Abstract
Cannabis has been widely used as a medicinal agent in Eastern medicine with earliest evidence in ancient Chinese practice dating back to 2700 BC. Over time, the use of medical cannabis has been increasingly adopted by Western medicine and is thus a rapidly emerging field that all pain physicians need to be aware of. Several randomized controlled trials have shown a significant and dose-dependent relationship between neuropathic pain relief and tetrahydrocannabinol – the principal psychoactive component of cannabis. Despite this, barriers exist to use from both the patient perspective (cost, addiction, social stigma, lack of understanding regarding safe administration) and the physician perspective (credibility, criminality, clinical evidence, patient addiction, and policy from the governing medical colleges). This review addresses these barriers and draws attention to key concerns in the Canadian medical system, providing updated treatment approaches to help clinicians work with their patients in achieving adequate pain control, reduced narcotic medication use, and enhanced quality of life. This review also includes case studies demonstrating the use of medical marijuana by patients with neuropathic low-back pain, neuropathic pain in fibromyalgia, and neuropathic pain in multiple sclerosis. While significant preclinical data have demonstrated the potential therapeutic benefits of cannabis for treating pain in osteoarthritis, rheumatoid arthritis, fibromyalgia, and cancer, further studies are needed with randomized controlled trials and larger study populations to identify the specific strains and concentrations that will work best with selected cohorts.
Keywords: randomized controlled trials, tetrahydrocannabinol, addiction, social stigma, fibromyalgia, neuropathic pain
Medical cannabis in history and society
Cannabis sativa (cannabis) has been used therapeutically for almost 5,000 years, beginning in traditional Eastern medicine.1 Some of the earliest evidence for this is found in the pen-ts’ao ching, the world’s first pharmacopeia, compiled based on ancient Chinese practices from as early as 2700 BC.1 It was not until 1841 that medical cannabis was introduced into Western medicine through the work of William O’Shaughnessy, an Irish physician, who encountered “Indian hemp” in Calcutta. By the late 19th century, medical cannabis became widely disseminated in the Americas; cannabis-based extracts, tinctures, cigarettes, and plasters produced by early prominent drug companies2,3 were indicated for a wide range of conditions, many of which were related to pain.1,4 Even Sir William Osler, the preeminent Canadian internist, wrote in The Principles and Practice of Medicine (1892) that “Cannabis Indica is probably the most satisfactory remedy for migraines”. However, the medical use of cannabis fell from favor in the 1930s and 1940s when fear escalated that recreational use of cannabis may be related to violence, crime, and other socially deviant behaviors. At that time, widespread prohibitive legislation banning the use of cannabis-based medicines occurred across the world.2 More recently, the medical use of cannabis has been reintroduced in a number of countries for the treatment of a variety of conditions, including pain.5,6
Indeed, support for medical cannabis appears to be on the rise. Overwhelmingly, patients prescribed medical cannabis for pain-related illnesses report being highly successful with pain reduction as well as with reducing their use of other medications. In a recent large survey of medical cannabis users in Arizona, 77% of fibromyalgia patients, 63% of patients with arthritis, and 51% of patients suffering from neuropathic pain reported experiencing “a lot or almost complete overall pain relief ”.7 Most patients with these conditions (94% of patients with fibromyalgia, 81% of arthritic patients, and 61% of patients with neuropathy) also found that they were able to lower their use of their other medications such as narcotic opioids.7 In fact, 75% of opioid-dependent medical cannabis users reported experiencing “a lot or almost complete overall relief ” from opioid dependency.7 Studies such as this shed light onto the wide range of clinical uses of medical cannabis, making it highly useful, since evidence from controlled clinical trials is still emerging.
Studies examining the characteristics of medical cannabis patients in the US have revealed that the majority medicate daily7–9 and consume 6–9 g of cannabis per week.8 In Canada, 42% of medical cannabis patients reported medicating two to three times per day, and 40% consume >14 g per week.10 In both Canada and the US, most patients choose inhalation as their preferred method of consumption.7,10
In addition to patients with access to prescribed medical cannabis, there is also a huge population of users who consume cannabis recreationally or for self-defined medical reasons. Cannabis is the most commonly used illicit drug in the world,11 with 7% of adults in the US reporting use within the last 30 days and 34% reporting having used in 2015.12 Interestingly, only 53% of adult cannabis users in the US consume cannabis exclusively for recreational purposes, while the other 47% of users consume cannabis “in part or entirely for medicinal purposes”, with 10% using solely for medicinal purposes.12 In Canada, ~4% of residents over the age of 14 reported at least one instance of past-year cannabis use to treat self-defined medical conditions in 2004.13,14
Cannabis and pain: mechanistic considerations
The cannabis plant contains many biologically active chemicals, including ~60 cannabinoids.15 The cannabinoids are a group of molecules that bind to cannabinoid receptors and include three varieties: phytocannabinoids, which are derived from cannabis plants; synthetic cannabinoids (such as nabilone [Cesamet] – a synthetic analog of Δ9-tetrahydrocannabinol [THC] with a high bioavailability [≥60%]16–19); and endogenous cannabinoid receptor ligands or endocannabinoids. THC is the primary psychoactive component found within cannabis, and has been shown to have analgesic effects.20 However, increasing evidence has highlighted numerous roles for other phytocannabinoids, particularly cannabidiol (CBD), a non-psychoactive component with anti-inflammatory,21 analgesic,22,23 and antipsychotic24,25 properties. THC and CBD (Figure 1) are biosynthesized as delta-9-tetrahydrocannabinolic acid and cannabidiolic acid, respectively, from a common precursor,26 and require decarboxylation by heat or extraction to produce THC and CBD properties.27 Other phytocannabinoids with potential therapeutic applications include cannabigerol, cannabichromene, cannabinol, cannabidivarin, and tetrahydrocannabivarin.28
Figure 1.
