-second adapter, four trials of 1-second adapter, or 16 trials of the 0.25-second adapter all result in four seconds of exposure to the adapter stimuli).
Rather than running separate blocks for baseline and adaptation as in the first experiment, in Experiment 2 the PSE was measured continuously throughout testing. Each condition began with 60 baseline trials (in which the timing of the trial was identical but the adapters were not shown) followed by a variable number of adapter trials depending on the condition. These baseline trials provided a measurement of the variability in numerosity perception prior to adaptation and allowed any residual biases to be observed. After the adapter period the experiment entered the test period, which proceeded identically to the baseline period (without any adapter presentations or top-up adaptation) with just the presentation of the test stimuli and the recording of response as shown in Figure 3A.
The experiment employed a 3 × 3 design wherein the number of adapter trials (one, four, or 16 trials) and the length of the adaptation (0.25-second adapter, 1-second adapter, or 4-second adapter) were varied to yield nine different conditions (Figure 3B). These parameters were picked to allow examination of the total amount of adaptation (as the multiplication of the number of trials by their length) irrespective of the number of trials or the length of each individual trial. All participants performed all nine conditions twice (on separate days) in a randomized order with the location of the high and low adapter within each pair switched between repetitions and conditions. During the test period, the standard was presented at the primarily adapted location (the “A” location) while the probe was presented at the secondary position (the “B” location). In each session one of the locations in each pair was the high adapter while the other was the low adapter. This meant that the change needed in the numerosity of the probe so that it appeared to perceptually match the standard would be either a decrease or increase in numerosity, depending on whether the standard was at the adapt high or adapt low location. In other words, since each pair underwent adaptation to low at one location and to high at the other, the effects observed were identical regardless of which location was adapted in which direction. As such, in this experiment the sign of the adaptation effect would change depending on which location contained the test stimuli. Thus in the second part of the analyses the upward and downward adaptation effects were combined. Instead of using the QUEST algorithm, a simple one-up one-down staircase with 5% steps was utilized in order to enhance the ability to track the changes in perceived numerosity across time. However, as in the first experiment, these steps were also perturbed with a random number drawn from a Gaussian distribution with standard deviation 0.15 log-units.
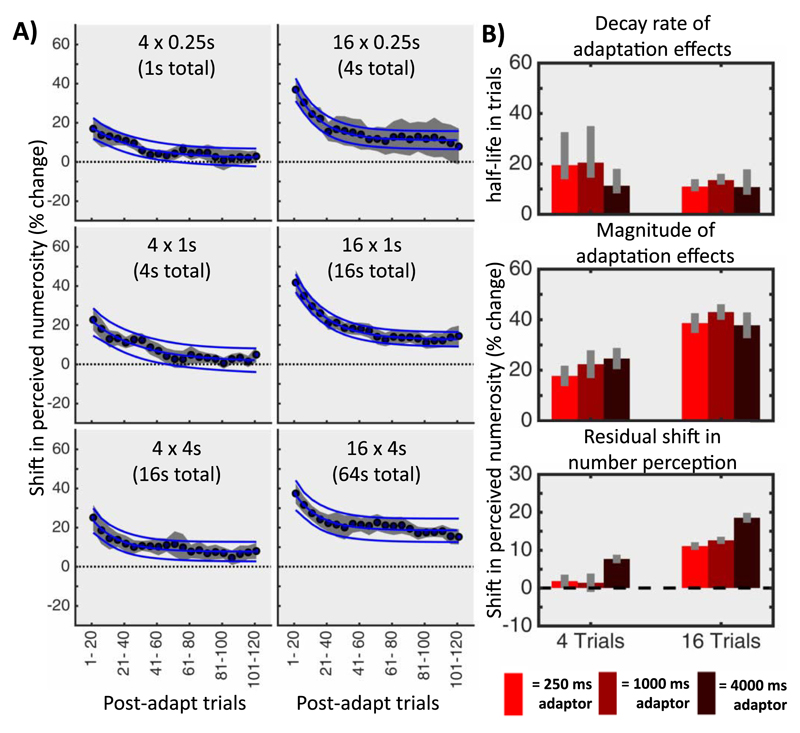
The timecourse was initially divided into nine separate nonoverlapping bins of 20 trials (three pre-adapt and six post-adapt). Within each bin the proportion of trials in which the test appeared more numerous than the probe was plotted against the test numerosity and fitted with a cumulative Gaussian to yield an estimate of the PSE. The fitting of the curve to the 20-trial window was bootstrapped 1000 times to reduce outlier noise and generate estimates of the standard deviation of the PSE fit. Data for both individual participants and the group means were then expressed as a percentage change in numerosity (Figure 5) and whether this change was greater than zero (i.e., if there was a significant influence of the adapter) was evaluated using a bootstrap sign test, corrected for multiple comparisons. In the second part of the analysis the post-adapt trials were analyzed using a sliding window of 20 trials (shifted in five trial increments). The data was then fit with a three-parameter exponential to produce estimates for the adaptation magnitude, adaptation decay rate (in trials), and an estimate of the magnitude of any residual adaptation effects (Figure 6).
