ABSTRACT
Staufen2 (Stau2) is a double-stranded RNA-binding protein involved in cell fate decision by regulating mRNA transport, mRNA stability, translation, and ribonucleoprotein assembly. Little is known about Stau2 expression and function in mammalian oocytes during meiosis. Herein we report the sub-cellular distribution and function of Stau2 in mouse oocyte meiosis. Western blot analysis revealed high and stable expression of Stau2 in oocytes from germinal vesicle (GV) to metaphase II (MII). Immunofluorescence showed that Stau2 was evenly distributed in oocytes at GV stage, and assembled as filaments after germinal vesicle breakdown (GVBD), particularly, colocalized with spindle at MI and MII. Stau2 was disassembled when microtubules were disrupted with nocodazole, on the other hand, when MTs were stabilized with taxol, Stau2 was not colocalized with the stabilized microtubules, but aggregated around the chromosomes array, indicating Stau2 assembly and colocalization with microtubules require both microtubule integrity and its normal dynamics. During interphase and mitosis of BHK and MEF cells, Stau2 was not distributed on microtubules, but colocalized with cis-Golgi marker GM130, implying its association with Golgi complex but not the spindle in fully differentiated somatic cells. Specific morpholino oligo-mediated Stau2 knockdown disrupted spindle formation, chromosome alignment and microtubule-kinetochore attachment in oocytes. The majority oocytes were arrested at MI stage, with bright MAD1 at kinetochores, indicating activation of spindle assembly checkpoint (SAC). Some oocytes were stranded at telophase I (TI), implying suppressed first polar body extrution. Together these data demonstrate that Stau2 is required for spindle formation and timely meiotic progression in mouse oocytes.
KEYWORDS: 1st PBE, meiosis; mouse, oocyte, Stau2, spindle formation
Introduction
Chromosome segregation errors in oocyte meiosis usually result in aneuploidy in embryos, causing infertility, spontaneous abortion and congenital defects in human.1,2 Paired chromosomes are equally pulled apart by forces from opposite poles of spindle, under the strict surveillance of spindle assembly checkpoint (SAC), which suppresses anaphase onset until all the chromosomes are properly aligned and attached by microtubules from poles of a well-formed spindle, thus ensuring equal division of chromosomes into daughter cells. Any defects in spindle structure or SAC function may impair the accuracy of chromosome separation, giving rise to genetic instability.3,4
A normal, delicately-regulated spindle organization is key for checkpoint inactivation during metaphase (MI) and subsequent anaphase onset. In mitosis, spindle formation is guided by centrosome, and hundreds of proteins are involved in this process. Much less is known about molecules involved in meiotic spindle organization, ascribed to the lack of centrosome structure in oocytes, but largely these proteins can be classified into several groups.5 At spindle poles, centrosomal components like γ-tubulin, pericentrin, and pole matrix molecules together to form unique microtubule organizing centers (MTOCs), promoting microtubule assembly in an acentrosomal way,6 At kinetochores, microtubules from opposite poles of spindle have to amphitelicly attach to each pair of homologous chromosomes to achieve the correct bi-orientation of chromosomes, the resulting tension across the kinetochores quenches the ‘wait-anaphase’ signal generated by the SAC;4 and microtubules themselves have to remain dynamic, and microtubule polymerization and depolymerization have to be well-balanced, which ensure that the spindle can be formed in proper structure and have effective stretching force that drives the move, alignment and separation of chromosomes.7,8
Most of proteins required for spindle organization and anaphase onset are translated by maternal mRNAs, which are synthesis and stored before meiosis entry and gradually decayed after fertilization.9 Multiple RNA binding proteins (RBPs) control the transport, stability or decay of maternal mRNAs and consequently affect protein translation,10 so RBPs are very important for normal meiosis progression. Staufen is a family of RBPs, which is first discovered in Drosophila, and has 2 newly identified mammalian homologs, Staufen1 (Stau1) and Staufen 2 (Stau2 ).11 Stau proteins directly combine with and transports target mRNAs along microtubules, leading to asymmetric localization of specific mRNAs, thereby regulating their cellular sites of translation.12-14 In particular, Stau2 is required for neurogenesis during early development and, also dendrite development, synapse function and plasticity in the mature nervous system.10,13 Both Stau1 and Stau2 are also implicated in mRNA decay by enhancing the activity of RNA helicase UPF1.15,16 Some proteins, including microtubule-associated protein 1 b (MAP1B), lim kinase 2 ( LIMK2), Sirtuin 3 (SIRT3), Rab GTPase, cell division cycle 42 (Cdc42) and small ubiquitin-like modifier 1 (SUMO1), etc., are all involved in spindle formation, chromosome alignment and cytokinesis during cell division,7,17-24 interestingly, mRNAs endocing these proteins are targets of Stau2,25 however, direct evidences about Stau2 association with mitotic or meiotic progression are still scarce.
In the present study, we demonstrate for the first time that Stau2 is stably expressed in mouse oocytes during meiotic division, and symmetrically distributed on spindle structure and colocalized with microtubules. In contrast, Stau2 is localized on Golgi complex in somatic cells during mitotic progression. Morpholino oligo-mediated Stau2 depletion affects the meiotic spindle organization, microtubule attachment to kinetochore and extrusion of first polar body during meiotic progression in oocytes.
Results
Stable expression of Stau2 in oocytes and its different subcellular distribution between oocyte meiosis and somatic mitosis
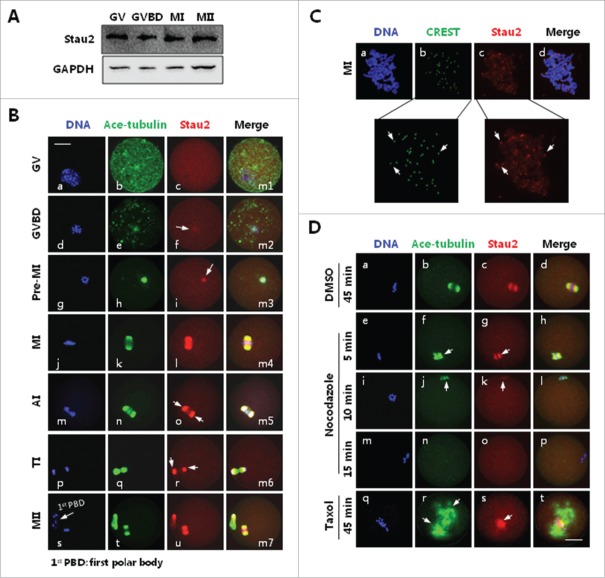
To examine the function of Stau2 in mouse oocytes, we first analyzed the protein expression pattern of Stau2 in mouse oocytes during meiotic division. As showed in Figure 1A, western blot approach revealed a high level of Stau2 protein at germinal vesicle stage (GV), which was sustained stably after the germinal vesicle breakdown (GVBD), even up to the metaphase I (MI) and metaphase II (MII) stages. This specific protein expression pattern of Stau2 promoted us to track Stau2 distribution mode in meiosis, and as displayed by immunofluorescence analysis (Fig. 1B), Stau2 was evenly distributed in oocytes at GV stage, with no special accumulation in both nuclear and cytoplasm (Fig. 1B, c). Stau2 began to emerge as filamentous aggregation after GVBD, and was colocalized with newly polymerized microtubule, both Stau2 and microtubules were gradually organized into bipolar structure along with the meiotic progression from pro-metaphase I (pro-MI) to MI stage (Fig. 1B, e-f, g-i, j-l). During the transition from anaphase I (AI) to telophase I (TI), Stau2 was localized on the lateral portions of the spindle but excluded in the midbody area (Fig. 1B, m-o, p-r: arrows). Filamentous Stau2 was again shaped into “spindle-like” structure at MII stage, with some Stau2 colocalized with microtubules in the area of first polar body (Fig. 1B, s-u, m7). Further immunostaining on chromosome spreads showed that Stau2 was also accumulated across the chromosome body, and particularly concentrated on the centromere areas (Fig. 1C: arrows).
