Abstract
This review focuses on the diagnosis and management of Parkinson-related pain which is one of the more frequently reported nonmotor symptoms in Parkinson’s disease (PD), which is the second most common neurodegenerative disease after Alzheimer’s disease. Pain is ranked high by patients as a troublesome symptom in all stages of the disease. In early-stage PD, pain is rated as the most bothersome symptom. Knowledge of the correct diagnosis of pain origin and possible methods of treatments for pain relief in PD is of great importance. The symptoms have a great negative impact on health-related quality of life. Separating PD-related pain from pain of other origins is an important challenge and can be characterized as “many syndromes under the same umbrella”. Among the different forms of PD-related pain, musculoskeletal pain is the most common form, accounting for 40%–90% of reported pain in PD patients. Augmentation by pathophysiological pathways other than those secondary to rigidity, tremor, or any of the other motor manifestations of the disease seems most probable. In PD, the basal ganglia process somatosensory information differently, and increased subjective pain sensitivity with lower electrical and heat-pain thresholds has been reported in PD patients. The mechanism is assumed to be diminished activity of the descending inhibitory control system of the basal ganglia. PD pain, like many of the nonmotor symptoms, remains underdiagnosed and, thus, poorly managed. A systematic collection of patient descriptions of type, quality, and duration of pain is, therefore, of utmost importance. Recent studies have validated new and more specific and dedicated pain scales for PD-related symptoms. Symptomatic treatments based on clinical pain classification include not only pharmacological but also nonpharmacological methods and, to some degree, invasive approaches. In the clinic, pharmacological and nonpharmacological interventions can be effective to varying degrees – as single therapies or in combination – and should be employed, because no therapeutic strategies have been validated to date for managing PD pain. Multimodal approaches should always be considered, dopamine replacement therapies should be adjusted, and analgesics and/or antidepressants should be considered, including the use of different forms of complementary therapies.
Keywords: basal ganglia, complementary therapies, nonmotor symptoms, pain, Parkinson’s disease, quality of life
Background
James Parkinson described the phenomenon of pain in Parkinson’s disease (PD) in 1817 in his original work An Essay on the Shaking Palsy:
[…] the writer of these lines was called to a female about forty years of age, complaining of great pain in both the arms, extending from the shoulder to the finger ends. She stated, that she […] was not benefited by any of the medicines which had been employed […] leaving both the arms and hands in a very weakened and trembling state.1
Now, almost 200 years later, the distressing and frequently occurring Parkinson-related chronic pain remains an under-appreciated and underdiagnosed symptom in PD.
Incidence and prevalence of PD, general pain, and PD-related pain
Estimates of PD prevalence and incidence have provided conflicting estimates. In Europe, the annual incidence estimates range from 5/100,000 to 346/100,000.2 Approximately 60,000 Americans are diagnosed with PD each year.3 The challenges involved with differential diagnoses and other forms of Parkinsonism, as well as the long time course from initial PD-like symptoms to a correct diagnosis, are likely responsible for the discrepancy in numbers.4
The reported prevalence of pain in PD and PD-related pain also varies between studies. In 2008, Negre-Pages et al5 estimated the prevalence of chronic pain in PD to be >60%. PD pain is often reported as heterogeneous in its clinical presentation, with a disabling effect on quality of life assessments. In 1998, the Swedish Parkinson Association reported on a survey of nonmotor symptoms (NMS) comprising almost 1,000 PD respondents, revealing that pain was more common in females than males (54% and 45%, respectively).6 However, general pain is also common in the population, with 18%–19% in a general adult population according to the prevalence data.7,8
In early-stage PD, pain is rated as one of the most troublesome NMS,9 and it seems to affect the side of the body that was initially worst impacted by motor symptoms (MS) of the disease (Table 1).10
Table 1.
Ranking of the ten most bothersome PD-related symptoms (MS and NMS) in 92 early-onset patients with up to 6 years of disease duration
| Rank | Symptom/condition | First choice (%) | Second choice (%) | Third choice (%) |
|---|---|---|---|---|
| 1 | Slowness | 33 | 5 | 13 |
| 2 | Tremor | 30 | 9 | 4 |
| 3 | Stiffness | 6 | 26 | 11 |
| 4 | Pain | 10 | 10 | 5 |
| 5 | Loss of smell/taste | 3 | 10 | 3 |
| 6 | Mood | 4 | 6 | 4 |
| 7 | Handwriting | 2 | 3 | 6 |
| 8 | Bowel problem | 2 | 3 | 5 |
| 9 | Sleep | 2 | 4 | 1 |
| 10 | Appetite/weight | 0 | 3 | 8 |
Notes: Data adapted from Politis et al9
Abbreviations: PD, Parkinson’s disease; MS, motor symptoms; NMS, nonmotor symptoms.
Pathophysiological pathways of pain in PD
The origin of pain in PD remains poorly understood. At times, it appears as dystonia when the dopaminergic (DA) effects wear off. The pathophysiological mechanisms behind this phenomenon are most probable by which dopamine, in the network with other monoamines such as noradrenaline and 5-hydroxytryptamine (5-HT), interacts through inhibitory and excitatory pathways. Abnormalities in descending pathways affect central pain processing. In addition, clinically registered neuropathic pain and other muscular pain sensations are described by PD patients. This has led to the exploration of pathways other than those secondary to rigidity, tremor, or any other motor manifestations of the disease, with abnormal nociception processing in PD patients suffering from pain as the most likely suspect.10 The basal ganglia (BG) process somatosensory information in different ways, and increased subjective pain sensitivity with lower electrical and heat pain thresholds has been reported in PD patients.11 This abnormal processing also comprises PD-related disorders such as multiple system atrophy, which exhibits almost the same prevalence of pain as PD.12
The functional–anatomical arrangements of BG are characterized by multiple interconnected structures and networks.13 BG serve as an integrating point for afferent fibers in the organization of behavioral responses to stimuli.14,15 Influx from cortical and subcortical brain regions contributes to the network between thalamus, cortex, and BG.16 The involved cortical areas also play important roles in the process and modulation of pain. These territories include the frontal and parietal lobes, the insula, and the hippocampus. Electrical stimulation of the substantia nigra, one of the nuclei in the BG, modulates impending pain within the dorsal horn of the spinal cord, which is probably mediated by a DA descending inhibitory pathway originating in the midbrain (Figure 1).11,12
Figure 1.
