Introduction
KEY TEACHING POINTS
|
The feasibility of resynchronizing ventricular activation by permanent pacing of the His bundle region has been previously described, and has clinical advantages over traditional right ventricular (RV) apical pacing.1, 2, 3, 4 The physiologic benefit of permanent His bundle pacing (HBP) is the ability to stimulate the ventricles through the intrinsic His-Purkinje system, which results in synchronous electrical and mechanical activation. It also has theoretical advantages over cardiac resynchronization therapy (CRT) using a coronary sinus lead, which is associated with limited coronary venous anatomy and complications that include coronary sinus dissection, venous perforation, and the potential for proarrhythmia.
Hyper-response, typically described as a patient showing functional recovery and left ventricular ejection fraction (LVEF) ≥50%, has been reported with CRT5, 6 and similar recovery has been seen with HBP, after restoration of normal intrinsic conduction.7, 8, 9 In the latter 3 patients, there was normalization of ventricular activation with HBP, and QRS durations ranged from 80 to 100 ms in these patients.
In this report, we present a case of a hyper-responder to HBP (LVEF 15%–55%) with parahisian capture that resulted in incomplete normalization of the QRS complex. We review the putative mechanisms of HBP, and the necessity of complete normalization of the QRS complex to achieve resynchronization with HBP.
Case report
A 74-year-old woman with hypertension, hypercholesterolemia, diabetes mellitus, symptomatic severe aortic valve stenosis (valve area 0.8 cm2, peak velocity 4.9 m/s), and asymmetric septal hypertrophy (thickness 1.5 cm) underwent aortic valve replacement (23 mm Carpentier-Edwards pericardial bioprosthesis) and septal myomectomy to relieve exertional symptoms of chest pain and shortness of breath. The surgical procedure was complicated by postoperative complete heart block with a ventricular escape (40 beats/minute), with subsequent recovery of atrioventricular conduction on postoperative day 5 and development of left bundle branch block (LBBB). Her LVEF immediately after surgery remained at 60%.
Over the ensuing 6 months, the patient developed progressively worsening dyspnea, initially on exertion and subsequently at rest. She developed signs of volume overload, and presented for medical evaluation. She exhibited NYHA class III–IV symptoms, and an LVEF of 20% was seen on echocardiography. She was started on diuretics and medical therapy for heart failure with a beta blocker, angiotensin receptor blocker, and aldosterone antagonist. Despite these interventions, her LVEF remained severely depressed (15%, Simpson’s biplane method), although her symptoms stabilized at NYHA class III. A coronary angiogram demonstrated no significant coronary lesions.
After 3 months of optimal medical therapy, given her depressed LVEF, LBBB (QRS duration of 198 ms), and NYHA class III symptoms, she was referred for consideration of resynchronization therapy and defibrillator. Magnetic resonance imaging was performed, which did not show any regions of delayed enhancement.
The patient was consented for resynchronization therapy and owing to the high clinical suspicion that her systolic dysfunction was induced by left bundle dyssynchrony, both HBP and implantation of a standard left ventricular (LV) lead were discussed in detail. The patient opted for an attempt at HBP prior to LV lead placement. During the procedure, a diagnostic His catheter (CRD2; St Jude Medical, Minneapolis, MN) was placed to serve as a fluoroscopic landmark. The AH interval and HV intervals were 88 ms and 64 ms, respectively. The patient underwent implantation of standard atrial lead and RV defibrillator lead (single coil). A Medtronic Select Secure lead (Model number 3830) was advanced through a Medtronic C315HIS sheath to the region of the His bundle. The lead was connected to an analyzer, and the high septal region adjacent to the site marked by the CRD2 catheter was mapped for a His bundle electrogram. The lead was fixated to a site with a near-field His recording (Figure 1A and B) with an acute capture threshold at this site of 2 V at 0.6 ms pulse width. With His bundle pacing, the QRS narrowed from 198 ms to 123 ms (with paced “HV” interval of 52 ms) (Figure 2). The His lead was placed into the LV port of the CRT device and because the device could not be programmed to pace the LV port only, it was programmed with a zero LV→RV offset with RV pacing output below the RV capture threshold to prevent fusion between RV pacing with HBP The patient tolerated the procedure well, and was discharged home the following day.
