Dear editor
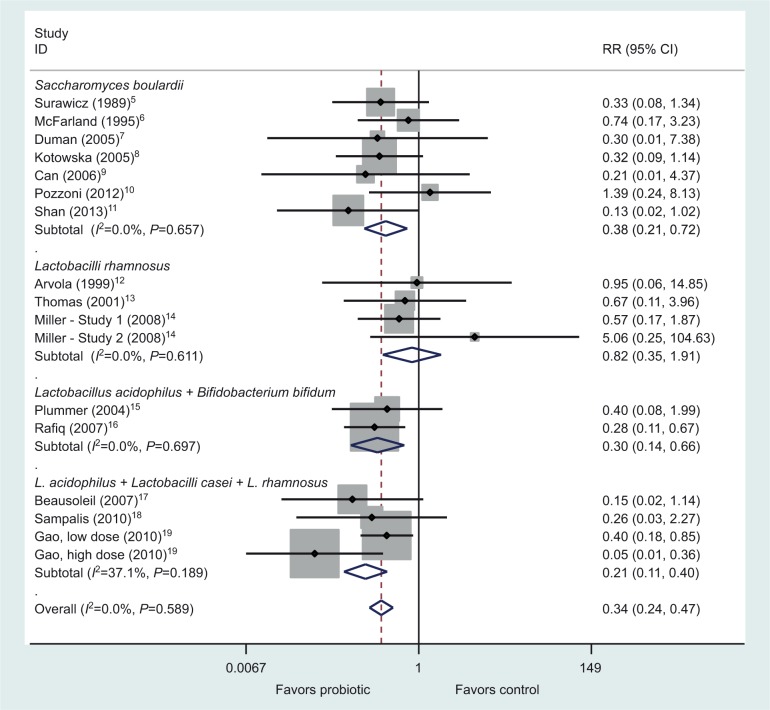
I read with great interest the systematic review of meta-analysis assessing probiotics for the prevention of Clostridium difficile-associated diarrhea (CDAD) published in the International Journal of General Medicine.1 These authors pooled 26 randomized controlled trials (RCTs) and concluded that Lactobacilli, mixtures, and Saccharomyces probiotics were effective in preventing CDAD. However, the meta-analysis by Lau and Chamberlain is flawed due to improper classification by the types of probiotics. It is important to recognize that the efficacy of probiotics for various diseases has been shown to be strain specific for each probiotic product, and thus the data should only be pooled for probiotics that are of the identical type.2,3 In their analysis of probiotic subgroups by various species, the authors have inappropriately merged different types of Lactobacilli into one subgroup “Lactobacilli” and different types of mixtures into one group classified as “Mix”. The Lactobacilli subgroup actually contains RCTs using six different types of Lactobacilli: Lactobacilli rhamnosus GG (three RCTs), one mixture of three Lactobacilli strains (Lactobacilli acidophilus CL1285, Lactobacilli casei LBC80R, and L. rhamnosus CLR2) (three RCTs), L. acidophilus (one RCT), one mixture of three strains of L. rhamnosus (E/n, Oxy, and Pen) (one RCT), L. casei Shirota (one RCT), and Lactobacilli plantarum 299v (one RCT). The Mix subgroup contains RCTs of five different types of strains, and only two trials evaluated the same type of probiotic mixture (L. acidophilus and Bifidobacterium bifidum). Only the Saccharomyces subgroup correctly pooled seven RCTs using the same strain of probiotic (Saccharomyces boulardii CNCM I-745). When these subgroups are pooled appropriately, as shown in Figure 1, only three types of probiotics have efficacy for the primary prevention of CDAD: S. boulardii (P=0.003) and two mixtures (BioK+, a mixture of three Lactobacilli strains4 [P<0.001] and the mixture of L. acidophilus with B. bifidum [P=0.002]). L. rhamnosus GG did not significantly prevent CDAD (P=0.65), a finding that is not apparent in the meta-analysis presentation of Lau and Chamberlain. No other probiotic strains had a second confirmatory RCT, and hence it is inappropriate to pool these different strains together. Only by appropriately combining and pooling data using the same strain or strains of probiotics can practical clinical guidance be determined as to which specific probiotic strains can be used to prevent CDAD.
Figure 1.
Forest plot of 15 randomized controlled trials by four probiotic subgroups for the primary prevention of Clostridium difficile disease. Adapted from Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016;9:27–37.1
Abbreviations: RR, relative risk; CI, confidence interval.
Footnotes
Disclosure
The author is on the Scientific Advisory Board of Biok+ and a paid lecturer of Biocodex. The author reports no other conflicts of interest in this communication.
