Abstract
Central nervous system (CNS) infection continues to be an important cause of mortality and morbidity, necessitating new approaches for investigating its pathogenesis, prevention and therapy. Escherichia coli is the most common Gram-negative bacillary organism causing meningitis, which develops following penetration of the blood–brain barrier (BBB). By chemical library screening, we identified epidermal growth factor receptor (EGFR) as a contributor to E. coli invasion of the BBB in vitro. Here, we obtained the direct evidence that CNS-infecting E. coli exploited sphingosine 1-phosphate (S1P) for EGFR activation in penetration of the BBB in vitro and in vivo. We found that S1P was upstream of EGFR and participated in EGFR activation through S1P receptor as well as through S1P-mediated up-regulation of EGFR-related ligand HB-EGF, and blockade of S1P function through targeting sphingosine kinase and S1P receptor inhibited EGFR activation, and also E. coli invasion of the BBB. We further found that both S1P and EGFR activations occurred in response to the same E. coli proteins (OmpA, FimH, NlpI), and that S1P and EGFR promoted E. coli invasion of the BBB by activating the downstream c-Src. These findings indicate that S1P and EGFR represent the novel host targets for meningitic E. coli penetration of the BBB, and counteracting such targets provide a novel approach for controlling E. coli meningitis in the era of increasing resistance to conventional antibiotics.
Author Summary
Escherichia coli is the most common Gram-negative bacillary organism causing meningitis, and E. coli meningitis continues to be an important cause of mortality and morbidity. E. coli penetration of the blood–brain barrier (BBB) is essential for the development of E. coli meningitis, but the underlying mechanisms remain incompletely understood. Recent reports of E. coli strains producing CTX-M-type or TEM-type extended-spectrum β-lactamases, including antimicrobial-resistant E. coli sequence type 131 (ST131) are of particular concern. These findings necessitate searches for new targets for investigating the pathogenesis and therapeutic development of E. coli meningitis. Our work demonstrated for the first time that sphingosine 1-phosphate (S1P) activation of epidermal growth factor receptor (EGFR) represents a novel mechanism by which CNS-infecting E. coli strains penetrate the BBB, and that blockade of S1P and EGFR prevented E. coli penetration of the BBB. We also determined that the specific E. coli factors contributing to penetration of the BBB exploit S1P-EGFR signaling, and that c-Src is downstream of S1P-EGFR. Our findings reveal a novel mechanism by which meningitic E. coli penetrates the BBB, and also demonstrate the novel targets for investigating the pathogenesis, prevention, and therapy of E. coli meningitis.
Introduction
Bacterial meningitis is currently recognized as one of the top ten leading causes of global deaths from infectious diseases. Case fatality rates range from 5–25%, and approximately 25–50% of survivors sustain neurologic sequelae [1–4]. The morbidity and mortality rates of bacterial meningitis vary, depending on age, immune state, patient location, and causative organism. Patient groups at risk of high rates of mortality and morbidity include newborns, the elderly, and those living in developing countries, while the infections with higher rates of mortality and morbidity are those caused by Gram-negative bacilli [2,3].
Escherichia coli is the most common Gram-negative bacillary organism causing meningitis [1–4]. Most cases of E. coli meningitis develop from hematogenous spread [5,6], and occur as a result of the bacterial penetration of the blood–brain barrier (BBB), which is a prerequisite for the development of central nervous system (CNS) infection [1–4].
The BBB consists of brain microvascular endothelial cells, astrocytes and pericytes, and is a structural and functional barrier that maintains the neural microenvironment by regulating the passage of molecules into and out of brain, and prevents circulating microbes from penetrating into the brain [1,2]. Meningitis isolates of E. coli, however, have been shown to invade the BBB [1–4], but it remains incompletely understood how circulating E. coli strains penetrate the BBB.
Several lines of evidence from human cases and experimental animal models of E. coli meningitis indicate that E. coli penetration into the brain follows a high level of bacteremia, and that cerebral capillaries are the portal of entry into the brain [1–6]. Since E. coli penetration into the brain occurred in the cerebral microvasculature [5], we developed the in vitro BBB model with human brain microvascular endothelial cells (HBMEC) to investigate E. coli invasion of the BBB [7,8]. We also developed the in vivo animal model of experimental hematogenous meningitis to mimic E. coli penetration into the brain that occurs in neonatal meningitis [5]. We have shown with both in vitro and in vivo models that E. coli invasion of HBMEC is directly correlated with its penetration into the brain [9–15], suggesting that elucidation of the mechanisms involved in E. coli invasion of HBMEC is likely to enhance our knowledge on the pathogenesis of E. coli meningitis.
We took advantage of genome sequencing information available from meningitis isolates of E. coli (e.g., strains IHE3034, S88, RS218) to study E. coli penetration of the BBB. Using functional genomics studies (e.g., transposon and signature-tagged mutagenesis, DNA microarray and comparative genome hybridization), we have identified several microbial factors contributing to meningitic E. coli invasion of HBMEC, which include OmpA, FimH, NlpI, IbeA, IbeB, IbeC and CNF1 [9–12,15–22]. We have also shown that these microbial factors exploit specific host receptors and host cell signaling molecules for bacterial invasion of HBMEC [2,3]. For example, OmpA interacts with gp96 on HBMEC, resulting in activation of focal adhesion kinase (FAK), while FimH interaction with CD48 and CNF1 interaction with 37 kDa laminin receptor precursor (37LRP) lead to activation of RhoGTPases [23–27]. Biological relevance of these microbial-host interactions in the pathogenesis of E. coli meningitis is shown by the demonstrations that (a) exogenous OmpA and gp96 and anti-gp96 antibodies block E. coli invasion of HBMEC, but do not exhibit any blocking effect on the OmpA mutant [23,24], (b) addition of exogenous FimH- or CD48- antibodies inhibits E. coli invasion of the HBMEC [25], and (c) expression levels of 37LRP dictates the ability of E. coli to invade HBMEC, but exhibited no effect on the CNF1 mutant [26,27]. Despite the extensive information available on microbial and host factors as well as host cell signaling molecules contributing to E. coli invasion of HBMEC [2–4], the mechanisms involved in E. coli penetration of the BBB remain incompletely understood.
Since meningitic E. coli invasion of HBMEC is correlated with its penetration into the brain, we used E. coli invasion of HBMEC as a biologically relevant model in the present study for screening of a chemical library to discover novel targets affecting E. coli penetration of the BBB. In our screen, we identified that gefitinib, a selective inhibitor of epidermal growth factor receptor (EGFR) tyrosine kinase [28], significantly inhibited E. coli invasion into HBMEC monolayers. EGFR belongs to the ErbB family of receptor tyrosine kinases (RTKs), consisting of four closely-related members (ErbB1/EGFR, ErbB2, ErbB3, ErbB4) [29–31]. EGFR is initially expressed in the plasma membrane in an inactive form, and becomes activated through certain kinases and/or after binding to its specific ligands, which are produced as transmembrane precursors and released by proteolytic cleavage [29–32]. To date, several bacterial pathogens have been reported to target EGFR through different mechanisms to facilitate their infection of host cells, including Neisseria gonorrhoeae, Neisseria meningitidis, Helicobacter pylori, Haemophilus influenzae, and Klebsiella pneumoniae [33–38]. However, to our knowledge, it is unknown whether EGFR is involved in meningitic E. coli invasion of the BBB. In the present study, we reported for the first time that sphingosine 1-phosphate (S1P)-mediated activation of EGFR represents a novel mechanism exploited by meningitic E. coli for penetration of the BBB, the essential step in the development of E. coli meningitis. EGFR as well as S1P are, therefore, likely to represent the novel targets for investigating the pathogenesis, prevention and therapy of E. coli meningitis.
Results
Meningitic E. coli exploits EGFR for its penetration of the BBB
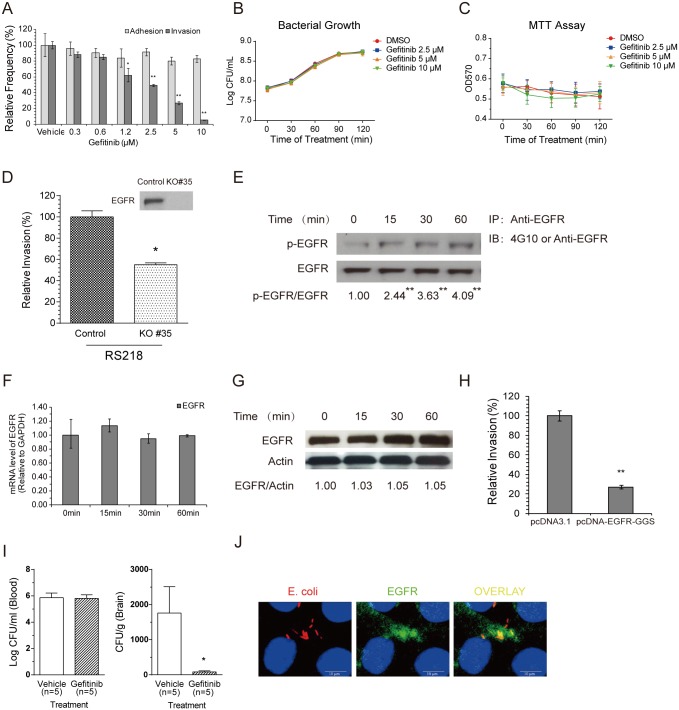
Our chemical screen identified gefitinib as an inhibitor of meningitic E. coli invasion of HBMEC monolayer. Gefitinib is a low-molecular-weight anilinoquinazoline that selectively inhibits EGFR [28]. EGFR has been shown to play essential roles in cell proliferation, survival, and migration as well as in carcinogenesis and cancer progression, and is considered an attractive target for anticancer therapies [29–31]. However, the role of EGFR in E. coli meningitis is unknown. To address this issue, we first determined the effect of gefitinib on meningitic E. coli strain RS218 binding to and invasion of HBMEC. We found that gefitinib inhibited RS218 invasion of HBMEC in a dose-dependent manner without affecting E. coli adhesion to HBMEC (Fig 1A), suggesting that its target, EGFR, is likely to be involved in E. coli invasion of the BBB. Gefitinib did not affect bacterial growth, as assessed by determination of colony-forming units (CFUs) in the presence and absence of gefitinib (Fig 1B). The cytotoxicity and proliferation of the HBMEC, as assessed by live/dead stain (Molecular Probes) and MTT assays, were not affected by gefitinib (Fig 1C). EGFR was subsequently knocked out from HBMEC via CRISPR-Cas9 editing approach, and bacterial invasion of the EGFR knock-out cells (KO#35) were compared with that of the control cells. The EGFR was not detectable in the KO#35 HBMEC by the Western blotting (Fig 1D), and RS218 invasion of the KO#35 cells was significantly decreased compared with that of the control cells (Fig 1D). We next examined the contribution of EGFR tyrosine kinase activity to E. coli invasion of HBMEC. We showed a time-dependent tyrosine phosphorylation of EGFR in response to meningitic E. coli RS218 infection (Fig 1E) but no change in EGFR transcription or expression (Fig 1F and 1G). E. coli invasion of HBMEC transfected with dominant-negative EGFR, pcDNA-EGFR-GGS, encoding EGFR without tyrosine kinase activity [39], was significantly decreased compared with that of control vector-transfected cells (Fig 1H). Moreover, we examined the role of EGFR in meningitic E. coli penetration into the brain in a neonatal animal model, involving intraperitoneal administration of gefitinib to 1-week-old mice. The results, as determined by bacterial counts (CFUs) recovered from the blood and brain specimens of mice receiving gefitinib or vehicle control, showed that gefitinib did not affect the level of bacteremia, but was efficacious in preventing E. coli penetration into the brain (Fig 1I). In addition, we showed a co-localization of EGFR with meningitic E. coli strain RS218 in HBMEC monolayer (Fig 1J). Together, these data support our novel concept that EGFR contributes to meningitis-causing E. coli penetration of the BBB.