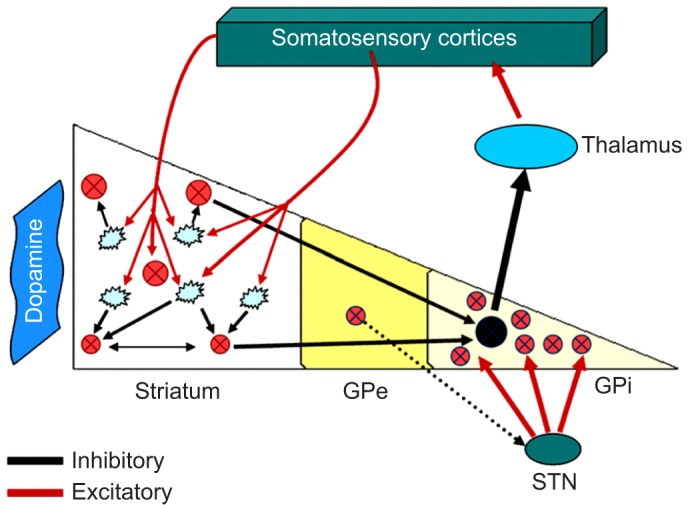
The pathophysiological basis of sensory disturbances in PD, the so-called “pain matrix” with information from different loci, processed in the BG.
Notes: Reprinted from Journal of the Neurological Sciences; 289(1–2); Juri C, Rodriguez-Oroz M, Obeso JA; The pathophysiological basis of sensory disturbances in Parkinson’s disease; 60–65; Copyright 2010, with permission from Elsevier.67 The blue dashed line indicates proposed modulator function from STN. Lewy bodies are observed in the vagal nucleuus and locus coeruleus during early stages of PD. These areas contribute to the so-called “pain matrix”. Sensory information of the integrational sensory information in the basal ganglia. Afferent sensory information; different regions of somatosensory cortices converge into striatum where it would interfere with information processing.
Abbreviations: PD, Parkinson’s disease; BG, basal ganglia; GPe, globus pallidus externa, GPi, globus pallidus interna; STN, subthalamic nucleus.
The two main DA pathways are well recognized. The nigrostriatal DA course projects from the substantia nigra to dorsal striatal structures in corpus striatum. This route has an established function in the sensorimotor integration and control.17 Subcortical structures, such as amygdala, thalamus, and nucleus accumbens, are reached by neurons with an origin in the ventral tegmental area.18 Distinct projections from the ventral tegmental area also innervate specific cortical regions, such as motor cortex and the prefrontal cortex.19 Consequently, there is considerable overlap between the DA system and these mentioned brain regions involved in pain processing, and interference in DA concentrations in these areas could lead to motor and sensory disturbances.20
Much evidence suggests that other areas such as brain-stem nuclei and diencephalic structures are affected,21 as extra-encephalic structures, the spinal cord and autonomic enteric plexus, also seem to be involved.22 Moreover, because DA drugs might be efficacious for many NMS, including PD pain, evidence suggests that these symptoms are associated with DA denervation in brain areas not primarily related to MS.
Physiological pathways of pain relief
In the early 1960s, theories initially developed by Melzack and Wall23 were introduced. They proposed three features of afferent input that were signed for pain: the ongoing activity that precedes the stimulus, the stimulus-evolved activity, and the relative balance of activity in large versus small fibers. The concept of the “gate control theory” was introduced. Pain messages encounter “nerve gates” in the spinal cord that open or close depending upon a number of factors (possibly including instructions received from the brain). When the gates are open, pain messages “pass” more easily and pain can be intense. When the gates are closed, pain messages are prevented from reaching the brain and may not even be experienced. Although the details of this process remain poorly understood, it can help to explain why various treatments are effective.
The existence of low-threshold mechanoreceptive C-tactile (CT) afferents was initially described by Vallbo et al.24 These afferents comprise a second anatomically and functionally distinct system that signals touch in human beings. The activation of these fibers is more closely related to limbic functions rather than cognitive and motor functions. Although rapid, accurate, and informative Aβ touch acutely reflects the external world through cutaneous events in an exteroceptive manner, CT activation shares more characteristics with interceptive modalities. This slow, affective nature is likely to be involved in the maintenance of physical well-being.
Measurement of pain
Pain, stress, and biomarkers of stress
Stress and pain are often closely linked. Each has an impact on the other, creating a vicious cycle that sets the stage for chronic pain and chronic stress. Therefore, stress management should be a component in pain therapy.
The Merriam-Webster Encyclopedia® defines the term stress as a “physical, chemical, or emotional factor that causes bodily or mental tension and may be a factor in disease causation”. The result of stress can be explained as physical or mental tension resulting from factors that tend to alter an existing equilibrium.
In human beings, stress is often a reaction to difficult and possibly dangerous situations. The search for humoral substrates that reflect bodily experiences of stress is an area that is attracting scientific curiosity. The “fight or flight” response occurs when a person perceives a threat and the body exerts energy to fight or to run away “to live another day”. This response is characterized by the release of epinephrine from the adrenal glands, causing blood vessels to constrict and the heart rate to increase. Measures of cortisol concentration in saliva have been shown to be a simple and useful indirect biomarker of stress. By studying hypothalamic–pituitary–adrenal axis function and cortisol secretion in PD patients as a surrogate marker for stress and indirect pain, it is possible to study and objectify intervention effects with the purpose of stress/pain relief.25 Other biomarkers are epinephrine, norepinephrine, and oxytocin concentrations. Blood pressure and heart and respiratory rates are examples of other markers for evaluating stress reduction.