Figure 1.
Expression and subcellular localization Stau2 in mouse oocyte during meiosis. Mouse oocytes were cultured in vitro in MEM medium with 10 % FBS for 0, 2, 4, 8, 12,17 h, corresponding to meiotic stages of germinal vesicle (GV), germinal vesicle breakdown (GVBD), pro-metaphase I (pro-MI), metaphase I (MI), anaphase I/telophase I (AI/TI) and metaphase II (MII), respectively, and collected for analysis with western blot and immunofluorescence. A. Western blot analysis revealed stable expression of Stau2 in mouse oocytes during meiotic division. Protein samples of 50 oocytes, at GV, GVBD, MI and MII stages, respectively, were prepared and segregated on 10% SDS-PAGE separation gel and immunoblotted with Stau2, GAPGH was placed as a loading control. B. Immunofluorescence analysis detected dynamic subcellular distribution in oocytes during meiosis. No special accumulation of Stau2 was detected at GV stage (c). Upon GVBD, Stau2 was aggregated as filaments and colocalized with microtubules around condensing chromosomes (g: arrow). During the meiotic progression from pro-MI to MI, Stau2 was gradually organized into bipolar structure and precisely overlapped with the meiotic spindle assembled from microtubules (j, k, n, o). During AI to TI transition, Stau2 remained colocalized with microtubules but excluded in the midbody structure (r, s: arrows). As oocytes developed to MII stage, Stau2 was assembled into “spindle-like” organization again and overlapped with the MII spindle, Stau2 was also colocalized with microtubules in first polar body (u-x). Stau2 was visualized in red, microtubule was labeled with antibody against acetylated-tubulin (ace-tubulin) and visualized in green, and DNA was visualized in blue. Scale bar = 20 μm. C. Immunostaining analysis revealed Stau2 accumulation across chromosomes, with particular concentration on the centromere areas (arrows in boxes). Stau2 was visualized in red, centromere was probed with CREST auto serum and visualized in green, and DNA was visualized in blue. Scale bar = 20 μm. D. Stau2 distribution was associated with microtubule integrity and stability in oocytes. MI oocytes were treated with 20 μg/ml nocodazole for 5, 10 and 15 min or 10 μM taxol for 45 min, then fixed for immunostaining with acetylated-tubulin (ace-tubulin) and Stau2. During incubation with nocodazole, Stau2 was gradually disassembled (f, g, n) together with microtubules (g, k, o). After taxol treatment, microtubules were stabilized in spindle and cytoplasmic microtubule organizing centers (MTOCs) (r: arrows), meanwhile Stau2 was congressed in the vicinity around chromosome alignment and absent on microtubules (s, t). Microtubules were visualized in green, Stau2 was visualized in red and DNA was labeled in blue. Scale bar = 20 μm.
To assess the possible association between Stau2 and microtubule dynamics, we analyzed changes of Stau2 distribution in MI oocytes treated with spindle-perturbing agents. The spindle microtubules were gradually depolymerized with the increase in incubation with nocodazole, a spindle depolymerizing agent, simultaneously Stau2 filaments also underwent disassembly but remained colocalized with microtubules (Fig. 1D, f-h, j-l: arrows). At the end of 20 min treatment, the spindle completely disappeared, both microtubules and Stau2 filaments totally vanished in oocytes (Fig. 1D, n-p). In contrast, after treatment with taxol, a potent microtubule-stabilizing reagent, an expanded spindle with broad poles was observed, and many microtubule asters were also labeled in cytoplasmic area (Fig. 1D, r: arrows). Interestingly, Stau2 was not “stabilized,” but concentrated on both sides of chromosome array, no filamentous Stau2 was found co-localized with spindle microtubules and cytoplasmic microtubule asters in taxol-treated oocytes (Fig. 1D, s: arrow, t). These data demonstrate that the subcellular distribution of Stau2 is tightly associated with microtubule integrity and normal dynamics.
It is worthy to point out that Stau2 is symmetrically distributed across the spindle structure in meiotic oocytes, this is different from the previous report that Stau2 is asymmetrically assigned in neural stem cells.13 We clarified the subcellular localization of Stau2 in fully differentiated somatic cells during mitotic progression. The immunofluorescence results showed that Stau2 was mainly localized as one large patch on one side of nucleus at interphase in both BHK-21 (Fig. S1 A, a-d: arrows) and MEF cells (Fig. S1 A, a′-d′: arrows), such congression was fragmented into small foci as cells entered mitosis (Fig. S1 A, g, g′: arrows), notably, these foci still remained asymmetrically distributed during cell cycle progression to metaphase and telophase (Fig. S1 A, i-l, m-p, I′-l′, m′-p′: arrows). Importantly, Stau2 was only aggregated in the peripheral area of spindle, but not overlapping with microtubule array, it is precisely colocalized with cis-Golgi marker GM130 (Fig. S1 B: arrows), implying Stau2 is associated with Golgi complex in conventional somatic cells. In general, the subcellular distribution pattern of Stau2 in mitotic cells is totally different from that in meiotic oocytes, suggesting different functional aspects of Stau2 between mitosis and meiosis.
Disrupted spindle formation and chromosome alignment in oocytes with depleted Stau2
In order to determine whether Stau2 is involved in meiotic spindle formation and maintenance in mouse oocytes, we analyzed the spindle structure when Stau2 protein expression was depleted by microinjection of Stau2-specific morpholino oligo. Western blot and statistical analysis showed that the total protein level of Stau2 was significantly reduced in oocytes injected with Stau2 morpholino when compared with that in uninjected oocytes and those injected with control morpholino (Fig. 2A, D), that is to say, Stau2 protein expression was successfully knocked down, this was further proved by the markedly weakened fluorescence intensity of Stau2 in oocytes treated with Stau2 morpholino (Fig. 2B, k, o, s), and confirmed by the statistically significant decrease in the number of MI oocytes with bright Stau2 in Stau2 morpholino group (P < 0.05) (Fig. 2E). In control oocytes checked at 8 h of maturation culture after morpholino treatment, all the chromosomes were arranged in a linear array within a bipolar MI spindle, simultaneously, bright Stau2 filaments were perfectly overlapped with microtubules and symmetrically assembled into a “spindle-like” structure (Fig. 2B, a-d, e-h). In contrary, the spindle was not properly organized in Stau2-depleted oocytes, mainly manifested with reduced microtubule density, unsymmetrical spindle structure and even no special microtubule organization (Fig. 2B, j, n, r), in the meantime, the chromosomes were not aligned in an orderly manner, and scattered in a pretty large area or even decondensed into configuration specific for chromatin at prophase (Fig. 2B, i, m: arrows, q). By statistical analysis, the number of oocytes with abnormal spindle was significantly higher in Stau2 morpholino group (56.26 ± 3.011) than that in uninjection (26.61 ± 1.038) and control morpholino groups (30.11 ±1.370) (P < 0.05) (Fig. 2F), concomitantly, the percentage of oocytes with misaligned chromosomes was also obviously higher in Stau2 morpholino group (P < 0.05) (Fig. 2G). Western blot analysis showed that the protein expression of LIMK2, a molecule associated with microtubule stability,17 was obviously reduced in oocytes treated with Stau2 morpholino oligo, quantitative analysis further confirmed that LIMK2 expression was significantly less in Stau2 morpholino group than that in 2 controls (Fig. 2H). These data suggest that the protein expression of Stau2 can be effectively knocked down in mouse oocytes by microinjection of Stau2-specific morpholino sequence, and consequently, leading to abnormal spindle formation and chromosome alignment.
