Abstract
Tetrahydrobiopterin (BH4) is an essential cofactor of nitric oxide synthase (NOS) and aromatic amino acid hydroxylases. BH4 and 7,8-dihydrobiopterin (BH2) are metabolically interchangeable at the expense of NADPH. Exogenously administered BH4 can be metabolized by the body, similar to vitamins. At present, synthetic BH4 is used as an orphan drug for patients with inherited diseases requiring BH4 supplementation. BH4 supplementation has also drawn attention as a means of treating certain cardiovascular symptoms, however, its application in human patients remains limited. Here, we tracked biopterin (BP) distribution in blood, bile, urine, liver, kidney and brain after BH4 administration (5 mg/kg rat, i.v.) with or without prior treatment with probenecid, a potent inhibitor of uptake transporters particularly including organic anion transporter families such as OTA1 and OAT3. The rapid excretion of BP in urine was driven by elevated blood concentrations and its elimination reached about 90% within 120 min. In the very early period, BP was taken up by the liver and kidney and gradually released back to the blood. BH4 administration caused a considerable decrease in the BH4% in blood BP as an inevitable compensatory process. Probenecid treatment slowed down the decrease in blood BP and simultaneously inhibited its initial rapid excretion in the kidney. At the same time, the BH4% was further lowered, suggesting that the probenecid-sensitive BP uptake played a crucial role in BH2 scavenging in vivo. This suggested that the overproduced BH2 was taken up by organs by means of the probenecid-sensitive process, and was then scavenged by counter-conversion to BH4 via the BH4 salvage pathway. Taken together, BH4 administration was effective at raising BP levels in organs over the course of hours but with extremely low efficiency. Since a high BH2 relative to BH4 causes NOS dysfunction, the lowering of the BH4% must be avoided in practice, otherwise the desired effect of the supplementation in ameliorating NOS dysfunction would be spoiled.
Introduction
Tetrahydrobiopterin (BH4) is an essential cofactor for a group of aromatic amino acid hydroxylases [1–4]. Inherited BH4 deficiencies are characterized by hyperphenylalaninemia and defective biosynthesis of classic monoamines such as dopamine, noradrenaline, adrenaline, as well as serotonin. BH4 therapy using (6R)-L-erythro-5,6,7,8-tetrahydrobiopterin dihydrochloride (6RBH4) has been very successful in replacing peripheral BH4, but not BH4 in the brain. A guide for the therapeutic use of BH4 is available [5]. This compound ameliorates hyperphenylalaninemia in cases of inherited BH4 deficiency [6, 7] and benefits patients with BH4-responsive phenylketonuria [8]. The most common use of 6RBH4 to date is as an orphan drug for these inherited diseases [9–11]. Once 6RBH4 is administered, it replaces innate BH4 and is integrated through endogenous metabolic pathways, similar to the intake of vitamins. However, like most drugs or supplements, large amounts of exogenous BH4 bypass endogenous pathways and are eliminated in urine [12, 13] and feces [14], therefore it is desirable to minimize this useless exclusion.
We previously observed that 6RBH4 administration raised tissue BH4 levels, however, with a concomitant rise in 7,8-dihydrobiopterin (BH2) [12, 13]. Urinary excretion was suggested to be the process most responsible for the rapid loss of exogenous BH4. Furthermore, we demonstrated that urinary excretion of administered 6RBH4 at a pharmacological dose predominantly involved secretion across renal tubular epithelium, distinct from glomerular filtration. We observed that the tubular secretion was almost completely suppressed by cyclosporin A (CsA), an inhibitor of transporters with broad specificity in excretion of xenobiotics and metabolic wastes.
In order to obtain greater insight into the renal exclusion of BH4 and the relative increase in dihydrobiopterin (BH2), in this study, we analyzed the systemic distribution of biopterin (BP), the sum total of BH4 and BH2 (BP = BH2 + BH4), in the presence of probenecid (Pbc), a potent inhibitor of uptake transporters with a broad specificity [15]. We confirmed the active role played by the renal secretion of BP in the heavy loss of exogenous BH4. Furthermore, we revised the role of the salvage pathway of BH4 biosynthesis in scavenging BH2 in terms of its passage through the liver and kidney under the conditions of 6RBH4 administration.
Materials and Methods
Chemicals
6RBH4 was donated by Suntory (Asubio Pharma, Kobe, Japan). 6RBH4 (BH4•2HCl) was dissolved at 10 mg/mL in 0.9% NaCl before the start of the BH4 administration experiment. The BH4 solution was neutralized by 0.1 M Na2HPO4 and saline to prepare a 5 mg/mL concentration immediately before injection. Probenecid (Pbc: p-(dipropylsulfamoyl)benzoic acid) and cyclosporin A were obtained from Sigma-Aldrich (St. Louis, MO). Pbc was dissolved by dropwise addition of 2M NaOH, followed by the addition of N-2-hydroxyethylpiperazine-N'-2'-ethanesulfonic acid (HEPES) to neutralize the solution and adjusted the concentration to 40 mg/mL. CsA was dissolved in ethanol, then diluted in 9 vol of olive oil (final concentration, 5 mg/mL). “Gall Powder®” was purchased from Wako Pure Chemical Industries (Osaka, Japan).
Ethics statement
The animal experiments were conducted in accordance with the ethical guidelines of the Teikyo University of Science and Technology Animal Experimentation Committee, and guidelines of the Japanese Pharmacological Society. The protocol was approved by the Teikyo University of Science and Technology Animal Experimentation Committee (Permit Number: B-08005). All efforts were made to minimize suffering. Rats (SD: Sprague Dawley) were obtained from Japan SLC (Hamamatsu, Japan) and bred in our SPF-grade laboratory. The animals were maintained on a constant 12-h light-dark cycle at 21–24°C at 40–60% humidity and were provided with ordinary laboratory chow and sterile tap water ad libitum. For deep but a short period of anesthesia, rats were administered sodium pentobarbital (30 mg/kg for light anesthesia, 50 mg/kg for deep, both i.p.). For experiments taking a longer time (4 ~ 6 hours), rats were anesthetized with an i.p.-administered cocktail of pentobarbital (45 mg/kg), atropine (0.05 mg/kg), dimorpholamine (Theraptique, 1.2 mg/kg), and xylazine (9.5 mg/kg) as described previously [12, 13]. The anesthetized rats were kept on a warm gel pad (40°C) and given a 1/3 to 1/2 dose every hour to maintain them under anesthesia.
Experimental procedure
All rats (8–10 weeks old, male and female random mix) were loaded with 6RBH4 (5 mg/mL) at a dose of 5 mg/kg (i.v.) under anesthesia. Administration of Pbc (200 mg/kg, i.p.) or CsA (10 mg/kg, i.p.) was performed 30 min prior to 6RBH4 administration. Experiments were carried out by means of two different protocols; (A) by sequentially collecting blood, bile and urine from individual rats under long-lasting anesthesia on a warm gel pad, and (B) by removing the liver, kidney and brain after sacrificing the respective rats under the deep anesthesia at specified times after 6RBH4 administration.
(A) Rats were intraperitoneally administered Pbc (“BH4 + Pbc”, n = 5) or saline as a control (“BH4 alone”, n = 7) and were then anesthetized. All rats were given 6RBH4, then subjected to sampling at specified times as previously described [12, 13], except that bile was also taken and the animal was given saline containing 2% bovine bile powder (w/v), at a rate of 0.9 mL/hr, which was administered into the intestinal duct. The bovine bile powder did not contain any measurable BH2 or BH4. In the following sample acquisition, special care to ensure minimal oxidation of BH4 was taken throughout the following procedure. In brief, the abdomen was opened and cannulae were set in place for collecting bile and urine. The blood samples were drawn from the tail vein into a heparin-coated capillary containing a solution of 0.1 M ascorbic acid (less than 5% by volume), and they were then subjected to differential iodine oxidation for BP determination. The urine was collected at intervals from the bladder through an indwelling needle connected to an airtight syringe in which the dead space had been filled with saline containing 50 mM each of ascorbic acid and EDTA (pH 6.8). The urine was immediately mixed with 9 volumes of 10 mM HCl containing 5 mM antioxidant. The aliquots were then subjected to quantitation of BP and creatinine. BP excretion was expressed as BP/creatinine (μmol/mg creatinine) based on the BP concentration (nmol/mL) divided by the time-matched creatinine level (μg/mL). In calculating the cumulative total, the amounts of BP in each fraction were added. Calculations were based on the BP concentration (nmol/mL) multiplied by the respective urine volume (mL) produced between each sampling time point regardless of the creatinine concentration. The bile was also collected from individual rats through a cannula in the bile duct and was immediately subjected to BP determination. The cumulative BP amount was calculated by adding up all samples measured.