Examples of pain scales
Visual analog scale
A visual analog scale (VAS) measures a continuum of a chosen present characteristic.26 For example, the experienced pain that a patient feels extends over a continuum from no pain to an extreme intensity of pain. This range of perceived pain appears continuous for the patient. Pain does not appear as an ordinary scale with jumps between the values, such as discrete, moderate, or severe. Word descriptors (WDSs) are only used in both ends of the line, which is usually 100 mm in length. This valuation is very subjective and best used within an individual and not between groups of individuals at the same time point. Most experts argue that a VAS at best can produce data of ordinal type. This is important to consider in the statistical analysis of VAS data. Rank ordering of scores rather than the exact values might be the best way to handle patient registrations on the 100 mm line.
Brief Pain Inventory
The Brief Pain Inventory was initially created for the purpose of measuring pain in cancer patients. It measures pain relief, pain quality, and patient perception of the cause of pain in terms of pain intensity (sensory dimension) and pain interference (reactive dimension).27
Examples of pain scales in PD
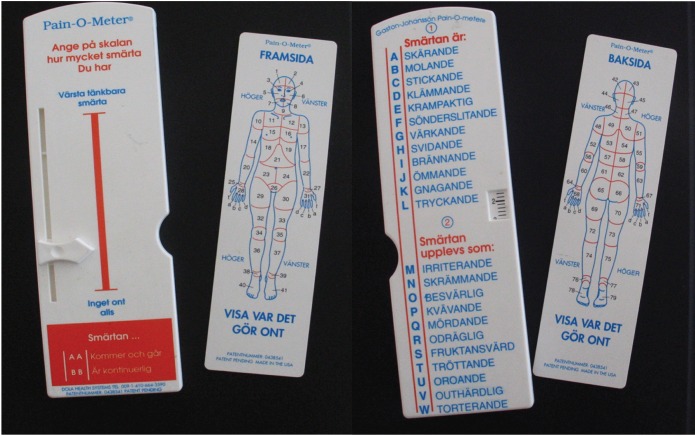
Pain-O-Meter
This is a self-administered pain assessment tool developed for the purpose of improving pain assessment and management in acute and chronic pain patients, not exclusively for PD pain. It is a hard, white, plastic tool. Two methods for assessing pain are located on the Pain-O-Meter (POM). The first is a 10-cm VAS with a moveable marker that patients use to rate their pain. The second is a list of 15 sensory and eleven affective WDSs. Each WDS is assigned an intensity value that can be as low as 1 or as high as 5 (Figure 2 and Table 2).28
Figure 2.
The Pain-O-Meter (Swedish Version).
Table 2.
Differentiation of experienced pain
| Sex | Durationa of disease, ≤5 years/>5 years | Pain before/after PD diagnosis | Durationb of pain/day, ≤10 h/>10 h | VAS,c ≤5 cm/>5 cm | Pain expressions by participants
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Migrating | Irritating | Worrying | Troublesome | Tiring | Suffocating | RLSd | |||||
| Females (28) | 11/16e | 10/17e | 20/8 | 13/14e | 13f | 5f | 3 | 20f | 20 | 1 | 5f |
| Males (16) | 9/7 | 6/10 | 9/7 | 9/7 | 4f | 9a | 5 | 5f | 9 | 0 | 10f |
Notes: POM results for PD and chronic pain patients. Data from Skogar et al.44
Lessor more than 5 years.
Maximal duration (less or more than 10 h) of pain in terms of days 1–5 of the period of pain measurement.
VAS maximal pain (number of patients reporting less or more than 5 cm) in terms of days 1–5 of the period of pain measurement.
Yes/No.
One missing data.
Statistically significant differences between sex, P≤0.05.
Abbreviations: PD, Parkinson’s disease; h, hours; VAS, visual analog scale; RLS, restless legs syndrome; POM, Pain-O-Meter.
King’s PD Pain Scale
To date, there is no specific validated scale that is widely used in the area of PD-related pain (PD pain). Therefore, it is important to describe the context of a study in this field. The King’s PD Pain Scale was very recently presented.29 The scale is easy to administer, requiring the investigator to ask the patient 14 questions and to score both severity and frequency of PD pain. The time required for the caregiver and patient is estimated to be ~10–15 minutes. Data from seven domains provide information on different types of PD pain, broadly classified as nociceptive and neuropathic patterns. Specifically, the scale captures pain ranging from the time-related pain correlated to the disappearance of antiparkinsonian drug effect, ie, wearing off-related pain to central, orofacial, and radicular pain.
Clinical diagnosis of PD-related pain
Classifications
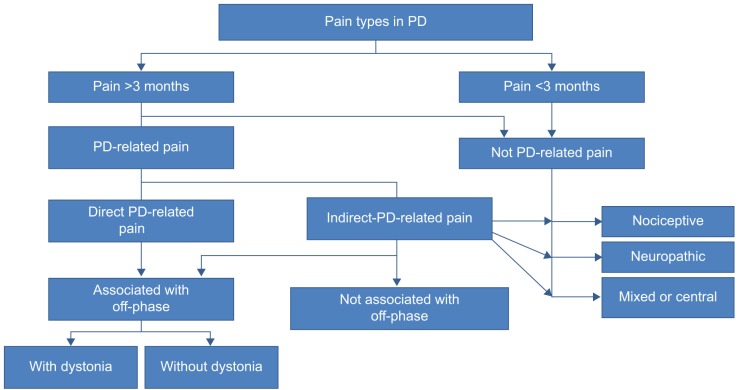
Traditionally, pain in PD is classified into five domains: musculoskeletal, radicular/neuropathic, dystonia-related, akathitic, and central pain.30 The most common pain syndromes are musculoskeletal and dystonic. Central PD pain is less common, but important to recognize; it can be intermittent or persistent in nature and is often described by patients as diffuse aching, burning, or cramping. It is not due to any lesion in the peripheral nervous system. Different parts of the body can be affected and is often combined with autonomic symptoms. No uncommon pain descriptions are from facial, abdominal, or even genital locations. However, the definition of central pain is not specific and the term is not seldom mistaken for central neuropathic pain that has another definition.31 Central mechanisms are almost always involved in the pain syndromes of PD, but this is not equal to fulfilling the criteria for central neuropathic pain (Figure 3).32
Figure 3.