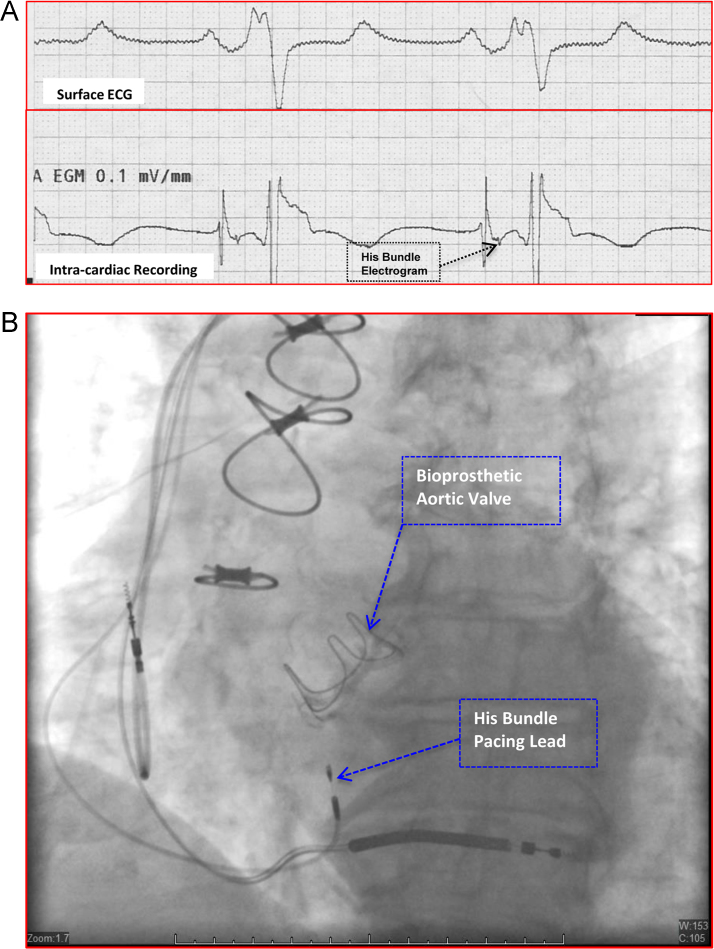
Figure 1.
Intracardiac recording and fluoroscopic appearance of parahisian pacing site. A: Intracardiac electrograms (lower tracing) obtained at a site in the high septum where the His bundle pacing lead was deployed. The His bundle electrogram is indicated by the arrow. The surface electrocardiogram (ECG; upper tracing) is also shown. B: Fluoroscopic appearance of the final position of the permanent His bundle pacing lead and the atrial and ventricular leads in a shallow right anterior oblique projection.
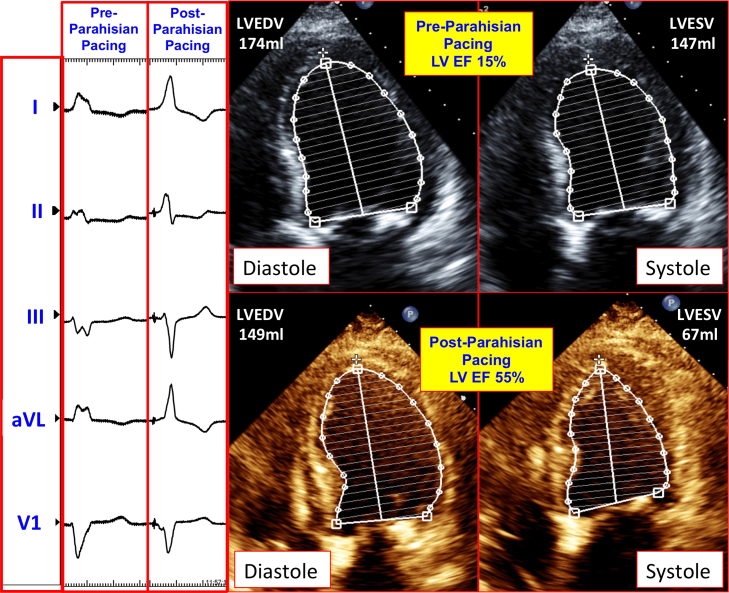
Figure 2.