(B) Rats were treated with 6RBH4 and drugs (Pbc and CsA), or saline as a vehicle control, and allowed to move freely after recovery from the light anesthesia. In the Pbc experiment, animal used numbered n = 4 each for the respective administration of “BH4 + Pbc”, and the vehicle control “BH4 alone”. In the CsA experiment, the numbers of rats for groups of “BH4 + CsA” and “BH4 alone” were n = 5, respectively. At 0, 30 and 120 min after 6RBH4 administration, blood samples were taken from the abdominal vein into a syringe in which the dead space was replaced with saline containing 50 mM each of ascorbic acid and EDTA (pH 6.8). Each blood sample was divided into three portions; one served as a “whole blood” specimen, the second was for the hematocrit determination, and the third was centrifuged (10,000 rpm, 4°C, 5 min) to separate red blood cells (RBCs) from the plasma. The whole blood, plasma, and RBCs were separately subjected to differential iodine oxidation for the BP determination described below. The results were expressed as the amount of BP per unit volume of the original blood sample (plasma + RBC, mL) based on the hematocrit value. The kidneys, liver, and whole brain were removed without prior perfusion, rinsed with saline, blotted, weighed, frozen in liquid nitrogen, and then stored at -80°C until BP determination. BP carryover with the blood was ignored in the liver and kidney. However, for the brain, the BP carryover was estimated using an average blood inclusion of 1.2% (v/w), which was determined by a comparison of the heme content of the blood and brain homogenates based on light absorbance at 415 nm [16].
Determinations
Biopterin was determined essentially according to Fukushima and Nixon [17] and modified as described previously [18, 19]. Animal tissues contain at least 4 chemical species of the reduced biopterin family; BH4-4a-carbinolamine (or 4a-hydroxy-tetrahydrobiopterin), quinonoid dihydrobiopterin and 7,8-dihydrobiopterin as well as BH4. The Fukushima and Nixon method includes tissue homogenization and extraction of pterin compounds followed by measurement of amount of fully oxidized biopterin after differential iodine oxidation under acid and alkaline conditions. With their method, the biopterin quantity remaining after the acid-oxidation was taken as the total biopterin (BP = “BH4” + “BH2” + “oxidized biopterin”) and that which remained after the alkaline-oxidation, as 7,8-dihydrobiopterin (BH2) plus oxidized biopterin. The amount of oxidized biopterin in extracts without the iodine oxidation was disregarded in this study since the observed amount was too small for the purpose of our work. Accordingly, in this study, the biopterin amount measured after the acid iodine oxidation was taken as the sum of “BH4” and “BH2” (“BP” = “BH2” + “BH4”), and that after the alkaline oxidation, as the amount of “BH2”. The difference was therefore taken as the “BH4” amount. The BH4 fraction (BH4%; the percentage of BH4 in BP) was calculated for each individual sample. The amounts of BH4 and BH2 were calculated based on molecular weights of 241 g/mol and 239 g/mol, respectively. The molecular mass of the reagent 6RBH4•2(HCl) was 315g.
The urinary creatinine concentration was measured using a creatininase-HMMPS method according to the manufacturer’s instructions (L-type Creatinine M; Wako Pure Chemical Industries, Osaka, Japan).
Data presentation
Data were expressed as means ±S.E. The statistical significance of two groups with non-repeated determination was analyzed by Student’s t-test. The significance of difference between determinations at different times with individual animal groups was analyzed by the Paired t-test. Significance of difference between three groups was analyzed by Tukey’s test. All the statistical analyses were performed with Pharmaco Basic Ver.15.0.1 (Scientist Co. Ltd., Tokyo). Using results of pterin kinetics, exponential least square approximation was performed with Excel software using Microsoft Office for Mac. For the best fit by a first-order equation, BPt = BP0 • e−kt, data were plotted on a semi-log scale of y-axis vs. time (min) on the linear x-axis, forming straight lines as Ln (BPt) = Ln (BP0) − kt, where BP0 represents the extrapolated initial concentration of BP and BPt at time t (in nmol/mL of bile or μmol/mg creatinine of urine). The slope of the plot, k, yields the fractional rate constant (min−1), and gives the corresponding decay half-time, T1/2 = Ln(2)/k (min).
Results
Rat biopterin levels in the blood, urine and bile after 6RBH4 administration
We tracked BP contents and BH4 fractions (BH4%) in blood, urine and bile after rats were administered 6RBH4 (5 mg/kg, i.v.) with or without the Pbc treatment (200 mg/kg, i.p.), denoted hereafter as “BH4 + Pbc” or “BH4 alone”, respectively. Experimental procedure (A) in the Methods section was used.
Blood and urine
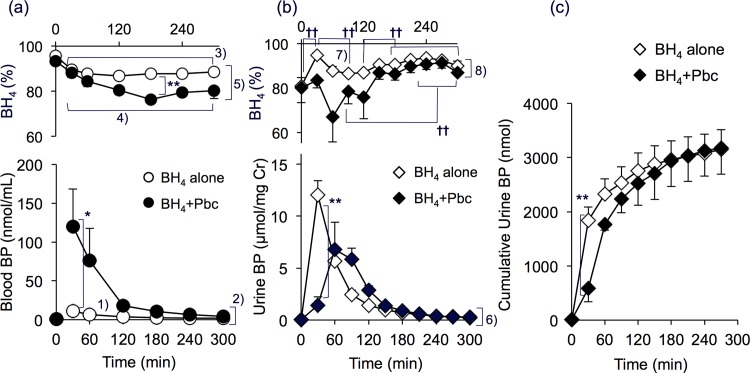
Prior to treatment, the combined BP level in the blood, plasma and red blood cells was 0.57 ± 0.08 nmol/mL and the BH4% was 90% to 95%. The BP level increased after receiving 6RBH4 “BH4 alone”, and gradually decreased to ca. 3.3-fold (P<0.005) of the initial level at 300 min (Fig 1A, lower panel). The blood BP content of the “BH4 + Pbc” group was much larger than that of the “BH4 alone” group. Reflecting this, the AUC0-120 of the “BH4 + Pbc” group was about 10-fold more than that of the group that received “BH4 alone”; 7,580 vs. 736 (nmol/mL)•min.
Fig 1. Effect of probenecid on biopterin levels in the blood and urine after 6RBH4 administration.
Rats were given 6RBH4 (5 mg/kg, i.v.) with probenecid (200 mg/kg, i.p., “BH4 + Pbc”) or without (“BH4 alone”). The blood and urine were collected as in experimental procedure (A) of Materials and Methods. (a) Blood BP (lower panel) and the BH4% (upper panel), “BH4 alone” (open circles) and “BH4 + Pbc” (closed circles). (b) Urinary BP relative to creatinine (μmol/mg creatinine, lower panel) and BH4% (upper panel), “BH4 alone” (open diamonds) and “BH4 + Pbc” (closed diamonds). (c) Cumulative BP content in the urine as a function of time after 6RBH4 administration, “BH4 alone” (open diamonds) and “BH4 + Pbc” (closed diamonds). All data are means ±S.E. of n = 5–7 for “BH4 alone” and n = 3–5 for “BH4 + Pbc”. Statistical significance: Student’s t-test (non-numbered) *P<0.05, **P<0.01; Paired t-test (non-numbered) ††P<0.01. Numbered significance: (a) 1) “BH4 alone”, increased (Paired t-test), 0-time vs. each data point from 30–300 min, P<0.005; in “BH4 + Pbc”, all greater than at 0-time, P<0.05. 2) “BH4 alone” vs. “BH4 + Pbc” (Student’s t-test), P = 0.03 at 30 min; P = 0.006 at 120 min; P = 0.02 at 180 min. 3) “BH4 alone”, decreased (Paired t-test), 0-time vs. each data point from 60–300 min, P<0.05. 4) “BH4 + Pbc”, decreased (Paired t-test), 0-time vs. each data point from 30–300 min, P<0.003. 5) “BH4 alone” vs. “BH4 + Pbc” (Student’s t-test): P = 0.05 at 60 min, P< 0.004 at each data point from 120–300 min. (b) 6) “BH4 alone” vs. “BH4 + Pbc” (Student’s t-test), P<0.001 at 30 min; P<0.006 at 90 and 120 min; P = 0.011 at 150 min. 7) “BH4 alone”, increased (Paired t-test), 0-time vs. 30 min, P = 0.005; 0-time vs. each data point from 150–270 min, P<0.04. 8) “BH4 alone” vs. “BH4 + Pbc” (Student’s t-test), P = 0.002 at 30 min; P = 0.04 at 30 min. (c) “BH4 alone” vs. “BH4 + Pbc” (Student’s t-test), P = 0.008 at 30 min.