A simplified scheme of pain evaluation and origin in PD.
Abbreviation: PD, Parkinson’s disease.
Clinical decisions
When PD patients express pain symptoms, the clinician should first determine whether the pain is PD related. Figure 2 can be used to assist in this determination. The associations between pain sensations and on/off symptomatology are crucial for determining pain origin and can support the use of dopamine substitution for pain relief. Mood evaluation of the patient is also crucial, because depression and anxiety require specific treatment options. However, the relationship between depression, chronic pain, and PD remains unclear.33–35 Depression may be secondary to PD pain or vice versa, or the two conditions may simply coexist. Neurotransmitter research suggests that the symptoms of disease, pain, and depression may have similar physiological substrates in PD patients (Figure 4).36
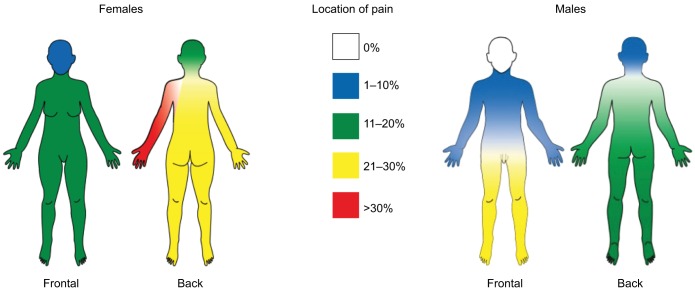
Figure 4.
Visualization of chronic pain localization for males and females.
Note: Adapted from Skogar O, Fall PA, Hallgren G, et al. Parkinson’s disease patients’ subjective descriptions of characteristics of chronic pain, sleeping patterns and health-related quality of life. Neuropsychiatr Dis Treat. 2012;8:435–442. © 2012 Skogar et al, publisher and licensee Dove Medical Press Ltd.44
Location-specific pain in PD
Abdominal pain
Abdominal pain and other types of gastrointestinal discomfort, such as dysphagia, are common in PD. Therefore, it is crucial to distinguish abdominal discomfort linked to fluctuations in DA load with other forms of abdominal pain, such as gastritis and esophageal reflux. The time correlations with DA intake and on–off symptoms may provide guidance.37
Back pain
One study has reported on the prevalence of back pain in PD patients, ranging up to74%, which is significantly higher than that in the general population.38
Shoulder pain
Shoulder pain has been observed in 11%, 43%, or 80% of PD patients in different studies and is easily mistaken for an orthopedic condition. Therefore, the clinician should be aware that this can be an early symptom of PD-related pain.39
Fluctuations of pain experiences in PD
Patterns of NMS fluctuations are heterogeneous and complex. Psychic NMS seem to fluctuate more frequently and severely than nonpsychic symptoms. A recent study of ten frequent NMS in advanced PD (100 participants) using VAS rating scales in motor-defined on- and off-states, as well as self-ratings at home, confirmed previous suspicions that increased pain in off-states and pain fluctuations correlate with a low health-related quality of life.40 Pain as NMS was more frequent in the off-state; more precisely, it was three to four times more common during the off-state than during the on-state.
Mental status and social networks
As previously mentioned, depression and pain are significant clinical problems that are comorbid with PD. Marital status, as well as other social networks, interferes with these conditions.41 The patient–spouse relationship, which indicates physical and emotional support, may have a mitigating effect on patient outcomes of depression prevalence and pain interference. Single PD patients seem to have greater pain interference scores than cohabiting PD patients.42
Treatment strategies
Pharmacological therapies
DA drugs
PD patients have a lack of DA tonus requiring treatment with DA drugs, such as levodopa and dopamine agonists. The efficacy of DA drugs has been determined in other NMS disorders, such as sleep and mood disorders; the DA drugs have the potential to reduce concurrent pain as well as direct antidystonic symptoms due to the existing dopamine deficit. Dystonia-related musculoskeletal pain often responds well to anti-PD medication.43
Paracetamol and nonsteroidal anti-inflammatory drugs, anxiolytics, and antidepressants
Polypharmacy is common in patients with PD pain. In a previous study, one-quarter of all participants were prescribed analgesics, with paracetamol the most common. However, only one-third reported pain relief with analgesics. Almost all (nine out of ten) used medication for anxiety/insomnia and one out of five used antidepressants.44
Opioid receptor antagonists
Pain is often the therapeutic target, but is seldom properly managed. The effects of the semisynthetic opioid receptor antagonist oxycodone were studied in 210 patients with various stages of PD.45 The participants included in the study presented with subjectively experienced severe pain and were followed for 16 weeks. The participants were randomly assigned (1:1) oral prolonged-release oxycodone and naloxone (5 mg and 2.5 mg, respectively) or placebo. Interestingly, the primary end point of mean 24-hour pain score at 16 weeks was not significantly different between the groups, which strengthens the theories of the unique characteristics of PD pain. Significant improvements in pain experience with the opioid receptor antagonist regimen were identified for only subgroups with severe musculoskeletal pain and severe nocturnal types of PD pain. Translated into everyday treatment efforts for PD pain patients, clinical improvements might only be achieved if the pain(s) type(s) is properly identified, characterized, and followed up.
Botulinum toxin and other pharmacotherapeutics with spasmolytic properties
One study has been performed using botulinum toxin for the treatment of dystonia.46 Of 30 patients treated for lower limb dystonia, pain was reported to disappear for 4 months in approximately two-thirds of the patients. The injection sites were in several lower limb muscles with a total median dose of 70 IU of botulinum toxin for each patient.