Figure 2.
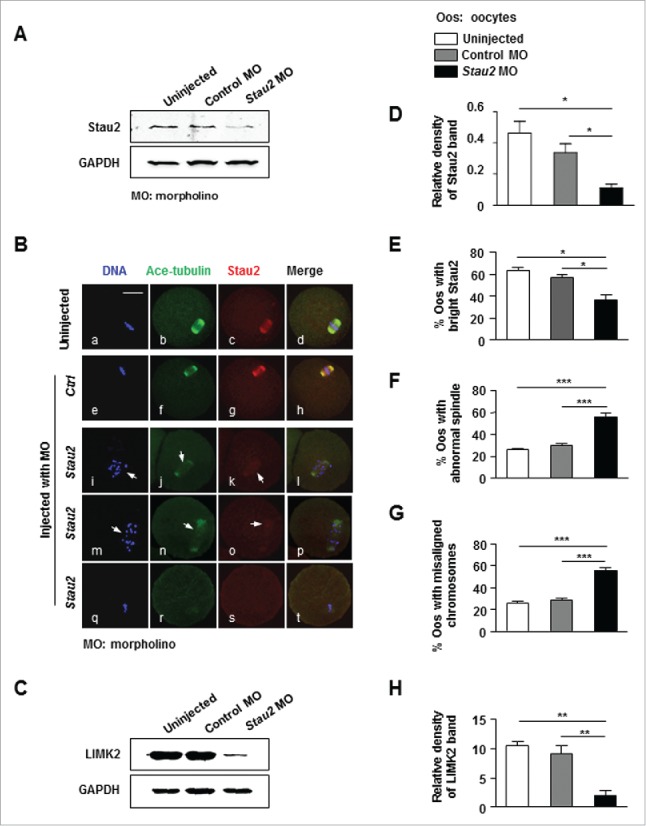
Knockdown of Stau2 disrupted the spindle structure and alignment of chromosome. Oocytes were cultured for 8 h in normal culture medium after microinjected with 1 mM Stau2 morpholino, and then collected for analysis with western blot and immunofluorescence. Oocytes uninjected or injected with 1 mM non-specific morpholino sequence were used as controls. A. The protein expression of Stau2 was effectively knocked down with morpholino oligo in mouse oocytes. Western blot analysis showed that Stau2 protein was significantly reduced in Stau2 morpholino group than that in 2 control groups. GAPGH was used as a loading control. B. Immunofluorescence analysis detected weak or no Stau2 expression after morphorlino treatment. In two control groups, the chromosomes were properly aligned (a, e) within a bipolar MI spindle (b, f), meanwhile Stau2 was pronouncedly labeled in symmetric organization (c, g) and overlapped with the meiotic spindles (d, h). In Stau2 morpholino group, the chromosomes were not aligned, but scattered or decondensed (i, m, q), spindle was abnormally formed (j, n, r) the fluorescence intensity of Stau2 was heavily reduced (k, o: arrows) or hardly detected (s). Microtubules were visualized in green, Stau2 was visualized in red and DNA was labeled in blue. Scale bar = 20 μm. C. The protein expression of LIMK2 was significantly reduced in mouse oocytes treated with Stau2 morpholino oligo. Western blot analysis indicated that LIMK2 protein was markedly decreased in Stau2 morpholino group than that in 2 control groups. GAPGH was used as a loading control. D. Quantitative analysis of Stau2 expression in control and Stau2 morpholino group. Analysis with Image J software and statistical procedure demonstrated the gray level of Stau2 was obviously decreased in Stau2 morpholino group than that in uninjected group and control morpholino-injected group. E. The percentage of oocytes with normal Stau2 intensity in control and Stau2 morpholino group. Statistical analysis, combined with fluorescence, showed that the proportion of oocytes with bright Stau2 intensity was significantly lower in Stau2 morpholino group than that in uninjected group and control morpholino-injected group. F. Percentage of oocytes with abnormal spindle in control and Stau2 morpholino group. Statistical analysis, combined with fluorescence, demonstrated that the propotion of oocytes with abnormal spindle was significantly higher in Stau2 morpholino group than in 2 control groups. G. Percentage of oocytes with misaligned chromosomes in control and Stau2 morpholino group. Statistical analysis, combined with fluorescence, illustrated that the proportion of oocytes with misaligned chromosomes was significantly higher in Stau2 morpholino group than in 2 control groups. H. Quantitative analysis of LIMK2 protein level in control and Stau2 morpholino group. Analysis with Image J software and statistical procedure showed that the gray level of LIMK2 was pronouncedly reduced in Stau2 morpholino group than that in control groups.
Abnormal microtubule-kinetochore attachments in Stau2-depleted oocytes
As described above, the configuration of meiotic spindle and chromosomes was severely damaged in oocytes with knockdown of Stau2, it is a considerable question whether the attachments between microtubules and kinetochores are impacted by the loss of Stau2 expression. To clarify this query, immunofluorescence staining was conducted by using acetylated-tubulin antibody and CREST serum in oocytes after incubated on ice for 5 min at the end of 8 h culture after microinjection of morpholino. Just as we expected, unattached kinetochores were more often observed in oocytes injected with Stau2 morpholino (Fig. 3A, h: arrows), by comparison, the obvious connection between kinetochores and microtubules was visualized in the majority control oocytes, with only few kinetochores were not attached (Fig. 3A, d). Further statistical analysis confirmed that the percentage of oocytes with abnormal microtubule-kinetochore attachments was significantly higher in Stau2 morpholino group (uninjection, 32.80 ± 2.153; control morpholino, 18.42 ± 9.310, Stau2 morpholino, 70.20 ± 10.06) (P < 0.05) (Fig. 3B). This data indicates Stau2 is involved in the establishment of connection between kinetochores and microtubules in oocytes during meiosis, and might be associated with its accumulation in centromere area.
Figure 3.