In the “BH4 alone” rats, the blood BH4% dropped significantly within 30 min and continued to drop over the experimental period (Fig 1A, upper panel). In the “BH4 + Pbc” rats, the BH4% was substantially decreased but the decrease proceeded gradually; its lowest value of 79% was reached at 180 min and remained low thereafter. The difference in the BH4% between the “BH4 alone” and “BH4 + Pbc” groups was most evident at 180 min (88% vs. 79%) (P = 0.001).
Urinary excretion of BP (relative to creatinine, μmol/mg Cr) after 6RBH4 administration in the presence or absence of Pbc is depicted in Fig 1B. A large amount of BP appeared soon after the 6RBH4 mono-loading of “BH4 alone” rats, with the peak appearing at around 30 min. On the other hand, the early excretion of BP in the “BH4 + Pbc” group at 30 min was strongly suppressed to 11.7% of the amount in the “BH4 alone” group (P<0.001) while the blood BP level of “BH4 + Pbc” rats was 8.5-fold higher than of the “BH4 alone” rats (P = 0.03) at the same time point. The peak was delayed and appeared between 60 and 90 min. Since glomerular filtration might not be inhibited by Pbc, the observed decrease in BP flow at 30 min was thought to be due to a transporter-mediated process across the tubular epithelial cell layer, consistent with our previous results using CsA [12, 13]. Despite the effective inhibition of the early outflow by Pbc, the outflow appeared to have been delayed, although the gross amount was not reduced. The creatinine-based AUC0–270 of the “BH4 alone” rats was 731 (μmol/mg Cr)•min, and that of the “BH4 + Pbc” rats was 608 (μmol/mg Cr)•min. The gross outflow was substantially completed by 270 min. The cumulative excretion calculated from the urine-volume-based BP reached 3,150 ±360 and 3,170 ±500 nmol in the “BH4 alone” and “BH4 + Pbc” groups, respectively, representing about 61% of the dose used (Fig 1C). We noted that at as early as 30 min, the BP outflow amounted to 1840 ± 250 nmol in the “BH4 alone” group; about 58% of the gross outflow obtained by 270 min. Similarly, it reached 87% of the gross urinary excretion by 120 min. Most of the remainder may have been broken down to pterin or xanthopterin. However, we have no information about the rest of the BP except for an observation with mice about BP movement in to the rumen of the GI tract in our previous study [14]. We observed that a considerable amount of administered 6RBH4 appeared in the rumen of mouse small intestine and moved to the caecum, where BP seemed not to be retrieved but essentially metabolized to pterin, a bare pterin-ring compound, by the cecal microflora.
The urinary BH4% before receiving 6RBH4 was 81 ± 4% (Fig 1B, upper panel). On BH4 mono-loading, the percentage had risen to 95 ± 1% at 30 min (Paired t-test, P = 0.005), presumably representing the amount that reached the kidney bypassing uptake by various organs. Subsequently, the BH4% was decreased to 87 ± 2% at 90 min (P = 0.01), followed by a gradual increase up to ca. 94% at 240 min (120 min vs. each data point from 150–240 min, P<0.005). Prior to administration of 6RBH4, but 30 min after receiving Pbc, the BH4% was 80 ± 7%. It remained relatively low for 120 min and then rose gradually (90 min vs. each data point from 210–270 min, P<0.005).
Bile
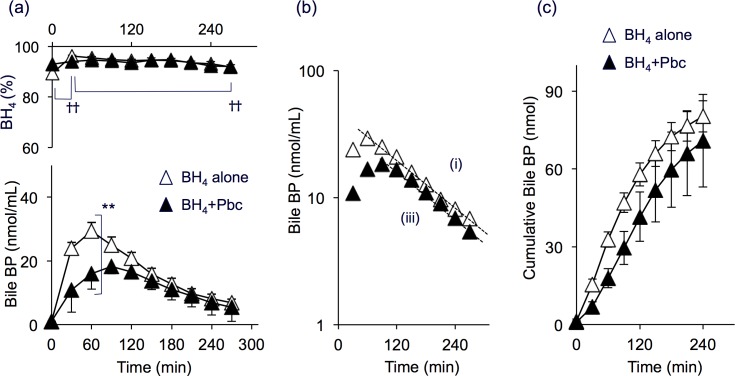
Excretion of BP in the bile was increased after administration of 6RBH4 either with or without Pbc. Levels of the bile BP were consistently higher than BP levels of time-matched blood samples taken from “BH4 alone” rats (compare Fig 2A with Fig 1A). At 30 min after 6RBH4 administration, for example, the BP level in the bile was 2.2-fold higher than that of time-matched blood (P = 0.001). The elevation of the BP content in the “BH4 + Pbc” group was 46% less than that of the “BH4 alone” group at 60 min (P = 0.006). On administration of 6RBH4, the biliary BH4% was maintained as high as 95% and was not affected by Pbc treatment over the course of the experiment (30–270 min). The bile BP profile appeared to have a limited correlation with that of the blood BP (compare upper panels in Fig 1A and Fig 2A).
Fig 2. Biliary biopterin excretion after loading rats with 6RBH4 with and without prior administration of probenecid.
Samples were collected from the same rats as those in Fig 1. The bile was collected from the bile duct, and BP levels and BH4 fractions (BH4%) were determined following experimental procedure (A) of Materials and Methods. (a) Biliary BP concentration (lower panel) and the BH4% (upper panel). One group of rats received “BH4 alone” (open triangles), and the other, “BH4 + Pbc” (closed triangles). (b) Exponential least-square fitting of biliary BP outflow: “BH4 alone”; BP(t) = 47.2 • e−0.007t, correlation coefficient |R| = 0.99. “BH4 + Pbc”; BP(t) = 37.2 • e−0.007t, |R| = 0.99. (c) Cumulative BP excretion in the bile. Data in (a) are means ±S.E. of n = 4–6 for “BH4 alone” and n = 5–7 for “BH4 + Pbc”. Some error bars are hidden behind symbols. Data in (b) are mean values in (a). Paired t-test, ††P<0.01; Student’s t-test, **P<0.01.
Fig 2B presents kinetics based on the same data (average) of Fig 2A. The BP concentration in both groups fitted two nearly parallel lines:
“BH4 alone”: BP(t) = 47.2 • e−0.007t (|R| = 0.99) (i)
“BH4 + Pbc”: BP(t) = 37.2 • e−0.007t (|R| = 0.99) (ii)
The common rate constant of 0.007 min-1 denoted a half-decay period of 99 min. This indicated that Pbc caused the BP pool size in bile to become smaller but did not change the rate constant of BP secretion, indicating that Pbc had little effect on the process. The BP pool might represent BP of the liver, the source of this fluid, suggesting that the liver supplied a smaller amount of BP in the presence of Pbc.
As shown in Fig 2C, the cumulative BP amount after administration of “BH4 alone” for 240 min was 80.3 nmoles per rat, representing 1.6% of the 6RBH4 dose. In addition, the cumulative BP excretion in the presence of Pbc, i.e., that of the “BH4 + Pbc” group, was not very different; 70.8 nmoles per rat for 240 min, which was 1.4% of the 6RBH4 dose. In either case, the amount of BP excreted into the bile was much less than that into the urine. We previously described excretion of BP in the bile as a route for its entero-hepatic circulation in mice, and a considerable amount of BP secreted in the small intestine was brought to the caecum [14]. In order to prevent bile BP to return to the circulation, we removed all the bile in the procedure (A). If BP was also excreted by the other route such as via the intestinal juice under the present experimental conditions, a certain amount of BP was also secreted to rumen of the small intestine and could have moved to the caecum. Possible loss of administered BP by this route remains elusive.
Organ distribution of biopterin after 6RBH4 administration to rats
Organ distribution of BP was examined after 6RBH4 administration (5 mg/kg, i.v.) with Pbc (200 mg/kg, i.p.) or CsA (10 mg/kg, i.p.) or without the inhibitor. In this experiment, rats were anesthetized for handling and allowed to move freely after recovery as described in experimental procedure (B).
