Abstract
The autophagy pathway is critical for the long-term homeostasis of cells and adult organisms and is often activated during periods of stress. Reduced pathway efficacy plays a central role in several progressive neurological disorders that are associated with the accumulation of cytotoxic peptides and protein aggregates. Previous studies have shown that genetic and transgenic alterations to the autophagy pathway impacts longevity and neural aggregate profiles of adult Drosophila. In this study, we have identified methods to measure the acute in vivo induction of the autophagy pathway in the adult fly CNS. Our findings indicate that the genotype, age, and gender of adult flies can influence pathway responses. Further, we demonstrate that middle-aged male flies exposed to intermittent fasting (IF) had improved neuronal autophagic profiles. IF-treated flies also had lower neural aggregate profiles, maintained more youthful behaviors and longer lifespans, when compared to ad libitum controls. In summary, we present methodology to detect dynamic in vivo changes that occur to the autophagic profiles in the adult Drosophila CNS and that a novel IF-treatment protocol improves pathway response in the aging nervous system.
Introduction
A complex mixture of genetic and environmental factors can influence the aging process in the nervous system, which in turn impacts the accumulation of protein aggregates and the maintenance of complex behaviors [1–3]. Age-dependent neural defects have been well characterized and have largely focused on the damage caused by reactive oxygen species (ROS), as well as the production of cytotoxic protein aggregates that disrupt neuronal function [2, 4–6]. The accumulation of neural aggregates, caused by alterations in protein homeostasis, has also been closely associated with several degenerative disorders and behavioral defects in people [1, 7, 8]. This includes neurological diseases that can be classified as genetic (familial) or sporadic in origin [7, 9, 10]. Genetic mutations that influence protein folding (i.e. alpha-synuclein) [7], aggregation tendencies (i.e. Poly-Q) [11, 12], or protein processing, resulting in the generation of cytotoxic peptides (i.e. amyloid-beta), have highlighted a genetic basis for some neurological diseases [13–16]. However, the most common progressive neurodegenerative disorders have not been linked to specific genetic defects (sporadic) and are often associated with progressive age-dependent changes to metabolic signaling (insulin/TOR) or cellular clearance pathways [9, 13, 14, 17–19].
One such clearance pathway is macroautophagy (autophagy), which is a highly conserved sequestration and vesicle transport system that intersects with the lysosomal and endosomal pathways [14, 18, 20–22]. Autophagy occurs at basal levels in most cells and tissues and requires the de novo formation of autophagosomes [21]. However, depending on changing physiological conditions, which influence multiple upstream signaling pathways, autophagic activity can be dramatically altered. This in turn impacts pathway flux and the subsequent degradation of material by the lysosome [23, 24]. While direct links between autophagic defects and neurological disorders are still being examined, mutagenesis studies have shown that loss of autophagic function accelerates protein aggregate accumulation and the decline of the nervous system [5, 18, 25]. In addition, a common feature of many human neural aggregates is the presence of the intracellular scaffolding protein p62 (SQSTM1) and ubiquitinated proteins [18, 26–28]. The p62 protein and its Drosophila homolog, Ref(2)P, are both autophagy components that facilitate the targeted selection and sequestration of substrates, which includes protein aggregates (aggrephagy) [1, 18, 23, 29]. As part of its interactions with targeted cargo and other pathway components, the p62 and Ref(2)P proteins are incorporated into new autophagosomes and degraded in the lysosome [18, 26]. Therefore, by taking advantage of this unique feature and by assessing total or insoluble p62/Ref(2)P levels, the relative in vivo activity and flux of targeted substrates through the pathway can be partly assessed in cells and whole tissues [30, 31].
Genetic and transgenic studies in Drosophila have been used extensively to model the complex in vivo cellular processes linked to human aging and neurological disorders. Previously, we have shown that adult flies containing autophagy mutations are short-lived, stress-sensitive and demonstrate a premature build-up of endogenous neural aggregates [5, 18, 25, 32]. Conversely, transgenic expression of the Atg8a protein in fly CNS tissues lowers protein aggregate profiles, preserves behaviors and promotes adult longevity [1, 5, 18]. Therefore, these findings indicate that the common practice of expressing tagged versions of the Atg8a or MAP-LC3 proteins to assess new autophagosome (Atg8a positive punctae) formation and pathway activity may actually alter the long-term in vivo profiles of the pathway [30, 33, 34]. Thus, in vivo studies focused on aging may require direct characterization of endogenous pathway components to better understand autophagic responses in complex tissues. Examining these dynamic processes in fly and mammalian nervous systems has remained a significant challenge [6, 34–39]. We have recently published on the response of the autophagy pathway occurring in the adult CNS following exposure to traumatic brain injury [24, 40, 41]. During these studies we determined there are dynamic changes in the pathway function that can be detected using confocal imaging and Western blot analysis [40].