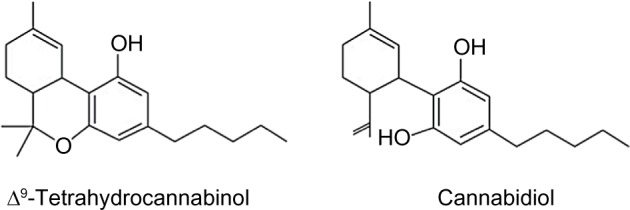
Diagram showing the chemical structure of Δ9-tetrahydrocannabinol and cannabidiol.
THC mimics the action of the endogenous cannabinoid receptor ligands anandamide and 2-arachidonylglycerol.29 Both THC and anandamide are partial agonists of CB1 receptors,27,29 which are primarily expressed in the central nervous system, especially in areas associated with pain, including the spinal trigeminal nucleus, amygdala, basal ganglia,30 and the periaqueductal gray.31,32 At the cellular level, centrally expressed CB1 receptors are localized on the terminals of presynaptic neurons.33 The endocannabinoids that bind these receptors act as retrograde signaling molecules; that is, they are synthesized postsynaptically and travel backward across the synapse to inhibit presynaptic neurotransmission.34 It is believed that, within regions associated with nociception, THC induces analgesia by binding presynaptic CB1 receptors, inhibiting neurons activated by pain in these areas.
CBD has intrinsic analgesic and anti-inflammatory properties of its own22,23,35 and antagonizes several adverse effects of THC, including sedation,27,36 tachycardia,27,37 and anxiety.38 CBD also ameliorates the psychoactive effects of THC,38 a concern for many medical cannabis patients. Unlike THC, CBD has low affinity for CB1 receptors39 and exerts analgesic actions by binding multiple proteins related to pain. For example, CBD has been shown to bind TRPV1 and mediate its desensitization39 and to inhibit inactivation of anandamide,39 both of which contribute to its analgesic actions. CBD also has potent anti-inflammatory properties,21 and may reduce pain by indirectly limiting inflammation at the site of injury.
Cannabis and pain: clinical evidence
Although significant preclinical data have highlighted the potential therapeutic benefits of smoked cannabis for pain relief in patients suffering from osteoarthritis, rheumatoid arthritis, fibromyalgia, and cancer, no randomized controlled trials (RCTs) have been carried out for these conditions.40 However, several RCTs have evaluated the analgesic efficacy of cannabis for patients with neuropathic pain.41–46 In a recent meta-analysis, the individual participant data from five of these studies were synthesized to determine the overall effect of inhaled cannabis on chronic neuropathic pain.47 All studies included in the meta-analysis compared the analgesic efficacy of cannabis with THC content ranging from 3.5% to 9.4% against that of placebo, and had periods of follow-up ranging from days to weeks.
The proportion of patients who participated in the five RCTs with a >30% improvement in chronic pain score following cannabis therapy was determined, and these patients were classified as “responders”, effectively creating a useful dichotomy for comparing response data between interventions. The meta-analysis concluded that inhaled cannabis results in a number needed to treat value to reduce chronic pain by >30% of 5.6. This needed to treat value rivals that of currently available therapeutics for chronic neuropathic pain,48 which is typically well above 8.47,49–51 The authors also found that the analgesia provided by cannabis was dose-dependent, with higher THC content producing more pronounced pain relief. This finding provides additional support for the notion that cannabis is an effective analgesic for chronic neuropathic pain.
Cannabis and cancer
Medical cannabis is also used for some cancer patients to relieve symptoms including nausea and vomiting (often caused by some cancer treatments such as chemotherapy and radiation therapy), loss of appetite, and pain. However, more research is required to identify strains and dose of medical cannabis that provide the optimal symptom relief with minimal side effects for this population.52
Pharmacokinetics
To date, most pharmacokinetic studies of cannabinoids have focused on the bioavailability of inhaled THC, which varies substantially in the literature, likely due to differences in factors such as breath-hold length, source of cannabis material, and method of inhalation.53,54 In general, 25%–27% of the available THC becomes available for the systemic circulation after smoking.55,56
The latency of effect onset for inhaled cannabis is shorter than that for cannabis consumed orally, requiring only minutes from the time of consumption to see observable changes, compared to hours when taken by the oral route.57–59 Furthermore, cannabis taken orally results in lower peak THC levels in the blood, but effects are observed for a longer period of time.53
Hepatic cytochrome p450 enzymes govern cannabinoid bioavailability. THC is metabolized primarily by CYP 2C9, 2C19, and 3A4,53 and drugs that inhibit these enzymes, including proton pump inhibitors, HIV protease inhibitors, macrolides, anti-mycotics, calcium antagonists, and some antidepressants, can increase the bioavailability of THC.60–62 Conversely, drugs that potentiate hepatic enzymes responsible for metabolism of THC will lower its bioavailability. Examples include phenobarbital, phenytoin, troglitazone, and St John’s wort.60,62
Common concerns regarding cannabis
Patients
When it comes to patient concerns, an acronym to remember is HASH.
“High” feeling
Contrary to common misconceptions about patients seeking access to medical cannabis, many patients prefer to avoid “feeling high”. This can be mitigated fairly easily through prescribing practice, since the psychoactive effects of cannabis are primarily associated with high-THC strains. Strains of cannabis containing high levels of CBD generally make patients feel less high, since CBD acts as an antagonist to the psychoactive effect of THC.38 A number of high-CBD, low-THC strains are available for patients concerned about feelings of highness and euphoria.
Acquisition cost
Medical cannabis is not typically covered by insurance plans in Canada. This can cause a significant concern for chronic pain patients who are often disabled, retired, or unable to work.63 Fortunately, special pricing is available through some licensed producers for individuals receiving federal and provincial financial assistance. Examples of financial assistance for medical cannabis pricing include the Canadian Pension Plan-Disability, the War Veterans allowance, and the Ontario Disability Support Program. The price of medical cannabis is not currently regulated in Canada. The price is set by the Health Canada-authorized licensed producers and generally ranges from $5 to $15 per gram.64 Generally, higher THC content is associated with a higher price per gram.