No published controlled studies seem to have been performed with baclofen or other spasmolytics.
Invasive therapies
The treatment of therapy-resistant PD pain with invasive therapies is rare, but knowledge of the effects on pain modulation reveals the complexity of PD pain origin.
Deep brain stimulation of the subthalamic nucleus and effects on pain
Deep brain stimulation of the subthalamic nucleus is commonly used to treat advanced phases of PD. However, pain modulation closely correlates with the inhibition of the pain experience. In an 8-year follow-up study on the effects of pain reduction in STN -treated PD patients, the results were stable over several years; 20 out of 24 patients with preoperative chronic pain reported pain only in the off-state condition. More than 80% of patients still experienced off-state pain 8 years after surgery, although the intensity and spread of pain registered at baseline, showing improvement.47 The number of body parts experiencing pain was reduced by ~40%, and the mean and median pain scores were improved. Even better results were observed in a subgroup of 12 patients with fluctuations in pain parallel to fluctuations in MS. Some of these patients reported an ongoing complete disappearance of pain in the on-state 8 years after surgery. However, the experience of pain complexes and other/new forms of pain evolved in most of these patients over the years studied, which should be considered.
Spinal cord stimulation
Implantation of electrodes close to the spinal cord has been described in a few cases (three) of PD patients with intractable pain. Because the pain in these patients was of non-parkinsonian origin, it is doubtful that these results will contribute to advances in invasive PD-related pain treatment.48
Complementary therapies
Complementary medicine (CAM) should not be mistaken for alternative medicine, where traditionally accepted treatments are replaced by a wide range of health care practices, products, and therapies, ranging from the biologically plausible, but not well tested, to directly contradicted by evidence and science or even harmful or toxic. CAMs are widely used; in Anglo-Saxon countries, four out of ten adults have used some type of CAM.49 In a telephone survey in 2000 with complete responses from 1,000 participants in Stockholm County, 57% had used massage therapy (MT).50 Adult women with high education levels are most represented in this consumption pattern.
Massage therapies
MT is one of the oldest forms of treatment; it was first described in the People’s Republic of China during the second century BC and soon after in India and Egypt. The American Massage Therapy Association defines massage as “manual soft tissue manipulation that includes holding, causing movement and/or applying pressure to the body. The practitioner applies manual techniques, and may apply adjunctive therapies with the intention of positively affecting the health and well-being of the client”.51
Music therapies
Music is a universal art form that exists in every culture in the world. Music may also be a means by which individuals can cope with emotional conflicts and increase self-awareness. Music has been shown to induce changes in heart and respiratory rates. In recent decades, studies have focused on the neural pathways involved in emotional and physical effects of music stimulation. Listening to music reduces pain intensity levels and opioid requirements, although the magnitude of these benefits is small and the clinical importance is unclear. In studies evaluating mean pain intensity, there are considerable variations in the effect of music, indicating statistical heterogeneity. Inconsistent results are also shown in the importance of personally independent choice of type of music in therapy situations.52,53 Liljeström et al found differences in the outcome measures, whereas another study measuring the effects on acute pain intensity up to 24 hours after surgery suggested no such differences. Independent of music selection, pain intensity was reduced and opioid requirements were decreased.54
Soft, slow music has, however, been shown to be effective in reducing depression and improving sleep quality, which is important for understanding the connections between depression, sleep patterns, and experiences of pain.55
Postulated mechanisms
Massage therapies
Interpersonal touch in the form of tissue manipulation has been postulated to trigger certain physiological responses, and much of the MT research has focused on measurable physiological parameters obtained in “positive directions” by MT. For short-term effects, the gate control theory is most often referred to.56 MT may also provide a shift in the autonomic nervous system from a state of sympathetic response to a parasympathetic state of response. A body faced with threat or challenge is associated with an increase in stress hormones, increased cardiovascular activity, and feelings of tension. The pressure applied during MT may stimulate vagal activity, suggesting that MT may promote reduced anxiety, depression, and pain, consistent with a state of calmness.57 Increased levels of serotonin have also been shown in some MT studies,58 and others have observed changes in endorphin release into the bloodstream following MT.59
Music therapies
Neuroimaging studies have shown the activation of specific pathways in several brain areas associated with emotional behaviors, such as the insular and cingulate cortex, hypothalamus, hippocampus, amygdala, and prefrontal cortex.60 Dopamine plays an important role as a biochemical mediator in the perceptual and emotional processing of music and is released from the ventral striatum and ventral tegmental areas in subjects listening to pleasant music.61,62
Effects of MT on endogenous cortisol concentrations
One of the first attempts to comprehensively review the effects of MT on human recipients of all ages was published by Field63 in 1998, and different theories were hypothesized to explain the effects. The study also included the potential effects of MT to facilitate growth in newborns, reduce pain, increase alertness, reduce depression, and enhance immune function. The decreasing effects of MT on human adrenocorticotropic hormone and cortisol concentrations are consistent across the range of studies reviewed and are strongly asserted as a precursor to the beneficial effects of MT.64
Although the acute effects of MT on cortisol concentration are prominent, they are not maintained in long-term follow-up studies.46,65 These results suggest that cortisol is not the direct mediator of the well-established and beneficial effects of MT on anxiety, depression, and pain, but rather a surrogate marker for stress reduction.
Clinical benefits of MT
Among the most prominent benefits of MT pain treatment is the lack of risk or harm to the patient. The side effects are rare, but occasionally involve tiredness, hypotension, and dizziness after the sessions. Alone or in combination with music therapy, MT has shown positive effects on the strength of the pain experience.66
Conclusion
The treatment strategies for PD pain require a deep knowledge of the mechanisms responsible for pain experiences in individual patients. Changes in the central pathways involved in sensory processing reduce pain thresholds in PD.66 Studies have confirmed the existence of pathways other than those secondary to rigidity, tremor, or any other motor manifestations of the disease.10 The BG has been shown to process somatosensory information through different methods, and recent pain inhibition studies have shown the presence of CT fibers with projections to the insular cortex.10,11,13,14 Activation of these fibers plays a role in pain inhibition and correlates to the effects observed with different forms of MT. Chronic PD-related pain is closely associated with stress, as well as other forms of chronic and/or acute pain. A decreased cortisol concentration in saliva/plasma is an example of biomarkers for stress reduction.