Figure 5.
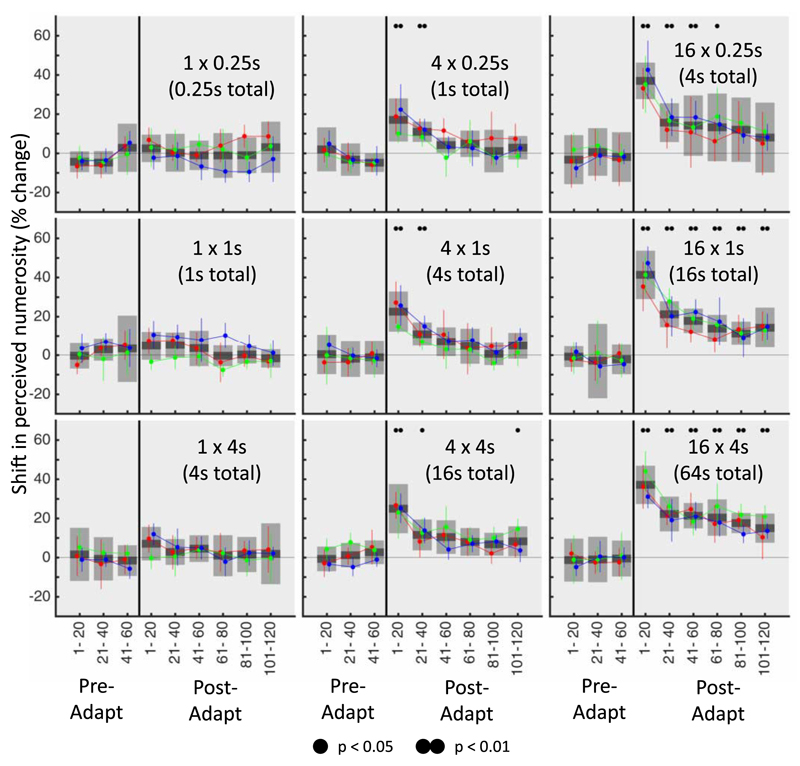
Shift in perceived numerosity for the different conditions. The percentage change in numerosity perception for each of the nine conditions. The number of adapter trials in each condition (one, four, or 16) changes along columns whereas the length of each individual adapter (0.25-second adapter, 1-second adapter, or 4-second adapter) changes along the rows. Here the timecourse was binned into nine nonoverlapping bins of 20 trials whereby the first three bins occurred before the adaptation period and the remaining six occurring after the adapter trials. Colored lines indicate the shift in individual subject’s numerosity perception (with 95% confidence intervals) whereas the gray bars denote the mean change across subjects (with shaded regions representing standard error). Significant changes in the mean perceived numerosity were determined by a bootstrap sign test (corrected for multiple comparisons) and are indicated by dots above each bin. There were no significant changes in numerosity perception in conditions with only a single adapter trial, indicating that the adapter had no effect on perception. In contrast, the first bin after adaptation was significant in all conditions with either four or 16 adapter trials. In the conditions with four 4-second adapter trials and all but the shortest of the conditions with 16 adapter trials, the final bin was also significantly above zero, indicating that even after 120 de-adapting trials there was still a significant shift in perception of numerosity.
Figure 6.
(A) Combined adaptation traces. The traces for both directions of adaptation were combined across participants to yield a more robust estimate of the temporal dynamics (with the standard error of these estimates represented by the shaded region). Each trace was fit with a three-parameter exponential function, as illustrated by the blue line, with the blue surrounding lines representing the 95% confidence intervals of the fit. (B) Quantitative fits to the combined adaptation traces. The three parameters of the exponential served as estimates for the decay rate of the adaptation, the magnitude of the effect, and the residual shift in the perception of numerosity. Error bars indicate the confidence intervals of the fits. The decay rate was lower for conditions with 16 adapter trials than with four adapter trials, while the magnitude of adaptation increased as the number of adapter trials increased. While there were no residual effects observed in the conditions with just four adapter trials (with the exception of the longest adapter), in all conditions with sixteen adapter trials a substantial numerosity adaptation effect was observed even after 120 post-adapt trials.