Social stigma
Many chronic pain patients considering medical cannabis anticipate disapproval from their friends and family. It is not uncommon for patients to avoid disclosing their medical cannabis use to their loved ones altogether, despite experiencing significant improvements in their pain management and quality of life. These concerns are rooted in societal stigmatization of cannabis and can often be mitigated by enabling patients to medicalize their approach to disclosure. By explaining to friends and family that cannabis has been prescribed to them as a medicine which is used to treat a variety of conditions, patients may avoid some of the stigmatization associated with use of medical cannabis. Empowering patients with evidence-based knowledge will significantly facilitate this process.
How to? Lack of understanding of route of administration
Many chronic pain patients have limited or no experience using cannabis. Certainly, some degree of education is required for inexperienced patients to become aware of their options for routes of administration and to understand how to exercise each method. This includes instructions for purchasing, grinding/milling, weighing, vaporization, joint rolling, and derivative making. Clinicians are not commonly familiar with these processes as it is not included in the current medical curriculums. However, it would be valuable for clinicians to gain knowledge in these matters to help answer patient questions and inform their prescribing practices. This education can be provided to clinicians in the future through Continuing Medical Education hours. Currently, this education is offered to patients by some licensed producers. Furthermore, clinics may have educators (ie, nurse educators and other scientifically trained staff) to educate patients on these matters. Newly prescribed patients should also be made aware of practical and legal limitations, including barriers to traveling with the medication.
Physicians
Credibility–criminality–clinical evidence
In 2014, upward of 1,500 studies were published on cannabinoids. Knowledge is rapidly expanding and has led to a change in attitudes toward medical cannabis. A popular example of this change was the apology by Dr Sanjay Gupta in 2009 for not better appreciating cannabis as a potential therapeutic drug.65 As we move toward greater acceptance of the medicinal benefits of cannabis, increasingly, there is a need for the establishment of evidence-based guidelines to assist clinicians in their prescribing practices in order to optimize patient care and quality of life.
Relatedly, some regional differences in the accessibility of medical cannabis have been reported.66 Accessing medical cannabis from a friend or acquaintance was more common in the Prairies and Maritimes compared to British Columbia and Ontario,66 suggesting reduced accessibility to authorized sources of medical cannabis in the Prairies and Maritimes. Examination of patient forums suggests that one reason for these regional differences may be a lack of physicians willing to prescribe medical cannabis in these regions. Providing physicians with evidence-based guidelines and training in prescribing practices will likely decrease such barriers to accessibility of medical cannabis.
Patient addiction
It has been shown that one in every eleven individuals (9% of individuals) consuming cannabis will become dependent on the drug.67 Unfortunately, this statistic is based on individuals consuming all types of cannabis, irrespective of purpose for consumption (ie, medical or nonmedical) and strain. Regardless, an incidence of dependency of one per eleven is still significantly lower than those of approved pharmaceuticals commonly used for chronic pain management.68 Monitoring for cannabis dependency is recommended for all patients.
Canadian medical cannabis regulations
In July 2001, Health Canada granted access to cannabis for medical purposes to Canadians with the support of their physicians under the Marihuana Medical Access Regulations (MMAR).69 Under this regulation, patients were given the following options: 1) applying to access Health Canada’s supply of dried marihuana under the MMAR, 2) applying for a personal-use production license, and 3) designating someone to cultivate on their behalf with a designated-person production license. The Medical Marihuana Access program was replaced by the Marihuana for Medical Purposes Regulations (MMPR) in April 2014.70 Following this change, production of legal medical marijuana is authorized to licensed producers. A list of the Health Canada-authorized licensed producers can be found on Health Canada’s website.71 A foreign corporation could operate as a licensed producer in Canada if the “corporation that has its head office in Canada or operates a branch office in Canada and whose officers and directors are all adults”.72
With the introduction of the MMPR in Canada, physicians are advised to follow the guidance set forth by their provincial college. The aim of the MMPR is to treat medical cannabis like other narcotics used for medicinal purposes whenever possible. Under the MMPR, the patient must consult with a medical doctor or a qualified nurse practitioner. A signed “medical document” is submitted to a Health Canada-approved licensed commercial producer of marijuana, granting the patient access to the program. These medical documents are treated similarly to prescriptions. They must meet specific requirements, including patient name, date of birth, physician information, including license number and signature, a daily allotment in grams, and a length of time for the access not exceeding 1 year. A physician may also indicate specific strains and/or an amount of THC allowed to the patient. While there is no legal requirement for licensed producers to follow strain and THC recommendations, many will abide by the request of the physician. Dispensaries and compassion clubs are not permitted under MMPR, so appropriate steps should be taken to ensure a patient is only being referred to Health Canada-approved organizations. Once the patients purchase the medication from the company, it is shipped to their home, or that of their caretaker. Alternatively, arrangements may be made for the licensed producer to transfer the drug to the health care prescriber, from which it can then be obtained by the patients. It should be noted that Health Canada neither approves nor regulates medical cannabis like it does pharmaceutical drugs. Thus, the medical document issued by physicians for medical cannabis is distinct from, and only partially analogous to, a prescription. Instead, the medical document can be viewed as a recommendation to the medical cannabis program. In Quebec, a distinction is made that physicians should not provide such a document unless it is part of a recognized research project and only for specified conditions. Other provincial colleges will have their own requirements. A recent decision by the Supreme Court of Canada has overturned the original requirements for licensed producers and patients to only sell and consume dried cannabis. This decision allows the sale of fresh, dried, and oil forms of cannabis to patients. Though, as of writing, no licensed producer has yet to be granted permission to sell fresh and oil alternative forms to patients.
Prescribing considerations
As mentioned, prescription and recommendation of medical cannabis at this point is largely nonspecific. Patients are recommended to the medical cannabis program but not necessarily a specific strain. Increasingly, an understanding of how specific strains of medical cannabis can offer benefit for specific ailments is appreciated by those recommending the use of medical cannabis. Unfortunately, the body of evidence supporting these practices is limited, due to an overall lack of investigation, which prevents physicians from making informed decisions to best improve the risk–benefit relationship of medical cannabis in their patients.
Many colleges recommend that Canadian physicians treat medical cannabis as they would any other prescribed narcotic drug. This often includes the use of patient–physician agreements on appropriate use and informed consent of the new medication. Physicians should also consider other following factors when recommending medical cannabis to their patients.