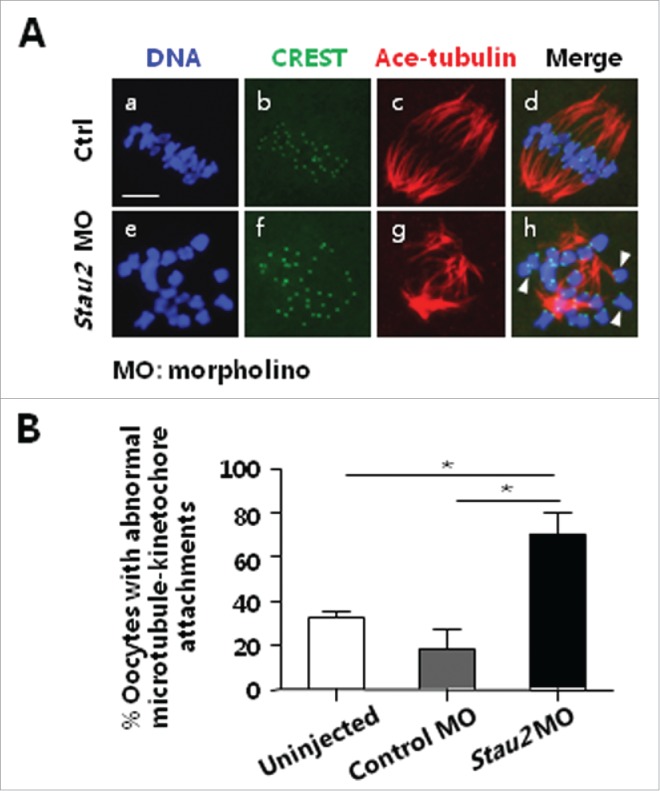
Knockdown of Stau2 impacted the attachment between microtubules and kinetochores. After morpholino treatment, MI oocytes were incubated on ice for 5 min and then processed with immunofluorescent staining using CREST serum and acetylated-tubulin antibody. The attachment between the microtubules and kinetochores was carefully analyzed. The microtubule-kinetochore attachment was frequently disrupted in oocytes treated with Stau2 morpholino oligo (A, h: arrows). Further statistical analysis demonstrated that the percentage of oocytes with abnormal microtubule-kinetochore attachment was significantly higher in Stau2 morpholino group than that in 2 control groups (P < 0.05) (B). Microtubules were visualized in red, CREST was visualized in green and DNA was labeled in blue. Scale bar = 20 μm.
Stau2 depletion blocked meiotic progression in oocytes
We believe that the defective spindle structure and microtubule-kinetochore attachment could trigger the activation of SAC system, a signal pathway blocking chromosome separation and anaphase onset until the spindle is properly formed and connected to chromosomes. We indeed found that the meiotic progression was arrested at MI stage after 17 h maturation culture in oocytes with disrupted expression of Stau2. Further quantitative analysis demonstrated that the percentage of oocytes at MI stage was pronouncedly higher in stau2 morpholino group after 17 h culture, only a few cells progressed into MII stage, while quite some oocytes were retarded at the late stage of telophase I (TI) (Fig. 4A, e-h, B). Immunofluorescence revealed no or very weak signal of Stau2 in the area of microtubules in these TI oocytes (Fig. 4A, g), and chromosomes were decondensed and presented in a configuration which should be typically observed at prophase stage (Fig. 4A, e). These data certainly indicate that Stau2 depletion resulted in defects in TI completion, and thus delayed cytokinesis progression and first polar body extrusion.
Figure 4.
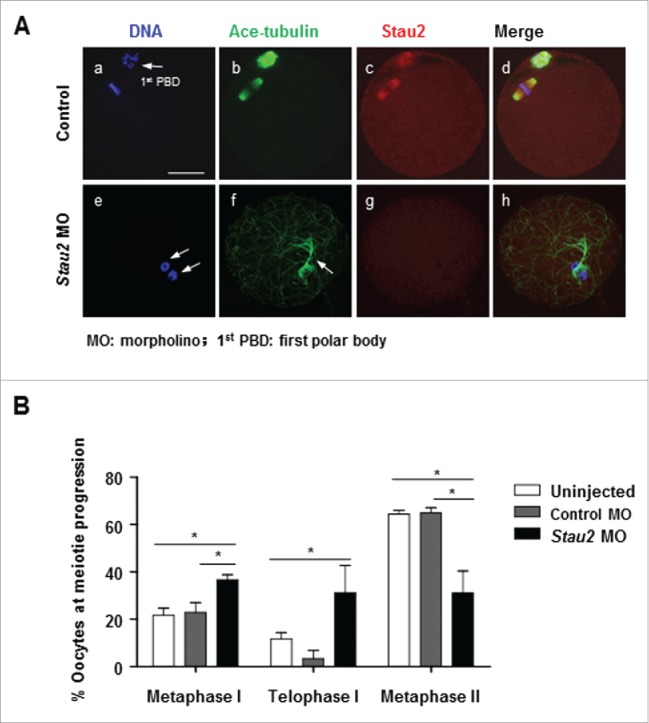
Stau2 depletion delayed meiosis I completion in oocytes. After morpholino treatment, oocytes were cultured in normal maturation medium for 17 h, and processed for immunostaining with acetylated-tubulin and Stau2. The meiotic progression was counted based on chromosome configuration and spindle structure. The percentage of oocytes at MI stage was significantly higher in Stau2 morpholino group, quite some oocytes were at the late stage of TI (A, e-h, B). Only faint Stau2 was detected with immunofluorescence on microtubules in TI oocytes (A, f, g, h), combined with decondensed chromosomes (A, e).
SAC activation in oocytes with depleted Stau2 expression
In logical consistence with the arrested meiotic progression, we found that SAC was definitely activated. When checked with immunostaining at 8 h of maturation culture, no or only weak signal of MAD1 was observed on chromosome centromeres which were labeled with CREST, in majority MI oocytes from 2 control groups (Fig. 5A, b-d, f-h: boxes), implying that SAC is quiescent and these oocytes might be ready for the transition from meiosis I to meiosis II.26 However, bright signal of MAD1 was detected on kinetochores in more than 50% oocytes in Stau2 morpholino group (Fig. 5A, j-l: boxes) and significantly higher than that in 2 controls (uninjection, 26.47 ± 3.61; control morpholino, 27.17 ± 4.86; Stau2 morpholino, 51.36 ± 6.41) (P < 0.05) (Fig. 5B), this suggests the abnormal spindle structure and disrupted microtubule-chromosome connection could bring about persistent activation of SAC, thus blocked the cell cycle progression through meiosis.
Figure 5.