Fig 1. Meningitic E. coli exploits EGFR for its penetration of the BBB in vitro and in vivo.
(A) The EGFR-selective inhibitor gefitinib inhibits meningitic E. coli RS218 invasion of HBMEC in a dose-dependent manner, but does not affect its adhesion. * p<0.05, ** p<0.01. (B) Bacterial growth was not affected by the treatment with gefitinib. Overnight bacterial culture was 1:100 transferred into fresh medium with or without gefitinib at indicated concentrations, and further incubated for 2 h. Viable bacterial counts were determined by series dilution and plating at 30 min interval. (C) Gefitinib did not lead to an inhibition of cell proliferation when used at the indicated concentrations. (D) Meningitic E. coli invasion of the EGFR knock-out HBMEC (KO#35) was significantly decreased compared to the invasion of the control cells. * p<0.05. (E) A time-dependent activation of EGFR occurs in response to E. coli RS218 in HBMEC. The ratio of p-EGFR and EGFR was calculated based on densitometry analysis. ** indicates the difference was significant compared to time 0 (p<0.01). (F) EGFR mRNA transcription levels did not change in response to E. coli RS218 in HBMEC, as assessed by real-time PCR analysis. GAPDH was used as the endogenous reference. Representative results from three individual experiments are shown. (G) EGFR protein expression levels were not affected in response to E. coli RS218 in HBMEC. Actin was probed in the same lysate and used as a loading control. (H) RS218 invasion was significantly reduced in HBMEC transfected with the dominant-negative EGFR construct pcDNA-EGFR-GGS compared with pcDNA3.1 control vector-transfected HBMEC. ** p<0.01. (I) E. coli RS218 penetration into the brain was significantly inhibited by administration of gefitinib (10 mg/kg) compared with vehicle treatment. In contrast, the magnitudes of bacteremia did not differ between the recipients of gefitinib and vehicle control. * p <0.05. (J) Co-localization of E. coli strain RS218 and EGFR is demonstrated in HBMEC. Scale bar = 10 μm.
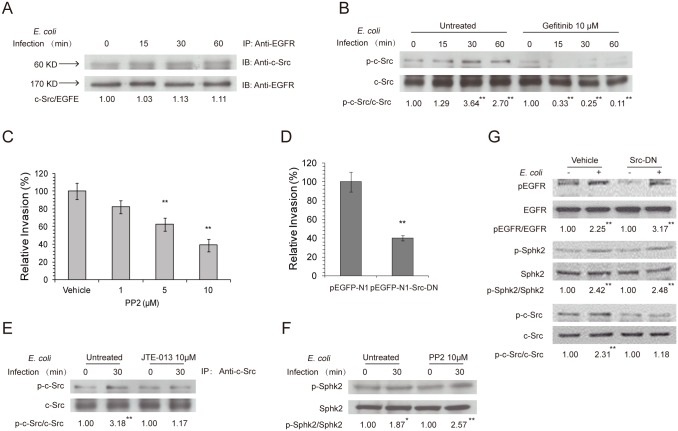
As indicated above, we demonstrated both in vitro and in vivo that EGFR is involved in meningitic E. coli RS218 penetration of the BBB, but there was no information on how EGFR is activated in response to E. coli invasion. Since E. coli penetration of the BBB requires specific microbial factors that contribute to HBMEC invasion [1–4], we examined whether EGFR activation occurred in response to those E. coli factors contributing to HBMEC invasion. The wild-type strain RS218 and its mutants with deletion of ompA, cnf1, fimH, ibeA, ibeB, ibeC or nlpI were examined for their involvement in EGFR tyrosine phosphorylation in HBMEC. We found that the mutants deleted of ompA, fimH or nlpI exhibited a significantly lower level of EGFR activation compared with wild-type strain RS218 (Fig 2A), suggesting that E. coli OmpA, FimH, and NlpI proteins are likely to contribute to EGFR activation in HBMEC. In contrast, the mutants deleted of ibeA, ibeB, ibeC or cnf1 did not show the decrease in EGFR activation (Fig 2A). The involvement of OmpA, FimH and NlpI in EGFR activation was further supported by the demonstration that antibodies directed against OmpA, FimH and NlpI inhibited EGFR activation in response to strain RS218 in HBMEC (Fig 2B). As expected, the triple mutant deleted of ompA, fimH and nlpI could not induce a discernible EGFR activation, similar to that of uninfected HBMEC (Fig 2C). The exploitation of EGFR by OmpA, FimH and NlpI in E. coli invasion of the cells was further examined by using another selective EGFR inhibitor, erlotinib [40], as well as the EGFR KO#35 cells. The results showed that erlotinib inhibited invasion of the wild-type strain RS218 in a dose-dependent manner, while it did not affect the HBMEC invasion by the triple mutant deleted of ompA, fimH, and nlpI (Fig 2D). Similarly, the triple mutant’s invasion of the KO#35 cells did not differ from that of the control cells (Fig 2E). Taken together, these findings indicate that E. coli virulence factors OmpA, FimH, and NlpI are likely to be involved in exploitation of EGFR in meningitic E. coli invasion of HBMEC.
Fig 2. OmpA, FimH, and NlpI proteins are involved in meningitic E. coli-induced activation of EGFR.
(A) E. coli mutants with deletion of ompA, fimH or nlpI exhibited lower EGFR activation compared with wild-type RS218 in HBMEC monolayer. * p<0.05 compared to wild type bacteria. (B) Antibodies directed against OmpA, FimH, and NlpI decreased EGFR activation in response to E. coli in HBMEC. The bacteria were preincubated with the antibodies (with 1:10 dilution) individually for 1 h, and then added to HBMEC and incubated for 30 min for assessment of EGFR activation. * p<0.05, ** p<0.01 compared to E. coli infection without antibody incubation. (C) EGFR activation in response to E. coli was not discernible with the triple deletion mutant (RS218ΔompAΔfimHΔnlpI), similar to that of the uninfected control HBMEC. * p<0.05, ** p<0.01 compared with the E. coli RS218 wild-type infection. (D) Erlotinib inhibited the wild-type strain RS218 invasion of HBMEC in a dose-dependent manner, while it did not affect the HBMEC invasion by the triple mutant strain with deletion of ompA, fimH, and nlpI. * p<0.05, ** p<0.01. (E) E. coli wild-type strain RS218 invasion was significantly decreased in EGFR knock-out HBMEC (KO#35 cells) (* p<0.05), while the triple deletion mutant’s invasion did not differ between knock-out and control cells.
SphK2-S1P-S1P2 signaling cascade contributes to E. coli penetration of the BBB
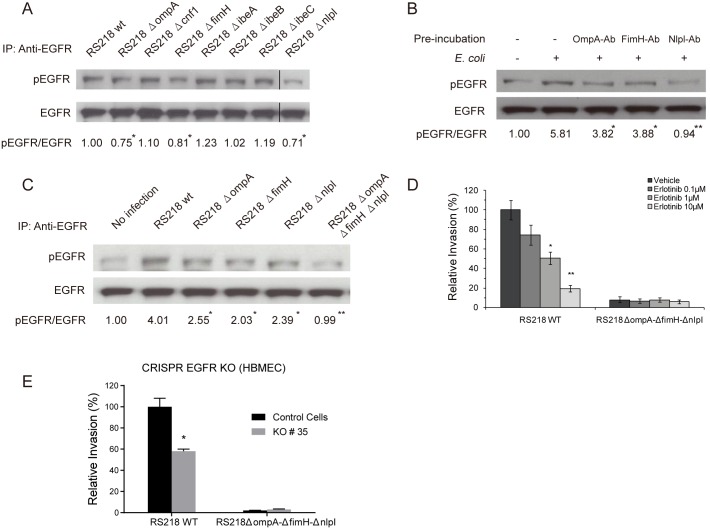
Recent studies have shown that S1P acts as a multifunctional bioactive sphingolipid metabolite implicated in a wide range of biological effects, such as cell proliferation, immune and allergic reactions, and regulation of the vascular cell function [41–44]. The role of S1P in E. coli meningitis, however, has not been previously appreciated. In our study, we found that the same microbial factors involved in EGFR activation also contributed to S1P generation in response to E. coli infection in HBMEC. S1P levels were significantly higher in HBMEC infected with meningitic E. coli wild-type strain RS218 compared to those infected with the triple mutant deleted of ompA, fimH and nlpI, as measured by LC-MS/MS after lipid extraction of HBMEC [45]. The S1P content in HBMEC was normalized to lipid phosphate in the extracted samples and expressed as pmol/nmol lipid phosphate (mean ± SD of three samples), being 2.45 ± 0.35 after 30 min at 37°C in HBMEC incubated with wild-type RS218 vs. 1.29 ± 0.36 in cells incubated with the triple deletion mutant (Fig 3A, p<0.05). Accordingly, a trend for the decreased amount of sphingosine was observed in HBMEC incubated with wild-type RS218 compared to that incubated with the triple mutant (Fig 3A). Since the same microbial factors contributing to HBMEC invasion are involved in EGFR activation and S1P generation, we hypothesized that the contribution of EGFR to E. coli penetration of the BBB is likely to be related to that of S1P.
Fig 3. SphK2-S1P-S1P2 mediates meningitic E. coli penetration of the BBB in vitro and in vivo.
(A) S1P generation was significantly higher in HBMEC incubated with wild-type RS218 compared with the triple mutant deleted of ompA, fimH and nlpI. Correspondingly, the sphingosine level was lower in HBMEC incubated with wild-type RS218 than those incubated with the triple deletion mutant. * p<0.05. (B) Structures of (S)-FTY720-vinylphosphonae (SphK1 and SphK2 inhibitor), (R)-FTY720-methyl ether (selective SphK2 inhibitor), RB-032 and RB-033 (selective SphK1 inhibitors), and RB-034 (inactive analogue). (C) Both (S)-FTY720-vinylphosphonae (SphK1 and SphK2 inhibitor, shown as (S)-FTY-Pn) and (R)-FTY720-methyl ether (SphK2 inhibitor, shown as ROME) significantly inhibited RS218 invasion of HBMEC, while the SphK1 inhibitors (RB-032 and RB-033) and inactive analogue (RB-034) did not exhibit any inhibition. ** p<0.01. The inhibitors were all used at 10 μM. (D) (R)-FTY720-methyl ether inhibited E. coli RS218 invasion of HBMEC in a dose-dependent manner. ** p<0.01. (E) E. coli RS218 activated SphK2 in a time-dependent manner in HBMEC, while such activation was abolished by pretreatment with 10 μM (R)-FTY720-methyl ether. ** p<0.01. (F) E. coli penetration into the brain was significantly less in SphK2 −/− mice compared with wild-type mice. In contrast, the levels of bacteremia did not differ between the two groups of mice. (G) JTE-013 (S1P2 antagonist) significantly inhibited E. coli invasion of HBMEC, while VPC23019 (S1P1 and S1P3 antagonist) did not exhibit any inhibition. ** p<0.01. (H) The mutants with deletion of ompA, fimH, or nlpI as well as the triple mutant (ΔompAΔfimHΔnlpI) induced significantly lower levels of SphK2 activation in HBMEC, compared with wild-type RS218. ** p<0.01.