Amount
MMPR requires the recommending physician allot a set amount of cannabis to which a patient will have access on a daily basis. Medical cannabis programs report average patient use of between 0.68 and 1.5 g per day.40,73 As a physician increases the amount of medical cannabis a patient is allowed access to, so too does the risk for diversion. However, patients report using up to 10 g of cannabis per day for self-medication purposes. Both the amount the patients currently use for self-identified medical reasons and their preferred route of administration should be taken into consideration when recommending an amount of medical cannabis.
Strain selection and recommendation
Given that evidence supporting the use of specific medical cannabis strains for various pain ailments is lacking, recommending a strain type to a patient can be difficult. The decision is often determined by a number of factors, including financial concerns, potential risk to the patient, and specific goals of the patient (such as to improve sleep or to avoid feeling high). Typically, recommendations are made based on medical history, cannabis use history, and financial barriers. Once all of these factors have been considered, a strain is selected by the clinician from a range of varieties recommended for medical use by Health Canada from authorized licensed producers. Each licensed producer produces different strains suitable for various medical purposes. Using the principles of “start low, go slow” titration, individuals with little or no experience, histories of bipolar disorder, strong familial schizophrenia, and/or a history of substance abuse begin their process with medical cannabis on a CBD-dominant strain. Patients with a history of cannabis use and no significant risk factors are initially prescribed a strain with higher THC content and maximal CBD content. If patients fail to get relief from their initial strains, an increase in the THC content is recommended in a stepwise fashion, as long as serious risk factors are not present. If risk factors are present, the risk–benefit analysis for this patient must be readdressed. Many colleges recommend indicating an amount of THC a patient would be permitted to access with a licensed producer. Unfortunately, the current regulatory environment in Canada does not require a licensed producer adhere to the recommendation. Likewise, there is rarely any guidance on prescribing strains with CBD content.
Route of administration
Many patients have concerns about medical cannabis smoke, which contains many of the same carcinogenic chemicals as tobacco smoke.74 Ultimately, the optimal route of administration to be recommended will depend largely on the desires and capabilities of the patient.
Inhalation by vaporization is the most effective route at delivering the medicinal cannabinoid content of medical cannabis,75 and both dried and extracted medical cannabis can be used in a vaporizer. Sometimes, vaporization can be burdensome for patients. Indeed, loading a vaporizer requires some degree of dexterity, which may be limited in certain populations of pain patients, such as those with rheumatoid arthritis and osteoarthritis. Patients may also complain of the temperature of vapor created by vaporization. Many patients require fairly extensive education regarding the use of a vaporizer.
Oral ingestion of medical cannabis typically refers to consumption of cannabis oils or edibles. These are generally produced by infusing a lipophilic substance, like an oil or butter, with cannabis, which is then used in drops or in food. Indeed, a number of recipes have become available online for the use of cannabis oil and butter in food, though some patients dislike the strong flavor. For patients with respiratory illnesses, the oral route is preferable. This method is limited, however, by lower absorption and bioavailability than for inhaled cannabis. Another potential concern is a lack of research on the effectiveness and safety of orally consumed cannabis for pain conditions. Given the increased latency of effect onset from orally consumed medical cannabis, patients should be cautioned to wait an adequate amount of time to feel the effects of the cannabis before readministering. While issues of dosing and effectiveness exist for orally administered cannabis, it is typically well tolerated by patients.
Sublingual tinctures are another, less common, route of administration for medical cannabis. Typically, these tinctures are extracted with ethanol, but vinegars and glycerine may also be used. The extracts are dropped under the tongue and held for a period of time sufficient to permit absorption by the branches of the lingual artery, including the sublingual and deep lingual arteries. If used properly, onset of action and bioavailability may be faster and higher for this route compared with oral administration, as is often observed with other drugs.76 Tinctures may be a favorable option in the future, as they mitigate the dosing and bioavailability issues associated with orally ingested cannabis and eliminate issues of tolerability with inhaled cannabis. However, the use of tinctures is not widespread today, and evidence supporting the therapeutic use of tinctures is limited. Moreover, patients often complain of the taste. In Canada, there is currently a sublingual cannabinoid pharmaceutical known as Sativex. This is approved for multiple sclerosis (MS)-related neuropathic pain or spasticity and for cancer-related pain. A case series has also been published on its effectiveness for fibromyalgia.77
Alternative routes of administration include transdermal ointments and balms, ophthalmic drops, and rectal suppositories. While rarely used, all of these routes may have therapeutic potential for patients, though little research has been done to assess this likelihood.
Follow-up frequency
When introducing a patient to medical cannabis for the first time, it is important to schedule frequent follow-ups until a strain has been selected that meets the treatment goals of both patient and physician. Since this process may require changes such as route of administration, an active follow-up schedule may be required to provide the patient with adequate knowledge to continue safely and confidently. Once a patient has been stabilized, follow-up visits should focus on monitoring for adverse reactions, including dependence.
In Canada, the medical document that is produced to allow a patient access to cannabis acts as a license. Thus, if the patients’ medical document expires while they are in possession of medical cannabis, they may be open to criminal charges. The timing of a patient’s follow-ups is an important nonmedical issue as well.
Contraindications
Several contraindications have been identified for medical cannabis recommendations. Due in part to the illicit nature of cannabis, research is lacking and there is a significant knowledge gap in this area, and medical cannabis recommendations should always be made with careful consideration of the current health status of the patient.
Psychosis
As previously mentioned, individuals suffering from, or at a high risk of developing, schizophrenia or other psychotic illnesses should only be recommended the use of cannabis under well-monitored conditions. The use of strains with minimal or no THC content is recommended.
Bipolar disorder
Recently, Kim et al found that cannabis use was significantly associated with lower rates of remission of bipolar spectrum patients over a 2-year follow-up period.78 Studies have also found an association between cannabis misuse and earlier onset of bipolar disorder.79 Thus, the use of low-THC content strains is recommended for these patients.
