Abstract
Introduction
Bisphenol A (BPA) and Nonylphenol (NP) have estrogen-like activity, and some of their adverse biological effects have been demonstrated. This study was designed to determine the association of plasma and tissue concentrations of BPA and NP and changes in the parameters of the reproductive system in rats.
Methods
Male Wistar rats were administered three doses of BPA and NP (5, 25, and 125 μg/kg) by gavage for 35 consecutive days in 2014–2015, and a 2-ml blood sample was taken from each treated rat. Concentrations of BPA and NP in the blood were determined using the HPLC-fluorescence detection method. The sperm are produced in the epididymis and vas deferens, and they swim up in Ham’s F10 solution, and, then, various parameters were evaluated using an invert microscope, and they included the count, motility, and morphology of the sperm.
Results
The weight of the testes and prostate in the rats receiving BPA and NP treatment showed significant decreases compared to the control group. Similarly, NP created higher concentration than BPA in the serum (e.g., 5.48 ± 0.65 vs. 1.36 ± 0.25, at 125 μg/kg). Compared to the control group, dose-dependent significant decreases in count and motility in the sperm were observed following the administration of BPA (25 and 125 μg/kg) and NP (all three doses). Morphologic aspects of the rats’ sperm were changed in various doses of BPA and NP
Conclusions
According to our findings, BPA and NP induced dose-dependent toxic effects on various parameters, i.e., sperm toxicity, weight of the testes, and weight of the prostate gland.
Keywords: Bisphenol A (BPA), Nonylphenol (NP), Spermatogenesis, HPLC, Fluorescence detector, Prostate
1. Introduction
Most of chemicals used be people are unavoidably destructive to the environment. Among them, plasticizers, such as nonylphenol (NP) and bisphenol A (BPA) are used largely in the plastics industry in which many of their harmful effects have been reported (1). NP is a yellow, sticky substance that has a molecular weight of 220.35 g/mol and a density of 0.953 g/cm3. This chemical is hydrophobic and relatively insoluble in water, and its solubility depends on the temperature and pH of the solution (2). BPA also is a product of the condensation reaction between two moles of phenol and one mole of acetone in the presence of concentrated sulfuric acid. This chemical has a molecular weight of 228.29 g/mol and a density of 1.2 g/cm3 at 20 °C. Its solubility in water is 120–300 ppm (3). These chemicals are used extensively in epoxy resins, polycarbonates, dental fillings, food preserving containers, baby bottles, and mineral water containers. NP and BPA have little stability in acid or alkaline conditions, and they are unstable upon exposure to UV radiation. These chemicals have the ability to transfer from polymers to foods, and they can contaminate foods at high or low temperatures (4). BPA and NP can be leached from plastic wastes and enter rivers in the form of landfill leachate, which is considered to be a serious threat to aquatic life. Relatively high concentrations of these chemicals have been found in marine organisms, and these concentrations indicate the high solubility of these compounds in water (2–5). BPA and NP have estrogenic characteristics (3). These chemicals have a variety of adverse effects on people, among which is the reduction of the fertility of men (6). Exposure of rats to very low concentrations of BPA and NP can cause adverse effects, including decreases in the daily production of sperm and acceleration of growth and maturity (7, 8). The detected amounts of BPA and NP in groups exposed to these chemicals are different. For example, a range of 10–20 μg of BPA was observed in individuals who use canned foods, and 20–30 μg of BPA were detected among those who used dental filling substances (9). The standard reference dose established by the U.S. Environmental Protection Agency (EPA) is 50 μg/kg/day, which is equal to 1/1000 its threshold dose (50 mg/kg/day) recommended by EPA (10). However, studies have shown that even doses less than the safe dose can disturb the physiological function of the human reproductive system (11). It has been reported that the exposure of rats to 25 ng/kg of BPA and NP can impair spermatogenesis and reduce sperm counts (12). BPA used in food packaging materials increases the development of a specific type of prostate cancer with mutant androgen receptors. For management of most prostate cancers, testosterone is required to develop, and inhibition of the production of this hormone is one of the methods used in the treatment of prostate cancer (13, 14). It also has been shown that BPA affects the quality of sperm (15) and reduces the weight of the prostate gland, as well as other reproductive organs (8, 16, 17).