S1P synthesis is catalyzed by sphingosine kinases 1 and 2 (SphK1 and SphK2) [41–44]. We, therefore, examined the role of S1P in E. coli invasion of the BBB by determining the involvement of SphK1 and SphK2 using specific inhibitors against SphK1 and/or SphK2, and an inactive analogue (Fig 3B) [46–50]. Both (S)-FTY720-vinylphosphonate (inhibitor of SphK1 and SphK2, abbreviated as (S)-FTY-Pn) and (R)-FTY720-methyl ether (selective inhibitor of SphK2, abbreviated as ROME) significantly inhibited E. coli RS218 invasion of HBMEC, while SphK1 inhibitors (RB-032 and RB-033) and the inactive analogue (RB-034) did not exhibit any inhibition (Fig 3C), suggesting the involvement of SphK2, not SphK1, in meningitic E. coli invasion of HBMEC. This finding was further supported by the dose-dependent inhibition of E. coli RS218 invasion by the SphK2 inhibitor (Fig 3D), as well as the time-dependent activation of SphK2 (analyzed as the ratio of p-SphK2/SphK2) in response to strain RS218 in HBMEC, which was abolished in HBMEC pretreated with the SphK2 inhibitor (Fig 3E). Next, the role of SphK2 in E. coli penetration into the brain was examined in SphK2−/− mice compared with wild-type C57BL/6j mice [42]. We found that the magnitudes of bacteremia did not differ between the two groups of mice, as shown by the similar bacterial counts in the blood of wild-type and SphK2−/− mice (Fig 3F). However, the bacterial counts in the brains of SphK2−/− mice were significantly lower than those in the brains of wild-type mice (Fig 3F), indicating that deletion of SphK2 resulted in decreased E. coli penetration into the brain without affecting the magnitude of bacteremia. These in vitro and in vivo findings demonstrate the novel concept that SphK2 contributes to meningitic E. coli penetration of the BBB.
S1P is known to exhibit diverse activities by binding to and signaling through its specific cell-surface receptors, which are members of the G protein-coupled receptors (GPCR) family [41,43,44]. We next determined the role of S1P receptors in meningitic E. coli invasion of HBMEC using selective receptor antagonists (VPC23019 for S1P1 and S1P3, and JTE-013 for S1P2) [41,44]. We found that pretreatment of HBMEC with VPC23019 at indicated concentrations had no effect on E. coli invasion (Fig 3G), suggesting that receptors S1P1 and S1P3 are not likely to be involved in E. coli invasion of the BBB. In contrast, HBMEC pretreated with S1P2-specific antagonist JTE-013 displayed a dose-dependent decrease in E. coli invasion (Fig 3G), suggesting that S1P2 plays a role in meningitic E. coli invasion of HBMEC. Taken together, our findings thus far support the concept that meningitic E. coli infection of HBMEC increases the generation of S1P through SphK2 activation, and that the interaction of S1P with S1P2 is involved in E. coli invasion of the BBB.
As indicated above, we showed that S1P levels were significantly less in HBMEC incubated with the triple mutant deleted of ompA, fimH and nlpI, compared to wild type strain. To further support our hypothesis that the contribution of EGFR to E. coli penetration of the BBB is related to that of S1P, the E. coli factors involved in EGFR activation (OmpA, FimH, NlpI) were examined for their contributions to SphK2 activation in HBMEC. The results revealed that phosphorylation of SphK2 was decreased in HBMEC infected with the mutants with deletion of ompA, fimH and nlpI, individually and in combination, compared with HBMEC infected with wild-type RS218 (Fig 3H). Therefore, these findings support that E. coli factors OmpA, FimH and NlpI, the key contributors to bacterial adhesion and invasion, participate in both EGFR activation and SphK2-S1P-S1P2 signaling cascade, and suggest a linkage between EGFR and SphK2-S1P-S1P2 in meningitic E. coli penetration of the BBB.
Meningitic E. coli-induced activation of EGFR requires SphK2-S1P-S1P2 signaling and SphK2-S1P-S1P2-mediated upregulation of HB-EGF in HBMEC
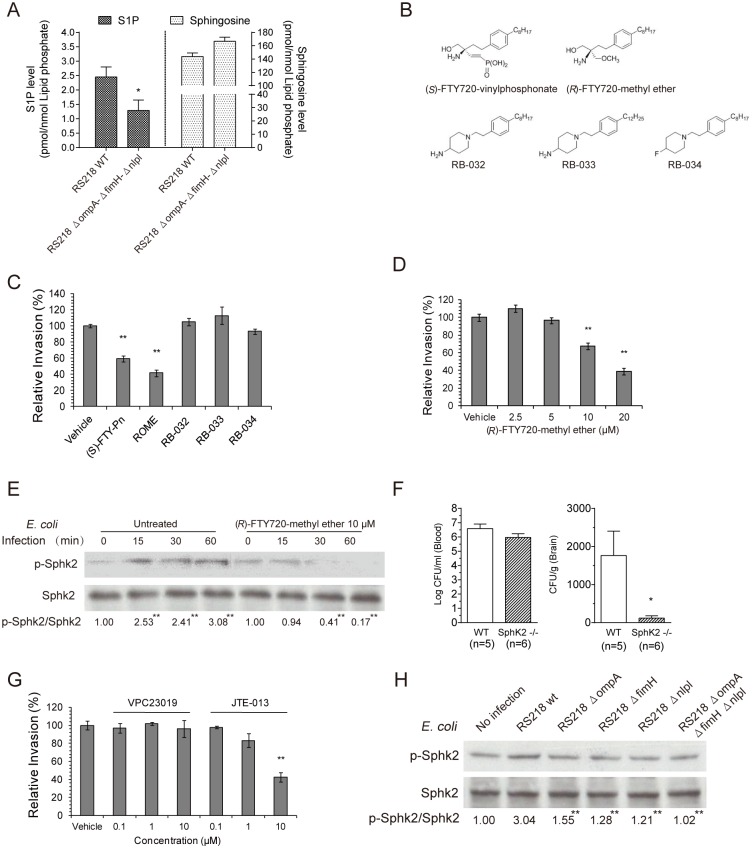
Since the same microbial factors (OmpA, FimH, NlpI) are involved in SphK2 activation, S1P generation, and EGFR activation, we examined the potential relationship between the SphK2-S1P-S1P2 cascade and EGFR activation in meningitic E. coli invasion of the BBB. We found that pharmacological inhibition of EGFR with gefitinib did not affect E. coli-induced phosphorylation of SphK2, as shown by the time-dependent activation of SphK2 in response to meningitic E. coli in HBMEC with and without gefitinib treatment (Fig 4A). We next compared E. coli-induced SphK2 phosphorylation in HBMEC transfected with EGFR dominant-negative construct pcDNA-EGFR-GGS with that in cells transfected with the vehicle control pcDNA3.1. The results showed that the pattern of increased SphK2 phosphorylation upon infection with meningitic E. coli was similar between HBMEC expressing dominant-negative EGFR and HBMEC expressing the control vector (Fig 4B). These findings indicate that pharmacological inhibition and dominant-negative expression of EGFR did not interfere with SphK2 activation by meningitic E. coli. Next, we examined and compared the EGFR activation upon E. coli infection in HBMEC, with and without inhibition of S1P function. As shown in Fig 4C, blockade of the S1P signaling cascade by the S1P2 antagonist JTE-013 was effective in inhibiting EGFR activation in response to meningitic E. coli. These findings demonstrate the novel concept that both SphK2-S1P-S1P2 and EGFR contribute to meningitic E. coli invasion, and that the SphK2-S1P-S1P2 signaling cascade is likely to act upstream of EGFR in meningitic E. coli penetration of the BBB.
Fig 4. SphK2-S1P-S1P2 is upstream of EGFR activation in meningitic E. coli invasion of HBMEC and contributes to HB-EGF mediated transactivation of EGFR.
(A) Activation of SphK2 in response to RS218 did not differ between HBMEC with and without gefitinib pretreatment. * p<0.05, ** p<0.01. (B) SphK2 activation was not affected in HBMEC expressing dominant-negative EGFR, while EGFR activation was, as expected, abolished in HBMEC expressing dominant-negative EGFR. Activation of c-Src occurred in response to E. coli in vector-transfected HBMEC, but did not occur in HBMEC expressing dominant-negative EGFR. ** p<0.01. (C) JTE-013 (S1P2 antagonist) inhibited EGFR activation in response to E. coli in HBMEC. ** p<0.01. (D) Real-time PCR analysis of the expression of EGFR ligands in response to wild-type E. coli RS218 or the triple deletion mutant in HBMEC. Representative results from three independent assays are shown. GAPDH was used as an endogenous reference. (E) Pretreatment of HBMEC with JTE-013 or (R)-FTY720-methyl ether (shown as ROME) prevented HB-EGF up-regulation (analyzed by real-time PCR) in response to RS218. (F) Pretreatment of HBMEC with CRM197 prevented EGFR activation in response to RS218. ** p<0.01. (G) CRM197 dose-dependently inhibited RS218 invasion of HBMEC, while only the highest dosage of CRM197 significantly affected HBMEC invasion by the triple mutant. ** p<0.01. (H) The release of HB-EGF from HBMEC infected with the triple deletion mutant for up to 4 h was below the detection limit, while HB-EGF release was significantly increased by approximately 3-fold from the cells infected with wild-type RS218 at 4 h, ** p<0.01.
EGFR consists of an extracellular ligand-binding domain, a single membrane-spanning region, and a cytoplasmic kinase domain [31,51,52]. It is known that EGFR activation occurs via kinases and/or transactivation through binding to specific ligands. At present, several EGFR-related ligands are known, including EGF, transforming growth factor α (TGFα), heparin-binding EGF-like ligand (HB-EGF), amphirugulin (AREG), betacellulin (BTC), epiregulin (EREG), epigen and neuregulin family members [31,51,52]. We next performed quantitative real-time PCR to investigate whether expressions of the above-mentioned ligands are affected in response to meningitic E. coli. We selected six ligands, EGF, AREG, BTC, EREG, HB-EGF, and TGFα, representing the EGFR-related ligands previously examined in a study with N. gonorrhoeae, and determined their transcriptional levels in response to meningitic E. coli infection. Our quantitative PCR data showed that three ligands, HB-EGF, AREG and EREG, displayed significant up-regulation at 60 min after infection with strain RS218, while the transcriptional levels of EGF, BTC and TGFα remained unchanged during the infection (Fig 4D). Since the E. coli factors OmpA, FimH, and NlpI were shown to be important in EGFR activation, we examined whether the transcriptional levels of those ligands were changed in response to the triple deletion mutant (ΔompAΔfimHΔnlpI), compared with the wild-type strain. The results showed that the triple deletion mutant was able to induce significant up-regulation of AREG and EREG after 60 min, while there was no up-regulation of HB-EGF, as well as EGF, BTC, and TGFα, after infection with the triple deletion mutant (Fig 4D). These findings demonstrate that HB-EGF is the ligand up-regulated in response to meningitic E. coli strain RS218, compared with the triple mutant with deletion of ompA, fimH, and nlpI, suggesting that increased expression of HB-EGF might be involved in the increased activation of EGFR by meningitic E. coli strain. As shown above, the SphK2-S1P-S1P2 signaling cascade is shown to be upstream of EGFR, and we determined the effect of SphK2-S1P-S1P2 blockade on up-regulation of HB-EGF in response to E. coli. As expected, we observed that HB-EGF up-regulation in response to strain RS218 was abolished in HBMEC pretreated with SphK2 inhibitor ROME and S1P2 antagonist JTE-013 (Fig 4E). Taken together, these findings support that the SphK2-S1P-S1P2 cascade exploits EGFR activation via up-regulation of HB-EGF in response to meningitic E. coli, and that SphK2-S1P-S1P2 is upstream of EGFR in meningitic E. coli invasion of HBMEC.