Cannabis allergies
It is estimated that C. sativa allergies are found in 8% of the general population, although the incidence may be higher among individuals who identify as users of cannabis.80 Avoidance is recommended for patients with cannabis allergies to avoid potentially lethal anaphylaxis. However, mild rhinoconjunctivitis symptoms can be treated with antihistamines, intranasal steroids, and nasal decongestants.81 Immunotherapy has been used to treat cannabis allergies,82,83 but this is not common practice.
Adverse effects
Findings from the currently available research suggest that the safety profile of the short-term use of medical cannabis is acceptable.84 A systematic review of 23 RCTs and eight observational studies of medical cannabis found that 96.6% of the adverse effects reported in the trials were not serious. The most commonly reported adverse effect was dizziness (15.5%). Rates of serious adverse effects did not vary between the group of participants assigned to medical cannabis and controls.84 Other commonly reported adverse effects are drowsiness, feeling faint or light headed, fatigue, headache, impaired memory, and disturbances in attention, concentration, and ability to think and make decisions.85 However, further research on the long-term safety profile of medical cannabis use is required.86,87
Case studies
Neuropathic low-back pain
A 49-year-old, single male patient reporting chronic lower back pain due to diagnoses of spinal stenosis, degenerative disc disease, and neuropathic pain including sciatica for over 20 years presented at our clinic. In-clinic recorded pain score for the patient was 9/10 on a numerical rating scale. Positive DN4 (>4/10) and Freynhagen Pain Detect Questionnaire (>19/35) scores were recorded. The patient also had diagnoses of gastroesophageal reflux disease, irritable bowel syndrome, and anxiety. At the time of meeting, the patient was using nabilone 0.25 mg daily, pregabalin 300 mg daily, ibuprofen 400–600 mg daily, omeprazole 40 mg daily, baclofen 20 mg daily, and clonazepam 0.5 mg daily. After several unsuccessful attempts at pain control using physiotherapy, chiropractic, osteopathy, acupuncture, corticosteroid injections, oxycodone, and Percocet, the patient confided he turned to illicit cannabis for pain relief on a daily basis, primarily in the evening after work.
The patient was prescribed 1 g per day of a cannabis strain containing 9% THC and 13% CBD to be administered by a vaporizer. At 60 days of follow-up, the patient’s pain was lowered to a weekly average of 3/10 on a numerical rating scale. The patient also indicated he did not see a need for pregabalin, and had begun the process of lowering his daily dose. Surprisingly, the patient also reported far fewer symptoms of his irritable bowel syndrome, claiming near-remission.
Fibromyalgia – widespread neuropathic pain
A 57-year-old, married male patient reporting fibromyalgia for 5 years, and osteoarthritis, torn shoulder tendon, and spinal stenosis for over 20 years was referred to our clinic. His initial in-clinic recorded pain score was 8/10 on a numerical rating scale. The patient also had a history of severe obesity, sleep apnea, restless legs syndrome, and anxiety. Signs of neuropathic pain included widespread allodynia and positive DN4 score. At the time of meeting, the patient was taking several prescribed pain medications, including Percocet 5/325 mg as needed and Oxyneo 40 mg daily. Physiotherapy, corticosteroid injections, codeine, and a number of anti-inflammatory medications were unsuccessful at achieving adequate analgesia. The patient was inexperienced with cannabis, except for intermittent use on weekends.
The patient was prescribed 1.5 g per day of a strain of cannabis containing 5% THC and 8% CBD to be administered by a vaporizer. After 2 weeks of trial, the patient reported a lack of success, and a strain of 12% THC was added to the other strain, with instructions to mix the strains in equal parts. At 60 days of follow-up, the patient’s pain was lowered to a weekly average of 3/10 on a numerical rating scale, and he lowered his use of Percocet from four pills per day to three pills per week, on average.
MS-related neuropathic pain
A 67-year-old, single female patient reporting neuropathic pain secondary to MS diagnosis of over 20 years was referred to our clinic by her pain intervention physician. In-clinic pain score was recorded at 9/10 on a numeric rating scale. The patient was actively taking gabapentin 2,200 mg daily and celecoxib 200 mg daily. The patient could not tolerate the use of opiate medications, claiming dissatisfaction with their sedative effects. Failed pain interventions included IV lidocaine and lumbar radiofrequency ablation. The patient was naïve to cannabis.
The patient was prescribed 1 g per day of cannabis containing 2.5% THC and 5% CBD to be administered by a vaporizer. After failing to achieve adequate analgesia, a strain of 9% THC and 13% CBD was recommended to the patient. At 60 days of follow-up, the patient’s pain subsided to a moderate 5/10 on a numeric rating scale, and she is planning to lower the dose of her other medications.