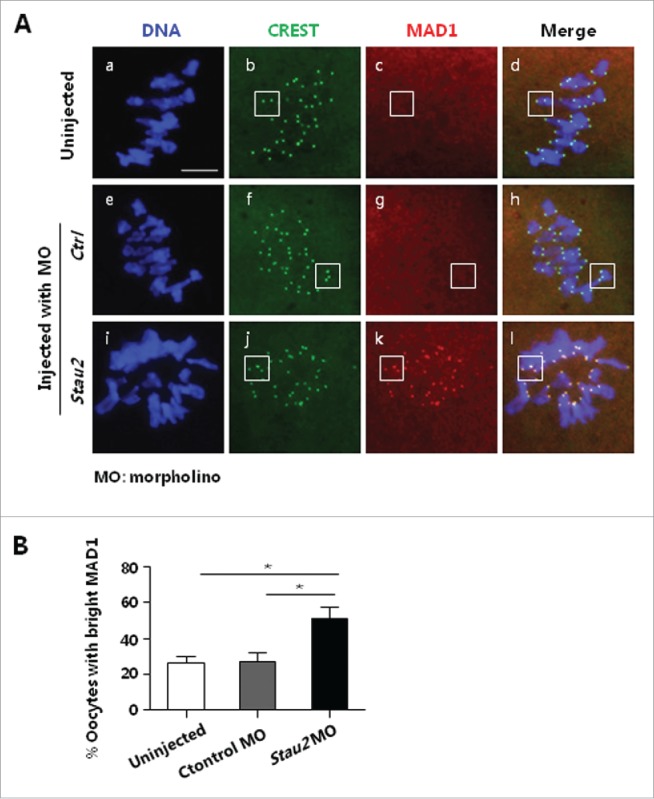
SAC activation in MI oocytes with depleted Stau2 expression. At the end of 8 h maturation culture, MI oocytes were processed for immunostaining with SAC protein MAD1, the proportion of oocytes with weak or no MAD1 signal was statistically analyzed. MAD1 was faintly detected in control oocytes (A, b-d, f-h: boxes), but obviously probed on centromere area in oocytes treated with Stau2 morpholino (A, j-l: boxes).Statistical analysis indicated that the number of oocytes with bright MAD1 was significantly higher in Stau2 morpholino group than that in 2 controls (B). CREST was visualized in green, MAD1 was visualized in red and DNA was labeled in blue. Scale bar = 20 μm.
Discussion
In the present study, we examined the expression and function of Stau2 in mouse oocytes during meiotic division. We demonstrate for the first time that Stau2 protein is stably expressed in mouse oocytes, with consistent accumulation on chromosome centromeres and symmetrical distribution across the spindle structure during meiotic progression, totally different from its Golgi localization during somatic mitosis. Stau2 depletion by specific morpholino oligo destroyed the meiotic spindle formation and the attachment between chromosomes and spindle microtubules, as well as the timely cell cycle progression through oocyte meiosis.
Stau2 is initially identified as a double-stranded mRNA binding protein, being a core component of RNA granules, it promotes mRNA transport and localization along microtubules, importantly, and this protein itself is asymmetrically localized and, thus induces asymmetrical distribution of key mRNA transcripts, especially those of fate determinants.11,12,27 Previous studies indicate the localized translation of such mRNAs is essential for neural stem cell self-renewal and differentiation, synaptic plasticity, cell motility, and asymmetric cell division.27-29 It is still unknown about Stau2 subcellular distribution and potential functions in conventional somatic cells during mitosis, and in mammalian oocytes during meiosis. Herein we found that Stau2 is localized in Golgi complex, without any association with microtubules, in BHK-21 and MEF cells, this distribution pattern is totally different from that in stem cells,13 implying Stau2 plays distinct roles in somatic cells, and maybe involve in the regulation of vesicle activity, this is supported, at least partially, by the information that Stau2 regulates the localization of mRNA encoding Rab11a (RAB11A, member RAS oncogene family),25 which is a key regulator of Golgi-derived vesicle formation and trafficking.22 In contrast, Stau2 is assembled as filaments and perfectly colocalized with microtubules on spindle in mouse oocytes. Stau2 distribution across the spindle structure is dependent on microtubule integrity, obviously, this aggregation completely disappears upon microtubule depolimerization by spindle poison nocodazole; interestingly, Stau2 is disassociated from microtubules when they are stabilized by taxol, but concentrated in the vicinity surrounding the chromosome array. This data suggests that Stau2 migrates dynamically along microtubules from the plus end to the minus, and such action is dependent on microtubule normal dynamics, but blocked by microtubule stabilization. Actually, Stau2 is found associated with dynein light chain-1 (Ddlc-1), a component of the minus-end-directed microtubule motor cytoplasmic dynein, promotes dynein-driven mRNA transport along microtubules to their minus ends in neurons and Drosophila oocytes.30,31 It is still to be explored about the exact interaction between Stau2 and motor proteins in mouse oocytes during meiotic division.
The stable expression of Stau2 is required for microtubule stability and organization in oocytes. Specific morpholino sequence-mediated Stau2 depletion reduced microtubule density on spindle, which means microtubule stability might be impaired. Abnormal spindle structure and chromosomes alignment were frequently observed in Stau2-depleted oocytes. As aforementioned, mRNAs of MAP1B, Rab5 (RAB5, member RAS oncogene family), Rab11, LIMK2, SIRT3 and SUMO1 are targets of Stau2,25 coincidently, these proteins are all colocalized with microtubules on spindle, and required for microtubule stability, spindle formation, as well as chromosome alignment and separation in somatic cells and oocytes,7,17-19,20,21,24 and our data clearly showed that LIMK2 protein expression was significantly reduced in Stau2-depleted oocytes, so it may be plausible that Stau2 participates in spindle assembly and chromosome division through regulating the expression and localization of these microtubule-related proteins, and Stau2 depletion may affect their function, causing abnormal spindle formation and chromosome congression, just as exhibited in the present study. As reported previously, in Stau2-deficient neurons, the majority of Stau2 targets are noticeably downregulated and, only a small fraction of them increase in level, implying Stau2 plays predominant role in mRNA stabilization other than its degradation.10,15,16
Besides localization on microtubules, Stau2 is also concentrated on chromosome centromere area in oocytes, this is similar with dynein congression on centromeres in somatic cells and oocytes.32,33 It is proved that dynein motor is required for stable kinetochore-microtubule attachment, and chromosome orientation and alignment in mitosis.33 In the present study, disrupted kinetochore-microtubule attachments were obviously found in oocytes with depleted Stau2, this indicates a direct role of Stau2 in the establishment and maintenance of attachments between kinetochore and microtubules in oocyte meiosis. Since Stau2 and dynein exhibit similar localization on microtubules and centromeres,32,33 it may be reasonable to propose that Stau2 regulates kinetochore-microtubule attachment through its cooperation with dynein. In addition, Rab5 mRNA is a target of Stau2,25 this small GTPase is newly found localized in centromeres and required for proper setup of attachment between microtubules and kinetochores in mouse oocytes,19 so in Stau2-depleted oocytes, the abnormal kinetochore-microtubule attachment may be, in some extent, contributed to defects in Rab5 localization and function. Further studies are needed to reveal the exact function of Stau2 in the establishment of connection between kinetochores and microtubules.
The abnormal spindle organization and its attachment to kinetochores are sensed by SAC in Stau2- depleted oocytes. The SAC activation, manifested with persistent congression of MAD1, the core member of SAC, on centromeres, prevented meiotic progression and arrested oocytes at MI stage.26 Notably, some oocytes left MI but not developed to MII stage, instead, stayed at late stage of TI with decondensed chromosomes. Stau2 association with microtubule during TI process may be required for microtubule sliding and contracting, which is essential for TI completion and the start of first polar body extrusion. It has been proved that Rab21 (RAB21, member RAS oncogene family) and NEDD8 (neural precursor cell expressed, developmentally down-regulated gene 8) are required for successive cytokinesis during somatic mitosis,34,35 and that Cdc42 is indispensable for cytokinesis completion during meiosis I in mouse oocytes.23 These information, together with the fact that Stau2 targets mRNAs of Rab21, NEDD8 and Cdc42,25 suggest that Stau2 may regulate cytokinesis progression by being an upstream regulator of these molecules.