Electrocardiographic and echocardiographic impact of parahisian pacing. The left panel shows the surface QRS before and after permanent His bundle pacing (PHBP) was implemented. The QRS duration narrowed from 198 ms to 123 ms following PHBP. The right panel demonstrates the diastolic and systolic transthoracic echocardiographic images obtained in the apical 4-chamber projection before and after PHBP, with respective endocardial tracings using Simpson’s method.
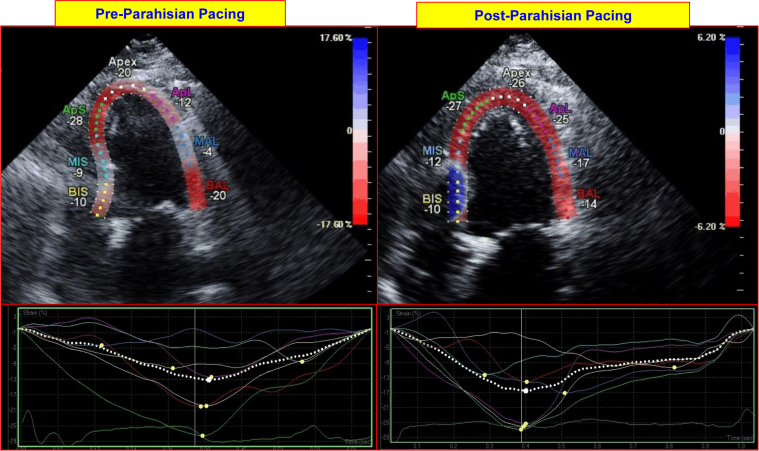
Over the subsequent 3 months, the patient noted marked improvement in her symptoms and functional status (NYHA class III to class I). Echocardiography at 6 weeks demonstrated improvement in her LVEF from 15% to 40%–45%, and at 3 months, her LVEF improved to 55%. It also demonstrated that the LV end-diastolic volume decreased from 174 ml to 147 ml, while the end-systolic volume decreased from 147 ml to 67 ml. Echocardiography also showed a reduction in LV end-diastolic dimension from 59 mm to 47 mm, and reductions in left and right atrial volumes from 90 ml and 37 ml, respectively, to 75 ml and 30 ml at 3 months post-procedure. Diastolic function also improved, from grade III to II. Echocardiographic strain imaging showed significant improvements in LV longitudinal deformation in the apex, apicolateral, mid anterolateral. and mid inferoseptal regions, with values increasing from -20%, -12%, -4%, and -9% before parahisian pacing, to -26%, -25%, -17%, and -19% after parahisian pacing, respectively, in these myocardial regions (Figure 3).
Figure 3.
Impact of parahisian pacing on regional left ventricular (LV) deformation on echocardiographic strain imaging. The top panels on the left and right show longitudinal (3-chamber) images of the left ventricle at peak contraction before and after initiation of parahisian pacing. Regional LV deformation is shown for each region (expressed in negative % deformation). The bottom panels show regional strain profiles of each region before and after parahisian pacing; these are more uniform following pacing. BIS = basal inferoseptal; MIS = mid inferoseptal; ApS = apicoseptum, ApL = apicolateral; MAL = mid anterolateral; BAL = basal anterolateral.
Discussion
Reports of “hyper-response” to HBP, defined as normalization of LVEF to ≥50%, are few in the literature.7, 8, 9 Dabrowski et al8 report a patient with normalization of LVEF and NYHA symptoms in a patient with complete (LVEF 28% to 50%, paced QRS 100 ms); Wu et al9 describe a similar patient with complete LBBB, and normalization of LVEF (25% to 50%, paced QRS 90 ms); and Manovel et al7 describe a patient whose LVEF improved to 57% from 30% (paced QRS 80 ms) following HBP for complete 3LBBB2. A unifying theme for these patients is pure His bundle pacing with complete normalization for the QRS duration (≤100 ms). To our knowledge, this patient is the first case reported to have a dramatic recovery of ejection fraction (15% to 55%) with HBP despite incomplete QRS normalization (parahisian pacing). Hyper-response in our patient may have important implications for the use and objective of HBP for cardiac resynchronization for a number of reasons.