The first action that the clinician should take is to exclude other possible sources for the pain experiences than PD. The dynamics over time and the correlation with on/off symptomatology and levodopa/DA agonist therapy should be evaluated, followed by the establishment of a program to follow up on the pain experiences. These should be measured in terms of strength, as well as by some form of the VAS and in terms of verbal description, which can be performed with the POM. CAMs are frequently used in this population, although this is in addition to the regular medical care and its cost.
Patient self-reported changes with regard to pain experiences should be considered when choosing future treatment strategies. The effects on pain relief in PD by analgesics and NSAIDs are not absent, but the limited effects have to be taken into account by understanding the partially different pain mechanisms due to the neurodegenerative disorder.38
There are several indications that physical therapy might be effective in PD when studying broad outcome measures not only for treatment of pain. Concerning allieviation of chronic PD-related pain, there is a need for further controlled studies. Subgroups of PD patients must be defined more thoroughly. Who benefits most of tactile touch or MT strategies, which type of standardized methods are the best, the relevant and optimal doses, and the duration and intervals of sessions all have to be evaluated properly.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Parkinson J. An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci. 2002;14(2):223–236. doi: 10.1176/jnp.14.2.223. discussion 222. [DOI] [PubMed] [Google Scholar]
- 2.von Campenhausen S, Bornschein B, Wick R, et al. Prevalence and incidence of Parkinson’s disease in Europe. Eur Neuropsychopharmacol. 2005;15(4):473–490. doi: 10.1016/j.euroneuro.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Parkinson’s Disease Foundation [webpage on the internet] Parkinson’s Disease Foundation, Inc. updated ©2016. [Accessed January 2016]. Available from: http://www.pdf.org/en/parkinson_statistics.
- 4.Brooks DJ. Parkinson’s disease: diagnosis. Parkinsonism Relat Disord. 2012;18(suppl 1):S31–S33. doi: 10.1016/S1353-8020(11)70012-8. [DOI] [PubMed] [Google Scholar]
- 5.Negre-Pages L, Regragui W, Rascol O, DoPaMiP Study Group Chronic pain in Parkinson’s disease: the cross-sectional French DoPaMiP survey. Mov Disord. 2008;23(10):1361–1369. doi: 10.1002/mds.22142. [DOI] [PubMed] [Google Scholar]
- 6.Borg A, Borgman A. “Parkitouch”-studien, Parkinsons sjukdom och effekten av beröringsmassage [The Parkitouch Study: The effects of tactile touch in Parkinson’s disease] The Swedish Parkinson Foundation; 2009. [Accessed July 19, 2016]. Available from: http://www.parkinsonstiftelsen.se/parkitouch.html. [Google Scholar]
- 7.Bekkering GE, Bala MM, Reid K, et al. Epidemiology of chronic pain and its treatment in The Netherlands. Neth J Med. 2011;69(3):141–153. [PubMed] [Google Scholar]
- 8.Reid KJ, Harker J, Bala MM, et al. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin. 2011;27(2):449–462. doi: 10.1185/03007995.2010.545813. [DOI] [PubMed] [Google Scholar]
- 9.Politis M, Wu K, Molloy S, G Bain P, Chaudhuri KR, Piccini P. Parkinson’s disease symptoms: the patient’s perspective. Mov Disord. 2010;25(11):1646–1651. doi: 10.1002/mds.23135. [DOI] [PubMed] [Google Scholar]
- 10.Schestatsky P, Kumru H, Valls-Solé J, et al. Neurophysiologic study of central pain in patients with Parkinson disease. Neurology. 2007;69(23):2162–2169. doi: 10.1212/01.wnl.0000295669.12443.d3. [DOI] [PubMed] [Google Scholar]
- 11.Mylius V, Engau I, Teepker M, et al. Pain sensitivity and descending inhibition of pain in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2009;80(1):24–28. doi: 10.1136/jnnp.2008.145995. [DOI] [PubMed] [Google Scholar]
- 12.Tison F, Wenning GK, Volonte MA, Poewe WR, Henry P, Quinn NP. Pain in multiple system atrophy. J Neurol. 1996;243(2):153–156. doi: 10.1007/BF02444007. [DOI] [PubMed] [Google Scholar]
- 13.Chudler EH, Dong WK. The role of the basal ganglia in nociception and pain. Pain. 1995;60(1):3–38. doi: 10.1016/0304-3959(94)00172-B. [DOI] [PubMed] [Google Scholar]
- 14.Markus Z, Eordegh G, Paroczy Z, Benedek G, Nagy A. Modality distribution of sensory neurons in the feline caudate nucleus and the substantia nigra. Acta Biol Hung. 2008;59(3):269–279. doi: 10.1556/ABiol.59.2008.3.1. [DOI] [PubMed] [Google Scholar]
- 15.Baev KV. Disturbances of learning processes in the basal ganglia in the pathogenesis of Parkinson’s disease: a novel theory. Neurol Res. 1995;17(1):38–48. doi: 10.1080/01616412.1995.11740285. [DOI] [PubMed] [Google Scholar]
- 16.Berger K, Przedborski S, Cadet JL. Retrograde degeneration of nigrostriatal neurons induced by intrastriatal 6-hydroxydopamine injection in rats. Brain Res Bull. 1991;26(2):301–307. doi: 10.1016/0361-9230(91)90242-c. [DOI] [PubMed] [Google Scholar]