In conclusion, Stau2 is required for meiotic spindle formation and maintenance, suitable attachment between spindle and chromosome kinetochores, as well as timely cell cycle progression in mammalian oocytes during meiotic division, implying its essential involvement in mechanism ensuring accurate chromosome separation.
Materials and methods
Oocyte and somatic cell culture
All the procedures of animal care and use were approved and conducted in accordance with Animal Care Commission policies of Capital Medical University. Oocytes were isolated from CB6F1 (C57BL/6 ♂ × BALB/C ♀ F1) female mice. As previously reported,36 21-23 day-old mice were injected with 10 IU PMSG (Beijing XinHuiZeAo Science and Technology) to stimulate pre-ovulatory follicle development, and then euthanatized with CO2 44-48 h later, cumulus cell-oocyte complexes (COCs) were harvested from inflated ovaries, and incubated in Minimal Essential Medium (MEM) containing 3 mg/ml bovine serum albumin (BSA, Sigma) and 10% fetal bovine serum (FBS, Gibco) at 37°C with 5% CO2 and saturated humidity. Oocytes at the stages of germinal vesicle (GV), germinal vesicle breakdown (GVBD), prometaphase (pro-MI), metaphase I (MI) and metaphase II (MII) were collected after culture for 0, 2, 4, 8 and 17 h, respectively. In order to be arrested at GV stage, oocytes were cultured in maturation medium supplemented with 3.3 μM milrinone (Sigma). After culture, oocytes were processed for subsequent experiments of drug treatment, or immunofluorescence and western blot.
In addition, baby hamster Syrian kidney-21 cells (BHK-21) and mouse embryonic fibroblast cells (MEF) were cultured at 37°C in 5% CO2 in DMEM supplemented with 10% fetal bovine serum for appropriate time, and then fixed for imunofluorescence analysis.
Immunofluorecence
Oocytes were fixed in 1% paraformaldehyde (PFA) in PEM buffer (100 mM Pipes, pH 6.9,1 mM MgCl2, 1 mM EGTA) with 0.2% Triton X-100 for 1 h at room temperature, then washed and blocked for 1 h in phosphate buffer saline (PBS) added with 10% normal goat serum(NGS). The oocytes were incubated at 4°C, overnight, in blocking solution containing specific primary antibodies: Stau2 antibody (1:1000, Cat#: GTX116458, GeneTex), monocloal anti-acetylated tubulin antibody (1:3000, Cat#: T7451, Sigma-Aldrich) or human auto anti-centromere serum (CREST) (1:1000, Cat#: 90C-CS1058, Fitzgerald). After three washes in PBS with 0.02% Triton X-100, the oocytes were treated with secondary antibodies for 1 h in the dark at room temperature, and then washed and mounted on glass slides in VECTASHIELD mounting medium containing DAPI (Cat#: H-1200, Vector Laboratories) and examined using an upright fluorescent microscope (Olympus Microsystems).
For immunofluorescence staining on chromosome spreads, oocytes were incubated in pre-warmed acid Tyrode's solution (Cat#: T1788, Sigma) for a short while, to get rid of the encompassing zona pellucida. After a short recovery time in MEM/BSA, a group of 10 oocytes each time were carefully shifted to 100 μl drops of fixation solution (1% paraformaldehyde in distilled water with 0.1% Triton-X 100) on glass slides, these oocytes dilated and ruptured after a few seconds, and gradually “thawed” on the slides. The slides were air-dried and stored at −20°C before use. Prior to 1 h incubation in blocking solution, the slides were immersed in PBS to wash off any salt mixed in the chromosome samples. The samples were immunolabeled with MAD1 antibody (1:100, Cat#: SC-137025, Santa Cruz) and CREST serum for 2 h at 37°C, and then visualized with Alexa 594-conjugated goat anti-mouse IgG and Alexa 488-conjugated goat anti-human IgG. Chromosomes were counterstained with DAPI (Vector Laboratories) and then assessed with fluorescent microscope (Olympus Microsystems). The fluorescent intensity of MAD1 was analyzed using Image J software (National Institutes of Health, Washington, DC).
For immunostaining in BHK-21 and MEF cells, the cells were plated on coverslips and fixed in cold methonal at −20°C for 20 min, then washed and treated for 1 h in blocking solution as above. Cells were incubated in blocking solution containing anti-Stau2 antibody (1:2000), anti-GM130 antibody (1:1000, Cat#: ab169276, abcam) or anti-acetylated tubulin antibody (1:10000) at 4°C, overnight. After being washed with PBS, cells were labeled with secondary antibodies and examined using a fluorescent microsystems.
Western blot analysis
A total of 50 oocytes were collected and frozen in laemlli buffer (Cat#: 161-0737, BIO-RAD) added with protease inhibitor cocktail (P2714, Sigma). The protein samples were boiled for 5 min before separated on 10% SDS-PAGE gel and transferred to polyvinylidene fluoride membrane (PVDF) (Cat#: IPVH00010, Millipore). The membrane was blocked in 5% non-fat dried milk dissolved in Tris-buffered saline (TBS) containing 0.1% Tween-20 (TBST), and incubated in anti-Stau2 antibody (1:3000) or anti-LIMK2 antibody (1:3000) (Cat#: aw5105, Abgent, Inc.) at 4°C, overnight. After thoroughly washed in TBST for 3 times, each for 20 min, the membrane was treated with horseradish peroxidase-conjugated secondary antibody (1:4000) (Cat#: LP1002a, ABGENT) for 1 h at room temperature. The membrane was again carefully washed 3 times in TBST, and illuminated with an enhanced chemiluminescence detection system (Cat#: P1010, Applygen Technologies Inc.). The level of GAPDH was used as a loading control, and detected with a rabbit monoclonal anti-GAPDH (1:2000) (Cat#: G9545, Sigma) antibody. The immunoreactive bands were quantified by densitometry analysis using Image J software (National Institutes of Health, Washington, DC), and normalized to GAPDH.
Nocodazole and taxol treatment
MI oocytes were treated with specific microtubule toxins, nocodazole (Cat#: M1404, Sigma-Aldrich) or taxol (Cat#:S1150, Selleck). The procedure was essentially same as that reported previously.24 Nocodazole was dissolved in dimethylsulfoxide (DMSO) to make a stock solution at 20 mg/ml, similarly, 10 mM taxol stock was also prepared. Prior to use, the stocks were further diluted in culture medium for a final concentration of 20 μg/ml and 10 μM, respectively, for nocodazole and taxol. Oocytes were incubated in 20 μg/ml nocodazole for 5, 10, 15 and 20 min or in10 μM taxol for 45 min at 37°C in an atmosphere of 5% CO2 in air. After treatment, the oocytes were washed thoroughly and fixed for immunofluorescence. Control oocytes were incubated with the same concentration of DMSO under same culture conditions. The final concentration of DMSO was not more than 0.1% (v/v) in culture medium.