Results
The traces of the PSE across time are shown in Figure 4. For conditions with a single adapter trial there were no adaptation effects regardless of how long the trial was shown. Indeed the point-of-subjective equality (PSE) can be seen to fluctuate around 40 (the same magnitude as the standard). However, for conditions with either four or 16 adapter trials, there was a substantial (up to 40%) shift in the perceived numerosity, with a substantial departure from baseline evident after the adapter trials were shown. The magnitude of the adaptation effect appeared to increase as the number of adapter trials increased (with a clear effect visible along the columns), yet remained relatively unchanged when the length of each individual adapter was increased (with little observable effect along different rows).
Figure 4.
Traces of the shift in PSE before and after numerosity adaptation. The traces for cyan and orange represent downward and upward adaption effects respectively. The abscissa for each plot shows the 60 pretrial baseline (in which no adapter was shown) followed by a varying number of adapter trials (indicated by the dark shading). Immediately following the adapter trials were the 120 post-adapt trials, which were identical to the pre-adapt trials and consisting of only the presentation of the test stimuli and the response (with no additional presentations of the adapter stimuli). The layout of the plots corresponds to the table in Figure 3B, with the columns representing the number of adapter trials (one, four, or 16 adapter trials from left to right) and the rows representing the length of each adapter (0.25-second adapter, 1-second adapter, or 4-second adapter from top to bottom). The duration of total exposure to adapter stimuli is equal along the diagonals.
To quantify these changes the data for downward and upward shifts in magnitude were combined and expressed as a percentage change in perceived numerosity. Figure 5 shows the timecourse data for each individual subject (indicated by different colored lines and each plotted with their respective 95% confidence intervals) as well as the mean shift in perceived numerosity across subjects. A bootstrap sign test was performed (corrected for multiple comparisons) to determine whether each bin was reliably above zero (indicating a significant shift in perceived numerosity). Critically, none of the bins with a single adapter trial were found to be significant regardless of either the length of each individual adapter presentation or the total adaptation time. However, for conditions with either four or 16 adapter trials, the first post-adapt bins were found to reliably differ from zero, indicating a significant adaptation effect. Furthermore, in conditions with 16 adapter trials, the perceived numerosity was found to still be significantly shifted even after 120 post-adapt trials.
To further understand the timecourse of adaptation, a more densely sampled timecourse of the post-adapt trials was computed for each condition (Figure 6A) and fit with exponential curves so that the magnitude of adaptation, the rate of adaptation decay, and the strength of any long-term baseline shifts could be estimated. These parameters, as well as their 95% confidence intervals, are shown in Figure 6B. The results show that there was a significantly faster rate of adaptation decay for conditions with four adapter trials (adapter trial duration: Mean + [95% CI]; 0.25 s: 19.45 [13.87, 32.58]; 1 s: 20.47 [14.47, 34.98]; 4 s: 11.33 [8.26, 18.00]) than for conditions with 16 adapter trials (0.25 s: 10.97 [9.06, 13.90]; 1 s: 13.55 [11.74, 16.03]; 4 s: 10.76 [7.71, 17.82]). However, this was notably affected by the magnitude of the adaptation effect, which was substantially higher in conditions with 16 adapter trials (0.25 s: 38.61 [34.70, 42.53]; 1 s: 42.99 [39.90, 46.08]; 4 s: 37.75 [32.63, 42.87]) compared with those with only four adapter trials (0.25 s: 17.71 [13.65, 21.77]; 1 s: 22.34 [16.82, 27.85]; 4 s: 24.59 [20.38, 28.80]). Although there appears to be some slight trend for the magnitude of adaptation to increase with longer individual adapter durations, the confidence intervals are widely overlapping, suggesting that any role is relatively minor (and likely related to the shifts in residual baseline). Finally, while there was very little residual shift in the PSE for conditions with only four adapter trials (0.25 s: 1.82 [0.10, 10.04]; 1 s: 1.39 [−1.05, 3.82]; 4 s: 7.67 [6.54, 8.80]), there was a significantly larger and longer lasting shift after 16 adapter trials (0.25 s: 11.07 [10.04, 12.09]; 1 s: 12.58 [11.63, 13.54]; 4 s: 18.54 [17.22, 19.877]). Interestingly, when there were 16 adapter trials, there was a significant increase in the size of the residual baseline depending on the duration of each individual adapter. This suggests that the length of the adapter did play some role in the adaptation effect but, rather than influencing the magnitude per se, it instead prevented the adaptation from fully returning to baseline. However, in order to distinguish whether this subtle effect is related to increasing the total adapter exposure or just the length of each individual trial would require additional conditions and further experimentation.