In this report, we demonstrate that the majority of Atg8a-positive vesicles, or autophagosomes, within the fly brain are primarily detected in the soma and projections of mature neurons. Further, our detection methodologies are expanded to assess the acute induction of the autophagy pathway in the adult fly nervous system following a fast. From these studies, we identify dynamic age, genetic and gender-specific functional alterations to autophagy. In addition, by utilizing a novel intermittent fasting (IF) protocol, we demonstrate IF-treatment also improves the long-term responses of the autophagy pathway following a fast, as well as promotes longevity and maintains behaviors.
Material and Methods
Fly stocks, culturing conditions, weight-loss and IF treatment protocol
Canton-S (CS), w1118, Atg8a1, and Atg8a2 flies have been previously described and were obtained from the Bloomington Stock center (Bloomington, IN, USA) [5, 18]. The chico1/Cy stock was a gift from Dr. Montminy’s group (Salk Institute) [18, 42, 43]. Wild-type (WT) control flies were F1 offspring generated from crosses between CS females and w1118 male flies. The other genotypes used in this study were also F1 individuals produced through crosses with either CS or w1118 fly strains, as previously described [1, 5, 40]. Adult flies were collected within four hours of eclosion, aged in same-sex cohorts (25 flies per vial), and maintained on standard fly media (agar, molasses, yeast, cornmeal, propionic acid, nipagin) and culturing conditions (25°C, 65% humidity, 12-hour light:dark cycle) [1, 18, 40].
To assess changing weight profiles, four gender-specific cohorts containing 25 young flies (1-week) were weighed four times, starting at 9:00 am. Flies were placed in fasting vials (1% agar, wet fast) and each fly cohort re-weighed after 4, 8 or 24 hours of fasting. To measure weight recovery, fasted male and female flies (8 hours) were returned to standard fly media and permitted to recover overnight (re-feeding 16-hours) before being re-weighed at 9:00 am the following morning [44, 45]. The data is presented as weight (mg) per fly. For all IF studies, flies were maintained using standard conditions until 1-week of age before being exposed to either IF or ad libitum treatment conditions. IF-treated flies were turned onto fasting vials (1% agar) three times per week from 9:00 am to 5:00 pm (8-hours). IF-treated flies were placed on ad libitum conditions for two full days prior to the initiation of behavioral or protein assays [46]. Longevity profiles were established by counting the number of dead flies three times per week [32]. IF-treated flies were exposed to IF conditions for the duration of longevity studies. Average lifespans and SEM values were generated using MS Excel and P-values were calculated using GraphPad software [5, 25].
Confocal imaging
The heads from young male and female WT flies (w1118/+, 1-week) were collected and the surrounding cuticle and tracheal tissues were dissected away from the adult CNS. Brains were fixed on ice for 45 min (4% paraformaldehyde, 1x PBS), washed in PBST (0.1% Triton X-100 in 1x PBS) and blocked in 5% normal goat serum (5% NGS) [32, 40]. Brains were incubated overnight (4°C) in anti-Elav (1:500 dilution, 9F8A9, Developmental Studies Hybridoma Bank [DSHB], Iowa City, IA, USA) [40, 47] or anti-Repo (1:400 dilution, 8D12, DSHB) [40, 48] antibodies and co-stained with an antibody specific for the fly Atg8a protein (1:250 dilution, E1J4E, human GABARAP, Cell Signaling Technology [CST], Danvers, MA, USA) [40]. Brains were washed with PBST, blocked in 5% NGS and incubated for 3 hours at room temperature with Alexa Fluor-488 (1:250 anti-mouse) or Cy3 (1:250 anti-rabbit) secondary antibodies (Jackson ImmunoReseach Labs, Inc., West Grove, PA, USA) as previously described [1, 18, 40]. Autophagosome counts were obtained from the brains (n = 4) of 1-week old male flies that were maintained on ad libitum conditions or fasted for 4-hours. Images from individual confocal Z series (1.5 μm optical section) were taken of the dorsal cortical regions of the adult CNS. The Atg8a-positive punctae were counted in multiple 20 μm2 area image fields (see Fig 1B and 1C) from flies on ad libitum or fasted conditions (4 to 8 hours) [40].
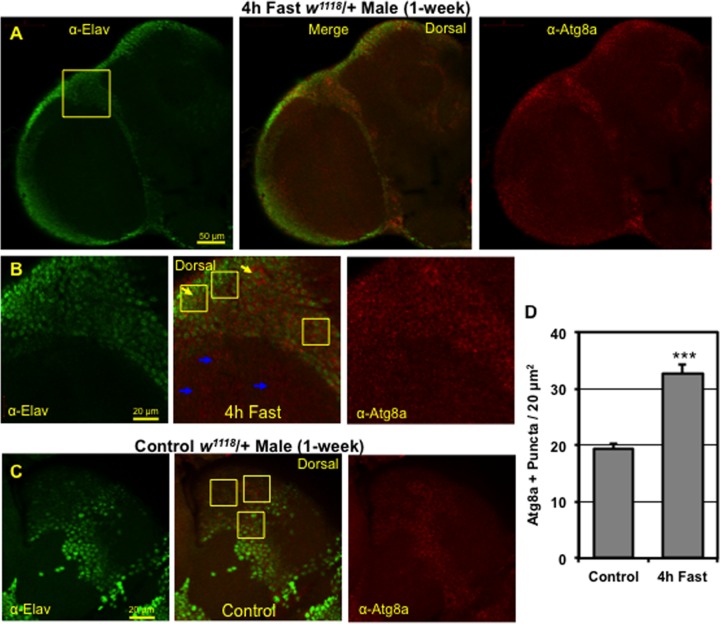
Fig 1. Distribution of Atg8a positive punctae in the adult Drosophila CNS.