Stau2 knockdown by microinjection of gene specific morpholino oligo
The micromanipulation process was conducted according to previous report.24 stau2-specific morpholino sequence (TTGCCATTTTATCGTGGAGAGAAGC) (Cat#: 33-29Apr14A, Gene Tool), 25 nucleotides in length, was used to knock down Stau2 translation level in oocytes. Control groups included: (i) noninjected oocytes that were subject to the same culture conditions and (ii) oocytes injected with morpholino oligo standard control (CCTCTTACCTCAGTTACAATTTATA) (Gene Tool). For each group, 10 pl of 1 mM morpholino solution was microinjected directly into the cytoplasm of denuded, fully grown oocytes arrested at GV in medium supplemented with 3.3 μM milrinone. Sterile Femtotip capillaries (Eppendorf) and a FemtoJet microinjector (Eppendorf) were used to standardize the injection volumes. Following oocyte microinjection, oocytes were maintained in GV arrest for 24 h. The oocytes were subsequently washed thoroughly, transferred to fresh medium and cultured for an additional 8 or 17 h. Finally, the oocytes were collected for further western blot and immunostaining analysis.
Cold treatment
In order to explore the effects of Stau2 depletion on the connection between microtubules and chromosome kinetochores, MI oocytes were collected after cultured for 8 h after morpholino treatment as stated above, and incubated in M2 medium (M7167, Sigma) on ice for 5 min, then immediately fixed for immunostaining with CREST serum and acetylated-tubulin antibody for the analysis of the attachment between the microtubules and kinetochores.
Statistical analysis
The data are expressed as the mean ± SEM of a minimum of 3 independent experimental replicates. Differences between treated groups were analyzed by t-test using GraphPad Prism 5 software (Hallogram Publishing, USA), and P < 0.05 is considered significant.
Supplementary Material
Abbreviations
- 1st PBE
first polar body extrution
- GV
germinal vesicle
- GVBD
germinal vesicle breakdown
- MI
metaphase I
- MII
metaphase II
- MTOC
microtubule organizing center
- PCM
pericentriolar material
- pro-MI
pro-metaphase I
- SAC
spindle assembly checkpoint
- Stau2
Staufen2
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors gratefully thank Xin Li and Haojie Wei for their critical technical assistance during this study.
Funding
This study was supported by grants from National Natural Science Foundation of China (31471108, 31271253 and 31500942).
References
- [1].Sakakibara Y, Hashimoto S, Nakaoka Y, Kouznetsova A, Hoog C, Kitajima TS. Bivalent separation into univalents precedes age-related meiosis I errors in oocytes. Nature 2015; 6:7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Herbert M, Kalleas D, Cooney D, Lamb M, Lister L. Meiosis and maternal aging: Insights from aneuploid oocytes and trisomy births. Cold Spring Harb Perspect Biol 2015; 7(4):a017970; PMID:25833844; http://dx.doi.org/ 10.1101/cshperspect.a017970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kuznetsova AY, Seget K, Moeller GK, Pagter M, de Roos J, Dürrbaum M. Chromosomal instability, tolerance of mitotic errors and multidrug resistance are promoted by tetraploidization in human cells. Cell Cycle 2015; 14(17):2810-20; PMID:26151317; http://dx.doi.org/ 10.1080/15384101.2015.1068482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Miller MP, Ünal E, Brar GA, Amon A. Meiosis I chromosome segregation is established through regulation of microtubule–kinetochore interactions. Elife 2012; 1:e00117; PMID:23275833; http://dx.doi.org/ 10.7554/eLife.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ma W, Koch JA, Viveiros MM. Protein kinase C delta (PKCδ) interacts with microtubule organizing center (MTOC)-associated proteins and participates in meiotic spindle organization. Dev Biol 2008; 320(2):414-25; PMID:18602096; http://dx.doi.org/ 10.1016/j.ydbio.2008.05.550 [DOI] [PubMed] [Google Scholar]
- [6].Dumont J, Desai A. Acentrosomal spindle assembly and chromosome segregation during oocyte meiosis. Trends Cell Biol 2012; 22(5):241-9; PMID:22480579; http://dx.doi.org/ 10.1016/j.tcb.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fanarraga ML, Villegas JC, Carranza G, Castaño R, Zabala JC. Tubulin cofactor B regulates microtubule densities during microglia transition to the reactive states. Exp Cell Res 2009; 315:535-41; PMID:19038251; http://dx.doi.org/ 10.1016/j.yexcr.2008.10.045 [DOI] [PubMed] [Google Scholar]
- [8].Gadde S, Heald R. Mechanisms and molecules of the mitotic spindle. Curr Biol 2004; 14:R797-805; PMID:15380094; http://dx.doi.org/ 10.1016/j.cub.2004.09.021 [DOI] [PubMed] [Google Scholar]
- [9].Li L, Zheng P, Dean J. Maternal control of early mouse development. Development 2010; 137(6):859-70; PMID:20179092; http://dx.doi.org/ 10.1242/dev.039487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Heraud-Farlow JE, Kiebler MA. The multifunctional Staufen proteins: conserved roles from neurogenesis to synaptic plasticity. Trends Neurosci 2014; 37(9):470-9; PMID:25012293; http://dx.doi.org/ 10.1016/j.tins.2014.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Furic L, Maher-Laporte M, Desgroseillers L. A genome-wide approach identifies distinct but overlapping subsets of cellular mRNAs associated with Staufen1- and Staufen2-containing ribonucleoprotein complexes. RNA 2008; 14(2):324-35; PMID:18094122; http://dx.doi.org/ 10.1261/rna.720308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Micklem DR, Adams J, Grünert S, St Johnston D. Distinct roles of two conserved Staufen domains in oskar mRNA localization and translation. EMBO J 2000; 19(6):1366-77; PMID:10716936; http://dx.doi.org/ 10.1093/emboj/19.6.1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vessey JP, Amadei G, Burns SE, Kiebler MA, Kaplan DR, Miller FD. An asymmetrically localized Staufen2-Dependent RNA complex regulates maintenance of mammalian neural stem cells. Cell Stem Cell 2012; 11:517-28; PMID:22902294; http://dx.doi.org/ 10.1016/j.stem.2012.06.010 [DOI] [PubMed] [Google Scholar]
- [14].Riechmann V, Ephrussi A. Axis formation during Drosophila oogenesis. Curr Opin Genet Dev 2001; 11(4):374-83; PMID:11448623; http://dx.doi.org/ 10.1016/S0959-437X(00)00207-0 [DOI] [PubMed] [Google Scholar]
- [15].Park E, Maquat LE. Staufen-mediated mRNA decay. Wiley Interdiscip Rev RNA 2013; 4(4):423-35; PMID:23681777; http://dx.doi.org/ 10.1002/wrna.1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Heraud-Farlow JE, Sharangdhar T, Li X, Pfeifer P, Tauber S, Orozco D, Hörmann A, Thomas S, Bakosova A, Farlow AR. Staufen2 regulates neuronal target RNAs. Cell Rep 2013; 5(6):1511-8; PMID:24360961; http://dx.doi.org/ 10.1016/j.celrep.2013.11.039 [DOI] [PubMed] [Google Scholar]