These results suggest that sixteen 0.25-second adapter trials were just as effective at inducing numerosity adaptation as sixteen 4-second adapter trials. Furthermore, despite the total duration that the participant saw the adapter being equivalent, it was found that sixteen 0.25-second adapter trials (for a total of 4-s exposure to adapter stimuli) were far more effective at inducing strong adaptation effects than either four 1-second adapter trials or a single 4-second adapter trial. This strongly suggests that it was the number of adapter trials, and not the total length of adaptation time or the length of each individual adapter, that was critical in driving the adaptation effects observed. Furthermore, in conditions in which there were 16 adapter trials, we observed a residual, persistent adaptation effect that had not returned to baseline even at the end of the post-adapt period. Thus, even in the current paradigm in which each post-adapt trial could be considered a de-adapting stimulus, when participants were exposed to a sufficient number of adapter trials, the effect of numerosity adaptation could still be seen to persist even after 120 trials.
Discussion
In the present study we examined the temporal dynamics of numerosity adaptation by investigating how the duration and frequency of adapters influenced both the magnitude and rate of decay of numerosity adaptation. The first experiment verified that our novel multiple locations, pair-based testing paradigm was able to induce adaptation effects of a similar magnitude to that reported by Ross and Burr (2008a), in the vicinity of 30% of the test stimulus, despite using a substantially reduced period of adaptation. Furthermore, we were able to simultaneously induce adaptation to different numerosities at different locations in the visual field, suggesting that numerosity adaptation is spatially specific. It was also found that a five-fold increase in the length of the adaptation period had a negligible effect on the magnitude of the effect, suggesting that a single second of exposure to an adapter every 9.5 s was able to accumulate across trials and saturate the adaption effect. In the second study we examined the temporal dynamics of numerosity adaptation by varying the number of adapter trials, the length of each individual adapter trial and the total duration of exposure to adapter stimuli within each condition. The magnitude, decay and presence of any residual effects was then measured across 120 post-trials and quantified with a simple three-parameter exponential curve model. Across all the conditions it was consistently demonstrated that the number of adapter trials was critical in the generation of the adaptation effect. Thus, provided that there were multiple discrete presentations, even 0.25-second adapter stimuli were sufficient to induce substantial adaptation effects.
Several studies have suggested that numerosity adaptation is spatially selective (Burr & Morrone, 2012; Burr & Ross, 2008a, 2008b). Recently, this spatial selectivity was also demonstrated for sequential numerosity displays. Arrighi and colleagues (2014) showed that adaptation to the temporal sequence of items in a particular region of the visual field produced an adaptation effect only when the subsequent test was presented at the same spatial location. Furthermore, when an eye-movement was introduced between adaptation and testing blocks, adaptation was found to be present only at the spatiotopic location in space. They concluded that this was consistent with the numerosity adaptation effects originating at relatively high levels of the visual hierarchy. In the current experiment we were able to generate substantial adaptation effects in response to both high and low numbers presentations, while keeping adaptation effects at other locations neutral, within the same interleaved paradigm. This adds to previous investigations of the spatial-specificity of numerosity adaptation by demonstrating that, even within a single block of trials, distinct adaptation effects can be generated at different locations in the visual field based on the history of stimuli presented at that specific location.