- 17.Le Moal M, Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev. 1991;71(1):155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- 18.Simon H, Le Moal M, Stinus L, Calas A. Anatomical relationships between the ventral mesencephalic tegmentum – a 10 region and the locus coeruleus as demonstrated by anterograde and retrograde tracing techniques. J Neural Transm. 1979;44(1–2):77–86. doi: 10.1007/BF01252703. [DOI] [PubMed] [Google Scholar]
- 19.Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47(6):787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Porro CA, Cavazzuti M, Baraldi P, Giuliani D, Panerai AE, Corazza R. CNS pattern of metabolic activity during tonic pain: evidence for modulation by beta-endorphin. Eur J Neurosci. 1999;11(3):874–888. doi: 10.1046/j.1460-9568.1999.00494.x. [DOI] [PubMed] [Google Scholar]
- 21.Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rub U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages) J Neurol. 2002;249(suppl 3):III, 1–5. doi: 10.1007/s00415-002-1301-4. [DOI] [PubMed] [Google Scholar]
- 22.Gold A, Turkalp ZT, Munoz DG. Enteric alpha-synuclein expression is increased in Parkinson’s disease but not Alzheimer’s disease. Mov Disord. 2013;28(2):237–240. doi: 10.1002/mds.25298. [DOI] [PubMed] [Google Scholar]
- 23.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 24.Vallbo AB, Olausson H, Wessberg J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. J Neurophysiol. 1999;81(6):2753–2763. doi: 10.1152/jn.1999.81.6.2753. [DOI] [PubMed] [Google Scholar]
- 25.Skogar Ö, Borg A, Larsson B, et al. “Effects of tactile touch on pain, sleep and health related quality of life in Parkinson’s disease with chronic pain”: a randomized, controlled and prospective study. Eur J Integr Med. 2013;5(2):141–152. [Google Scholar]
- 26.Huskinson E. Visual analogue scale. In: Melzack R, editor. Measurement and Assessment. New York, NY: Raven Press; 1983. [Google Scholar]
- 27.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 28.Gaston-Johansson F. Measurement of pain: the psychometric properties of the Pain-O-Meter, a simple, inexpensive pain assessment tool that could change health care practices. J Pain Symptom Manage. 1996;12(3):172–181. doi: 10.1016/0885-3924(96)00128-5. [DOI] [PubMed] [Google Scholar]
- 29.Chaudhuri KR, Rizos A, Trenkwalder C, et al. King’s Parkinson’s disease pain scale, the first scale for pain in PD: an international validation. Mov Disord. 2015;30(12):1623–1631. doi: 10.1002/mds.26270. [DOI] [PubMed] [Google Scholar]
- 30.Ford B. Pain in Parkinson’s disease. Clin Neurosci. 1998;5(2):63–72. [PubMed] [Google Scholar]
- 31.Jensen TS, Baron R, Haanpaa M, et al. A new definition of neuropathic pain. Pain. 2011;152(10):2204–2205. doi: 10.1016/j.pain.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 32.Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 33.Snider SR, Fahn S, Cote LJ, Isgreen WP. Letter: pain, paresthesia and parkinsonism. N Engl J Med. 1975;293(4):200. doi: 10.1056/nejm197507242930418. [DOI] [PubMed] [Google Scholar]
- 34.Stein WM, Read S. Chronic pain in the setting of Parkinson’s disease and depression. J Pain Symptom Manage. 1997;14(4):255–258. doi: 10.1016/s0885-3924(97)00176-0. [DOI] [PubMed] [Google Scholar]
- 35.Shang AB, King SA. Parkinson’s disease, depression, and chronic pain. Hosp Community Psychiatry. 1991;42(11):1162–1163. doi: 10.1176/ps.42.11.1162. [DOI] [PubMed] [Google Scholar]
- 36.King SA. Pain in depression and Parkinson’s disease. Am J Psychiatry. 1993;150(2):353–354. doi: 10.1176/ajp.150.2.353. [DOI] [PubMed] [Google Scholar]
- 37.Rana AQ, Depradine J. Abdominal pain: a symptom of levodopa end of dose wearing off in Parkinson’s disease. West Indian Med J. 2011;60(2):223–224. [PubMed] [Google Scholar]
- 38.Broetz D, Eichner M, Gasser T, Weller M, Steinbach JP. Radicular and nonradicular back pain in Parkinson’s disease: a controlled study. Mov Disord. 2007;22(6):853–856. doi: 10.1002/mds.21439. [DOI] [PubMed] [Google Scholar]
- 39.Kim YE, Jeon BS. Musculoskeletal problems in Parkinson’s disease. J Neural Transm (Vienna) 2013;120(4):537–542. doi: 10.1007/s00702-012-0960-2. [DOI] [PubMed] [Google Scholar]
- 40.Storch A, Schneider CB, Wolz M, et al. Nonmotor fluctuations in Parkinson disease: severity and correlation with motor complications. Neurology. 2013;80(9):800–809. doi: 10.1212/WNL.0b013e318285c0ed. [DOI] [PubMed] [Google Scholar]
- 41.Rana AQ, Qureshi AR, Mumtaz A, et al. Associations of pain and depression with marital status in patients diagnosed with Parkinson’s disease. Acta Neurol Scand. 2016;133(4):276–280. doi: 10.1111/ane.12454. [DOI] [PubMed] [Google Scholar]
- 42.Rana AQ, Qureshi AR, Rahman L, Jesudasan A, Hafez KK, Rana MA. Association of restless legs syndrome, pain, and mood disorders in Parkinson’s disease. Int J Neurosci. 2016;126(2):116–120. doi: 10.3109/00207454.2014.994208. [DOI] [PubMed] [Google Scholar]
- 43.Ha AD, Jankovic J. Pain in Parkinson’s disease. Mov Disord. 2012;27(4):485–491. doi: 10.1002/mds.23959. [DOI] [PubMed] [Google Scholar]