Two modes of local tissue capture are operative in His bundle pacing: direct (or pure) Hisian and indirect (or fused) parahisian capture.1, 10 In pure Hisian pacing, depolarization is restricted to the His bundle, with no capture of local myocardium. Fused parahisian capture, as the name implies, indicates depolarization of the Hisian trunk, as well as the local myocardium. Pure His pacing results in latency between the pacing spike and the QRS complex; however, depending on the mechanism of LBBB, it may or may not result in QRS narrowing.1, 2 Compared to the baseline HV interval, the paced “HV” (pacing spike to QRS) remains unchanged with pure Hisian pacing,2, 3 there is concordance of QRS and T-wave complexes, and there is no widening of the QRS at lower output.2, 3 Fused Hisian pacing typically results in shortening of the paced “HV” interval compared to baseline HV (52 ms vs 64 ms in this patient), with a pre-excited electrocardiogram pattern, and relative narrowing of the QRS complex.3 Owing to the capture of local myocardium, depolarization consists of a fusion of basal septal myocardium and ventricular activation via the intrinsic conduction system; hence, a completely normal QRS (<100 ms) is unlikely.
Depending on the mechanism of complete LBBB (central, ie, longitudinal dissociation;11, 12 proximal; or distal), a completely narrow QRS may or may not be achievable. In the case of longitudinal dissociation or proximal block, a pacing electrode situated distal to the site of block with intact distal conduction will likely normalize the QRS complex, while distal block, or complex or multiple anatomic sites of block, is unlikely to result in complete normalization. In cases of proximal block, the virtual pacing electrode may capture the conduction distally when the pacing output is high. Other mechanisms of QRS narrowing include activation of the left septal subendocardium with fusion of wavefronts from septal pacing and Purkinje activation,4 and theoretical entry of the propagating paced wavefront into the conduction system, with distal spread.
In our patient, the site of LBBB is likely proximal but also complex, as it resulted from septal myomectomy and probable resection of a segment of the proximal left bundle. Complete narrowing of the QRS complex is unlikely to occur with HBP in this patient and indicates that the likely mechanism of QRS narrowing is pre-excitation of the left septal subendocardium. Although a major goal of HBP is local capture of the Hisian trunk and normalization of the QRS duration, this may be neither feasible in certain patients nor necessary in others, as the mechanism of LBBB may not be central or proximal. In these patients, extensive mapping and lead deployment to achieve a completely narrow QRS may unnecessarily prolong the procedure and increase the risk of complications. The mechanism of LBBB is also worth considering a priori, as the objective of HBP may differ whether complete QRS narrowing is achievable or not. An electrocardiographic LBBB pattern results from a variety of ventricular activation patterns13 that may affect the ability to effectively resynchronize the ventricles with pure Hisian or parahisian pacing. A strategy in HBP may be an initial goal for pure Hisian pacing, with parahisian as an acceptable alternate result. Other clinical factors that favorably contributed to the observed hyper-response in our patient include nonischemic etiology, short duration of cardiomyopathy (<24 months), and LBBB etiology of intraventricular conduction delay, which have been demonstrated to be predictive in CRT.5, 6, 14
Potential limitations of HBP include elevated thresholds at or following successful implantation, concerns regarding lead stability, feasibility, and ease of lead placement. Although historically, these concerns have been valid, increasing experience suggests that these limitations can be overcome.15 The availability of deflectable sheaths, leads that are particularly amenable for deployment at the atrioventricular junction, and generators with expanded battery capacity suggest the perceived limitations of HBP are gradually being overcome.
In conclusion, this first case report of a hyper-responder to parahisian pacing and incomplete QRS normalization highlights the concept that depending on the mechanism of LBBB, parahisian pacing may be an acceptable method to achieve resynchronization. Further studies are warranted to better understand the applicability of pure Hisian or parahisian (fused) pacing for resynchronization therapy, and other pacing indications.