In Experiment 2 it was found that adaptation was predominantly driven by the number of adapter events and not by length of time that the adapter had been shown. Furthermore, the strength of numerosity adaptation was also found to be unrelated to the total amount of adaptation received during the adapting window. Although sixteen 0.25-second adapter trials, four 1-second adapter trials, and a single 4-second adapter trial all expose the participant to the adapting stimulus for a total of four seconds, the condition with sixteen trials yielded a substantially stronger adaptation effect, whereas a single 4-second adapter trial produced no measured effect. Thus it appears that even exceptionally brief periods of adaptation are sufficient to induce substantial adaptation. Although the number of presentations was found to be the main factor determining the magnitude of the numerosity adaptation, there was some evidence that either the length of each adapter or the total adapter exposure also had some (albeit smaller) influence in the residual baseline effect and decay rate. Indeed, the results for four presentations of a 4-second adapter strongly resemble those found with sixteen presentations of the 0.25-second adapter. This suggests that, although the initial appearance of a new event conveys the greatest contribution to the adaption effects, there may additionally be some small influence of the adapter duration. This dependency on the number of discrete adaptation events suggests that when viewing an adapting stimulus, after the first initial moments of novelty, prolonged exposure produces rapidly diminishing returns. This idea fits well with the claims of other adaptation studies in which the majority of adaptive changes were found to occur within the initial 100–1000 ms after presentation with very little additional changes occurring after this window (Fairhall, Lewen, Bialek, & de Ruyter van Steveninck, 2001; Nagel & Doupe, 2006). Similarly it may explain the surprisingly large adaptation effects, such as the tilt aftereffect found by Kosovicheva and colleagues (2012), that have been observed following very brief (but frequently repeated) adaptation periods. Whereas this could be rephrased to say that it is the number of discrete stimulus onsets that is critical to the magnitude of adaptation, this description of adaptation conflicts with the more traditional idea that the magnitude of the effect depends on the duration of exposure to the adapting stimulus.
Although linking the strength of adaptation to the number of adapting events rather than to the duration of adapter exposure is unusual for an adaptation study, it fits well with a recently proposed theory of adaptation in which adaptation effects are related to stimulus predictability. Chopin and Mamassian (2012) suggest that we estimate the statistics of the current environmental over a large time frame, and adaptation effects result from attempts to reconcile variance in the recent history (what you have just observed) with the variance observed in a longer, more encompassing history. Importantly, this framework places an emphasis on events rather than duration as the critical component guiding adaptation. In the case of numerosity, after substantial experience with stimuli whose numerosity was in the vicinity of 40 (in the pre-adapt trials) participants were then subjected to relatively few trials of a substantially different magnitude (i.e., an adapter of 80). This would then cause the perception of subsequent patches of 40 dots to be perceived lower (i.e., as approximately 30), so that the statistics of the recent past would better approximate the average statistics acquired over a longer history. Under this explanation the number of adapter events becomes critical to the degree of shift observed in the current perception. One way in which this model could be explicitly tested would be to extend the number of post-adapt trials beyond 120, as in this case the model would predict that with a sufficient number of post-trials (so that the adapter trials themselves became part of the longer history) the effect should switch and perception would instead be attracted towards the previously shown adapters. As several other studies have observed this eventual positive aftereffect (Cicchini, Anobile, & Burr, 2014; Fischer & Whitney, 2014; Liberman, Fischer, & Whitney, 2014), it would be particularly interesting to see whether the considerable residual effect observed in the 16 adapter trials conditions would also eventually reverse.
Whereas the results here suggest a critical role of events in the generation of numerosity adaptation effects, precisely what constitutes an “event” remains an unanswered question. While in the current experiment each adapter event was separated by a substantial period of time (9.5 s), it is possible that events could have occurred much closer together. Indeed, at the extreme of this idea, simply flickering or refreshing the stimuli may have been sufficient to register as a new event. As such, it may have been possible that a “single” trial of 4 s that was refreshed every 0.25-seconds could have functioned similarly to the 16 0.25-second adapter condition in the current experiment. Another possibility is that, as each adapter was randomly generated at the time of presentation, it was important that each event was not only temporally distinct but also represented a new variant of the adapter magnitude. In the case of numerosity, this could have been particularly critical as, although many low-level properties of the adapter stimulus may have varied on each presentation, the target feature (in this case numerosity) would remain the same. This feature-selective consistency could potentially allow numerosity to be specifically adapted without also inducing adaptation to other features. Additionally, it may be that in the same way that visual objects represent a collection of features over a discrete portion of space, an event could be thought of as a collection of features over a discrete portion of time. Future studies are needed in order to investigate precisely what is considered an event and whether some stages of adaptation may depend solely on the number of events (i.e., the magnitude of the numerosity adaptation effect) while others are also influenced by the total duration of exposure to adapter stimuli (i.e., the residual shift in numerosity perception).