Blood
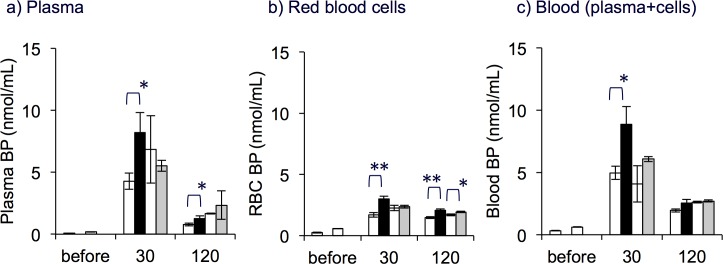
The initial BP level in the plasma was 0.13 ± 0.02 nmol/mL. After rats were administered 6RBH4, the plasma BP of all groups, “BH4 alone”, “BH4 + Pbc” and “BH4 + CsA”, was 30- to 50-fold over the initial value (Fig 3A). The elevated BP level in “BH4 + Pbc” rats at 30 min was 1.8-fold higher than that in “BH4 alone” (P<0.05). The difference between BP levels in “BH4 + CsA” and “BH4 alone” was not clear. Plasma BP levels in both groups of rats returned to around 1 nmol/mL at 120 min after 6RBH4 administration but they were about 10-fold greater than the initial value. The initial BP level in RBCs was higher than in plasma (0.43 ± 0.06 nmol/mL) and roughly 77% of the blood BP was localized in these cells. The amount of BP in RBCs changed more slowly than that in plasma (Fig 3A and 3B).
Fig 3. Effect of probenecid and cyclosporin A on the biopterin distribution in blood after 6RBH4 administration.
Panels show BP levels (nmol/mL) in plasma (a), red blood cells (b), and whole blood (c). The blood was collected as described in experimental procedure (B) of Materials and Methods. The amount of 6RBH4 administered was 5 mg/kg, i.v. (“BH4 alone”, open bars), 6RBH4 plus probenecid, 200 mg/kg, i.p. (“BH4 + Pbc”, closed bars), or 6RBH4 plus cyclosporin A, 10 mg/kg, i.p. (“BH4 + CsA”, grey bars). The open bars on the left represent the results of “BH4 alone” in experiments performed at the same time with “BH4 + Pbc”, and the open bars on the right represent “BH4 + CsA” at the respective time points. In all panels, the rat group “before” received no treatment in all respective drug-administered groups. “BP” denotes BH2 + BH4. The calculation of the amount of BP per volume of original blood (mL) was based on the hematocrit value. Data are means ± S.E. (n = 4 in “BH4 + Pbc” and n = 5 in “BH4 + CsA”). Student's t-test, “BH4 alone” vs. “BH4 + Pbc”, or “BH4 alone” vs. “BH4 + CsA”, *P<0.05, **P<0.01.
Increase in BP contents in the liver, kidney and brain
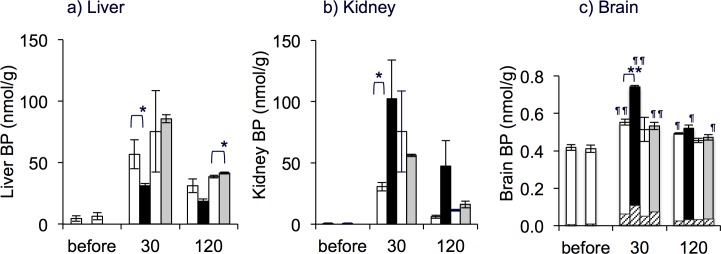
The BP contents increased in the liver, kidney and brain after 6RBH4 administration as shown in Fig 4. The initial BP levels in the organs were as follows: liver, 5.86 ± 0.47; kidney, 0.68 ± 0.04; and brain, 0.41 ± 0.01 nmol/g. The protocol used was experimental procedure (B) in the Materials and Methods section.
Fig 4. Organ distribution of biopterin after 6RBH4 administration and the effect of probenecid and cyclosporin A.
BP levels in the liver (a), kidney (b), and brain (c) were determined. Rats were given 6RBH4 alone (5 mg/kg, i.v., “BH4 alone”, open bars) or 6RBH4 with prior administration 30 min earlier of probenecid (“BH4 + Pbc”, 200 mg/kg, i.p., closed bars) or cyclosporin A (“BH4 + CsA”, 10 mg/kg, i.p., grey bars). The open bars on the left represent the results of “BH4 alone” in experiments performed at the same time as with “BH4 + Pbc”, and the open bars on the right were those with “BH4 + CsA” at respective time points. The hatched bars in (c) indicate blood carryover of biopterin based on estimated blood inclusion (1.2%) in the brain. After the indicated periods, rats were sacrificed, and the organs were dissected as described in experimental procedure (B) of Materials and Methods. Samples were taken from the same rats as those in Fig 3. Student’s t-test (“BH4 alone” vs. BH4 + drug at respective time points), *P<0.05; Tukey’s test (“before” vs. 30 or 120 min), ¶P<0.05, ¶¶P<0.01; data are means ± S.E., (n = 4 for Pbc series, n = 5 for CsA series).
Liver
The liver took up a large amount of BP within the short term; the BP content increased to 53.3 nmol/g (9.1-fold over the initial value) in the “BH4 alone” rats within the first 30 min (Fig 4A). With Pbc and CsA, the effect was different; the increase in the “BH4 + Pbc” group (5.3-fold) was considerably less than in the “BH4 alone” group (P<0.05 at 30 min), while that of “BH4 + CsA” was rather increased (P<0.05 at 120 min). Two interesting findings emerged: 1) the net uptake by the liver was suppressed considerably, 9.1-fold down to 5.3-fold, even though the elevation of the plasma BP doubled in the presence of Pbc (Fig 3A), suggesting that the rapid uptake was strongly inhibited to nearly one quarter by the drug. This was in sharp contrast to “BH4 + CsA” rats in which the BP accumulation was elevated to a level corresponding to that in the blood, suggesting that CsA did not inhibit BP uptake in this organ. 2) the release process was not greatly inhibited by the drugs either Pbc or CsA; the decrease in liver BP levels in the three groups seemed proportional over the 30–120 min period. If the BP uptake was mediated by a transporter, the fact that Pbc inhibited BP uptake by the liver suggested that the putative transporter was Pbc-sensitive and was somehow inward-directional for BP transport from the blood. BP uptake by the liver in comparison to uptake by the other organs is summarized in Table 1.
Table 1. Biopterin distribution in organs after 6RBH4 administration.
| 0-time | 30 min | 120 min | ||||||
|---|---|---|---|---|---|---|---|---|
| organ weight (g) | relative1)to body | BP (nmol) | relative2)to liver | BP (nmol) | relative3)to dose | BP (nmol) | relative3)to dose | |
| Liver | 6.9 | 2.8% | 40.4 | (100%) | 367 | 7.1% | 268 | 5.2% |
| Blood | 17 | 6.8% | 8.32 | 21% | 76.8 | 1.5% | 39.5 | 0.76% |
| Kidneys | 1.8 | 0.72% | 1.22 | 3.0% | 70.6 | 1.4% | 17.3 | 0.3% |
| Brain | 1.5 | 0.60% | 0.62 | 1.5% | 0.8 | 0.15% | 0.71 | 0.14% |
| Sub-total | 27 | 11% | 50.6 | n.a. | 515 | 9.9% | 325 | 6.3% |
Biopterin distribution in liver compared with the other organs. Data apply to Figs 3 and 4. BP contents (nmol/organ) are expressed as the mean values and are multiplied by the respective organ weights (g).
1) Relative to a 250 g body weight, adapted from “SD-Rat Control Data 2009” of Charles River Laboratories Japan, Inc.
2) Relative to the amount of BP in whole liver (nmol)
3) Relative to dose: (increase in organ BP over 0-time) / (5180 nmol: 6RBH4 dose per 250 g).
Kidney
Although the endogenous BP in the kidney was only 3% of that in the liver, this organ had a relatively high capacity to uptake exogenous BP. The amount of tissue BP was greatly increased (58-fold over the initial value) in the “BH4 alone” rats at 30 min (Fig 4B). The BP rise in the “BH4 + Pbc” rats was even greater (150-fold), as was the rise in “BH4 + CsA” rats (about 80-fold), both corresponding to the respective rises in the plasma BP (compare Fig 4B with Fig 3A). The decrease in BP in all three groups during the period between 30 and 120 min was quite rapid, similar to the change noted in plasma. Taking into account that at 30 min, the peak amount of BP in the kidney was only 4–5% of the urinary BP outflow, in which renal tubular secretion was dominant as mentioned earlier (cf. Fig 1C), it is reasonable to assume that the BP in kidney tissue transited from the plasma to the urine across the tubular epithelium. This is in remarkable contrast to the liver BP which was stored in a large quantity then released gradually into the plasma.