References
- 1.Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016;9:27–37. doi: 10.2147/IJGM.S98280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill C, Guarner F, Reid G, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 3.McFarland LV. Importance of subgroup analysis in probiotic meta-analyses. Letter to the Editor. Br J Nutr. 2016;116:375–376. doi: 10.1017/S0007114516002026. [DOI] [PubMed] [Google Scholar]
- 4.Auclair J, Frappier M, Millette M. Lactobacillus acidophilus CL1285, L. casei LBC80R and L. rhamnosus CLR2 (Bio-K+): characterization, manufacture, mechanisms of action and quality control of a specific probiotic combination for primary prevention of Clostridium difficile infections. CID. 2015;60(suppl 2):S135–S144. doi: 10.1093/cid/civ179. [DOI] [PubMed] [Google Scholar]
- 5.Surawicz CM, Elmer GW, Speelman P, et al. Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: a prospective study. Gastroenterology. 1989;96:981–988. doi: 10.1016/0016-5085(89)91613-2. [DOI] [PubMed] [Google Scholar]
- 6.McFarland LV, Surawicz CM, Greenberg RN, et al. Prevention of -lactam-associated diarrhea by Saccharomyces boulardii compared to placebo. Am J Gastroenterol. 1995;90:439–448. [PubMed] [Google Scholar]
- 7.Duman DG, Bor S, Ozütemiz O, et al. Efficacy and safety of Saccharomyces boulardii in prevention of antibiotic-associated diarrhoea due to Helicobacter pylori eradication. Eur J Gastroenterol Hepatol. 2005;17(12):1357–1361. doi: 10.1097/00042737-200512000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Kotowska M, Albrecht P, Szajewska H. Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea in children: a randomized double-blind placebo-controlled trial. Alimentary Pharmacology and Therapeutics. 2005;21(5):583–590. doi: 10.1111/j.1365-2036.2005.02356.x. [DOI] [PubMed] [Google Scholar]
- 9.Can M, Beirbellioglu BA, Avci IY, Beker CM, Pahsa A. Prophylactic Saccharomyces boulardii in the prevention of antibiotic-associated diarrhea: a prospective study. Med Sci Monit. 2006;12(4):119–122. [PubMed] [Google Scholar]
- 10.Pozzoni P, Riva A, Bellatorre AG, et al. Saccharomyces boulardii for the prevention of antibiotic-associated diarrhea in adult hospitalized patients: a single-center, randomized, double-blind, placebo-controlled trial. Am J Gastroenterol. 2012;107(6):922–931. doi: 10.1038/ajg.2012.56. [DOI] [PubMed] [Google Scholar]
- 11.Shan L, Hou P, Wang Z, et al. Prevention and treatment of diarrhea with Saccharomyces boulardii in children with acute lower respiratory tract infections. Beneficial Microbes. 2013;4(4):329–334. doi: 10.3920/BM2013.0008. [DOI] [PubMed] [Google Scholar]
- 12.Arvola T, Laiho K, Torkkeli S, et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104(5):e64. doi: 10.1542/peds.104.5.e64. [DOI] [PubMed] [Google Scholar]
- 13.Thomas MR, Litin SC, Osmon DR, Corr AP, Weaver AL, Lohse CM. Lack of effect of Lactobacillus GG on antibiotic-associated diarrhea: a randomized, placebo-controlled trial. Mayo Clin Proc. 2001;76:883–889. doi: 10.4065/76.9.883. [DOI] [PubMed] [Google Scholar]
- 14.Miller M, Florencio S, Eastmond J, Reynolds S. Results of 2 prospective randomized studies of Lactobacillus GG to prevent C. difficile infection in hospitalized adults receiving antibiotics. Abstract of the Interscience Conference on Antimicrobial Agents and Chemotherapy. 2008;48:578–579. [Google Scholar]
- 15.Plummer S, Weaver MA, Harris JC, Dee P, Hunter J. Clostridium difficile pilot study: effects of probiotic supplementation on the incidence of C. difficile diarrhoea. Int Microbiol. 2004;7:59–62. [PubMed] [Google Scholar]
- 16.Rafiq R. Prevention of Clostridium difficile (C. difficile) diarrhea with probiotic in hospitalized patients treated with antibiotics. Gastroenterol. 2007;132(4):187. Abstract S1167. [Google Scholar]
- 17.Beausoleil M, Fortier N, Guénette S, et al. Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: a randomized, double-blind, placebo-controlled trial. Can J Gastroenterology. 2007;21(11):732–736. doi: 10.1155/2007/720205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sampalis J, Psaradellis E, Rampakakis E. Efficacy of BIO K+ CL1285 in the reduction of antibiotic-associated diarrhea – a placebo controlled double-blind randomized, multi-center study. Arch Med Sci. 2010;6(1):56–64. doi: 10.5114/aoms.2010.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao XW, Mubasher M, Fang CY, Reifer C, Miller LE. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105(7):1636–1641. doi: 10.1038/ajg.2010.11. [DOI] [PubMed] [Google Scholar]