HB-EGF is synthesized as a membrane-spanning precursor molecule and proteolytically processed by metalloproteinases of the ADAM (a disintegrin and metalloproteinase) family to be involved in binding to and activation of EGFR [53,54]. To further examine the contribution of HB-EGF to EGFR activation and E. coli invasion of the BBB, we investigated the effect of HB-EGF shedding on EGFR activation and E. coli invasion of HBMEC using the diphtheria toxin mutant Cross-Reacting Material 197 (CRM197), a nontoxic mutant of the diphtheria toxin that retains the ability to bind pro-HB-EGF and prevent its shedding from EGFR stimulation [55]. The results showed that CRM197 was effective in preventing EGFR activation in response to E. coli strain RS218 (Fig 4F), and also significantly inhibited RS218 invasion of HBMEC in a dose-dependent manner. The triple deletion mutant, as expected, exhibited significantly decreased HBMEC invasion and CRM197 did not decrease the triple mutant’s invasion, and significant inhibition was demonstrated at the highest concentration tested (Fig 4G). As shown previously [1–4], HBMEC invasion frequency of less than 5% of wild type strain’s invasion, however, is less likely to be biologically relevant. We next examined and compared the release of secretory HB-EGF in the supernatants of HBMEC in response to infection with wild-type strain RS218 or its triple deletion mutant by ELISA. The HB-EGF levels in HBMEC infected with the triple deletion mutant for up to 4 h were below the detection limit (16 pg/mL), similar to those of the uninfected control. In contrast, the HB-EGF levels in HBMEC infected with wild-type RS218 were increased by approximately 3-fold at 4 h of infection (p<0.01) (Fig 4H).
Taken together, the above findings demonstrate that meningitic E. coli RS218, with the help of specific microbial factors OmpA, FimH and NlpI, up-regulates the expression and release of HB-EGF, resulting in the transactivation of EGFR, and that this transactivation is dependent on the SphK2-S1P-S1P2 signaling cascade.
c-Src tyrosine kinase participates in E. coli invasion of HBMEC and is downstream of the S1P-EGFR pathway
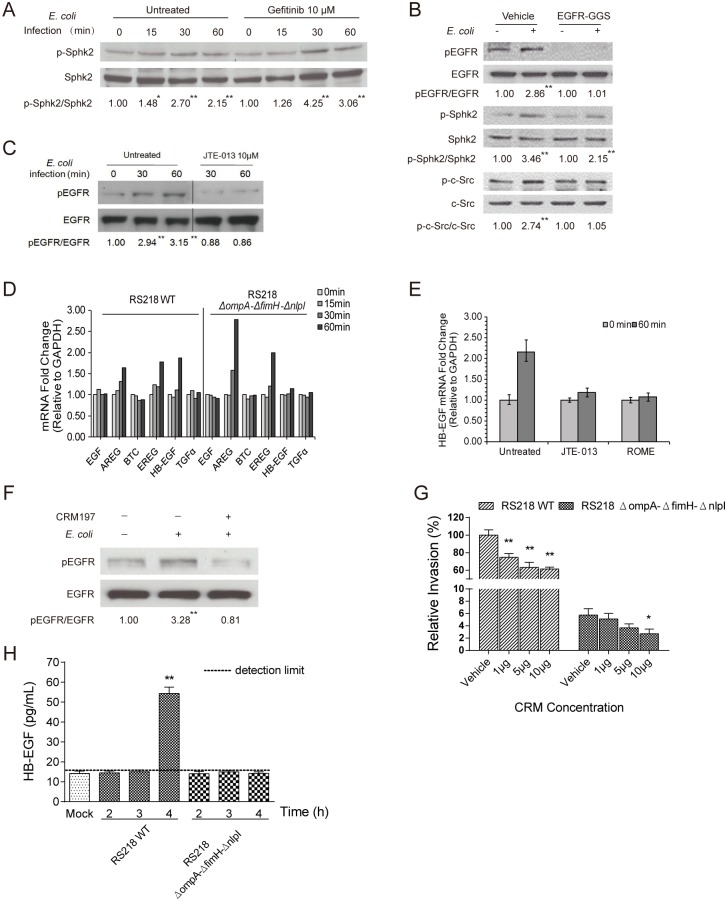
Meningitic E. coli strains exploit specific host cell signaling molecules to promote their invasion of the BBB [1–4]. The phosphotyrosine residues in the cytoplasmic domain of EGFR can serve as bait for recruitment of proteins containing SH2 domains or certain phosphotyrosine-binding domains, depending on stimuli, and can act as a switch to assist and extend the signal transduction of RTK pathways [29]. The c-Src tyrosine kinase was shown to be recruited by the phosphotyrosine residues of EGFR upon activation and to function as a mediator of EGFR signaling, e.g., ligand-independent activation of EGFR [56]. Moreover, c-Src tyrosine kinase was reported to regulate host cell actin cytoskeleton rearrangement and contribute to E. coli invasion of HBMEC [57]. To examine whether c-Src tyrosine kinase is involved in EGFR signaling in response to meningitic E. coli invasion, we performed co-immunoprecipitation and Western blotting to assess the possible recruitment and activation of c-Src by EGFR. Our co-immunoprecipitation experiments with an anti-EGFR antibody showed that c-Src tyrosine kinase (60 kD) was associated with EGFR and that this association was maintained in HBMEC during 60 min incubation with meningitic E. coli RS218 (Fig 5A). After stripping, the membrane was re-probed for EGFR, showing an unchanged level of EGFR upon E. coli invasion (Fig 5A), consistent with our earlier demonstration of no change in EGFR expression in response to meningitic E. coli infection. We subsequently examined the activation of c-Src tyrosine kinase in response to E. coli RS218 infection, and showed that c-Src activation occurred in a time-dependent manner, but was abolished in HBMEC pretreated with the EGFR kinase inhibitor gefitinib (Fig 5B) as well as in HBMEC expressing the EGFR dominant-negative construct (Fig 4B). These findings suggest that c-Src tyrosine kinase acts downstream of EGFR in meningitic E. coli invasion of HBMEC.
Fig 5. S1P and EGFR promote meningitic E. coli invasion of HBMEC monolayer via exploiting c-Src.
(A) Association of c-Src with EGFR in response to E. coli in HBMEC, as shown by co-immunoprecipitation of HBMEC lysates with an anti-EGFR antibody. (B) c-Src activation occurred in response to E. coli in a time-dependent manner in HBMEC, but was abolished by pretreatment with gefitinib. (C) Pretreatment of HBMEC with PP2 (Src inhibitor) exhibited a dose-dependent inhibition of E. coli RS218 invasion. ** p<0.01. (D) E. coli RS218 invasion was significantly reduced in HBMEC expressing the dominant-negative Src construct, pEGFP-N1-Src-DN, compared with the vector (pEGFP-N1)-transfected HBMEC. ** p<0.01. (E, F) Pretreatment of HBMEC with JTE-013 (S1P2 antagonist) inhibited c-Src activation in response to E. coli (E), while pretreatment with PP2 (Src inhibitor) did not affect SphK2 activation (F). * p<0.05, ** p<0.01. (G) E. coli activation of EGFR and SphK2 was not affected in HBMEC expressing dominant-negative c-Src, while c-Src activation was, as expected, abolished in HBMEC transfected with dominant-negative c-Src compared with vector control-transfected HBMEC. ** p<0.01.
We next examined the contribution of c-Src to E. coli invasion of HBMEC. As expected from the c-Src association with EGFR, E. coli invasion of HBMEC was significantly decreased by pretreatment of HBMEC with the c-Src tyrosine kinase inhibitor PP2 compared with the vehicle control, as well as by transfection of HBMEC with c-Src dominant-negative construct compared with the control vector (Fig 5C and 5D). In support of our demonstration that c-Src is downstream of EGFR activation, which is in turn a downstream event of SphK2-S1P-S1P2 signaling in E. coli invasion of HBMEC, we found that pretreatment of HBMEC with the S1P2 antagonist JTE-013 inhibited c-Src activation in response to E. coli RS218 (Fig 5E), while HBMEC pretreated with the c-Src kinase inhibitor PP2 or transfected with the c-Src dominant-negative construct did not affect SphK2 and EGFR activation in response to RS218 infection in HBMEC (Fig 5F and 5G). Taken together, these findings demonstrate that c-Src tyrosine kinase is downstream of the SphK2-S1P-S1P2-EGFR signaling cascade and that SphK2-S1P-S1P2-EGFR-c-Src contribute to meningitic E. coli invasion of the BBB.
Discussion
Bacterial pathogens, including meningitis-causing pathogens, exploit host cell signaling molecules to promote their infections, but the underlying mechanisms vary depending upon the types of pathogens and host tissues. Recent studies have shown that EGFR plays a role for several pathogenic organisms in the pathogenesis of their infections. For example, Pseudomonas aeruginosa and H. pylori were shown to induce transactivation of EGFR to prevent epithelial cell apoptosis during the early stage of infection, thereby facilitating their survival in the host cells [36,58]. EGFR activation by nontypeable H. influenzae attenuates host defensive and immune responses by negatively regulating the expression of Toll-like receptor 2 [37]. K. pneumoniae induces EGFR and its downstream signaling cascades to attenuate the activation of NF-κB, thereby modulating host immune responses [38]. Pasteurella multocida toxin exploits the transactivation of EGFR and subsequent mitogen-activated protein kinase activation to induce fibroblast proliferation [59]. In addition, N. gonorrhoeae modulates the activity and cellular distribution of host EGFR to facilitate its invasion and transmigration across the epithelium [33,34].
Meningitic E. coli strains have been shown to penetrate the BBB via a transcellular mechanism [2], but the underlying mechanisms remain incompletely understood. In this report, we demonstrate a novel role of EGFR in E. coli penetration of the BBB, a prerequisite for the development of E. coli meningitis. Noticeably, our chemical library screen using a model of E. coli invasion of HBMEC identified gefitinib, a selective inhibitor of EGFR tyrosine kinase, as an effective inhibitor of E. coli invasion of HBMEC. We, then, showed that EGFR contributed to E. coli penetration of the BBB, indicating that our approach of targeting E. coli invasion of HBMEC is likely to identify targets involved in the pathogenesis of E. coli meningitis.
Here, our demonstration of EGFR contribution to meningitic E. coli penetration of the BBB is provided by several lines of evidence. These include (a) pharmacological inhibition and knock-out of EGFR inhibited meningitic E. coli strain RS218 invasion of HBMEC in vitro, (b) administration of gefitinib effectively inhibited the penetration of circulating E. coli into the brain of neonatal mice, and (c) the co-localization of EGFR with the E. coli strain in HBMEC. These findings support the novel concept that EGFR is a biologically relevant host factor affecting E. coli penetration of the BBB, but the underlying mechanisms remain unclear.
We have previously shown that meningitic E. coli exploits specific host factors for invasion of the BBB, and that host factors contribute to invasion of the BBB by functioning as receptors for specific microbial factors and/or exploiting specific host cell signaling molecules [1–4]. If EGFR functions as a cell surface receptor for specific microbial factors, then blockade of EGFR would have affected E. coli binding to and invasion of the BBB. However, we found that pharmacological inhibition of EGFR blocked E. coli invasion of HBMEC in a dose-dependent manner and there was no effect on bacterial adhesion, indicating that EGFR is likely to contribute to E. coli invasion of the BBB by affecting host cell signaling molecules. This concept was also supported by our demonstration that three bacterial determinants contributing to E. coli invasion of the BBB (OmpA, FimH, NlpI) were involved in EGFR activation. Since these E. coli proteins were shown to interact with different host receptors [2,23–25], it is unlikely that EGFR functions as a co-receptor for different microbial-host factors. Studies are needed to elucidate how different microbial-host interactions exploit EGFR activation for E. coli penetration of the BBB.