Conclusion
This review documents some of the relevant history and current research literature on medical cannabis. It draws to attention the key concerns in the Canadian medical system and provides updated treatment approaches to help clinicians work with their patients in achieving adequate pain control, reduced narcotic and other medication use (and their adverse effects), and enhanced quality of life. RCTs using large population samples are needed in order to identify the specific strains and concentrations that will work best with selected cohorts. Cannabis-based medicine is a rapidly emerging field of which all pain physicians need to be aware.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zuardi AW. History of cannabis as a medicine: a review. Rev Bras Psiquiatr. 2006;28(2):153–157. doi: 10.1590/s1516-44462006000200015. [DOI] [PubMed] [Google Scholar]
- 2.Turcotte D, Le Dorze J-A, Esfahani F, Frost E, Gomori A, Namaka M. Examining the roles of cannabinoids in pain and other therapeutic indications: a review. Expert Opin Pharmacother. 2010;11(1):17–31. doi: 10.1517/14656560903413534. [DOI] [PubMed] [Google Scholar]
- 3.Hirst RA, Lambert DG, Notcutt WG. Pharmacology and potential therapeutic uses of cannabis. Br J Anaesth. 1998;81(1):77–84. doi: 10.1093/bja/81.1.77. [DOI] [PubMed] [Google Scholar]
- 4.Aldrich M. History of therapeutic cannabis. In: Mathre ML, editor. Cannabis in Medical Practice: A Legal, Historical, and Pharmacological Overview of the Therapeutic Use of Marijuana. Jefferson, NC: McFarland & Co.; 1997. pp. 35–55. [Google Scholar]
- 5.Boychuk DG, Goddard G, Mauro G, Orellana MF. The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache. 2015;29(1):7–14. doi: 10.11607/ofph.1274. [DOI] [PubMed] [Google Scholar]
- 6.Tramèr MR, Carroll D, Campbell FA, Reynolds DJ, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ. 2001;323(7303):16–21. doi: 10.1136/bmj.323.7303.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Troutt WD, DiDonato MD. Medical cannabis in Arizona: patient characteristics, perceptions, and impressions of medical cannabis legalization. J Psychoactive Drugs. 2015;47(4):259–266. doi: 10.1080/02791072.2015.1074766. [DOI] [PubMed] [Google Scholar]
- 8.Bonn-Miller MO, Boden MT, Bucossi MM, Babson KA. Self-reported cannabis use characteristics, patterns and helpfulness among medical cannabis users. Am J Drug Alcohol Abuse. 2014;40(1):23–30. doi: 10.3109/00952990.2013.821477. [DOI] [PubMed] [Google Scholar]
- 9.Ilgen MA, Bohnert K, Kleinberg F, et al. Characteristics of adults seeking medical marijuana certification. Drug Alcohol Depend. 2013;132(3):654–659. doi: 10.1016/j.drugalcdep.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Walsh Z, Callaway R, Belle-Isle L, et al. Cannabis for therapeutic purposes: patient characteristics, access, and reasons for use. Int J Drug Policy. 2013;24(6):511–516. doi: 10.1016/j.drugpo.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Di Forti M, Morrison PD, Butt A, Murray RM. Cannabis use and psychiatric and cognitive disorders: the chicken or the egg? Curr Opin Psychiatry. 2007;20(3):228–234. doi: 10.1097/YCO.0b013e3280fa838e. [DOI] [PubMed] [Google Scholar]
- 12.Schauer GL, King BA, Bunnell RE, Promoff G, McAfee TA. Toking, vaping, and eating for health or fun: marijuana use patterns in adults, U.S., 2014. Am J Prev Med. 2016;50(1):1–8. doi: 10.1016/j.amepre.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 13.Belle-Isle L, Hathaway A. Barriers to access to medical cannabis for Canadians living with HIV/AIDS. AIDS Care. 2007;19(4):500–506. doi: 10.1080/09540120701207833. [DOI] [PubMed] [Google Scholar]
- 14.Canadian Centre on Substance Abuse . Canadian addiction survey 2004: microdata eguide. Ottawa, ON: Canadian Centre on Substance Abuse; [Accessed August 20, 2015]. Available from: http://www.ccsa.ca/Resource%20Library/ccsa-004028-2005.pdf. [Google Scholar]
- 15.Ebert T, Zolotov Y, Eliav S, Ginzburg O, Shapira I, Magnezi R. Assessment off Israeli physicians’ knowledge, experience and attitudes towards medical cannabis: a pilot study. Isr Med Assoc J. 2015;17(7):437–441. [PubMed] [Google Scholar]
- 16.Frank B, Serpell MG, Hughes J, Matthews JN, Kapur D. Comparison of analgesic effects and patient tolerability of nabilone and dihydrocodeine for chronic neuropathic pain: randomised, crossover, double blind study. BMJ. 2008;336(7637):199–201. doi: 10.1136/bmj.39429.619653.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glass RM, Uhlenhuth EH, Hartel FW. The effects of nabilone, a synthetic cannabinoid, on anxious human volunteers [proceedings] Psychopharmacol Bull. 1979;15(2):88–90. [PubMed] [Google Scholar]
- 18.Lemberger L, Rubin A, Wolen R, et al. Pharmacokinetics, metabolism and drug-abuse potential of nabilone. Cancer Treat Rev. 1982;9(Suppl B):17–23. doi: 10.1016/s0305-7372(82)80031-5. [DOI] [PubMed] [Google Scholar]
- 19.McGilveray IJ. Pharmacokinetics of cannabinoids. Pain Res Manag. 2005;10(Suppl A):15A–22A. doi: 10.1155/2005/242516. [DOI] [PubMed] [Google Scholar]
- 20.Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63(5):569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Shabat S, Hanuš LO, Katzavian G, Gallily R. New cannabidiol derivatives: synthesis, binding to cannabinoid receptor, and evaluation of their antiinflammatory activity. J Med Chem. 2006;49(3):1113–1117. doi: 10.1021/jm050709m. [DOI] [PubMed] [Google Scholar]
- 22.Maione S, Piscitelli F, Gatta L, et al. Non-psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of action. Br J Pharmacol. 2011;162(3):584–596. doi: 10.1111/j.1476-5381.2010.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costa B, Trovato AE, Comelli F, Giagnoni G, Colleoni M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol. 2007;556(1–3):75–83. doi: 10.1016/j.ejphar.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Niesink RJ, van Laar MW. Does cannabidiol protect against adverse psychological effects of THC? Front Psychiatry. 2013;16(4):130. doi: 10.3389/fpsyt.2013.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iseger TA, Bossong MG. A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophr Res. 2015;162(1–3):153–161. doi: 10.1016/j.schres.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 26.De Meijer EPM, Bagatta M, Carboni A, et al. The inheritance of chemical phenotype in Cannabis sativa L. Genetics. 2003;163(1):335–346. doi: 10.1093/genetics/163.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russo E, Guy GW. A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses. 2006;66(2):234–246. doi: 10.1016/j.mehy.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 28.Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30(1):515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Fride E, Mechoulam R. Pharmacological activity of the cannabinoid receptor agonist, anandamide, a brain constituent. Eur J Pharmacol. 1993;231(2):313–314. doi: 10.1016/0014-2999(93)90468-w. [DOI] [PubMed] [Google Scholar]