There are different methods for assaying BPA and NP in biological samples, e.g., spectrophotometry and gas chromatography-mass spectrometry (GC-MS). GC-MS, which is widely used for this purpose, has high accuracy (18–20). Another analytical method for assaying these compounds is high performance liquid chromatography (HPLC) with different detectors, such as ECD, UV, and fluorescence, that have high accuracy and are used for biological samples, such as plasma and tissues (19, 21, 22). In this study, HPLC with a fluorescence detector was used as a simple, cost-effective, and accurate technique for the detection of BPA and NP. According to the literature and based on the increased use of BPA and NP, we attempted to study the effects of low doses of these compounds on reproductive parameters, such as sperm count and sperm morphology. Moreover, the absorbed concentrations of these compounds in the serum and testicular tissue of adult rats was investigated using HPLC.
2. Material and Methods
2.1. Animals
In this study, which was performed in 2014–2015, we used male Wistar rats that weighed 150–200 g. The study was approved by the Ethics Committee at Babol University of Medical Sciences (Babol, Iran), and the investigation was conducted under the guidelines for safe animal research. The animals were housed under standard laboratory conditions, i.e., temperature of 22±2 °C, 12 h light and 12 h dark, and free access to tap water and chow ad libitum. The rats were divided into control and treatment groups. The treatment group received three doses (5, 25, and 125 μg/kg) of BPA and NP by gavage for 35 consecutive days. The BPA and NP were dissolved in corn oil as a vehicle and then used. The control group only received corn oil. On day 36, the rats were anesthetized using sodium thiopental and blood samples were taken from the axillary vein for assay of BPA and NP concentration in the serum. The epididymis and vas deferens were removed to obtain sperm parameters.
2.2. Chemicals
The NP was obtained from Kento, Japan, and the BPA was purchased from Daejung, Korea; both were analytical grade. HPLC solvent, acetonitrile, methanol, and water for extraction were purchased from Merck, Germany. SPE disposable columns (INOPAK, Korea) were used for washing and filtering the tissue samples. Standard solutions of BPA and NP prepared using HPLC grade acetonitrile were used in the experiments.
2.3. Blood samples
Immediately after anesthetizing the rats with sodium thiopental, the axillary plexus was opened and after cutting axillary vessels, a 2-ml blood sample was taken. The blood samples were transferred to 5-ml Eppendorf tube, and the serum was separated by centrifugation (15 min at 3500 rpm) and kept in a freezer at −20 °C for the next analysis. A 100-μl aliquot of the serum was transferred to a 5-ml vial, and then we added 100 μl of ammonium acetate buffer with a pH of 4.5 and 4 ml of solvent containing n-hexane and diethyl ether (70:30) to the vial, after which the mixture was vortexed for 30 minutes. Then, the sample was centrifuged at 10,000 rpm for 10 minutes; the organic phase was separated and placed in the refrigerator at 5 °C to remove the solvent. After extracting the solvent, 100 μl of the mobile phase solvent were added to the vial, and the mixture was vortexed again. An aliquot of 20-ml was injected into the HPLC, and the concentrations of BPA and NP in the serum were measured using a standard calibration curve that had been prepared earlier.
2.4. Testis samples
After treatment for each experimental group, testis tissue was removed, weighed, and kept at −20 °C to assay concentrations of BPA and NP. Then, for analysis of the BPA and NP concentrations, the testis samples were removed from the freezer and allowed to thaw at ambient temperature. Then, 1 g of testicular tissue was put in the mortar, and, while adding liquid nitrogen, the tissue was ground using a pestle and then transferred to a 15 ml centrifuge vial. Then, 20 ml of ammonium acetate buffer (0.01 M) with pH 4.5, 8 ml of methanol, and 100 μl of perchloric acid (4 M) were added to the vial. The mixture was vortexed for 30 seconds for homogenization and then centrifuged at 14,000 rpm at 4 °C. An SPE C18 cartridge was used to filter the sample using solvents, including 10 ml of methanol, 5 ml of water, 5 ml of ammonium acetate buffer, and another 5 ml of water; a vacuum pump was used to accelerate the filtration process. The filtrate was poured in a 50-ml vial (with open lid) and put in the refrigerator at 4 °C until the solvent was removed. Then, 100 μl of mobile phase solvent were added to the vial; 20 μl of the sample was injected directly into the HPLC, and concentrations of BPA and NP in the testicular tissue samples were determined.