S1P is recognized as a novel bioactive lipid mediator involved in physiological and pathogenic vascular functions [60]. For example, S1P regulates angiogenesis by activating the S1P1 and S1P3 receptors (GPCRs) on endothelial cells, which is required for endothelial cell morphogenesis into capillary-like networks [61]. S1P was also shown to induce adherens junction assembly through the Gi/MAPK pathway and the small GTPase Rho and Rac pathways [61]. The S1P receptor S1P1 was shown to be essential for vascular maturation, and S1P1-mediated migration was defective in S1P1 mutant cells through their inability to activate the small GTPase Rac [62]. In addition, the S1P receptor S1P2 regulates vascular inflammation and atherosclerosis by inducing the release of inflammatory cytokines IL-1β and IL-18 and retaining macrophages in plaques [63]. Since various lines of evidence have shown that S1P functions in the vasculature and vascular-related cells [41,60], we investigated whether S1P is involved in microbial penetration of the BBB. Our data demonstrate for the first time a novel role of S1P and a potential crosstalk between EGFR and SphK2-S1P-S1P2 signaling in HBMEC upon meningitic E. coli infection. This novel concept was shown by our demonstrations (a) that S1P levels were significantly increased in HBMEC in response to meningitic E. coli infection, (b) that SphK2 inhibitors, but not SphK1 inhibitors, and S1P2 antagonist, but not S1P1/3 antagonist, inhibited E. coli invasion of HBMEC and (c) that the E. coli factors contributing to EGFR activation (OmpA, FimH, NlpI) were also shown to be involved in the activation of SphK2, and subsequent S1P generation in response to E. coli RS218. Moreover, S1P was shown to be an upstream signaling molecule of EGFR, by demonstrations that blockade of S1P function inhibited EGFR activation in response to meningitic E. coli, while blockade of EGFR function did not affect SphK2 activation. These findings prompted us to investigate how EGFR activation occurs, and to determine the role of S1P signaling in EGFR transactivation in meningitic E. coli invasion of the BBB.
To address these questions, we investigated the mechanisms of EGFR activation in response to meningitic E. coli in HBMEC. Activation of EGFR can occur upon binding of a specific ligand to its extracellular ligand-binding domain, leading to EGFR homo- and hetero-dimerization, and tyrosine phosphorylation of the cytoplasmic tyrosine kinase domain [31,51,52], and is classified as a ligand-dependent transactivation. A previous study on N. gonorrhoeae invasion of genital epithelial cells showed that EGFR transactivation occurred through up-regulation of several ligands including HB-EGF, AREG, and TGFα [33]. Here, we examined whether EGFR activation occurred in response to meningitic E. coli in a ligand-dependent manner by analyzing the expression levels of EGFR-related ligands. Our quantitative real-time PCR analysis revealed that upregulation of HB-EGF differed between HBMEC infected with wild-type meningitic E. coli and those infected with its triple mutant deleted of ompA, fimH and nlpI. Additionally, we were able to detect significant levels of HB-EGF in the supernatants of HBMEC infected with meningitic E. coli strain RS218, but not with the triple deletion mutant, suggesting that HB-EGF is likely to be involved in the activation of EGFR. In addition, HB-EGF is shown to bind to EGFR and ErbB4 [64], and our experiments here with EGFR knock-out and dominant-negative construct supported the involvement of EGFR in E. coli invasion of HBMEC. The contribution of HB-EGF was also shown by our demonstration that blockade of HB-EGF with CRM197 [55], which prevents ectodomain shedding of proHB-EGF, significantly inhibited EGFR activation as well as E. coli invasion of HBMEC. These findings support that the ligand-dependent action involving OmpA/FimH/NlpI exploitation of HB-EGF is likely to contribute to transactivation of EGFR in meningitic E. coli invasion of HBMEC.
Our time-dependent mRNA up-regulation of HB-EGF, however, did not follow the same time-dependent kinetics as EGFR activation. We showed that EGFR activation occurred in HBMEC as early as 15 min after infection with meningitic E. coli, while our real-time PCR analysis revealed that the mRNA level of HB-EGF was up-regulated at 60 min after infection, implying that EGFR activation in response to meningitic E. coli may occur via two mechanisms, comprising an early activation (perhaps representing a ligand-independent activation) and a late ligand-dependent activation involving HB-EGF. Previous studies have reported the crosstalk between GPCRs and EGFR, in which both ligand-independent and ligand-dependent activation of EGFR by GPCRs was demonstrated [65]. Here, our data showed that SphK2-S1P-S1P2 was upstream of EGFR in meningitic E. coli invasion, and more importantly that the EGFR activation at 30 min after infection was abolished by treatment with the S1P2 antagonist JTE-013, implying that the early EGFR activation might arise from the S1P-S1P2 pathway, which involves GPCR-related signaling. While S1P signaling occurred, it also trigged the late ligand-dependent activation of EGFR by regulating the expression of the EGFR-associated ligand HB-EGF. This is supported by the demonstration that blockade of S1P function with the SphK2 inhibitor and the S1P2 antagonist effectively prevented HB-EGF mRNA up-regulation at 60 min.
The ligand-dependent transactivation of EGFR by GPCRs is shown to occur by proteolytic processing of the transmembrane pro-EGFR-ligand precursor followed by paracrine activation of EGFR [53–55,65]. This process requires the participation of metalloproteinase activity, the so-called “Triple Membrane Passing Signal” (TMPS) mechanism. Therein, GPCRs stimulation induces metalloproteinase activity that cleaves the EGF-like ligand precursor and allows shedding of the ligand to bind to the extracellular ligand-binding domain of the receptor, thus transactivating EGFR signaling [65]. Therefore, the metalloproteinase-mediated shedding of EGF-like ligands might serve as a key step in GPCR-induced EGFR transactivation. In the present study, our finding for SphK2-S1P-S1P2 signaling to EGFR via HB-EGF was compatible with this TMPS mechanism. Noticeably, our results supported the exclusive involvement of HB-EGF, rather than other ligands, in EGFR activation in response to meningitic E. coli, and that is the reason for using CRM197 to specifically block HB-EGF function, rather than using a broad inhibitor of metalloproteinases. Nevertheless, several questions concerning the TMPS mechanism remain to be clarified for complete elucidation of the mechanisms underlying EGFR activation in response to meningitic E. coli invasion, such as identification of the specific member in the metalloproteinase family, the functional domain of metalloproteinases required for ligand precursor cleavage, and the manner in which activation occurs through GPCR signaling in HBMEC upon infection. At the present time, our findings support the involvement of S1P signaling in both early (ligand-independent) and late (ligand-dependent) activation of EGFR in response to meningitic E. coli. Further studies are needed to elucidate the mechanisms involved in the S1P-mediated early activation of EGFR in response to meningitic E. coli invasion of the BBB.
Meningitic E. coli triggers the activation of multiple host cell signal transduction pathways for invasion of the BBB [1–4]. The signaling molecules such as FAK and its associated cytoskeletal protein paxillin, phosphatidylinosital 3-kinase (PI3K), RhoGTPases, cytosolic phospholipase A2 (cPLA2) and ERM (ezrin, radixin, and moesin) protein family have all been identified to be involved in this process, mostly likely through their promoting actin cytoskeleton rearrangements in HBMEC [11,13,14,23,25–27,66,67]. Tyrosine-phosphorylated Src is found to be associated with EGFR upon stimulation of certain GPCRs. It has been shown to function as a mediator of the EGFR signaling pathway and can also be recruited by the activated EGFR phosphotyrosine domain [65]. The implication of host cell Src family tyrosine kinase in bacterial internalization has been reported in several pathogens. For example, ErbB2 phosphorylation results in recruitment and activation of the tyrosine kinase Src, which is dependent on the intrinsic kinase activity of ErbB2, and selective inhibitors of both ErbB2 and Src inhibited N. meningitidis internalization [35]. In Opa52-mediated phagocytosis of N. gonorrhoeae by human neutrophils, the activity of the Rho-family member Rac was controlled by a Src-like tyrosine kinase for efficient uptake [68]. Likewise, Src, together with Rho family GTPases, are involved in the internalization of Shigella into epithelial cells [69,70], and in triggering the formation of actin polymerization foci induced by Shigella [71]. Previously, c-Src tyrosine kinase was shown to regulate host cell actin cytoskeleton rearrangement and PI3K activation in HBMEC by E. coli, and contributed to E. coli invasion of HBMEC [57]. Here, we showed an association of c-Src with EGFR by co-immunoprecipitation analysis, and demonstrated that c-Src activation followed the SphK2-S1P-S1P2-EGFR signaling cascade and played an important role in meningitic E. coli invasion of the BBB. Additional studies are needed to elucidate the mechanisms involved with EGFR-c-Src signaling in E. coli invasion of the BBB.
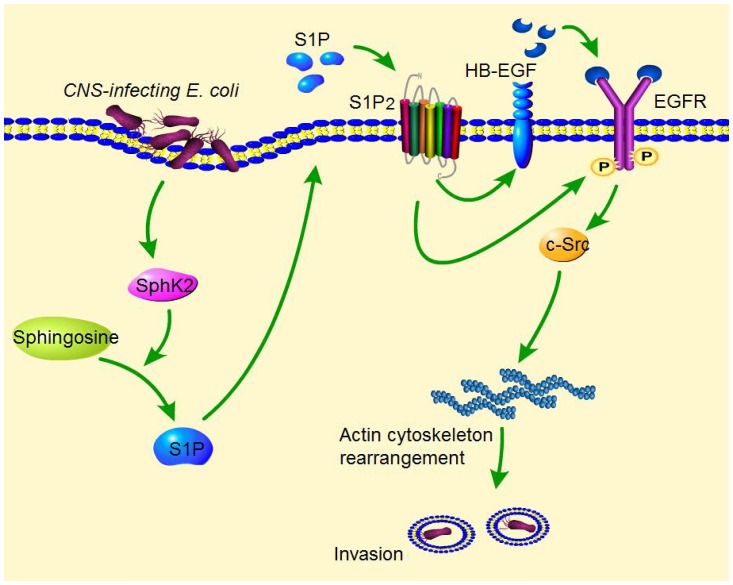
In summary, our findings report a novel mechanism exploited by meningitic E. coli for penetration of the BBB. Via its OmpA, FimH, and NlpI proteins, meningitic E. coli induces activation of SphK2, leading to increased generation of S1P that interacts with its receptor S1P2. This SphK2-S1P-S1P2 signaling is involved in early activation of EGFR, and also induces up-regulation and release of the EGFR-related ligand HB-EGF, which is responsible for transactivation of EGFR. Activated EGFR subsequently recruits c-Src and induces tyrosine phosphorylation of c-Src kinase, which promotes reorganization of the actin cytoskeleton in HBMEC and E. coli entry of the BBB (Fig 6). Hijacking of this SphK2-S1P-S1P2-EGFR-c-Src signaling cascade will facilitate meningitic E. coli to penetrate the BBB, the essential step in the development of E. coli meningitis. Recent reports have indicated that antimicrobial resistance is an emerging problem in E. coli causing meningitis [3,4], necessitating the development of novel targets for effective therapy. To our knowledge, this is the first demonstration that meningitic E. coli exploits S1P activation of EGFR for penetration of the BBB in vivo and in vitro, suggesting that S1P-EGFR represents a novel target for therapeutic development of E. coli meningitis.
Fig 6. Schematic representation of the S1P-EGFR signaling pathway in meningitic E. coli invasion of HBMEC.
Meningitic E. coli penetration of the BBB follows the microbial-host interactions contributing to HBMEC invasion, via exploiting specific host cell signaling molecules. During the HBMEC invasion, meningitic E. coli strains activate SphK2, which catalyzes the synthesis of S1P from sphingosine. S1P is then secreted outside and acts on S1P receptor S1P2. S1P interaction with S1P2 is involved in the activation of EGFR, as well as the up-regulation and release of EGFR-related ligand HB-EGF, which is proteolytically processed by metalloproteinases. The released HB-EGF binds to the extracellular ligand-binding domain of EGFR and leads to tyrosine phosphorylation of the EGFR cytoplasmic kinase domain. This SphK2-S1P-S1P2-EGFR cascade induces the activation of c-Src tyrosine kinase, an intracellular mediator that has been shown to regulate host cell actin cytoskeleton rearrangements, leading to E. coli invasion of HBMEC.
Materials and Methods
Bacterial strains and cell culture
E. coli strain RS218 (O18:K1:H7) was obtained from cerebrospinal fluid of a neonate with meningitis [15]. All the mutants used in this study were derived from strain RS218 as previously described [9–11,15,20–22]. E. coli strains were cultured at 37°C overnight in brain heart infusion broth with appropriate antibiotics unless otherwise specified.