Brain
The brain BP was significantly increased in all three groups, although much less than that of the other organs (Fig 4C). The BP contents of “BH4 alone” rats were about 43% higher after 30 min than those of untreated rats at (P<0.01). At this time point, the increase in “BH4 + Pbc” rats was significantly larger, as much as 65% (P<0.01), compared to that of “BH4 alone” rats (P<0.05). Since the blood content included in the brain samples was about 1.2% (v/w), it was uncertain whether the increase was caused by a blood carryover which contained increased the amount of exogenous BP (cf. Fig 3C). The BP carryover was calculated and compared with the respective BP contents of each group using mean values of BP in time-matched blood (Fig 4C). When the observed increases in BP were adjusted by subtracting the carryover, the net increase in the “BH4 alone” rats for the first 30 min was roughly 15%. Similarly, in “BH4 + Pbc” and in “BH4 + CsA” rats, BP levels were elevated by 41% and 13%, respectively. Therefore, a greater increase in BP content was verified in “BH4 + Pbc” rats than in “BH4 alone” rats (P<0.05).
The increase in the brain BP observed in this study might not be sufficient for stimulation of monoamine biosynthesis as reported [20, 21]. In fact, Miwa et al [22] reported that a 50- to 200-fold increase in BH4 was needed, when the preparation was injected directly into the brain ventricle for significant stimulation of monoamine biosynthesis. Although the elevation observed in this study seems to be insufficient for stimulating the biosynthesis of monoamines, it could be beneficial for neural stimulation of their release [23].
The BH4 fraction (BH4%) in organ biopterin and the effect of probenecid
Alterations in the BH4% along with the BP distribution profiles in respective organs (Figs. 3 and 4) are depicted in Fig 5.
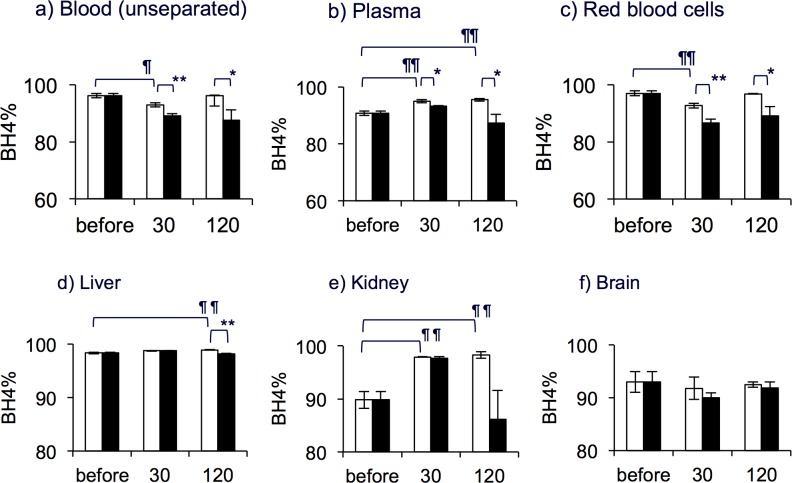
Fig 5. Effect of probenecid on the BH4 fraction (BH4%) in blood biopterin.
Panels show levels in whole blood in which the plasma and RBCs were not separated (a), plasma (b) and RBCs (c), liver (d), kidney (e) and brain (f). Rats were given 6RBH4 alone (5 mg/kg, i.v., “BH4 alone”, open bars) or 6RBH4 with prior administration 30 min earlier of probenecid (“BH4 + Pbc”, 200 mg/kg, i.p., closed bars). In all panels, the “before” rat group did not receive either 6RBH4 or Pbc. The experimental procedure was the same as described in Figs 3 and 4. The BH4% was determined as described in Materials and Methods. Data are means ± S.E. (n = 4). Tukey’s test for multiple comparison, “before” vs. 30 min and 120 min, ¶P<0.05, ¶¶P<0.01. Student’s t-test for two groups, “BH4 alone” vs. “BH4 + Pbc”, *P<0.05, **P<0.01.
The BH4% in untreated whole blood, on which the plasma and RBCs were not separated, was 96.2 ±0.2%, and it decreased significantly along with time (Fig 5A, P<0.05). Although the BH4% in the plasma showed a significant but slight increase at 30 and 120 min, the lowering of the BH4% in RBCs was relatively large (Fig 5C, P<0.01) and pulled the blood BH4% down. All samples, plasma, RBCs as well as the unseparated blood, taken from “BH4 + Pbc” rats showed a significantly lower BH4% than those of “BH4 alone” rats.
The BH4% in the liver, kidney and brain are shown in Fig 5D–5F. The initial BH4% in these organs were: liver, 98.3 ±0.1%; kidney, 89.8 ±1.6%; and brain, 93.0±2.0%. In the liver, the BH4% was quite stable at levels higher than 98% in all groups. In the presence of Pbc, however, namely in “BH4 + Pbc” rats, the BH4% was lowered slightly but significantly at 120 min (Fig 5D, “BH4 alone” vs. “BH4 + Pbc”, P<0.01). The kidney BP showed a considerable increase in its BH4% after the 6RBH4 administration (Fig 5E, “before” as the reference, P<0.01). It was noted that profiles of the BP content in the blood and kidney looked quite similar (compare Fig 3A and Fig 4B) in the period after the 6RBH4 administration. The brain took up a small amount of blood BP as seen in Fig 4C, and no significant change in the BH4% was observed in this study (Fig 5F). The significance of correlation between the BH4% of blood, liver and kidney is listed in Table 2. In this Table, the BH4% of all organs after 6RBH4 administration with or without Pbc or CsA are listed and compared with respect to the statistical significance of differences between the organs. The BH4% in the liver and kidney was persistently higher than that of blood BP, while the data showed no statistically significant difference between those of liver and kidney.
Table 2. The BH4% in the blood, liver and kidney after 6RBH4 administration.
| Time | Rat group | Blood | Liver 1) | Kidney 1), 2) | ||
|---|---|---|---|---|---|---|
| % | % | % | ||||
| 30 min | BH4 alone | 93.9 ±0.6 | 98.7 ±0.1 | ** | 98.0 ±0.2 | **, n.s. |
| BH4 + Pbc | 89.2 ±0.7 | 98.7±0.1 | ** | 97.7 ±0.3 | **, n.s. | |
| BH4 + CsA | 94.4 ±0.1 | 98.6 ±0.1 | ** | 98.1 ±0.4 | **, n.s. | |
| 120 min | BH4 alone | 96.2 ±0.1 | 98.8 ±0.1 | ** | 98.6 ±0.3 | **, n.s. |
| BH4 + Pbc | 87.6 ±3.7 | 98.1 ±0.1 | n.s. | 89.2 ±5.5 | n.s., n.s. | |
| BH4 + CsA | 95.3 ±0.1 | 98.5 ±0.1 | ** | 98.4 ±0.1 | **, n.s. | |
The BH4% in organs 30 min and 120 min after administration of 6RBH4 with or without prior treatment with Pbc or CsA. For each rat group, differences in the organ BH4% between blood, liver and kidney were analyzed by Tukey’s multiple testing method. BH4% values are means ±S.E. and apply to Fig 5.
1) Difference between the BH4% of time-matched blood vs. liver or kidney. **P<0.01 significance, suggesting the BH4% is higher than that of blood; “n.s.”, not significant.
2) Difference between the BH4% of time-matched liver vs. kidney.
Discussion
Urinary excretion was the major route of BP loss while bile was a minor route
Renal exclusion of BH4 immediately after 6RBH4 administration was remarkably rapid. The major portion of the exclusion, up to ca. 87% of the apparent elimination observed within 270 min, took place in the first 120 min in healthy rats. Kidney exclusion of xenobiotics and metabolic waste generally takes two paths from plasma to urine; glomerular filtration and tubular secretion [24]. The tubular secretion of exogenously administered BH4 was effectively suppressed by prior administration of Pbc, as shown in this report, providing confirmative evidence supporting a previous observation using CsA [12, 13]. The previous report demonstrated that the urinary BP flow involved at least two processes, glomerular filtration with a rate constant of k = 0.013 min-1, and tubular secretion with a 2.3-fold more rapid rate constant of k = 0.030 min-1, which represented the difference between the observed gross rate constant of k = 0.043 min-1 minus the glomerular rate constant. The present data on “BH4 + Pbc” rats after 90 min (Fig 1B, lower panel) also fitted the equation BP(t) = 9.76 • e−0.012t (|R| = 0.92), suggesting that the urinary flow was essentially from glomerular filtration at 90 min or later after 6RBH4 administration. The treatment with Pbc greatly increased the blood BP by inhibiting BP uptake by various organs including the kidney. Despite the substantial suppression, BP exclusion within 270 min after 6RBH4 administration was almost the same in “BH4 alone” and “BH4 + Pbc” rats. This was explained by the fact that the concomitantly high concentration of plasma BP caused by the Pbc treatment speeded up the glomerular filtration. It should be noted that the tubular secretion was enhanced when mediated by a drug-sensitive transporter(s) presumably with large capacity but low affinity. This infers that the putative transporter only functions when the plasma BP concentration is high. Actually, it was demonstrated that the dominancy of epithelial secretion over glomerular filtration occurred only when the plasma BP concentration was 10 times higher than ordinary levels [12, 13]. In this context, adjusting the formula or recipe for 6RBH4 administration so as to avoid the tubular secretion by maintaining the plasma BP level around the critical concentration (~ 10-fold) may result in a much higher efficiency in bodily replacement of BH4.