2.5. HPLC analysis
High performance liquid chromatography (HPLC, Knauer, Germany) equipped with fluorescent detector (Shimadzu, Japan) was used to measure the concentrations of BPA and NP in the serum and testis tissue samples. The working conditions were as follows: the absorption and emission wavelengths were 227 and 313 nm, respectively. The column was C18 (Eurospher), and the mobile phase was a mixture of acetonitrile and water in the ratio of 70 to 30 (v/v %) for 0 to 6 min, 6.0–6.2 min mobile phase A increased from 70 to 100% and continued to minute 12. The temperature was 25 °C, and the flow rate of the mobile phase was 0.8 ml/min. Ezchrome software was used to extract area and height of the peaks. For this analysis, nonylphenol (NP) was used as the internal standard for bisphenol A (BPA) and vice versa. Peak area ratio (area of each compound divided by the area of the internal standard) was calculated for each analysis, and the standard curve for BPA and NP was prepared by plotting the ratio versus serum or tissue concentration of BPA and NP. The slope, intercept, and R2 coefficient were calculated. The coefficient variation (CV) for this analysis was 3.7%.
2.6. Sperm parameters
After anesthetizing the rats, the epididymis and vas deferens were isolated and put in Ham’s F10 medium. The samples were incubated at 37 °C in a 5% CO2 atmosphere for at least 30 minutes until the sperm were released from the epididymis and deferens ducts and suspended in the culture. Then, all of the supernatant of the culture was collected. The obtained liquid was transferred to a tube, and the same volume of fresh Ham’s F10 medium was added to it. Then, a smear slide was prepared from the liquid specimen and analyzed for sperm parameters, including sperm count, motility, and morphology, using an invert microscope (Olympus). This procedure was followed for all groups receiving BPA and NP as well as for the controls.