HBMEC were isolated and characterized as described previously [7]. Cells were routinely grown in RPMI 1640 containing 10% heat-inactivated fetal bovine serum, 10% Nu-Serum, 2 mM L-glutamine, 1 mM Sodium pyruvate, nonessential amino acids, vitamins, and penicillin and streptomycin (100 U/ml) in 37°C incubator under 5% CO2 until they reached confluence. In some experiments, confluent HBMEC were washed thrice with Hanks’ Balanced Salt Solution (Corning Cellgro, Manassas, VA, USA) and starved in serum-free medium (1:1 mixture of Ham’s F-12 and M-199) for 16–18 h before further treatment.
Reagents, antibodies and plasmids
The EGFR tyrosine kinase inhibitor gefitinib, S1P receptors antagonists VPC23019 and JTE-013, and c-Src kinase inhibitor PP2 were purchased from Cayman Chemical Company (Ann Arbor, MI, USA). Other inhibitors of EGFR and ErbB were purchased from Selleck (Houston, TX, USA). The inhibitors of SphK1 and SphK2 and inactive analogue, including (S)-FTY720-vinylphosphonae, (R)-FTY720-methyl ether, RB-032, RB-033, and RB-034, were described previously [46–50] and used at 10 μM. The diphtheria toxin mutant CRM197 was obtained from BioAcademia Inc. (Osaka, Japan). Protein G Agarose Fast Flow beads and anti-phosphotyrosine (4G10) horseradish peroxidase (HRP)-conjugate antibody (used at 1:1000 for EGFR and c-Src phosphorylatyion detection) were purchased from EMD Millipore Corporation (Temecula, CA, USA). Anti-EGFR, anti-c-Src, and anti-c-Src-HRP conjugated antibodies were from Santa Cruz Biotechnology (1:1000) (Santa Cruz, CA, USA). A sphingosine kinase activation antibody kit was obtained from ECM Biosciences (Versailles, KY, USA). Cy3-conjugated antibody were purchased from Abcam (Cambridge, MA, USA). Anti-rabbit IgG HRP-conjugate antibody and Alexa Fluor 488-conjugated antibody were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-actin antibody was from Sigma-Aldrich (1:3000) (St. Louis, MO, USA). Preparations of OmpA-, FimH-, and NlpI-specific antibodies were previously described [20–22]. The TurboFect transfection reagent was purchased from Thermo Scientific (Suwanee, GA, USA) and used according to the instructions. G418 sulfate solution was from Corning Cellgro. The EGFR and c-Src dominant-negative constructs, pcDNA-EGFR-GGS and pEGFP-N1-Src-DN, along with their vector controls pcDNA3.1 and pEGFP-N1 were obtained from Drs. Hristova and Taylor, respectively [39,72].
Chemical library screening
We used the Johns Hopkins Drug Library (JHDL), which is comprised of 3,400 chemicals that are approved by the US Food and Drug Administration and entered phase 2 clinical trials or approved for use abroad [73], for discovery of novel targets affecting E. coli invasion of HBMEC, as follows. Drugs were arrayed in 96-well plates and screened at a final concentration of 10 μM in DMSO (solvent). HBMEC grown in 96-well tissue culture plates were incubated with the JHDL for 60 min at room temperature, and then, examined for E. coli invasion, by a modification of the HBMEC invasion assay [9,10,15]. Briefly, 10 μl containing approximately 1×106 CFUs of E. coli strain RS218 were inoculated into each well of HBMEC in the plate. The plates were incubated at 37°C for 90 min for bacterial invasion to occur, and then intracellular CFUs were determined. This screening assay always included E. coli strain RS218 in vehicle (DMSO)-treated HBMEC as a positive control for invasion, while bacteria without HBMEC were used as a control for assessing any inhibitory effect of the chemicals on the growth of E. coli. Since this JHDL contains antibiotics, those wells exposed to antibiotics were used as a positive control for identification of chemicals that inhibit E. coli growth. The assay was highly reproducible, and the coefficient of correlation from at least two separate experiments was r = 0.98 (p<0.0001). From this assay, we identified gefitinib, which inhibited meningitic E. coli invasion of HBMEC greater than 90%. It is important to note that gefitinib did not affect bacterial growth, as assessed by comparing CFUs in experimental medium with or without the drug and also did not affect HBMEC viability, as assessed by live/dead stain (Molecular Probes). It is also important to note that EGFR has not been previously appreciated to affect E. coli penetration of the BBB.
Bacterial adhesion and invasion assays in HBMEC
The ability of E. coli to bind to and invade HBMEC was determined as previously described [9–12,15,20–22]. Briefly, E. coli strains were grown overnight in brain heart infusion broth with streptomycin (50 μg/ml). Bacteria were resuspended in experimental medium (M199-Ham F12 [1:1] medium containing 5% heat-inactivated FBS) and added into the confluent HBMEC monolayer grown in 24-well plate at MOI of 100. The plate was incubated at 37°C incubator with 5% CO2 for 90 min to allow binding. HBMECs were then washed three times to remove unbound bacteria, and lysed in 0.025% Triton X-100 buffer. Bacterial counts of adhesion were determined by plating with appropriate dilutions. For invasion assay, bacteria were added into HBMEC as described above for the adhesion assay. Subsequently, cells were washed three times to remove the unbound bacteria and incubated in experimental medium containing 100 μg/ml gentamicin for another 1 h to kill extracellular bacteria. HBMEC were washed and lysed as above mentioned. The released intracellular bacteria were quantified by appropriate dilutions and plating. As specified in some experiments, HBMEC were pretreated with various inhibitors for 1 h prior to addition of bacteria and then processed for bacterial invasion. The results were calculated as percentages of the initial inoculums, and presented as percent relative adhesion/invasion compared with that in the presence of the vehicle control (DMSO). Each assay was performed in triplicate.
MTT assay
MTT Cell Proliferation Assay Kit was purchased from BioVision (Milpitas, CA, USA) and used according to the instructions. HBMEC were seeded in 96 well plates at 5×103 per well in 100 μL culture medium and incubated for 24 h. Gefitinib was added as indicated in HBMEC binding and invasion assays. Supernatant of each well was removed and MTT dissolved in serum-free medium was added and further incubated for another 4 h. After incubation, 100 μL of MTT solvent was added into each well, and the plate was wrapped in a foil and shaked on an orbital shaker for 15 min. Absorbance of all wells at 570 nm were determined.
Mass spectrometry analysis of the sphingolipids in HBMEC
HBMEC monolayers grown in 100-mm dish were serum-starved overnight and incubated with E. coli strain RS218 or the triple deletion mutant at a MOI of 100 for 30 min at 37°C. Sphingolipids were extracted using acidified organic solvents and quantitated by HPLC electrospray ionization triple quadrupole mass spectrometry and quantitated using mass labeled internal standards [74]. Briefly, sphingolipids were extracted from cell lysates as previously described [74,75]. Prior to extraction, a mixture of C17 sphingolipids (125 pmol/sample) was added to each sample as the internal standards. Sphingolipids were quantitated by HPLC electrospray ionization tandem mass spectrometry using selected ion monitoring on an ABSciex 4000 Q-Trap instrument as described previously [76–78]. Total phospholipids for each sample were measured using modified Ames and Dubin assay as previously described [75,79].
Transfection
HBMEC were transfected with empty vectors control or expression vectors encoding EGFR or c-Src dominant-negative constructs using the TurboFect transfection reagent as described previously [66,80]. pcDNA3.1 cloned with green fluorescence protein (GFP) and pEGFP-N1 vectors were used to determine the transfection efficiency by fluorescence microscopy.
Genome editing via CRISPR-Cas9
A human codon-optimized Cas9 expression vector was obtained from Addgene, plasmid #41815 [81], and Cas9 was cloned into the pEF6 expression vector (Invitrogen, Carlsbad, CA, USA), downstream and in-frame with a nuclear-localized YFP, linked by a piconaviral 2A bicistronic peptide [82], such that nuclear localization signal (NLS)-YFP and Cas9 are expressed in approximate equimolar quantities. A hEGFR guide RNA (gRNA) construct, including the U6 promoter, was synthesized as a double stranded DNA fragment and cloned into the pEF6-nls-YFP-2A-Cas9 vector by InFusion Cloning (Clontech, Mountain View, CA, USA). This vector was used for transfection of HBMEC as mentioned above and clones resistant to blasticidin were identified and used for isolation of a single clone. Single clones were used for expression of EGFR by Western blot and bacterial invasion assay.
Immunoprecipitation and western blotting
HBMEC were seeded at 1×106 cells/100-mm dish and cultured until confluence. Cells were then serum-starved overnight, stimulated with E. coli strains at MOI of 100 for specified periods of time, and processed for immunoprecipitation and Western blotting analysis as previously described [66,80].
RNA isolation and real-time PCR
Confluent HBMEC grown in 100-mm dishes were serum-starved overnight and then infected with E. coli at a MOI of 100 for indicated periods of time. At each time point, the medium was removed and the cells were lyzed for total RNA preparation using the TRIzol reagent (Invitrogen). Contaminating DNA was removed by DNase I treatment (New England Biolabs, Ipswich, MA, USA). Aliquots (1 μg) of the total RNA in each sample were subjected to cDNA synthesis using ProtoScript Taq RT-PCR kit (New England Biolabs). Real-time PCR was performed with a QuantStudio 12K Flex Real-Time PCR System (Applied BioSystems, Foster City, CA, USA) using Power SYBR Green PCR master mix (Applied BioSystems), according to the manufacturers’ instructions. The primer sequences for human EGFR and its ligands were as follows: EGFR, 5'-CAAGTGCTGGATGATAGA-3' (forward) and 5'-GAAGTTGGAGTCTGTAGG-3' (reverse); EGF, 5'-GTTGGCAGGTGGTGAAGTTG-3' (forward) and 5'-CCACAGGAGCACAGTCATCT-3' (reverse); AREG, 5'-ATTATGCTGCTGGATTGG-3' (forward) and 5'-GAGGACGGTTCACTACTA-3' (reverse); BTC, 5'-CCAAGCAATACAAGCATTAC-3' (forward) and 5'-GTCCTCTGTCTCCTCTTAG-3' (reverse); EREG, 5'-AGTTCAGACAGAAGACAATC-3' (forward) and 5'-ACATCGGACACCAGTATA-3' (reverse); HB-EGF, 5'-TATACCTATGACCACACAAC-3' (forward) and 5'-CACATCATAACCTCCTCTC-3' (reverse); TGFα, 5'-GGCTGTCCTTATCATCAC-3' (forward) and 5'-AGACCACTGTTTCTGAGT-3' (reverse). Primers for human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were provided in the RT-PCR kit. The amplification conditions were: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The products were then applied to a melt curve stage with denaturation at 95°C for 15 s, anneal at 60°C for 1 min, and slow dissociation by ramping from 60°C to 95°C at 0.05°C/s to ensure the specificity of the PCR products.
Determination of secretory HB-EGF from HBMEC by ELISA
To determine the release of secretory HB-EGF from HBMEC, cells were infected with meningitic E. coli strain RS218 or its triple deletion mutant for varying time points, and the supernatants were discarded and cells were washed with 1.5 M NaCl/1×PBS/1% BSA to dissolve heparin-bound HB-EGF. Cleaved and secretory HB-EGF levels were then quantified from wash buffer using HB-EGF Human ELISA Kit, purchased from Abcam (Cambridge, MA, USA), according to the manufacturer’s instructions.
Animal infection assay
C57BL/6j mice were purchased from Jackson Laboratory (Bar Harbor, Maine). SphK2−/− mice in the background of C57BL/6 were described previously [42]. Male or female mice at 1 week of age were used for induction of hematogenous E. coli meningitis. All procedures and handling techniques were approved by the Animal Care and Use Committee of the Johns Hopkins University. Each mouse received approximately 3×105 CFU of E. coli strain RS218 in 50 μl sterile normal saline via intracardiac injection. At 1 h post-inoculation, the mice were euthanized and blood from the right ventricle was collected for quantitative bacterial cultures. Subsequently, the mice were perfused as previously described [14], and their brains were removed, weighed, homogenized, and plated to determine the bacterial counts, which were expressed as CFUs per gram. In some experiments, gefitinib, applied at therapeutic dosage (10 mg/kg) [83], was intraperitoneally administrated 2 h before bacterial challenge.