- 30.Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83(2):393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 31.Walker JM, Huang SM, Strangman NM, Tsou K, Sañudo-Peña MC. Pain modulation by release of the endogenous cannabinoid anandamide. Proc Natl Acad Sci U S A. 1999;96(21):12198–12203. doi: 10.1073/pnas.96.21.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson-Poe AR, Morgan MM, Aicher SA, Hegarty DM. Distribution of CB1 cannabinoid receptors and their relationship with mu-opioid receptors in the rat periaqueductal gray. Neuroscience. 2012;213:191–200. doi: 10.1016/j.neuroscience.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashimotodani Y, Ohno-Shosaku T, Kano M. Endocannabinoids and synaptic function in the CNS. Neuroscientist. 2007;13(2):127–137. doi: 10.1177/1073858406296716. [DOI] [PubMed] [Google Scholar]
- 34.Soltesz I, Alger BE, Kano M, et al. Weeding out bad waves: towards selective cannabinoid circuit control in epilepsy. Nat Rev Neurosci. 2015;16(5):264–277. doi: 10.1038/nrn3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richardson JD, Aanonsen L, Hargreaves KM. SR 141716A, a cannabinoid receptor antagonist, produces hyperalgesia in untreated mice. Eur J Pharmacol. 1997;319(2–3):R3–R4. doi: 10.1016/s0014-2999(96)00952-1. [DOI] [PubMed] [Google Scholar]
- 36.Nicholson AN, Turner C, Stone BM, Robson PJ. Effect of delta-9-tetrahydrocannabinol and cannabidiol on nocturnal sleep and early-morning behavior in young adults. J Clin Psychopharmacol. 2004;24(3):305–313. doi: 10.1097/01.jcp.0000125688.05091.8f. [DOI] [PubMed] [Google Scholar]
- 37.Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA. Cannabidiol interferes with the effects of delta 9-tetrahydrocannabinol in man. Eur J Pharmacol. 1974;28(1):172–177. doi: 10.1016/0014-2999(74)90129-0. [DOI] [PubMed] [Google Scholar]
- 38.Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology (Berl) 1982;76(3):245–250. doi: 10.1007/BF00432554. [DOI] [PubMed] [Google Scholar]
- 39.Bisogno T, Hanus L, De Petrocellis L, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134(4):845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Health Canada Controlled Substances Tobacco Directorate . Information for health care professionals: cannabis (marihuana, marijuana) and the cannabinoids. Ottawa, ON: Health Canada, Controlled Substances and Tobacco Directorate; 2013. [Accessed August 3, 2015]. Available from: http://www.hc-sc.gc.ca/dhp-mps/marihuana/med/infoprof-eng.php. [Google Scholar]
- 41.Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68(7):515–521. doi: 10.1212/01.wnl.0000253187.66183.9c. [DOI] [PubMed] [Google Scholar]
- 42.Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34(3):672–680. doi: 10.1038/npp.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ware MA, Wang T, Shapiro S, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ. 2010;182(14):E694–E701. doi: 10.1503/cmaj.091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9(6):506–521. doi: 10.1016/j.jpain.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14(2):136–148. doi: 10.1016/j.jpain.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corey-Bloom J, Wolfson T, Gamst A, et al. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ. 2012;184(10):1143–1150. doi: 10.1503/cmaj.110837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andreae MH, Carter GM, Shaparin N, et al. Inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain. 2015;16(12):1221–1232. doi: 10.1016/j.jpain.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia - an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013;11:CD010567. doi: 10.1002/14651858.CD010567.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Birse F, Derry S, Moore RA. Phenytoin for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2012;5:CD009485. doi: 10.1002/14651858.CD009485.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaparro LE, Wiffen PJ, Moore RA, Gilron I. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst Rev. 2012;7:CD008943. doi: 10.1002/14651858.CD008943.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corrigan R, Derry S, Wiffen PJ, Moore RA. Clonazepam for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2012;5:CD009486. doi: 10.1002/14651858.CD009486.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Canadian Cancer Society Medical marijuana and cannabinoids. 2015. [Accessed August 3, 2015]. Available from: http://www.cancer.ca/en/cancer-information/diagnosis-and-treatment/complementary-therapies/medical-marijuana-and-cannabinoids/?region=on.
- 53.Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770–1804. doi: 10.1002/cbdv.200790152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agurell S, Halldin M, Lindgren JE, et al. Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev. 1986;38(1):21–43. [PubMed] [Google Scholar]
- 55.Zuurman L, Ippel AE, Moin E, van Gerven JMA. Biomarkers for the effects of cannabis and THC in healthy volunteers. Br J Clin Pharmacol. 2009;67(1):5–21. doi: 10.1111/j.1365-2125.2008.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carter GT, Weydt P, Kyashna-Tocha M, Abrams DI. Medicinal cannabis: rational guidelines for dosing. IDrugs. 2004;7(5):464–470. [PubMed] [Google Scholar]
- 57.Hart CL, Ilan AB, Gevins A, et al. Neurophysiological and cognitive effects of smoked marijuana in frequent users. Pharmacol Biochem Behav. 2010;96(3):333–341. doi: 10.1016/j.pbb.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walsh D, Nelson KA, Mahmoud FA. Established and potential therapeutic applications of cannabinoids in oncology. Support Care Cancer. 2003;11(3):137–143. doi: 10.1007/s00520-002-0387-7. [DOI] [PubMed] [Google Scholar]
- 59.Cone EJ, Johnson RE, Paul BD, Mell LD, Mitchell J. Marijuana-laced brownies: behavioral effects, physiologic effects, and urinalysis in humans following ingestion. J Anal Toxicol. 1988;12(4):169–175. doi: 10.1093/jat/12.4.169. [DOI] [PubMed] [Google Scholar]
- 60.The Netherlands Ministry of Health Welfare and Sports . Medicinal Cannabis, Information for Health Care Professionals. Amsterdam, the Netherlands: The Netherlands Ministry of Health Welfare and Sports; 2008. [Google Scholar]
- 61.Li X-Q, Andersson TB, Ahlström M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome p450 activities. Drug Metab Dispos. 2004;32(8):821–827. doi: 10.1124/dmd.32.8.821. [DOI] [PubMed] [Google Scholar]
- 62.Spina E, Santoro V, D’Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30(7):1206–1227. doi: 10.1016/s0149-2918(08)80047-1. [DOI] [PubMed] [Google Scholar]
- 63.Karoly P, Ruehlman LS, Okun MA. Psychosocial and demographic correlates of employment vs disability status in a national community sample of adults with chronic pain: toward a psychology of pain presenteeism. Pain Med. 2013;14(11):1698–1707. doi: 10.1111/pme.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.The Arthritis Society Medical cannabis: a guide to access. 2015. [Accessed August 10, 2015]. Available from: http://arthritis.ca/getmedia/99682fb5-3992-4924-895a-d5f03d16f151/Medical-Cannabis-2015-a-Guide-to-Access.pdf.