- 44.Skogar O, Fall PA, Hallgren G, et al. Parkinson’s disease patients’ subjective descriptions of characteristics of chronic pain, sleeping patterns and health-related quality of life. Neuropsychiatr Dis Treat. 2012;8:435–442. doi: 10.2147/NDT.S34882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trenkwalder C, Chaudhuri KR, Martinez-Martin P, et al. Prolonged-release oxycodone-naloxone for treatment of severe pain in patients with Parkinson’s disease (PANDA): a double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2015;14(12):1161–1170. doi: 10.1016/S1474-4422(15)00243-4. [DOI] [PubMed] [Google Scholar]
- 46.Tornhage CJ, Skogar O, Borg A, et al. Short- and long-term effects of tactile massage on salivary cortisol concentrations in Parkinson’s disease: a randomised controlled pilot study. BMC Complement Altern Med. 2013;13:357. doi: 10.1186/1472-6882-13-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung YJ, Kim HJ, Jeon BS, Park H, Lee WW, Paek SH. An 8-year follow-up on the effect of subthalamic nucleus deep brain stimulation on pain in Parkinson Disease. JAMA Neurol. 2015;72(5):504–510. doi: 10.1001/jamaneurol.2015.8. [DOI] [PubMed] [Google Scholar]
- 48.Nishioka K, Nakajima M. Beneficial therapeutic effects of spinal cord stimulation in advanced cases of Parkinson’s Disease with intractable chronic pain: a case series. Neuromodulation. 2015;18(8):751–753. doi: 10.1111/ner.12315. [DOI] [PubMed] [Google Scholar]
- 49.Hunt KJ, Coelho HF, Wider B, et al. Complementary and alternative medicine use in England: results from a national survey. Int J Clin Pract. 2010;64(11):1496–1502. doi: 10.1111/j.1742-1241.2010.02484.x. [DOI] [PubMed] [Google Scholar]
- 50.Hanssen B, Grimsgaard S, Launso L, Fonnebo V, Falkenberg T, Rasmussen NK. Use of complementary and alternative medicine in the Scandinavian countries. Scand J Prim Health Care. 2005;23(1):57–62. doi: 10.1080/02813430510018419. [DOI] [PubMed] [Google Scholar]
- 51.American Massage Therapy Association . AMTA Definition of Massage Therapy. Evanston, IL: AMTA; 2002. Retrieved August 27, 1999. [Google Scholar]
- 52.Tan YZ, Ozdemir S, Temiz A, Celik F. The effect of relaxing music on heart rate and heart rate variability during ECG GATED-myocardial perfusion scintigraphy. Complement Ther Clin Pract. 2015;21(2):137–140. doi: 10.1016/j.ctcp.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 53.Pacchetti C, Mancini F, Aglieri R, Fundaro C, Martignoni E, Nappi G. Active music therapy in Parkinson’s disease: an integrative method for motor and emotional rehabilitation. Psychosom Med. 2000;62(3):386–393. doi: 10.1097/00006842-200005000-00012. [DOI] [PubMed] [Google Scholar]
- 54.Liljeström S, Juslin PN, Västfjäll D. Experimental evidence of the roles of music choice, social context, and listener personality in emotional reactions to music. Psychol Music. 2012;41:579–599. [Google Scholar]
- 55.Cepeda MS, Carr DB, Lau J, Alvarez H. Music for pain relief. Cochrane Database Syst Rev. 2006;(2):CD004843. doi: 10.1002/14651858.CD004843.pub2. [DOI] [PubMed] [Google Scholar]
- 56.Chan MF, Chan EA, Mok E. Effects of music on depression and sleep quality in elderly people: a randomised controlled trial. Complement Ther Med. 2010;18(3–4):150–159. doi: 10.1016/j.ctim.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 57.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 58.Field T, Diego M. Vagal activity, early growth and emotional development. Infant Behav Dev. 2008;31(3):361–373. doi: 10.1016/j.infbeh.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Field T, Hernandez-Reif M, Diego M, Schanberg S, Kuhn C. Cortisol decreases and serotonin and dopamine increase following massage therapy. Int J Neurosci. 2005;115(10):1397–1413. doi: 10.1080/00207450590956459. [DOI] [PubMed] [Google Scholar]
- 60.Morhenn V, Beavin LE, Zak PJ. Massage increases oxytocin and reduces adrenocorticotropin hormone in humans. Altern Ther Health Med. 2012;18(6):11–18. [PubMed] [Google Scholar]
- 61.Boso M, Politi P, Barale F, Enzo E. Neurophysiology and neurobiology of the musical experience. Funct Neurol. 2006;21(4):187–191. [PubMed] [Google Scholar]
- 62.Menon V, Levitin DJ. The rewards of music listening: response and physiological connectivity of the mesolimbic system. Neuroimage. 2005;28(1):175–184. doi: 10.1016/j.neuroimage.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 63.Field TM. Massage therapy effects. Am Psychol. 1998;53(12):1270–1281. doi: 10.1037//0003-066x.53.12.1270. [DOI] [PubMed] [Google Scholar]
- 64.Morhenn V, Beavin LE, Zak PJ. Massage increases oxytocin and reduces adrenocorticotropin hormone in humans. Altern Ther Health Med. 2012;18(6):11–18. [PubMed] [Google Scholar]
- 65.Zambito Marsala S, Tinazzi M, Vitaliani R, et al. Spontaneous pain, pain threshold, and pain tolerance in Parkinson’s disease. J Neurol. 2011;258(4):627–633. doi: 10.1007/s00415-010-5812-0. [DOI] [PubMed] [Google Scholar]
- 66.Pacchetti C, Albani G, Martignoni E, Godi L, Alfonsi E, Nappi G. “Off ” painful dystonia in Parkinson’s disease treated with botulinum toxin. Mov Disord. 1995;10(3):333–336. doi: 10.1002/mds.870100317. [DOI] [PubMed] [Google Scholar]
- 67.Juri C, Rodriguez-Oroz M, Obeso JA. The pathophysiological basis of sensory disturbances in Parkinson’s disease. J Neurol Sci. 2010;289(1–2):60–65. doi: 10.1016/j.jns.2009.08.018. [DOI] [PubMed] [Google Scholar]