2.7. Statistical analysis
The data were presented as mean ± standard deviation. The results were analyzed using t-test and ANOVA post-hoc Tukey tests, and the difference between the data was considered statistically significant at p-value less than 0.05.
3. Results
3.1. Plasma and tissue concentrations of the BPA and NP
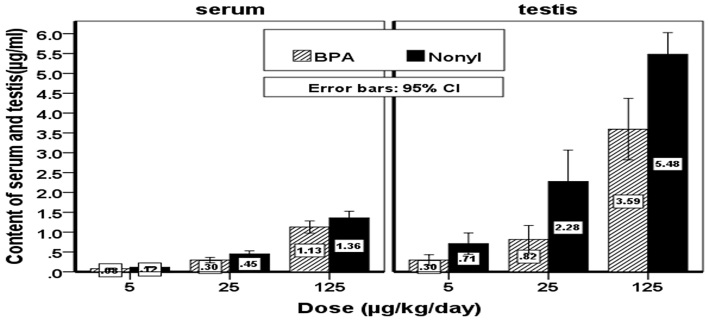
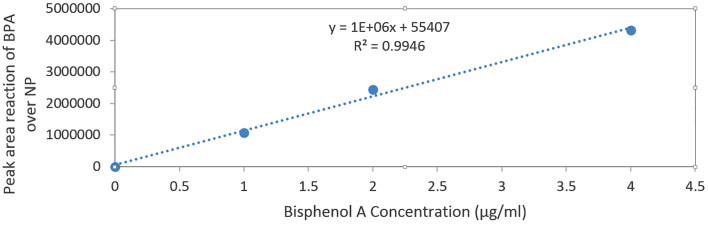
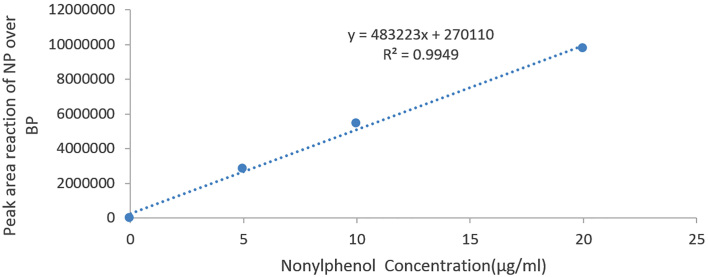
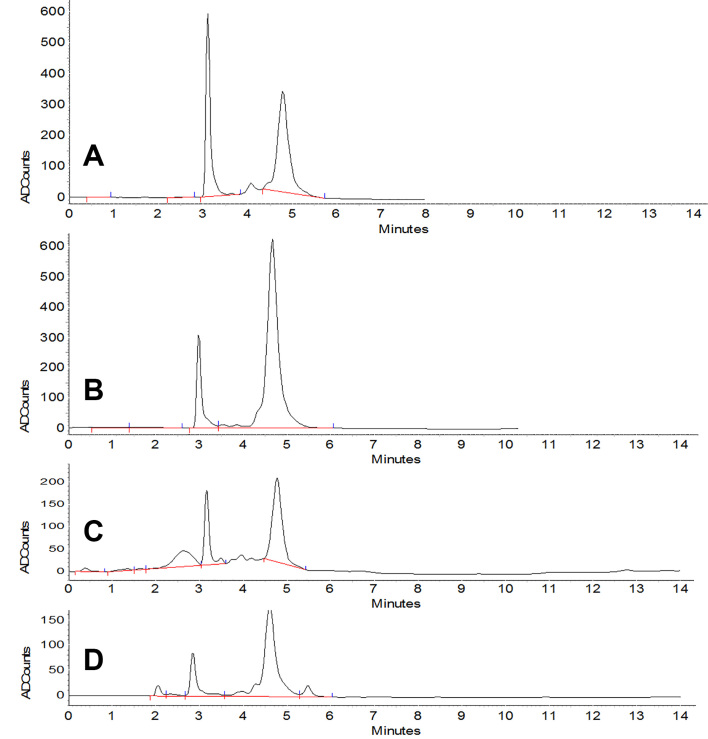
The mean and standard deviation of BPA and NP concentrations were measured, and there were significant concentrations in the rats in the NP group compared to BPA group (p < 0.05) (Figure 1). The difference also was significant between the groups receiving different doses of BPA, i.e., 5, 25, and 125 μg/kg body weight and those receiving NP (p < 0.05). The concentrations of BPA and NP in the serum and testicular tissues were determined based on the internal standard method using calibration curves. The calibration curves are presented in Figures 2 and 3 for BPA and NP, respectively. Figure 4 (chromatogram) also shows sample HPLC chromatograms obtained for BPA and NP analysis. According to Table 1, the NP at doses equal to the doses of BPA had higher concentrations.
Figure 1.
Content of serum and testis concentration of NP and BPA groups
Figure 2.
Standard curve of the BPA, peak area ratio of the BPA over the NP as internal standard vs. BPA concentration (μg/ml)
Figure 3.
Standard curve of the NP, peak area ratio of the NP over the BPA as internal standard vs. NP concentration (μg/ml)
Figure 4.
Sample peaks of BPA and NP, (3.3 min) BPA, (5 min) NP (A) standard BPA (4 μg/ml) & internal standard 20 μg/ml NP; (B) standard NP (20 μg/ml) & internal standard 2 μg/ml BPA; (C) tissue sample 25 μg/kg BPA & internal standard 20 μg/ml NP; (D) serum sample 25 μ/kg BPA & internal standard 20 μg/ml NP
Table 1.