Immunofluorescence of HBMEC
HBMEC was grown on collagen-coated glass slide to confluency. Cells were washed thrice with serum-free medium and then pre-incubated for 30 minutes in experimental medium. Cells were then incubated with E. coli containing a red fluorescence protein (RFP)-expressing plasmid (RS218-RFP), at an MOI of 1:100 for a period of 90 minutes at 37°C with 5% CO2. Cells were washed with PBS to remove the free, unbound bacteria, and then fixed with 4% paraformaldehyde, permeabilized with Triton X-100 solution, and blocked with 5% BSA in PBS. Cells were then incubated with EGFR antibody overnight at 4°C, washed, and incubated with Alexa Fluor 488-labeled secondary antibody (Life Technologies A11034), followed by nucleus staining with DAPI (Vector Laboratories H-1200). The glass slide was mounted and visualized using fluorescence microscopy.
Statistical analysis
Data were expressed as mean ± standard errors of the mean (SEM) unless otherwise noted. Differences of the bacterial counts in adhesion and invasion assays were determined by Student’s t-test. Differences of the bacterial counts between different treatments or groups of mice were determined by the Wilcoxon rank-sum test. P-values of < 0.05 were considered significant.
Ethics statement
This study was carried out in strict accordance with the current recommendations in the Guide for the Care and Use of Handling Animals, NIH publication DHHS/USPHS. The animal protocol was approved by The Johns Hopkins Animal Care and Use Committee (Animal Welfare Assurance Number: A3272-01). All efforts were made to provide the ethical treatment and minimize suffering of animals (mice) employed in this study.
Acknowledgments
We thank Donna Pearce at JHU for technical assistance for HBMEC preparations and HB-EGF ELISA, Dr. Proia at NIH for providing SphK2-/- mice and Dr. Shim at JHU for arranging the chemical library. We also thank Dr. Hristova at JHU and Dr. Taylor at Georgetown University for providing us with EGFR dominant negative construct pcDNA-EGFR-GGS and c-Src dominant negative construct pEGFP-N1-Src-DN, respectively. In addition, we thank Ruicheng Yang and Jiyang Fu at Huazhong Agricultural University for their technical assistance in the immunofluorescence assays.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported in part by the NIH grants (https://www.nih.gov/), NS91102 and AI84984 (KSK), R01NS072241 (MJW) and HL083187 (RB), and by grants from the National Natural Science Foundation of China (NSFC) (http://www.nsfc.gov.cn/) No.31502062 (XW). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kim KS (2003) Pathogenesis of bacterial meningitis: from bacteraemia to neuronal injury. Nat Rev Neurosci 4: 376–385. 10.1038/nrn1103 [DOI] [PubMed] [Google Scholar]
- 2. Kim KS (2008) Mechanisms of microbial traversal of the blood-brain barrier. Nat Rev Microbiol 6: 625–634. 10.1038/nrmicro1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim KS (2010) Acute bacterial meningitis in infants and children. Lancet Infect Dis 10: 32–42. 10.1016/S1473-3099(09)70306-8 [DOI] [PubMed] [Google Scholar]
- 4. Kim KS (2012) Current concepts on the pathogenesis of Escherichia coli meningitis: implications for therapy and prevention. Curr Opin Infect Dis 25: 273–278. 10.1097/QCO.0b013e3283521eb0 [DOI] [PubMed] [Google Scholar]
- 5. Kim KS, Itabashi H, Gemski P, Sadoff J, Warren RL, et al. (1992) The K1 capsule is the critical determinant in the development of Escherichia coli meningitis in the rat. J Clin Invest 90: 897–905. 10.1172/JCI115965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dietzman DE, Fischer GW, Schoenknecht FD (1974) Neonatal Escherichia coli septicemia—bacterial counts in blood. J Peds 85: 128–130. 10.1016/S0022-3476(74)80308-2 [DOI] [PubMed] [Google Scholar]
- 7. Stins MF, Gilles F, Kim KS (1997) Selective expression of adhesion molecules on human brain microvascular endothelial cells. J Neuroimmunol 76: 81–90. 10.1016/S0165-5728(97)00036-2 [DOI] [PubMed] [Google Scholar]
- 8. Stins MF, Badger J, Kim KS (2001) Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb Pathog 30: 19–28. 10.1006/mpat.2000.0406 [DOI] [PubMed] [Google Scholar]
- 9. Huang SH, Chen YH, Fu Q, Stins M, Wang Y, et al. (1999) Identification and characterization of an Escherichia coli invasion gene locus, ibeB, required for penetration of brain microvascular endothelial cells. Infect Immun 67: 2103–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y, Huang SH, Wass CA, Stins MF, Kim KS (1999) The gene locus yijP contributes to Escherichia coli K1 invasion of brain microvascular endothelial cells. Infect Immun 67: 4751–4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khan NA, Wang Y, Kim KJ, Chung JW, Wass CA, et al. (2002) Cytotoxic necrotizing factor-1 contributes to Escherichia coli K1 invasion of the central nervous system. J Biol Chem 277: 15607–15612. 10.1074/jbc.M112224200 [DOI] [PubMed] [Google Scholar]
- 12. Wang Y, Kim KS (2002) Role of OmpA and IbeB in Escherichia coli K1 invasion of brain microvascular endothelial cells in vitro and in vivo. Pediatr Res 51: 559–563. 10.1203/00006450-200205000-00003 [DOI] [PubMed] [Google Scholar]
- 13. Zhu L, Pearce D, Kim KS (2010) Prevention of Escherichia coli K1 penetration of the blood-brain barrier by counteracting the host cell receptor and signaling molecule involved in E. coli invasion of human brain microvascular endothelial cells. Infect Immun 78: 3554–3559. 10.1128/IAI.00336-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu L, Maruvada R, Sapirstein A, Malik KU, Peters-Golden M, et al. (2010) Arachidonic acid metabolism regulates Escherichia coli penetration of the blood-brain barrier. Infect Immun 78: 4302–4310. 10.1128/IAI.00624-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang SH, Wass C, Fu Q, Prasadarao NV, Stins M, et al. (1995) Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo: molecular cloning and characterization of invasion gene ibe10 . Infect Immun 63: 4470–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Badger JL, Wass CA, Weissman SJ, Kim KS (2000) Application of signature-tagged mutagenesis for identification of Escherichia coli K1 genes that contribute to invasion of human brain microvascular endothelial cells. Infect Immun 68: 5056–5061. 10.1128/IAI.68.9.5056-5061.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Badger JL, Wass CA, Kim KS (2000) Identification of Escherichia coli K1 genes contributing to human brain microvascular endothelial cell invasion by differential fluorescence induction. Mol Microbiol 36: 174–182. 10.1046/j.1365-2958.2000.01840.x [DOI] [PubMed] [Google Scholar]
- 18. Xie Y, Kolisnychenko V, Paul-Satyaseela M, Elliott S, Parthasarathy G, et al. (2006) Identification and characterization of Escherichia coli RS218-derived islands in the pathogenesis of E. coli meningitis. J Infect Dis 194: 358–364. 10.1086/505429 [DOI] [PubMed] [Google Scholar]
- 19. Prasadarao NV, Wass CA, Weiser JN, Stins MF, Huang SH, et al. (1996) Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect Immun 64: 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Teng CH, Xie Y, Shin S, Di Cello F, Paul-Satyaseela M, et al. (2006) Effects of ompA deletion on expression of type 1 fimbriae in Escherichia coli K1 strain RS218 and on the association of E. coli with human brain microvascular endothelial cells. Infect Immun 74: 5609–5616. 10.1128/IAI.00321-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Teng CH, Cai M, Shin S, Xie Y, Kim KJ, et al. (2005) Escherichia coli K1 RS218 interacts with human brain microvascular endothelial cells via type 1 fimbria bacteria in the fimbriated state. Infect Immun 73: 2923–2931. 10.1128/IAI.73.5.2923-2931.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Teng CH, Tseng YT, Maruvada R, Pearce D, Xie Y, et al. (2010) NlpI contributes to Escherichia coli K1 strain RS218 interaction with human brain microvascular endothelial cells. Infect Immun 78: 3090–3096. 10.1128/IAI.00034-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khan NA, Shin S, Chung JW, Kim KJ, Elliott S, et al. (2003) Outer membrane protein A and cytotoxic necrotizing factor-1 use diverse signaling mechanisms for Escherichia coli K1 invasion of human brain microvascular endothelial cells. Microb Pathog 35: 35–42. [DOI] [PubMed] [Google Scholar]
- 24. Shin S, Lu G, Cai M, Kim KS (2005) Escherichia coli outer membrane protein A adheres to human brain microvascular endothelial cells. Biochem Biophys Res Commun 330: 1199–1204. 10.1016/j.bbrc.2005.03.097 [DOI] [PubMed] [Google Scholar]
- 25. Khan NA, Kim Y, Shin S, Kim KS (2007) FimH-mediated Escherichia coli K1 invasion of human brain microvascular endothelial cells. Cell Microbiol 9: 169–178. 10.1111/j.1462-5822.2006.00779.x [DOI] [PubMed] [Google Scholar]
- 26. Chung JW, Hong SJ, Kim KJ, Goti D, Stins MF, et al. (2003) 37-kDa laminin receptor precursor modulates cytotoxic necrotizing factor 1-mediated RhoA activation and bacterial uptake. J Biol Chem 278: 16857–16862. 10.1074/jbc.M301028200 [DOI] [PubMed] [Google Scholar]
- 27. Kim KJ, Chung JW, Kim KS (2005) 67-kDa laminin receptor promotes internalization of cytotoxic necrotizing factor 1-expressing Escherichia coli K1 into human brain microvascular endothelial cells. J Biol Chem 280: 1360–1368. 10.1074/jbc.M410176200 [DOI] [PubMed] [Google Scholar]
- 28. Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, et al. (2002) ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res 62: 5749–5754. [PubMed] [Google Scholar]
- 29. Olayioye MA, Neve RM, Lane HA, Hynes NE (2000) The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 19: 3159–3167. 10.1093/emboj/19.13.3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schlessinger J (2000) Cell signaling by receptor tyrosine kinases. Cell 103: 211–225. 10.1016/S0092-8674(00)00114-8 [DOI] [PubMed] [Google Scholar]
- 31. Yarden Y, Sliwkowski MX (2001) Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2: 127–137. 10.1038/35052073 [DOI] [PubMed] [Google Scholar]
- 32. Tao RH, Maruyama IN (2008) All EGF(ErbB) receptors have preformed homo- and heterodimeric structures in living cells. J Cell Sci 121: 3207–3217. 10.1242/jcs.033399 [DOI] [PubMed] [Google Scholar]
- 33. Swanson KV, Griffiss JM, Edwards VL, Stein DC, Song W (2011) Neisseria gonorrhoeae-induced transactivation of EGFR enhances gonococcal invasion. Cell Microbiol 13: 1078–1090. 10.1111/j.1462-5822.2011.01603.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Edwards VL, Wang LC, Dawson V, Stein DC, Song W (2013) Neisseria gonorrhoeae breaches the apical junction of polarized epithelial cells for transmigration by activating EGFR. Cell Microbiol 15: 1042–1057. 10.1111/cmi.12099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoffmann I, Eugene E, Nassif X, Couraud PO, Bourdoulous S (2001) Activation of ErbB2 receptor tyrosine kinase supports invasion of endothelial cells by Neisseria meningitidis . J Cell Biol 155: 133–143. 10.1083/jcb.200106148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yan F, Cao H, Chaturvedi R, Krishna U, Hobbs SS, et al. (2009) Epidermal growth factor receptor activation protects gastric epithelial cells from Helicobacter pylori-induced apoptosis. Gastroenterology 136: 1297–1307, e1291–1293. 10.1053/j.gastro.2008.12.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mikami F, Gu H, Jono H, Andalibi A, Kai H, et al. (2005) Epidermal growth factor receptor acts as a negative regulator for bacterium nontypeable Haemophilus influenzae-induced Toll-like receptor 2 expression via an Src-dependent p38 mitogen-activated protein kinase signaling pathway. J Biol Chem 280: 36185–36194. 10.1074/jbc.M503941200 [DOI] [PubMed] [Google Scholar]