This study is the first formal examination of the temporal dynamics governing numerosity adaptation. We found that numerosity adaptation does not require the frequent and lengthy periods of adaptation periods that are typically used in the study of both numerosity and many other visual attributes (Burr & Ross, 2008a; Clifford & Rhodes, 2005). Instead substantial adaptation effects can be generated with very brief presentations, provided that there are several unique adapting events, supporting the idea that numerosity adaptation occurs within extrastriate areas (Henson, 2003). This itself fits well with the areas highlighted in electrophysiological and fMRI studies (Harvey et al., 2013; Kong et al., 2005; Nieder, Diester, & Tudusciuc, 2006; Nieder, Freedman, & Miller, 2002; Piazza et al., 2007) which mainly have suggested the intraparietal structure, as well as prefrontal cortex, as critical to numerosity judgments. The current study also has implications for how numerosity, as well as other stimulus attributes susceptible to adaptation, could be tested in future. The novel paradigms used in Experiment 1 and 2 allowed for the simultaneous measurement of multiple adaptation effects (and neutral controls) in a very rapid design that did not require prolonged viewing of static stimuli. Whereas this study focused on numerosity adaptation, the design could also be implemented in the examination of adaptation effects for other features. The ability to rapidly induce and maintain visual adaptation is critical, particularly when studying development or clinical groups, where compliance or attention resources are often limited or impaired in participants. Indeed, a modified version of this brief-adaptation task was recently used successfully to investigate numerosity adaptation among typical and autistic children (Turi et al., 2015) in which robust adaptation results were acquired for all the groups without the excessive attentional demands that traditional adaptation paradigms (using lengthy periods initial adaptation and frequent top-up periods) would have required.
Overall these results suggest that the mechanisms underlying numerosity perception are highly plastic and exhibit substantial, spatially specific adaptation in response to the repeated deviant number presentations. Critically, these adaptive changes appear to be driven not by the duration of exposure to an adapting event, but instead by the number of discrete adapting events that occurred at that location.
Acknowledgments
This study has been supported by the European Research Council projects “STANIB” (grant number 229445) and “ESCPLAIN” (grant number 338866).
Footnotes
Commercial relationships: none.
References
- Aagten-Murphy D, Attucci C, Daniel N, Klaric E, Burr D, Pellicano E. Numerical estimation in children with autism. Autism Research. 2015;8:668–681. doi: 10.1002/aur.1482. [DOI] [PubMed] [Google Scholar]
- Anobile G, Cicchini GM, Burr D. Separate mechanisms for perception of numerosity and density. Psychological Science. 2014;25:265–270. doi: 10.1177/0956797613501520. [DOI] [PubMed] [Google Scholar]
- Anobile G, Cicchini GM, Burr DC. Number as a primary perceptual attribute: A review. Perception. 2016;45:5–31. doi: 10.1177/0301006615602599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrighi R, Togoli I, Burr D. A generalized sense of number. Proceedings of the Royal Society of London B: Biological Sciences. 2014;281:20141791. doi: 10.1098/rspb.2014.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Burr D, Anobile G, Turi M. Adaptation affects both high and low (Subitized) numbers under conditions of high attentional load. Seeing and Perceiving. 2011;24:141–150. doi: 10.1163/187847511X570097. [DOI] [PubMed] [Google Scholar]
- Burr DC, Morrone MC. Constructing stable spatial maps of the world. Perception. 2012;41:1355–1372. doi: 10.1068/p7392. [DOI] [PubMed] [Google Scholar]
- Burr D, Ross J. A visual sense of number. Current Biology: CB. 2008a;18:425–428. doi: 10.1016/j.cub.2008.02.052. [DOI] [PubMed] [Google Scholar]
- Burr D, Ross J. Response: Visual number. Current Biology. 2008b;18:R857–R858. [Google Scholar]
- Chopin A, Mamassian P. Predictive properties of visual adaptation. Current Biology: CB. 2012;22:622–626. doi: 10.1016/j.cub.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Cicchini GM, Anobile G, Burr DC. Compressive mapping of number to space reflects dynamic encoding mechanisms, not static logarithmic transform. Proceedings of the National Academy of Sciences USA. 2014;111:7867–7872. doi: 10.1073/pnas.1402785111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford CWG, Rhodes G. Fitting the mind to the world: Adaptation and after-effects in high-level vision. Oxford, UK: Oxford University Press; 2005. [Google Scholar]