Body weight before and after experiments, weight of testes and prostate, ratio of testes and prostate over body weight of the rats in control and BPA and NP treatment groups
| Groups | (Initial/Final) Body weight (g) | Testes weight (g) | Testes wt/body wt ratio | Prostate weight (g) | Prostate wt/body wt ratio |
|---|---|---|---|---|---|
| Control | 6 | 1.419±0.075 | 0.238 | 0.596±0.076 | 0.099 |
| BPA 5 μg/kg | −6.4 | 1.357±0.139 | −0.212 | 0.269±0.086 | −0.042 |
| BPA 25 g/kg | −12.30 | 1.183±0.090 | −0.096 | 0.225±0.029 | −0.018 |
| BPA 125/kg | −6.00 | 1.166±0.056 | −0.194 | 0.159±0.072 | −0.027 |
| NP 5 μg/kg | −30.70 | 1.148±0.110 | −0.037 | 0.218±0.115 | −0.007 |
| NP 25 μg/kg | −29.60 | 1.101±0.060 | −0.037 | 0.166±0.034 | −0.006 |
| NP 125 g/kg | −41.80 | 0.991±0.183 | −0.024 | 0.096±0.069 | −0.002 |
Wt: weight
3.2. Weight changes
The rats’ weights were recorded before the start of the experiments and also at the end of the study. There were significant weight losses in the rats that received BPA and NP compared to the control (Table 1). This weight loss was considered to be in the testes of the BPA and NP groups when compared to the control, as well (p < 0.05). The difference was also significant between the groups receiving different doses of the BPA, i.e., 5, 25, and 125 μg/kg body weight, and those receiving the NP (p < 0.05). No significant differences were seen in the testes of the rats receiving various doses of BPA. The weight of prostate tissues in BPA and NP treatment groups showed a significant decrease in comparison to the control (p < 0.05). However, no significant decrease was seen between the treatments groups receiving BPA and NP. The results showed that contact with high doses of BPA and NP may cause a considerable decrease in the weight of testis, while the weight of the prostate gland showed a further decrease (Table 1).
3.3. Evaluation of sperm parameters
Sperm count, motility, and morphology were evaluated using an invert microscope, and the results are summarized in Table 2. There was a significant decrease in the percentage of sperm motility in groups receiving BPA and NP compared to the control (p < 0.05). The high-speed, direct movement and movements toward unknown directions were reduced. BPA and NP treatment induced slow forward movement of sperm and sperm in situ motility was decreased in the groups that received doses of BPA and NP compared to the control group (p < 0.05). At high dosages of NP, the sperm’s motility almost stopped (Table 2).
Table 2.
Sperm parameters in control, BPA and NP treatment groups: Grade 4 - Fast and forward progression where sperm move in a straight direction; Grade 3 - Sperm move forward but at a slower speed and/or in a curved direction; Grade 2 - Sperm move slowly and in a poorly-defined direction; Grade 1 - Sperm move but fail to progress forward.