We previously described excretion of BP in the bile as a route for its entero-hepatic circulation [14]. In the present experiment following experimental procedure (A) in the Materials and Methods section, we removed all the bile and did not let it return to the liver. After 6RBH4 administration, the bile BP increased along with the liver BP levels in the presence or absence of Pbc. Further, the BH4% of the bile BP was persistently high, similar to the high BH4% in the liver, either in the presence or absence of Pbc. Pbc might have inhibited BP uptake by the liver cells but might not have affected its release into the bile. We also noted that the cumulative excretion of BP in the bile was only about 3% that in the urine. Although it was reported that biliary excretion was a major route for the elimination of various drugs or their metabolites [24, 25], our findings revealed that it was rather a minor route with regard to the bodily loss of administered BH4. However, the fact that the liver excels in secreting BP with a high BH4% suggests that it has some role in BH4 secretion into the gut, however, the physiological function of the bile BH4 remains elusive.
Distribution and retention of administered 6RBH4 in the organs
Hoshiga et al. [26] described the body-wide distribution of exogenous BH4 which they detected by means of whole body autoradiography after i.v. injection of a tracer amount of (6R)-[U-14C]BH4. They found the densest deposition of radioactivity to be in the kidney at 2 hours. Among large organs, the liver had the next densest deposition, while deposition in the brain and striated muscles was unremarkable. Hayashi et al. (in a Japanese journal [27]) described BP distribution after administration of 6RBH4. According to their report, the BP contents of various organs including the liver were all highest at 30 min (the earliest sampling) and the BP amount in each organ decreased gradually over the course of the 24-hour experiment, suggesting that the uptake phase of BP, in terms of the net balance of uptake minus release, had been virtually completed in organs within the first 30 min. In our present research, we treated rats with Pbc or CsA prior to administration of 6RBH4 with the purpose of examining the effect of these inhibitors on BH4 uptake by various organs and the respective differences in the BH4%. We started sampling at 30 min, assuming that the net uptake had already been completed by the time that BP was circulating throughout the body based on the data reported by Hayashi et al. above. The retention of the BP accumulated in the liver was longer than that of the kidney and brain, and that of RBCs in the blood. Taking its large mass into account, the liver seemed to function as a large reservoir of the administered BH4 for gradual supply to peripheral tissues including the vasculature. This meant that, over the long period, the liver released BP gradually to the bile and presumably to the blood circulation.
Homeostasis of BH2/BH4 after administration of 6RBH4
We observed that the BH4% in the blood and urine was considerably decreased while the amount of tissue BP was prominently increased in the liver and kidney after 6RBH4 administration. Further, Pbc, an uptake inhibitor, enhanced the BH4% decrease in the blood and urine. These results raised two questions: first, why did the BH4% decrease after 6RBH4 administration and secondly, why did the uptake inhibitor promote a further decrease.
As to the first question, we consider that the answer lies in the mechanisms by which cells take up extracellular BH4. BH4 is barely able to cross the cell membrane in its tetrahydro-form because as an active coenzyme, it must remain inside the cell. Generally, the cell membrane works as a strong barrier against the in and out movement of such a hydrophilically active coenzyme as BH4. For the uptake of extracellular BH4, a complex series of events must occur [28]. First, BH4 is oxidized, the resultant BH2 is taken up by the cell, an action presumably mediated by transporters, and then BH2 is reduced back to BH4 through the salvage pathway. Although the location and manner of the rapid oxidation of the administered 6RBH4 was ambiguous, the systemic oxidation of BH4 to BH2 in vivo was clearly demonstrated by the appearance of a BH2 serge in the blood immediately after 6RBH4 administration in the mouse [19]. In the present study using rats under anesthesia, we did not observe such a prominent BH2 surge after 6RBH4 administration, but noted instead a significant decrease in the BH4%, in other words, an increase in BH2 relative to BH4, in the blood and urine. The moderate but long-lasting decrease in the BH4% in rats seemed to result from essentially the same process responsible for the rapid appearance of the BH2 serge in mice. We consider that in both rats and mice, these outcomes reflect an inevitable compensatory response of the animal body toward exogenous BH4.
As for the second question, the answer is contained in the response to the first. The reducing action of BH2 is an intracellular event but the BH2 has to be brought into the cell from the plasma. Therefore, BH2 uptake by the tissues is a prerequisite for scavenging blood BH2. Although the uptake transporters have not all been identified, at least we know that two equilibrative nucleoside transporters called ENT1 (SLC29A1) and ENT2 (SLC29A2) are able to mediate BH2 relocation favoring the other dihydropterin-compound, sepiapterin, but not BH4 [29]. Some portion of the BP uptake observed in this study was obviously mediated by another transporter(s) because ENT1 and ENT2 are sensitive to nitrobenzylthioinosine (NBMPR) but not to Pbc to our knowledge. The fact that the blockade of BP uptake by Pbc enhanced a further decrease in the blood BH4% suggests that BH2 was effectively or even preferentially taken up by the putative transporter(s) which was sensitive to Pbc. Since the salvage pathway is thermodynamically favored to proceed, the internalized BH2 is readily scavenged as in the push-pull accumulation of BH4 [18, 30]. As for extracellular BH4, however, we have not uncovered any form of active transport that would allow it to directly accumulate in the cell against a concentration gradient in a tetrahydro-form. A passive transport could not contribute to BH4 accumulation against the concentration gradient even if a transporter(s) could potentially move it across the plasma membrane.
The endogenous content and the capacity to uptake BH4 was prominent in the liver and kidney (Table 1). This suggested to us that most BH2 scavenging might have occurred in these organs. It was noted that the BH4% in these organs did not decrease when the BH4% was lowered in the blood and urine after 6RBH4 administration. BP uptake by the liver was considerably inhibited by prior treatment with Pbc and it was accompanied by an even greater decline in the BH4% of the blood and urine. This observation was explained by inhibition of the uptake process by the drug and the consequent inability of the liver to convert BH2 to BH4. Taking the liver’s large capacity for BP uptake into account, this organ might have played the greatest role in removing the scavenged BH2.
Considering that the kidney excreted urine with a consistently higher BH4% than that of the plasma, the strong ability of the kidney to scavenge BH2 was also noted. The pronounced decrease in the BH4% in the urine in the presence of Pbc suggested reduced BH2 scavenging in the kidney. This Pbc sensitivity indicates that the BH2 scavenging occurred during the transit across the tubular epithelium for secretion. In this manner, the kidney was able to scavenge BH2 from the plasma. Additionally, the kidney might have released a large amount of BH2-scavenged “clean” BP back to the plasma resulting in a rise in the plasma BH4%, but our experiment was not designed to show this explicitly. The details of tubular secretion with regard to the relevant transporter for BH2 scavenging remain elusive.
When our focus was limited to classic enzyme pathways, the salvage pathway of BH4 was seen as a mechanism for maintaining BH4 levels against a loss of BH4 cofactor activity. The salvage pathway, coupled with uptake of the extracellular precursor, should now be considered as an innate mechanism for scavenging BH2, a mechanism which covers a wide range of interactions between cells and their environment throughout the body. The liver and kidney are the major sites of BH2 scavenging under conditions of 6RBH4 administration owing to their large-scale uptake of blood BP.
Remarks concerning NOS dysfunction
In clinical practice, 6RBH4 has been prescribed to patients with an inherited BH4 deficiency as well as to many with BH4-responsive phenylketonuria. On a search of the literature, we did not encounter any mention of a serious adverse effect of long-term administration in these patients [31]. In another area of medicine, BH4 supplementation has also drawn increased attention with respect to whether it ameliorates NOS dysfunction in the cardiovascular system as a high BH2/BH4 ratio, representing a type of oxidative stress, is a great risk factor. Studies in this area are concerned with the homeostasis of the BH2/BH4 ratio, an inverse expression of the BH4%.