References
- 1.Lustgarten D.L., Calame S., Crespo E.M., Calame J., Lobel R., Spector P.S. Electrical resynchronization induced by direct his-bundle pacing. Heart Rhythm. 2010;7:15–21. doi: 10.1016/j.hrthm.2009.09.066. [DOI] [PubMed] [Google Scholar]
- 2.Deshmukh P., Casavant D.A., Romanyshyn M., Anderson K. Permanent, direct his-bundle pacing: a novel approach to cardiac pacing in patients with normal his-purkinje activation. Circulation. 2000;101:869–877. doi: 10.1161/01.cir.101.8.869. [DOI] [PubMed] [Google Scholar]
- 3.Sharma P.S., Dandamudi G., Naperkowski A., Oren J.W., Storm R.H., Ellenbogen K.A., Vijayaraman P. Permanent his-bundle pacing is feasible, safe, and superior to right ventricular pacing in routine clinical practice. Heart Rhythm. 2015;12(12):305–312. doi: 10.1016/j.hrthm.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Barba-Pichardo R., Manovel Sanchez A., Fernandez-Gomez J.M., Morina-Vazquez P., Venegas-Gamero J., Herrera-Carranza M. Ventricular resynchronization therapy by direct his-bundle pacing using an internal cardioverter defibrillator. Europace. 2013;15:83–88. doi: 10.1093/europace/eus228. [DOI] [PubMed] [Google Scholar]
- 5.Castellant P., Fatemi M., Orhan E., Etienne Y., Blanc J.J. Patients with non-ischaemic dilated cardiomyopathy and hyper-responders to cardiac resynchronization therapy: characteristics and long-term evolution. Europace. 2009;11:350–355. doi: 10.1093/europace/eup035. [DOI] [PubMed] [Google Scholar]
- 6.Castellant P., Fatemi M., Bertault-Valls V., Etienne Y., Blanc J.J. Cardiac resynchronization therapy: “nonresponders” and “hyperresponders.”. Heart Rhythm. 2008;5:193–197. doi: 10.1016/j.hrthm.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Manovel A., Barba-Pichardo R., Tobaruela A. Electrical and mechanical cardiac resynchronisation by novel direct his-bundle pacing in a heart failure patient. Heart Lung Circ. 2011;20:769–772. doi: 10.1016/j.hlc.2011.05.617. [DOI] [PubMed] [Google Scholar]
- 8.Dabrowski P., Kleinrok A., Kozluk E., Opolski G. Physiologic resynchronization therapy: a case of his bundle pacing reversing physiologic conduction in a patient with CHF and LBBB during 2 years of observation. J Cardiovasc Electrophysiol. 2011;22:813–817. doi: 10.1111/j.1540-8167.2010.01949.x. [DOI] [PubMed] [Google Scholar]
- 9.Wu G., Cai Y., Huang W., Su L. Hisian pacing restores cardiac function. J Electrocardiol. 2013;46:676–678. doi: 10.1016/j.jelectrocard.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Barba-Pichardo R., Manovel Sanchez A., Fernandez-Gomez J.M., Morina-Vazquez P., Venegas-Gamero J., Herrera-Carranza M. Ventricular resynchronization therapy by direct his-bundle pacing using an internal cardioverter defibrillator. Europace. 2013;15:83–88. doi: 10.1093/europace/eus228. [DOI] [PubMed] [Google Scholar]
- 11.Narula O.S. Longitudinal dissociation in the his bundle. Bundle branch block due to asynchronous conduction within the his bundle in man. Circulation. 1977;56:996–1006. doi: 10.1161/01.cir.56.6.996. [DOI] [PubMed] [Google Scholar]
- 12.El-Sherif N., Amay Y.L.F., Schonfield C., Scherlag B.J., Rosen K., Lazzara R., Wyndham C. Normalization of bundle branch block patterns by distal his bundle pacing. Clinical and experimental evidence of longitudinal dissociation in the pathologic his bundle. Circulation. 1978;57:473–483. doi: 10.1161/01.cir.57.3.473. [DOI] [PubMed] [Google Scholar]
- 13.Fung J.W., Yu C.M., Yip G., Zhang Y., Chan H., Kum C.C., Sanderson J.E. Variable left ventricular activation pattern in patients with heart failure and left bundle branch block. Heart. 2004;90:17–19. doi: 10.1136/heart.90.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adelstein E., Schwartzman D., Gorcsan J., 3rd, Saba S. Predicting hyperresponse among pacemaker-dependent nonischemic cardiomyopathy patients upgraded to cardiac resynchronization. J Cardiovasc Electrophysiol. 2011;22:905–911. doi: 10.1111/j.1540-8167.2011.02018.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee M.Y., Yeshwant S.C., Lustgarten D.L. Honing in on optimal ventricular pacing sites: an argument for his bundle pacing. Curr Treat Options Cardiovasc Med. 2015;17:372. doi: 10.1007/s11936-015-0372-3. [DOI] [PubMed] [Google Scholar]