| Groups | Sperm Count*106 | Motility % | IV % | III % | II % | I % |
|---|---|---|---|---|---|---|
| Control | 105 | 29 | 10 | 30 | 45 | 15 |
| BPA 5 μg/kg | 80 | 17 | 8 | 22 | 3 | 40 |
| BPA 25 μg/kg | 77 | 16 | 1 | 8 | 20 | 71 |
| BPA 125 μg/kg | 58 | 10 | 1 | 4 | 13 | 82 |
| NP 5 μg/kg | 69 | 16 | 0 | 1 | 5 | 94 |
| NP 25 μg/kg | 58 | 11 | 0 | 1 | 11 | 88 |
| NP 125 μg/kg | 54 | 2 | 0 | 1 | 2 | 97 |
4. Discussion
This study was conducted to determine the association between serum and testicular tissue concentrations of BPA and NP and sperm parameters of rats receiving bisphenol A and nonylphenol. The levels of BPA and NP in serum and testicular tissue samples were assayed using HPLC equipped with fluorescence detector. The count, motility, and grading of sperm, as well as the weight of the body, testes, and prostate gland were evaluated in the control, BPA, and NP groups. According to the results, HPLC-fluorescence detection method was very accurate, easy, and economical to measure the concentration of these compounds, and it is preferable to common laboratory methods. Although, various methods, such as GC-MS, LC-MS, and HPLC-FLD, have been reported to assay BPA and NP in water, soil, and plastic samples (23, 24), only a few methods for measuring BPA and NP in biological samples have been introduced. Mikaoda et al. determined the concentration of BPA in serum using the GC–MS technique, and they used the LC–MS method for detection of NP in tissue samples (25). The more convenient method for assaying NP is extraction by steam-distillation and then using HPLC with a UV detector (26). In the present study, retention times required for the BPA and NP peaks to appear were 3 and 5 min, respectively. These times were shorter than the previously-reported results (4.5 and 12 min for BPA and NP, respectively) (8). In our study, liquid–liquid extraction (LLE) was used to detect BPA and NP in serum. To avoid column obstruction, organic solvents were used to remove proteins and lipids of the samples and SPE (Solid phase extraction) column was recruited. Our study showed that 5, 25, and 125 μg/kg/day of both NP and BPA can significantly reduce the body weight, ratio of testes and prostate weights to body weight of the rats in the control and BPA and NP treatment groups (Figure 4). These results were similar to several previous studies. Similarly, the findings indicated that exposure to NP resulted in decreases in the weights of the rats’ testes, decreased epididymal sperm motility, and affected sperm morphology (27). In a previous study, it was shown that oral administration of various doses of PBA (0.2, 2 and 20 μg/kg in corn oil) for 45 consecutive days significantly reduced the weights of the epididymis and testes of the rats, while the weight of their prostates had significant increases compared to the control (17). Unlike the findings of our study, these doses of BPA did not show any effect on the body weight of the rats. Such an effect may be caused by oxidative stress reactions in the presence of BPA in the tissues (17). In the present study, the count and motility of sperm in rats treated with BPA and NP had a significant decrease compared to the control group while the morphology of sperm did not show any considerable change. A significant decrease in motility of sperm has been reported in the male offspring rats of mothers that had received high doses of the NP during their pregnancy. Also, at high doses, the sperm had abnormal movements as well (28). BPA and NP have been shown to have adverse effects on different species, including fish (29, 30), quail (31), and mice (30). In mammals, successful fertilization can occur only when the sperm have enough motility and normal structure (30), but any changes in sperm count, motility, and morphology may affect normal fertility adversely. It has been shown that NP (750 ng/ml) had a destructive effect on the sperm of Medaka fish.
5. Conclusions
This study showed that NP (1 μg/ml) can significantly change the acrosome of sperm. The movement of the sperm can stop at concentrations of 250 μg/ml and higher, and they may die at concentrations higher than 500 μg/ml (27). Based on the results, both BPA and NP have adverse effects on sperm parameters. These effects were greater for NP than BPA. This may be due to the higher concentration of NP in serum or testis tissue after administration of equal dosages of BAP and NP.
Acknowledgments
The authors thank Dr. Ali Bijani for his cooperation and assistance. This investigation was supported financially by the Research Affairs Division of Babol University of Medical Sciences.
Footnotes
iThenticate screening: April 19, 2016, English editing: May 10, 2016, Quality control: August 02, 2016
Conflict of Interest:
There is no conflict of interest to be declared.
Authors’ contributions:
All authors contributed to this project and article equally. All authors read and approved the final manuscript.