The administration of 6RBH4 is followed by a lowering of the blood BH4% as an inevitable compensatory reaction, as discussed above. In the case of the lowering of the BH4% in the blood, endothelial cells are the ultimate interface in all organs and are active sites of nitric oxide (NO) production regulating vascular contraction. In fact, we previously demonstrated that ENT1 and ENT2 were able to mediate cell membrane permeation of sepiapterin preferentially, permeation of BH2 moderately and that of BH4 only slightly [29]. Furthermore, we localized ENT2 on the vascular lumen side of endothelial cells [32], suggesting that these cells are ready to uptake increased amounts of BH2. Owing to two characteristic properties of the enzyme NOS, once in the cell, BH2 must be converted to BH4 promptly. 1) The affinity of NOS for BH2 is not particularly low compared to its affinity for BH4; in the case of an nNOS, an isoform of eNOS, the affinity to BH2 and to 6RBH4 was as close as 1: 10 [33]. 2) BH2-bound NOS is unable to maintain an active dimer and uncouples O2 reduction to produce superoxide [34–36]. In this context, dihydrofolate reductase is the key enzyme for maintaining a BH2/BH4 ratio sufficiently low for sound NO production. However, endothelial cell-derived culture cells, especially of human origin, are poorly furnished with dihydrofolate reductase [37–39], suggesting that the original endothelial cells in the human vasculature have the same vulnerability. Consequently, a high BH2/BH4 ratio in the plasma causes eNOS to uncouple locally in endothelial cells and leads to a negative spiral favoring BH4 oxidation [40]. In this study, we noticed that after 6RBH4 administration, the blood BH4% persistently dropped below 90%, that is, the BH2 to BH4 ratio became less than 1:10 even in the “BH4 alone” group, while it was maintained at ca. 95% in untreated rats. We also noted that Pbc, a widely prescribed anti-gout drug, further increased the blood BH2 when used with 6RBH4. It is interesting that many experimental studies support the use of 6RBH4 administration as a means of ameliorating cardiovascular symptoms, however, translation of these studies to human patients remains limited [41]. The stated vulnerability of endothelial cells suffering from a lowering of the blood BH4% might represent a great risk factor for possible exacerbation of cardiovascular dysfunction in cases in which 6RBH4 is regularly administered repeatedly at a dose of 5–20 mg/kg/day.
We conclude that supplementation with BH4 by means of administration of 6RBH4 has remarkably low efficacy due to its rapid loss through the urine, nonetheless it is still effective in raising BH4 levels in peripheral organs. However, considering the general vulnerability of the cardiovascular system to an increasing BH2/BH4 ratio, regular administration of 6RBH4 may spoil the desired effect of countering NOS dysfunction by lowering the BH4%. The compensatory lowering of the BH4% must be avoided in future improvements to the therapeutic composition. Take together improving 6RBH4 preparations to allow for sustained distribution while avoiding a steep elevation in plasma might help to realize the potential benefit of BH4 supplementation.
Acknowledgments
We wish to thank R Fujii, N Nishimoto, Y Suetaka, Y Aoshima, M Fukasawa, M Ishikawa, M Nito, T Okamoto, E Saito, K Sekihata, T Sumi, Y Taiga, R Tanaka, A Watanabe, and S Yamazaki for their efforts and collaboration in the Student Research Program 2007–2009, Department of Biosciences, Teikyo University of Science and Technology. We are grateful to Dr William Campbell and Catherine Campbell for editing our draft for correct English usage.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by Sato Fund, Nihon University School of Dentistry to AO; Dental Research Center, Nihon University of Dentistry to AO and MN; Uemura Fund, Nihon University School of Dentistry to TT; and JSPS KAKENHI JP16K20429 to AO. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kaufman S. The Structure of the phenylalanine-pydroxylation cofactor. Proc Natl Acad Sci U S A. 1963. December;50:1085–93. 10.1073/pnas.50.6.1085 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagatsu T, Levitt M, Udenfriend S. Tyrosine hydroxylase: the initial step in norepinephrine biosynthesis. J Biol Chem. 1964. September;239:2910–7. . [PubMed] [Google Scholar]
- 3.Lovenberg W, Jequier E, Sjoerdsma A. Tryptophan hydroxylation: measurement in pineal gland, brainstem, and carcinoid tumor. Science. 1967. January 13;155(759):217–9. 10.1126/science.155.3759.217 . Epub 1967/01/13. eng. [DOI] [PubMed] [Google Scholar]
- 4.Ichiyama A, Nakamura S, Nishizuka Y, Hayaishi O. Enzymic studies on the biosynthesis of serotonin in mammalian brain. J Biol Chem. 1970. April 10;245(7):1699–709. . Epub 1970/04/10. eng. [PubMed] [Google Scholar]
- 5.Blau N. Sapropterin dihydrochloride for the treatment of hyperphenylalaninemias. Expert Opinion on Drug Metabolism & Toxicology. 2013;9(9):1207–18. 10.1517/17425255.2013.804064 . [DOI] [PubMed] [Google Scholar]
- 6.Bartholome K, Byrd DJ, Kaufman S, Milstien S. Atypical phenylketonuria with normal phenylalanine hydroxylase and dihydropteridine reductase activity in vitro. Pediatrics. 1977. May;59(5):757–61. . Epub 1977/05/01. eng. [PubMed] [Google Scholar]
- 7.Schaub J, Daumling S, Curtius HC, Niederwieser A, Bartholome K, Viscontini M, et al. Tetrahydrobiopterin therapy of atypical phenylketonuria due to defective dihydrobiopterin biosynthesis. Arch Dis Child. 1978. August;53(8):674–6. 10.1136/adc.53.8.674 . Pubmed Central PMCID: 1545051. Epub 1978/08/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kure S, Hou DC, Ohura T, Iwamoto H, Suzuki S, Sugiyama N, et al. Tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. J Pediatr. 1999. September;135(3):375–8. 10.1016/S0022-3476(99)70138-1 . Epub 1999/09/15. [DOI] [PubMed] [Google Scholar]
- 9.Blau N, Burgard P. Disorders of phenylalanine and tetrahydrobiopterin metabolism In: Blau N, Hoffmann GF, Leonard J, Clarke JTR, editors. Physician's Guide to the Treatment and Follow-Up of Metabolic Diseases. 2006, XVIII, 416 p. 13 illus. With CD-ROM., Hardcover ed. Heidelberg: Springer-Verlag; 2006. p. 25–34. [Google Scholar]
- 10.Heintz C, Cotton RG, Blau N. Tetrahydrobiopterin, its mode of action on phenylalanine hydroxylase, and importance of genotypes for pharmacological therapy of phenylketonuria. Human Mutation. 2013. July;34(7):927–36. Epub 2013/04/06. eng. 10.1002/humu.22320 [DOI] [PubMed] [Google Scholar]
- 11.Wettstein S, Underhaug J, Perez B, Marsden BD, Yue WW, Martinez A, et al. Linking genotypes database with locus-specific database and genotype-phenotype correlation in phenylketonuria. European Journal of Human Genetics: EJHG. 2014. June 18 10.1038/ejhg.2014.114 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohashi A, Suetake Y, Saeki Y, Harada T, Aizawa S, Hasegawa H. Rapid clearance of supplemented tetrahydrobiopterin is driven by high-capacity transporters in the kidney. Mol Genet Metab. 2012;105:575–81. 10.1016/j.ymgme.2012.01.009 [DOI] [PubMed] [Google Scholar]
- 13.Ohashi A, Suetake Y, Saeki Y, Harada T, Aizawa S, Hasegawa H. Corrigendum to "Rapid clearance of supplemented tetrahydrobiopterin is driven by high-capacity transporters in the kidney" [Mol Genet Metab.105/4 (2012) 575–581]. Mol Genet Metab. 2013;108:107 10.1016/j.ymgme.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 14.Sawabe K, Saeki Y, Ohashi A, Mamada K, Wakasugi KO, Matsuoka H, et al. Tetrahydrobiopterin in intestinal lumen: Its absorption and secretion in the small intestine and the elimination in the large intestine. J Inherit Metab Dis. 2009. February;32(1):79–85. Epub 2008/11/26. Eng. 10.1007/s10545-008-0964-0 [DOI] [PubMed] [Google Scholar]