- [17].Po'uha ST, Shum MS, Goebel A, Bernard O, Kavallaris M. LIM-kinase 2, a regulator of actin dynamics, is involved in mitotic spindle integrity and sensitivity to microtubule-destabilizing drugs. Oncogene 2010; 29(4):597-607; PMID:19881550; http://dx.doi.org/ 10.1038/onc.2009.367 [DOI] [PubMed] [Google Scholar]
- [18].Choi BS, Park JE, Jang CY. Sirt3 controls chromosome alignment by regulating spindle dynamics during mitosis. Biochem Biophys Res Commun 2014; 444(4):662-9; PMID:24491532; http://dx.doi.org/ 10.1016/j.bbrc.2014.01.124 [DOI] [PubMed] [Google Scholar]
- [19].Ma R, Hou X, Zhang L, Sun SC, Schedl T, Moley K, Wang Q. Rab5a is required for spindle length control and kinetochore-microtubule attachment during meiosis in oocytes. FASEB J 2014; 28(9):4026-35; PMID:24876181; http://dx.doi.org/ 10.1096/fj.14-250886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Capalbo L, D'Avino PP, Archambault V, Glover DM. Rab5 GTPase controls chromosome alignment through Lamin disassembly and relocation of the NuMA-like protein Mud to the poles during mitosis. Proc Natl Acad Sci U S A 2011; 108(42):17343-8; PMID:21987826; http://dx.doi.org/ 10.1073/pnas.1103720108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hehnly H, Doxsey S. Rab11 endosomes contribute to mitotic spindle organization and orientation. Dev Cell 2014; 28(5):497-507; PMID:24561039; http://dx.doi.org/ 10.1016/j.devcel.2014.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Johnson JL, He J, Ramadass M, Pestonjamasp K, Kiosses WB, Zhang J, Catz SD. Munc13-4 Is a Rab11-binding protein that regulates Rab11-positive vesicle trafficking and docking at the plasma membrane. J Biol Chem 2016; 291(7):3423-38; PMID:26637356; http://dx.doi.org/ 10.1074/jbc.M115.705871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang ZB, Jiang ZZ, Zhang QH, Hu MW, Huang L, Ou XH, Guo L, Ouyang YC, Hou Y, Brakebusch C. Specific deletion of Cdc42 does not affect meiotic spindle organization/migration and homologous chromosome segregation but disrupts polarity establishment and cytokinesis in mouse oocytes. Mol Biol Cell 2013; 24(24):3832-41; PMID:24131996; http://dx.doi.org/ 10.1091/mbc.E13-03-0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yuan YF, Zhai R, Liu XM, Khan HA, Zhen YH, Huo LJ. SUMO-1 plays crucial roles for spindle organization, chromosome congression, and chromosome segregation during mouse oocyte meiotic maturation. Mol Reprod Dev 2014; 81(8):712-24; PMID:25123474 [DOI] [PubMed] [Google Scholar]
- [25].Maher-Laporte M, DesGroseillers L. Genome wide identification of Staufen2-bound mRNAs in embryonic rat brains. BMB Rep 2010; 43(5):344-8; PMID:20510018; http://dx.doi.org/ 10.5483/BMBRep.2010.43.5.344 [DOI] [PubMed] [Google Scholar]
- [26].Zhang D, Li M, Ma W, Hou Y, Li YH, Li SW, Sun QY, Wang WH. Localization of Mitotic Arrest Deficient 1 (MAD1) in mouse oocytes during the first meiosis and its functions as a spindle checkpoint protein. Biol Reprod 2005; 72:58-68; PMID:15342357; http://dx.doi.org/ 10.1095/biolreprod.104.032987 [DOI] [PubMed] [Google Scholar]
- [27].Kuse G, Campbell M, Doyle F, Tenenbaum SA, Kiebler M, Temple S. Asymmetric segregation of the double-stranded RNA binding protein Staufen2 during mammalian neural stem cell divisions promotes lineage progression. Cell Stem Cell 2012; 11(4):505-16; PMID:22902295; http://dx.doi.org/ 10.1016/j.stem.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wickham L, Duchaine T, Luo M, Nabi IR, Desgroseillers L. Mammalian Staufen is a double-stranded-RNA- and tubulin-binding protein which localizes to the rough endoplasmic reticulum. Mol Cell Biol 1999; 19(3):2220-30; PMID:10022909; http://dx.doi.org/ 10.1128/MCB.19.3.2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jia M, Shan Z, Yang Y, Liu C, Li J, Luo ZG, Zhang M, Cai Y, Wen W, Wang W. The structural basis of Miranda-mediated Staufen localization during Drosophila neuroblast asymmetric division. Nat Commun 2015; 6:8381; PMID:26423004; PMID:17587311; http://dx.doi.org/ 10.1038/ncomms9381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jeong JH, Nam YJ, Kim SY, Kim EG, Jeong J, Kim HK. The transport of Staufen2-containing ribonucleoprotein complexes involves kinesin motor protein and is modulated by mitogen-activated protein kinase pathway. J Neurochem 2007; 102(6):2073-84; http://dx.doi.org/ 10.1111/j.1471-4159.2007.04697.x [DOI] [PubMed] [Google Scholar]
- [31].Schnorrer F, Bohmann K, Nüsslein-Volhard C. The molecular motor dynein is involved in targeting swallow and bicoid RNA to the anterior pole of Drosophila oocytes. Nat Cell Biol 2000; 2(4):185-90; PMID:10783235; http://dx.doi.org/ 10.1038/35008601 [DOI] [PubMed] [Google Scholar]
- [32].Varma D, Monzo P, Stehman SA, Vallee RB. Direct role of dynein motor in stable kinetochore-microtubule attachment, orientation, and alignment. J Cell Biol 2008; 182(6):1045-54; PMID:18809721; http://dx.doi.org/ 10.1083/jcb.200710106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang D, Yin S, Jiang MX, Ma W, Hou Y, Liang CG, Yu LZ, Wang WH, Sun QY. Cytoplasmic dynein participates in meiotic checkpoint inactivation in mouse oocytes by transporting cytoplasmic mitotic arrest-deficient (Mad) proteins from kinetochores to spindle poles. Reproduction 2007; 133:685-95; PMID:17504913; http://dx.doi.org/ 10.1530/rep.1.01167 [DOI] [PubMed] [Google Scholar]
- [34].Högnäs G, Tuomi S, Veltel S, Mattila E, Murumägi A, Edgren H, Kallioniemi O, Ivaska J. Cytokinesis failure due to derailed integrin traffic induces aneuploidy and oncogenic transformation in vitro and in vivo. Oncogene 2012; 31(31):3597-606; http://dx.doi.org/ 10.1038/onc.2011.527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kurz T, Pintard L, Willis JH, Hamill DR, Gönczy P, Peter M, Bowerman B. Cytoskeletal regulation by the Nedd8 ubiquitin-like protein modification pathway. Science 2002; 295(5558):1294-8; PMID:11847342; http://dx.doi.org/ 10.1126/science.1067765 [DOI] [PubMed] [Google Scholar]
- [36].Du J, Cao Y, Wang Q, Zhang NN, Liu XY, Chen DD, Liu XY, Xu QY, Ma W. Unique subcellular distribution of phosphorylated Plk1 (Ser137 and Thr210) in mouse oocytes during meiotic division and pPlk1Ser137 involvement in spindle formation and REC8 cleavage. Cell Cycle 2015; 14(22):3566-79; PMID:26654596; http://dx.doi.org/ 10.1080/15384101.2015.1100770 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.