- 65.Gupta S. Why I changed my mind on weed. 2013. [Accessed July 30, 2015]. Available from: http://frogbuddha.com/?p=234.
- 66.Belle-Isle L, Walsh Z, Callaway R, et al. Barriers to access for Canadians who use cannabis for therapeutic purposes. Int J Drug Policy. 2014;25(4):691–699. doi: 10.1016/j.drugpo.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 67.Lopez-Quintero C, Pérez de los Cobos J, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug Alcohol Depend. 2011;115(1–2):120–130. doi: 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hood SD, Norman A, Hince DA, Melichar JK, Hulse GK. Benzodiazepine dependence and its treatment with low dose flumazenil. Br J Clin Pharmacol. 2014;77(2):285–294. doi: 10.1111/bcp.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Health Canada About the Marihuana Medical Access Program. 2014. [Accessed August 5, 2015]. Available from: http://www.hc-sc.gc.ca/dhp-mps/marihuana/about-apropos/index-eng.php.
- 70.Government of Canada Justice Laws Website. Marihuana for Medical Purposes Regulations. 2015. [Accessed August 25, 2015]. Available from: http://www.laws-lois.justice.gc.ca/eng/regulations/SOR-2013-119.
- 71.Health Canada Authorized licensed producers under the Marihuana for Medical Purposes Regulations. 2015. [Accessed August 5, 2015]. Available from: http://www.hc-sc.gc.ca/dhp-mps/marihuana/info/list-eng.php.
- 72.Government of Canada Justice Laws Website. Marihuana for Medical Purposes Regulations. 2015. [Accessed August 10, 2015]. Available from: http://www.laws-lois.justice.gc.ca/eng/regulations/SOR-2013-119/page-6.html.
- 73.Hazekamp A, Heerdink ER. The prevalence and incidence of medicinal cannabis on prescription in the Netherlands. Eur J Clin Pharmacol. 2013;69(8):1575–1580. doi: 10.1007/s00228-013-1503-y. [DOI] [PubMed] [Google Scholar]
- 74.Moir D, Rickert WS, Levasseur G, et al. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem Res Toxicol. 2008;21(2):494–502. doi: 10.1021/tx700275p. [DOI] [PubMed] [Google Scholar]
- 75.Pomahacova B, Van der Kooy F, Verpoorte R. Cannabis smoke condensate III: the cannabinoid content of vaporised Cannabis sativa. Inhal Toxicol. 2009;21(13):1108–1112. doi: 10.3109/08958370902748559. [DOI] [PubMed] [Google Scholar]
- 76.Scavone JM, Greenblatt DJ, Friedman H, Shader RI. Enhanced bioavailability of triazolam following sublingual versus oral administration. J Clin Pharmacol. 1986;26(3):208–210. doi: 10.1002/j.1552-4604.1986.tb02935.x. [DOI] [PubMed] [Google Scholar]
- 77.Ko G, Wine W, Tumarkin E. Case series of fibromyalgia patients with neuropathic pain improved with the sublingual cannabinoid Sativex. Eur J Pain. 2007;11(S1):145–146. [Google Scholar]
- 78.Kim S-W, Dodd S, Berk L, et al. Impact of cannabis use on long-term remission in bipolar I and schizoaffective disorder. Psychiatry Investig. 2015;12(3):349–355. doi: 10.4306/pi.2015.12.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leite RTP, Nogueira Sde O, do Nascimento JPR, et al. The use of cannabis as a predictor of early onset of bipolar disorder and suicide attempts. Neural Plast. 2015;2015:434127. doi: 10.1155/2015/434127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Larramendi CH, López-Matas MÁ, Ferrer A, et al. Prevalence of sensitization to Cannabis sativa. Lipid-transfer and thaumatin-like proteins are relevant allergens. Int Arch Allergy Immunol. 2013;162(2):115–122. doi: 10.1159/000351068. [DOI] [PubMed] [Google Scholar]
- 81.Ocampo TL, Rans TS. Cannabis sativa: the unconventional “weed” allergen. Ann Allergy Asthma Immunol. 2015;114(3):187–192. doi: 10.1016/j.anai.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 82.Gupta BN, Mehrotra NK, Clerk SH, et al. Immunotherapy in hemp workers having respiratory complaints. Indian J Med Sci. 1980;34(4):72–81. [PubMed] [Google Scholar]
- 83.Kumar R, Gupta N. A case of bronchial asthma and allergic rhinitis exacerbated during Cannabis pollination and subsequently controlled by subcutaneous immunotherapy. Indian J Allergy Asthma Immunol. 2013;27(2):143. [Google Scholar]
- 84.Wang T, Collet JP, Shapiro S, Ware MA. Adverse effects of medical cannabinoids: a systematic review. CMAJ. 2008;178(13):1669–1678. doi: 10.1503/cmaj.071178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Health Canada Consumer information – cannabis (marihuana, marijuana) 2015. [Accessed August 3, 2015]. Available from: http://www.hc-sc.gc.ca/dhp-mps/marihuana/info/cons-eng.php.
- 86.Degenhardt L, Hall WD. The adverse effects of cannabinoids: implications for use of medical marijuana. CMAJ. 2008;178(13):1685–1686. doi: 10.1503/cmaj.080585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Belendiuk KA, Baldini LL, Bonn-Miller MO. Narrative review of the safety and efficacy of marijuana for the treatment of commonly state-approved medical and psychiatric disorders. Addict Sci Clin Pract. 2015;10:10. doi: 10.1186/s13722-015-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