- 15.Hsu V, de LTVM, Zhao P, Zhang L, Zheng JH, Nordmark A, et al. Towards quantitation of the effects of renal impairment and probenecid inhibition on kidney uptake and efflux transporters, using physiologically based pharmacokinetic modelling and simulations. Clinical Pharmacokinetics. 2014 Mar;53(3):283–93. 10.1007/s40262-013-0117-y . Pubmed Central PMCID: PMC3927056. Epub 2013/11/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue F, Hasegawa H, Nishimura M, Yanagisawa M, Ichiyama A. Distribution of 5-hydroxytryptamine (5HT) in tissue of a mutant mouse deficient in mast cell (W/Wv). Demonstration of the contribution of mast cells to the 5HT content in various organs. Agents Actions. 1985. July;16(5):295–301. 10.1007/bf01982861 . Epub 1985/07/01. eng. [DOI] [PubMed] [Google Scholar]
- 17.Fukushima T, Nixon JC. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal Biochem. 1980. February;102(1):176–88. 10.1016/0003-2697(80)90336-x . Epub 1980/02/01. eng. [DOI] [PubMed] [Google Scholar]
- 18.Ohashi A, Sugawara Y, Mamada K, Harada Y, Sumi T, Anzai N, et al. Membrane transport of sepiapterin and dihydrobiopterin by equilibrative nucleoside transporters: a plausible gateway for the salvage pathway of tetrahydrobiopterin biosynthesis. Mol Genet Metab. 2011;102:18–28. 10.1016/j.ymgme.2010.09.005 [DOI] [PubMed] [Google Scholar]
- 19.Sawabe K, Wakasugi KO, Hasegawa H. Tetrahydrobiopterin uptake in supplemental administration: elevation of tissue tetrahydrobiopterin in mice following uptake of the exogenously oxidized product 7,8-dihydrobiopterin and subsequent reduction by an anti-folate-sensitive process. J Pharmacol Sci. 2004 Epub 2004. October 2;96(2):124–33. 10.1254/jphs.fp0040280 . [DOI] [PubMed] [Google Scholar]
- 20.Brand MP, Hyland K, Engle T, Smith I, Heales SJ. Neurochemical effects following peripheral administration of tetrahydropterin derivatives to the hph-1 mouse. J Neurochem. 1996. March;66(3):1150–6. 10.1046/j.1471-4159.1996.66031150.x . [DOI] [PubMed] [Google Scholar]
- 21.Levine RA, Zoephel GP, Niederwieser A, Curtius HC. Entrance of tetrahydropterin derivatives in brain after peripheral administration: effect on biogenic amine metabolism. J Pharmacol Exp Ther. 1987. August;242(2):514–22. . Epub 1987/08/01. eng. [PubMed] [Google Scholar]
- 22.Miwa S, Watanabe Y, Hayaishi O. 6R-L-erythro-5,6,7,8-tetrahydrobiopterin as a regulator of dopamine and serotonin biosynthesis in the rat brain. Arch Biochem Biophys. 1985. May 15;239(1):234–41. 10.1016/0003-9861(85)90831-8 . Epub 1985/05/15. eng. [DOI] [PubMed] [Google Scholar]
- 23.Koshimura K, Murakami Y, Tanaka J, Kato Y. The role of 6R-tetrahydrobiopterin in the nervous system. Prog Neurobiol. 2000. July;61(4):415–38. 10.1016/s0301-0082(99)00059-3 . Epub 2000/03/23. eng. [DOI] [PubMed] [Google Scholar]
- 24.Singhvi SM, Pan HY, Morrison RA, Willard DA. Disposition of pravastatin sodium, a tissue-selective HMG-CoA reductase inhibitor, in healthy subjects. Br J Clin Pharmacol. 1990. February;29(2):239–43. 10.1111/j.1365-2125.1990.tb03626.x . Pubmed Central PMCID: 1380090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagenbuch B, Gui C. Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica. 2008. July;38(7–8):778–801. 10.1080/00498250801986951 [DOI] [PubMed] [Google Scholar]
- 26.Hoshiga M, Hatakeyama K, Watanabe M, Shimada M, Kagamiyama H. Autoradiographic distribution of [14C]tetrahydrobiopterin and its developmental change in mice. J Pharmacol Exp Ther. 1993;267(2):971–8. [PubMed] [Google Scholar]
- 27.Hayashi T, Ogata A, Takehisa M, Komoriya K, Ohnuma N. Studies on metabolism and disposition of sapropterin hydrochloride (SUN 0588) L-erythro-tetrahydrobiopterin hydrochloride in rats. Kiso to Rinsho. 1992;26(8):3471–95. [Google Scholar]
- 28.Hasegawa H, Sawabe K, Nakanishi N, Wakasugi OK. Delivery of exogenous tetrahydrobiopterin (BH4) to cells of target organs: role of salvage pathway and uptake of its precursor in effective elevation of tissue BH4. Mol Genet Metab. 2005. December;86 Suppl 1:S2–10. 10.1016/j.ymgme.2005.09.002 . [DOI] [PubMed] [Google Scholar]
- 29.Ohashi A, Fukumuro M, Sawabe K, Mamada K, Sugawara Y, Matsuoka H, et al. Transcellular relocation of tetrahydrobiopterin across Caco-2 cells: A model study of tetrahydrobiopterin absorption through epithelial cells of intestinal mucosa. J Inherit Metab Dis. 2009. November 3Feb;32(1):73–8. Epub 2008/11/04. Eng. 10.1007/s10545-008-0961-3 [DOI] [PubMed] [Google Scholar]
- 30.Sawabe K, Yamamoto K, Harada Y, Ohashi A, Sugawara Y, Matsuoka H, et al. Cellular uptake of sepiapterin and push-pull accumulation of tetrahydrobiopterin. Mol Genet Metab. 2008. August;94(4):410–6. 10.1016/j.ymgme.2008.04.007 [DOI] [PubMed] [Google Scholar]
- 31.Blau N, Longo N. Alternative therapies to address the unmet medical needs of patients with phenylketonuria. Expert Opin Pharmacother. 2015. April;16(6):791–800. Epub 2015/02/11. eng. 10.1517/14656566.2015.1013030 [DOI] [PubMed] [Google Scholar]
- 32.Ohashi A, Mamada K, Tsuboi I, Aizawa S, Hasegawa H. Asymmetric uptake of sepiapterin and 7,8-dihydrobiopterin as a gateway of the salvage pathway of tetrahydrobiopterin biosynthesis from the lumenal surface of rat endothelial cells. Mol Genet Metab. 2011. June 12;104:404–6. Epub 2011/06/28. Eng. 10.1016/j.ymgme.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 33.Klatt P, Schmid M, Leopold E, Schmidt K, Werner ER, Mayer B. The pteridine binding site of brain nitric oxide synthase. Tetrahydrobiopterin binding kinetics, specificity, and allosteric interaction with the substrate domain. J Biol Chem. 1994. May 13;269(19):13861–6. . Epub 1994/05/13. eng. [PubMed] [Google Scholar]
- 34.Cosentino F, Katusic ZS. Tetrahydrobiopterin and dysfunction of endothelial nitric oxide synthase in coronary arteries. Circulation. 1995. January 1;91(1):139–44. 10.1161/01.cir.91.1.139 . Epub 1995/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 35.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, et al. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA. 1998. August 4;95(16):9220–5. 10.1073/pnas.95.16.9220 . Pubmed Central PMCID: 21319. Epub 1998/08/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stuehr D, Pou S, Rosen GM. Oxygen reduction by nitric-oxide synthases. J Biol Chem. 2001. May 4;276(18):14533–6. 10.1074/jbc.r100011200 . Epub 2001/03/30. eng. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt K, Kolesnik B, Gorren AC, Werner ER, Mayer B. Cell type-specific recycling of tetrahydrobiopterin by dihydrofolate reductase explains differential effects of 7,8-dihydrobiopterin on endothelial nitric oxide synthase uncoupling. Biochem Pharmacol. 2014. August 1;90(3):246–53. Pubmed Central PMCID: 4099517. 10.1016/j.bcp.2014.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitsett J, Rangel Filho A, Sethumadhavan S, Celinska J, Widlansky M, Vasquez-Vivar J. Human endothelial dihydrofolate reductase low activity limits vascular tetrahydrobiopterin recycling. Free Radic Biol Med. 2013. October;63:143–50. Pubmed Central PMCID: 3748942. 10.1016/j.freeradbiomed.2013.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crabtree MJ, Channon KM. Dihydrofolate reductase and biopterin recycling in cardiovascular disease. J Mol Cell Cardiol. 2009. December;47(6):749–51. Epub 2009/10/06. eng. 10.1016/j.yjmcc.2009.09.011 [DOI] [PubMed] [Google Scholar]
- 40.Crabtree MJ, Hale AB, Channon KM. Dihydrofolate reductase protects endothelial nitric oxide synthase from uncoupling in tetrahydrobiopterin deficiency. Free Radic Biol Med. 2011. June 1;50(11):1639–46. Epub 2011/03/16. eng. 10.1016/j.freeradbiomed.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moens AL, Kietadisorn R, Lin JY, Kass D. Targeting endothelial and myocardial dysfunction with tetrahydrobiopterin. J Mol Cell Cardiol. 2011. October;51(4):559–63. Epub 2011/04/05. eng. 10.1016/j.yjmcc.2011.03.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.