- Dakin SC, Tibber MS, Greenwood JA, Kingdom FAA, Morgan MJ. A common visual metric for approximate number and density. Proceedings of the National Academy of Sciences USA. 2011;108:19552–19557. doi: 10.1073/pnas.1113195108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Piazza M, Pinel P, Cohen L. Three parietal circuits for number processing. Cognitive Neuropsychology. 2003;20:487–506. doi: 10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]
- Dodwell PC, Humphrey GK. A functional theory of the McCollough effect. Psychological Review. 1990;97:78–89. doi: 10.1037/0033-295x.97.1.78. [DOI] [PubMed] [Google Scholar]
- Droit-Volet S, Clément A, Fayol M. Time, number and length: Similarities and differences in discrimination in adults and children. Quarterly Journal of Experimental Psychology. 2008;61:1827–1846. doi: 10.1080/17470210701743643. [DOI] [PubMed] [Google Scholar]
- Durgin FH. Texture density adaptation and visual number revisited. Current Biology. 2008;18:R855–R856. doi: 10.1016/j.cub.2008.07.053. [DOI] [PubMed] [Google Scholar]
- Durgin FH, Huk AC. Texture density aftereffects in the perception of artificial and natural textures. Vision Research. 1997;37:3273–3282. doi: 10.1016/s0042-6989(97)00126-0. [DOI] [PubMed] [Google Scholar]
- Fairhall AL, Lewen GD, Bialek W, de Ruyter van Steveninck RR. Efficiency and ambiguity in an adaptive neural code. Nature. 2001;412:787–792. doi: 10.1038/35090500. [DOI] [PubMed] [Google Scholar]
- Fang F, Murray SO, Kersten S, He S. Orientation-tuned fMRI adaptation in human visual cortex. Journal of Neurophysiology. 2005;94:4188–4195. doi: 10.1152/jn.00378.2005. [DOI] [PubMed] [Google Scholar]
- Fischer J, Whitney D. Serial dependence in visual perception. Nature Neuroscience. 2014;17:738–743. doi: 10.1038/nn.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebuis T, Reynvoet B. The neural mechanisms underlying passive and active processing of numerosity. NeuroImage. 2013;70:301–307. doi: 10.1016/j.neuroimage.2012.12.048. [DOI] [PubMed] [Google Scholar]
- Gepshtein S, Lesmes LA, Albright TD. Sensory adaptation as optimal resource allocation. Proceedings of the National Academy of Sciences USA. 2013;110:4368–4373. doi: 10.1073/pnas.1204109110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberda J, Mazzocco MMM, Feigenson L. Individual differences in non-verbal number acuity correlate with maths achievement. Nature. 2008;455:665–668. doi: 10.1038/nature07246. [DOI] [PubMed] [Google Scholar]
- Harvey BM, Klein BP, Petridou N, Dumoulin SO. Topographic representation of numerosity in the human parietal cortex. Science. 2013;341:1123–1126. doi: 10.1126/science.1239052. [DOI] [PubMed] [Google Scholar]
- Henson RNA. Neuroimaging studies of priming. Progress in Neurobiology. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Kleiner M, Brainard D, Pelli D, Ingling A, Murray R, Broussard C. What’s new in Psychtoolbox-3. Perception. 2007;36:1. [Google Scholar]
- Kohn A. Visual adaptation: Physiology, mechanisms, and functional benefits. Journal of Neurophysiology. 2007;97:3155–3164. doi: 10.1152/jn.00086.2007. [DOI] [PubMed] [Google Scholar]
- Kong J, Wang C, Kwong K, Vangel M, Chua E, Gollub R. The neural substrate of arithmetic operations and procedure complexity. Cognitive Brain Research. 2005;22:397–405. doi: 10.1016/j.cogbrainres.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Kosovicheva AA, Maus GW, Anstis S, Cavanagh P, Tse PU, Whitney D. The motion-induced shift in the perceived location of a grating also shifts its aftereffect. Journal of Vision. 2012;12(8):7, 1–14. doi: 10.1167/12.8.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold DA, O’Toole AJ, Vetter T, Blanz V. Prototype-referenced shape encoding revealed by high-level aftereffects. Nature Neuroscience. 2001;4:89–94. doi: 10.1038/82947. [DOI] [PubMed] [Google Scholar]
- Liberman A, Fischer J, Whitney D. Serial dependence in the perception of faces. Current Biology: CB. 2014;24:2569–2574. doi: 10.1016/j.cub.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollough C. Color adaptation of edge-detectors in the human visual system. Science. 1965;149:1115–1116. doi: 10.1126/science.149.3688.1115. [DOI] [PubMed] [Google Scholar]