References
- 1.Careghini A, Mastorgio AF, Saponaro S, Sezenna E. Bisphenol A, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: a review. Environ Sci Pollut Res Int. 2015;22(8):5711–41. doi: 10.1007/s11356-014-3974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vazquez Duhalt R, Marquez Rocha F, Ponce E, Liicea F, Viana MT. Nonylphenol, anintegrated vision of a pollutant. Applied Ecology and Environmental Research. 2005;4(1):1–25. doi: 10.15666/aeer/0401-001025. [DOI] [Google Scholar]
- 3.Ben Jonathan N, Steinmetz R. Xenoestrogens: the emerging story of bisphenol a. Trends Endocrinol Metab. 1998;9(3):124–8. doi: 10.1016/s1043-2760(98)00029-0. [DOI] [PubMed] [Google Scholar]
- 4.Talsness CE, Andrade AJ, Kuriyama SN, Taylor JA, vom Saal FS. Components of plastic: experimental studies in animals and relevance for human health. Philos Trans R Soc Lond B Biol Sci. 2009;364(1526):2079–96. doi: 10.1098/rstb.2008.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mortazavi S, Bakhtiari AR, Sari AE, Bahramifar N, Rahbarizade F. Phenolic endocrine disrupting chemicals (EDCs) in Anzali Wetland, Iran: elevated concentrations of 4-nonylphenol, octhylphenol and bisphenol A. Mar Pollut Bull. 2012;64(5):1067–73. doi: 10.1016/j.marpolbul.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Recchia AG, Vivacqua A, Gabriele S, Carpino A, Fasanella G, Rago V, et al. Xenoestrogens and the induction of proliferative effects in breast cancer cells via direct activation of oestrogen receptor alpha. Food Addit Contam. 2004;21(2):134–44. doi: 10.1080/02652030310001641177. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura D, Yanagiba Y, Duan Z, Ito Y, Okamura A, Asaeda N, et al. Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. Toxicol Lett. 2010;194(1–2):16–25. doi: 10.1016/j.toxlet.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Xiao Q, Li Y, Ouyang H, Xu P, Wu D. High-performance liquid chromatographic analysis of bisphenol A and 4-nonylphenol in serum, liver and testis tissues after oral administration to rats and its application to toxicokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;830(2):322–9. doi: 10.1016/j.jchromb.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 9.Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect. 2001;109(7):675–80. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin P, Wang X, Chang F, Bai Y, Li Y, Zhou R, et al. Low dose bisphenol A impairs spermatogenesis by suppressing reproductive hormone production and promoting germ cell apoptosis in adult rats. J Biomed Res. 2013;27(2):135–44. doi: 10.7555/jbr.27.20120076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derouiche S, Warnier M, Mariot P, Gosset P, Mauroy B, Bonnal JL, et al. Bisphenol A stimulates human prostate cancer cell migration via remodelling of calcium signalling. Springerplus. 2013;2(1):54. doi: 10.1186/2193-1801-2-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66(11):5624–32. doi: 10.1158/0008-5472.can-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salian S, Doshi T, Vanage G. Perinatal exposure of rats to Bisphenol A affects the fertility of male offspring. Life Sci. 2009;85(21–22):742–52. doi: 10.1016/j.lfs.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Gomella GL. Effective Testosterone Suppression for Prostate Cancer: Is There a Best Castration Therapy? Rev Urol. 2009;11(2):52–60. [PMC free article] [PubMed] [Google Scholar]
- 15.Timms BG, Howdeshell KL, Barton L, Bradley S, Richter CA, vom Saal FS. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc Natl Acad Sci U S A. 2005;102(19):7014–9. doi: 10.1073/pnas.0502544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prins GS, Ye SH, Birch L, Ho SM, Kannan K. Serum bisphenol A pharmacokinetics and prostate neoplastic responses following oral and subcutaneous exposures in neonatal Sprague-Dawley rats. Reprod Toxicol. 2011;31(1):1–9. doi: 10.1016/j.reprotox.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chitra KC, Latchoumycandane C, Mathur PP. Induction of oxidative stress by bisphenol A in the epididymal sperm of rats. Toxicology. 2003;185(1–2):119–27. doi: 10.1016/s0300-483x(02)00597-8. [DOI] [PubMed] [Google Scholar]