- 38. Frank CG, Reguerio V, Rother M, Moranta D, Maeurer AP, et al. (2013) Klebsiella pneumoniae targets an EGF receptor-dependent pathway to subvert inflammation. Cell Microbiol 15: 1212–1233. 10.1111/cmi.12110 [DOI] [PubMed] [Google Scholar]
- 39. He L, Hristova K (2012) Consequences of replacing EGFR juxtamembrane domain with an unstructured sequence. Sci Rep 2: 854 10.1038/srep00854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blumenberg M (2014) Differential transcriptional effects of EGFR inhibitors. PloS one 9: e102466 10.1371/journal.pone.0102466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maceyka M, Harikumar KB, Milstien S, Spiegel S (2012) Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol 22: 50–60. 10.1016/j.tcb.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, et al. (2005) Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol 25: 11113–11121. 10.1128/MCB.25.24.11113-11121.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pyne S, Bittman R, Pyne NJ (2011) Sphingosine kinase inhibitors and cancer: seeking the golden sword of Hercules. Cancer Res 71: 6576–6582. 10.1158/0008-5472.CAN-11-2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spiegel S, Milstien S (2011) The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol 11: 403–415. 10.1038/nri2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Selim S, Sunkara M, Salous AK, Leung SW, Berdyshev EV, et al. (2011) Plasma levels of sphingosine 1-phosphate are strongly correlated with haematocrit, but variably restored by red blood cell transfusions. Clin Sci (Lond) 121: 565–572. 10.1042/CS20110236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tonelli F, Lim KG, Loveridge C, Long J, Pitson SM, et al. (2010) FTY720 and (S)-FTY720 vinylphosphonate inhibit sphingosine kinase 1 and promote its proteasomal degradation in human pulmonary artery smooth muscle, breast cancer and androgen-independent prostate cancer cells. Cell Signal 22: 1536–1542. 10.1016/j.cellsig.2010.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baek DJ, MacRitchie N, Pyne NJ, Pyne S, Bittman R (2013) Synthesis of selective inhibitors of sphingosine kinase 1. Chem Commun (Camb) 49: 2136–2138. 10.1039/c3cc00181d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lu X, Sun C, Valentine WJ, Shuyu E, Liu J, et al. (2009) Chiral vinylphosphonate and phosphonate analogues of the immunosuppressive agent FTY720. J Org Chem 74: 3192–3195. 10.1021/jo900023u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Baek DJ, MacRitchie N, Anthony NG, Mackay SP, Pyne S, et al. (2013) Structure-activity relationships and molecular modeling of sphingosine kinase inhibitors. J Med Chem 56: 9310–9327. 10.1021/jm401399c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lim KG, Sun C, Bittman R, Pyne NJ, Pyne S (2011) (R)-FTY720 methyl ether is a specific sphingosine kinase 2 inhibitor: Effect on sphingosine kinase 2 expression in HEK 293 cells and actin rearrangement and survival of MCF-7 breast cancer cells. Cell Signal 23: 1590–1595. 10.1016/j.cellsig.2011.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Citri A, Yarden Y (2006) EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 7: 505–516. 10.1038/nrm1962 [DOI] [PubMed] [Google Scholar]
- 52. Linggi B, Carpenter G (2006) ErbB receptors: new insights on mechanisms and biology. Trends Cell Biol 16: 649–656. 10.1016/j.tcb.2006.10.008 [DOI] [PubMed] [Google Scholar]
- 53. Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, et al. (1999) EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402: 884–888. 10.1038/47260 [DOI] [PubMed] [Google Scholar]
- 54. Sanderson MP, Dempsey PJ, Dunbar AJ (2006) Control of ErbB signaling through metalloprotease mediated ectodomain shedding of EGF-like factors. Growth Factors 24: 121–136. 10.1080/08977190600634373 [DOI] [PubMed] [Google Scholar]
- 55. Mitamura T, Higashiyama S, Taniguchi N, Klagsbrun M, Mekada E (1995) Diphtheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity. J Biol Chem 270: 1015–1019. 10.1074/jbc.270.3.1015 [DOI] [PubMed] [Google Scholar]
- 56. Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, et al. (1999) c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem 274: 8335–8343. 10.1074/jbc.274.12.8335 [DOI] [PubMed] [Google Scholar]
- 57. Liu W, Zhao WD, Yan JC, Ren ZY, Fang WG, et al. (2010) Involvement of Src tyrosine kinase in Escherichia coli invasion of human brain microvascular endothelial cells. FEBS letters 584: 27–32. 10.1016/j.febslet.2009.10.090 [DOI] [PubMed] [Google Scholar]
- 58. Zhang J, Li H, Wang J, Dong Z, Mian S, et al. (2004) Role of EGFR transactivation in preventing apoptosis in Pseudomonas aeruginosa-infected human corneal epithelial cells. Invest Ophthalmol Vis Sci 45: 2569–2576. 10.1167/iovs.03-1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Seo B, Choy EW, Maudsley S, Miller WE, Wilson BA, et al. (2000) Pasteurella multocida toxin stimulates mitogen-activated protein kinase via G(q/11)-dependent transactivation of the epidermal growth factor receptor. J Biol Chem 275: 2239–2245. 10.1074/jbc.275.3.2239 [DOI] [PubMed] [Google Scholar]
- 60. Tamama K, Okajima F (2002) Sphingosine 1-phosphate signaling in atherosclerosis and vascular biology. Curr Opin Lipidol 13: 489–495. [DOI] [PubMed] [Google Scholar]
- 61. Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, et al. (1999) Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99: 301–312. 10.1016/S0092-8674(00)81661-X [DOI] [PubMed] [Google Scholar]
- 62. Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, et al. (2000) Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest 106: 951–961. 10.1172/JCI10905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Skoura A, Michaud J, Im DS, Thangada S, Xiong Y, et al. (2011) Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol 31: 81–85. 10.1161/ATVBAHA.110.213496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Taylor SR, Markesbery MG, Harding PA (2014) Heparin-binding epidermal growth factor-like growth factor (HB-EGF) and proteolytic processing by a disintegrin and metalloproteinases (ADAM): a regulator of several pathways. Semin Cell Dev Biol 28: 22–30. 10.1016/j.semcdb.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 65. Gschwind A, Zwick E, Prenzel N, Leserer M, Ullrich A (2001) Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene 20: 1594–1600. 10.1038/sj.onc.1204192 [DOI] [PubMed] [Google Scholar]
- 66. Reddy MA, Prasadarao NV, Wass CA, Kim KS (2000) Phosphatidylinositol 3-kinase activation and interaction with focal adhesion kinase in Escherichia coli K1 invasion of human brain microvascular endothelial cells. J Biol Chem 275: 36769–36774. 10.1074/jbc.M007382200 [DOI] [PubMed] [Google Scholar]
- 67. Baud O, Aujard Y (2013) Neonatal bacterial meningitis. Handbook of clinical neurology 112: 1109–1113. 10.1016/B978-0-444-52910-7.00030-1 [DOI] [PubMed] [Google Scholar]
- 68. Hauck CR, Meyer TF, Lang F, Gulbins E (1998) CD66-mediated phagocytosis of Opa52 Neisseria gonorrhoeae requires a Src-like tyrosine kinase- and Rac1-dependent signalling pathway. EMBO J 17: 443–454. 10.1093/emboj/17.2.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Adam T, Giry M, Boquet P, Sansonetti P (1996) Rho-dependent membrane folding causes Shigella entry into epithelial cells. EMBO J 15: 3315–3321. [PMC free article] [PubMed] [Google Scholar]
- 70. Dehio C, Prevost MC, Sansonetti PJ (1995) Invasion of epithelial cells by Shigella flexneri induces tyrosine phosphorylation of cortactin by a pp60c-src-mediated signalling pathway. EMBO J 14: 2471–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dumenil G, Olivo JC, Pellegrini S, Fellous M, Sansonetti PJ, et al. (1998) Interferon alpha inhibits a Src-mediated pathway necessary for Shigella-induced cytoskeletal rearrangements in epithelial cells. J Cell Biol 143: 1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pengetnze Y, Steed M, Roby KF, Terranova PF, Taylor CC (2003) Src tyrosine kinase promotes survival and resistance to chemotherapeutics in a mouse ovarian cancer cell line. Biochem Biophys Res Commun 309: 377–383. 10.1016/j.bbrc.2003.08.012 [DOI] [PubMed] [Google Scholar]
- 73. Chong CR, Chen X, Shi L, Liu JO, Sullivan DJ Jr (2006) A clinical drug library screen identifies astemizole as an antimalarial agent. Nat Chem Biol 2: 415–416. 10.1038/nchembio806 [DOI] [PubMed] [Google Scholar]
- 74. Siow D, Sunkara M, Dunn TM, Morris AJ, Wattenberg B (2015) ORMDL/serine palmitoyltransferase stoichiometry determines effects of ORMDL3 expression on sphingolipid biosynthesis. J Lipid Res 56: 898–908. 10.1194/jlr.M057539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Siow DL, Anderson CD, Berdyshev EV, Skobeleva A, Pitson SM, et al. (2010) Intracellular localization of sphingosine kinase 1 alters access to substrate pools but does not affect the degradative fate of sphingosine-1-phosphate. J Lipid Res 51: 2546–2559. 10.1194/jlr.M004374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Deevska GM, Sunkara M, Morris AJ, Nikolova-Karakashian MN (2012) Characterization of secretory sphingomyelinase activity, lipoprotein sphingolipid content and LDL aggregation in ldlr-/- mice fed on a high-fat diet. Bioscience reports 32: 479–490. 10.1042/BSR20120036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Salous AK, Panchatcharam M, Sunkara M, Mueller P, Dong A, et al. (2013) Mechanism of rapid elimination of lysophosphatidic acid and related lipids from the circulation of mice. J Lipid Res 54: 2775–2784. 10.1194/jlr.M039685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schneider G, Bryndza E, Abdel-Latif A, Ratajczak J, Maj M, et al. (2013) Bioactive lipids S1P and C1P are prometastatic factors in human rhabdomyosarcoma, and their tissue levels increase in response to radio/chemotherapy. Mol Cancer Res 11: 793–807. 10.1158/1541-7786.MCR-12-0600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ames BN, Dubin DT (1960) The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem 235: 769–775. [PubMed] [Google Scholar]
- 80. Shin S, Paul-Satyaseela M, Lee JS, Romer LH, Kim KS (2006) Focal adhesion kinase is involved in type III group B streptococcal invasion of human brain microvascular endothelial cells. Microb Pathog 41: 168–173. 10.1016/j.micpath.2006.07.003 [DOI] [PubMed] [Google Scholar]
- 81. Mali P, Yang L, Esvelt KM, Aach J, Guell M, et al. (2013) RNA-guided human genome engineering via Cas9. Science 339: 823–826. 10.1126/science.1232033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rodriguez S, Wolfgang MJ (2012) Targeted chemical-genetic regulation of protein stability in vivo. Chem Biol 19: 391–398. 10.1016/j.chembiol.2011.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang S, Guo P, Wang X, Zhou Q, Gallo JM (2008) Preclinical pharmacokinetic/pharmacodynamic models of gefitinib and the design of equivalent dosing regimens in EGFR wild-type and mutant tumor models. Mol Cancer Ther 7: 407–417. 10.1158/1535-7163.MCT-07-2070 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.