- Nagel KI, Doupe AJ. Temporal processing and adaptation in the songbird auditory forebrain. Neuron. 2006;51:845–859. doi: 10.1016/j.neuron.2006.08.030. [DOI] [PubMed] [Google Scholar]
- Nieder A. Counting on neurons: The neurobiology of numerical competence. Nature Reviews Neuroscience. 2005;6:177–190. doi: 10.1038/nrn1626. [DOI] [PubMed] [Google Scholar]
- Nieder A, Diester I, Tudusciuc O. Temporal and spatial enumeration processes in the primate parietal cortex. Science. 2006;313:1431–1435. doi: 10.1126/science.1130308. [DOI] [PubMed] [Google Scholar]
- Nieder A, Freedman DJ, Miller EK. Representation of the quantity of visual items in the primate prefrontal cortex. Science. 2002;297:1708–1711. doi: 10.1126/science.1072493. [DOI] [PubMed] [Google Scholar]
- Park J, DeWind NK, Woldorff MG, Brannon EM. Rapid and direct encoding of numerosity in the visual stream. Cerebral Cortex. 2016;26:748–763. doi: 10.1093/cercor/bhv017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Piazza M, Izard V. How Humans Count: Numerosity and the Parietal Cortex. The Neuroscientist. 2009;15:261–273. doi: 10.1177/1073858409333073. [DOI] [PubMed] [Google Scholar]
- Piazza M, Pinel P, Le Bihan D, Dehaene S. A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron. 2007;53:293–305. doi: 10.1016/j.neuron.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Pinel P, Piazza M, Le Bihan D, Dehaene S. Distributed and overlapping cerebral representations of number, size, and luminance during comparative judgments. Neuron. 2004;41:983–993. doi: 10.1016/s0896-6273(04)00107-2. [DOI] [PubMed] [Google Scholar]
- Priebe NJ, Churchland MM, Lisberger SG. Constraints on the source of short-term motion adaptation in macaque area MT. I. The role of input and intrinsic mechanisms. Journal of Neurophysiology. 2002;88:354–369. doi: 10.1152/.00852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe NJ, Lisberger SG. Constraints on the source of short-term motion adaptation in macaque area MT. II. Tuning of neural circuit mechanisms. Journal of Neurophysiology. 2002;88:370–382. doi: 10.1152/jn.2002.88.1.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A, MacLeod D. The McCollough effect with plaids and gratings: Evidence for a plaid-selective visual mechanism. Journal of Vision. 2011;11(1):26, 1–9. doi: 10.1167/11.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J, Burr D. Vision senses number directly. Journal of Vision. 2010;10(2):10, 1–8. doi: 10.1167/10.2.10. [DOI] [PubMed] [Google Scholar]
- Solomon SG, Kohn A. Moving sensory adaptation beyond suppressive effects in single neurons. Current Biology. 2014;20:1012–1022. doi: 10.1016/j.cub.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibber MS, Greenwood JA, Dakin SC. Number and density discrimination rely on a common metric: Similar psychophysical effects of size, contrast, and divided attention. Journal of Vision. 2012;12(6):8, 1–19. doi: 10.1167/12.6.8. [DOI] [PubMed] [Google Scholar]
- Turi M, Burr D, Igliozzi R, Aagten-Murphy D, Muratori F, Pellicano E. Children with autism spectrum disorder show reduced adaptation to number. Proceedings of the National Academy of Sciences USA. 2015;112:7868–7872. doi: 10.1073/pnas.1504099112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan P, Nieder A. Neuronal correlates of a visual “sense of number” in primate parietal and prefrontal cortices. Proceedings of the National Academy of Sciences USA. 2013;110:11187–11192. doi: 10.1073/pnas.1308141110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vul E, Krizay E, MacLeod DIA. The McCollough effect reflects permanent and transient adaptation in early visual cortex. Journal of Vision. 2008;8(12):4, 1–12. doi: 10.1167/8.12.4. [DOI] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. Quest: A Bayesian adaptive psychometric method. Perception & Psychophysics. 1983;33:113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- Webster MA. Pattern selective adaptation in color and form perception. The Visual Neurosciences. 2003;2:936–947. [Google Scholar]
- Webster MA. Adaptation and visual coding. Journal of Vision. 2011;11(5):3, 1–23. doi: 10.1167/11.5.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MA, Kaping D, Mizokami Y, Duhamel P. Adaptation to natural facial categories. Nature. 2004;428:557–561. doi: 10.1038/nature02420. [DOI] [PubMed] [Google Scholar]
- Xu F, Spelke ES. Large number discrimination in 6-month-old infants. Cognition. 2000;74:B1–B11. doi: 10.1016/s0010-0277(99)00066-9. [DOI] [PubMed] [Google Scholar]