- 18.Tsutsumi S, Yamaguchi Y, Nishida I, Akiyama K, Zakaria MP, Takada H. Alkylbenzenes in mussels from South and South East Asian coasts as a molecular tool to assess sewage impact. Mar Pollut Bull. 2002;45(1–12):325–31. doi: 10.1016/s0025-326x(02)00151-0. [DOI] [PubMed] [Google Scholar]
- 19.Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110(11):703–7. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katayama M, Sasaki T, Matsuda Y, Kaneko S, Iwamoto T, Tanaka M. Sensitive determination of bisphenol A and alkylphenols by high performance liquid chromatography with pre-column derivatization with 2-(4-carboxyphenyl)-5,6-dimethylbenzimidazole. Biomed Chromatogr. 2001;15(6):403–7. doi: 10.1002/bmc.88. [DOI] [PubMed] [Google Scholar]
- 21.Kvistad AM, Lundanes E, Greibrokk T. Determination of alkylphenols in water samples by solid-phase extraction on to poly(styrene-divinylbenzene) and quantification by liquid chromatography with UV-detection. Chromatographia. 1998;48(9):707–13. doi: 10.1007/BF02467603. [DOI] [Google Scholar]
- 22.Aly HA, Domenech O, Banjar ZM. Effect of nonylphenol on male reproduction: analysis of rat epididymal biochemical markers and antioxidant defense enzymes. Toxicol Appl Pharmacol. 2012;261(2):134–41. doi: 10.1016/j.taap.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Xiao QW, Li YQ, Zhang H, Liang JL, Wu DS. [Determination of 4-nonylphenol and bisphenol A in rat serum by high performance liquid chromatography with fluorescence detection]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2004;35(2):271–3. 6. [PubMed] [Google Scholar]
- 24.Miyakoda H, Tabata M, Onodera S, Takeda K. Comparison of Conjugative Activity, Conversion of Bisphenol A to Bisphenol A Glucuronide, in Fetal and Mature Male Rat. Journal of Health Science. 2000;46(4):269–74. doi: 10.1248/jhs.46.269. [DOI] [Google Scholar]
- 25.Jing X, Bing S, Xiaoyan W, Xiaojie S, Yongning W. A study on bisphenol A, nonylphenol, and octylphenol in human urine amples detected by SPE-UPLC-MS. Biomed Environ Sci. 2011;24(1):40–6. doi: 10.3967/0895-3988.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 26.McClusky LM, de Jager C, Bornman MS. Stage-related increase in the proportion of apoptotic germ cells and altered frequencies of stages in the spermatogenic cycle following gestational, lactational, and direct exposure of male rats to p-nonylphenol. Toxicol Sci. 2007;95(1):249–56. doi: 10.1093/toxsci/kfl141. [DOI] [PubMed] [Google Scholar]
- 27.Hara Y, Strussmann CA, Hashimoto S. Assessment of short-term exposure to nonylphenol in Japanese medaka using sperm velocity and frequency of motile sperm. Arch Environ Contam Toxicol. 2007;53(3):406–10. doi: 10.1007/s00244-006-0172-6. [DOI] [PubMed] [Google Scholar]
- 28.Jie X, Yang W, Jie Y, Hashim JH, Liu XY, Fan QY, et al. Toxic effect of gestational exposure to nonylphenol on F1 male rats. Birth Defects Res B Dev Reprod Toxicol. 2010;89(5):418–28. doi: 10.1002/bdrb.20268. [DOI] [PubMed] [Google Scholar]
- 29.Lahnsteiner F, Berger B, Grubinger F, Weismann T. The effect of 4-nonylphenol on semen quality, viability of gametes, fertilization success, and embryo and larvae survival in rainbow trout (Oncorhynchus mykiss) Aquat Toxicol. 2005;71(4):297–306. doi: 10.1016/j.aquatox.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Razia S, Maegawa Y, Tamotsu S, Oishi T. Histological changes in immune and endocrine organs of quail embryos: exposure to estrogen and nonylphenol. Ecotoxicol Environ Saf. 2006;65(3):364–71. doi: 10.1016/j.ecoenv.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 31.Wassarman PM. Contribution of mouse egg zona pellucida glycoproteins to gamete recognition during fertilization. J Cell Physiol. 2005;204(2):388–91. doi: 10.1002/jcp.20389. [DOI] [PubMed] [Google Scholar]