(A) Representative confocal image (1.0 μm optical section, top left) of adult male fly brains following a 4-hour fast. Adult brains were co-stained with the anti-Elav neuronal (green) and the anti-Atg8a autophagy (red) markers. (B) Higher magnification images (see Fig 1A inset) highlight areas enriched with Atg8a positive punctae, which primarily include neuronal soma (cell bodies) and regions of neuropil (blue arrows). Yellow boxes (20 μm2) show the location of regions in the CNS that primarily contain neuronal soma that were used to count and establish autophagosome punctae profiles that occur in the adult fly brain. Additional, higher magnification images are included in S1A and S1B Fig (regions highlighted by yellow arrows). (C) Magnified images from similar brain locations of non-fasted adult male flies stained with anti-Elav and anti-Atg8a antibodies. Yellow boxes indicate regions containing neuronal soma (green) that were used to count Atg8a positive punctae (red). (D) Average number of Atg8a positive punctae or autophagosomes in control (n = 53 fields) neural tissues and following a brief 4-hour fast (n = 66 fields). P*** ≤ 0.001.
Antibodies and protein analysis
For protein aggregate analysis, male flies from different ages, genotypes and treatment conditions were collected, flash frozen, and stored at -80°C. Adult head tissues were homogenized using a Bead Ruptor-24 System (Omni International, Kennesaw, GA, USA) for analysis of total proteins or for sequential detergent extraction in Triton X-100 (1.0%) and SDS (2.0%) buffers as described [1, 5, 32]. Protein concentrations for each sample were determined using the DC Protein assay (Bio-Rad, Hercules, CA, USA). For Western blots, 20 μg of protein was loaded per lane and resolved on a 12% Midi-Bis-Tris gel (Bio-Rad), followed by electro-blotting onto Immobilon-P membranes (Millipore Corp., Billerica, MA, USA) using the Trans Blot Turbo system (Bio-Rad). Blots were sequentially probed using anti-Atg8a (E1J4E, CST) [40], anti-Ref(2)P [1], anti-Ubiquitin (P4D1, CST) and anti-Actin (13E5, CST) antibodies at a 1:1,000 dilution [1, 18]. Blots were developed using Thermo Scientific West Dura Substrate (Thermo Scientific Pierce, Rockford, IL, USA) and the ChemiDoc digital Imaging System and Quantity One software (Bio-Rad). ImageJ software (imagej.nih.gov) was used to quantify relative intensity of different proteins, which are also normalized to Actin levels. For each condition a minimum of three replicate samples were used for Western blot analyses.
Quantitative PCR
Male flies (1-week) were fasted for 0, 4 or 8-hours, collected at each time-point, flash frozen and stored at -80°C. Replicate RNA samples were isolated from 25 heads using Trizol (Thermo Fisher Scientific) and cDNA libraries were generated using the RevertAid First Strand cDNA Synthesis kit, with a combination of random hexamer and oligo-dT primers (Thermo Fisher Scientific). Quantitative PCR was performed on a CFX Connect Real-Time PCR Detection System (Bio-Rad) and Universal PCR SYBR Mix reagents (Bio-Rad). Primer sequences for the Drosophila Atg8a gene are available upon request. Melt curve analyses of all qPCR products confirmed the production of a single DNA duplex. The Pfaffl method was used to quantitate expression profiles and Cyp1 used as a reference gene [1, 5, 40]. Relative mRNA levels of non-fasted control flies were set at 1.0 and subsequent expression levels from different time points were expressed as normalized values.
Geotaxis response
The Drosophila negative geotaxis response protocol and apparatus used in this study has been previously described [1]. Fly cohorts (15–25) were tapped down and digital images of climbing flies were recorded after 5-sec (w1118/+, Atg8a1) or 3-sec (chico1/+) using a Nikon Coolpix L18 camera. Flies were allowed to rest for 1-min between four replicate runs [1, 49]. Digital images were scored for the distance traveled within 5 or 3 seconds (bottom = 0 to top = 6). Replicate runs from each fly cohort were taken at weekly intervals and used to establish age and genotype-specific climbing indexes [1].
Statistical analysis
Statistical analyses between groups were performed using either Microsoft Excel or GraphPad software and Student’s t-tests (two-tailed, unpaired) were performed to establish P-values. All values are reported as means ± SEM [1].
Results
Assessing autophagy profiles in adult Drosophila neural tissues
For an initial assessment of autophagic profiles, we examined the distribution of Atg8a-positive punctae or organelles (autophagosomes) in CNS tissues from adult flies fasted for 4 hours (Fig 1) [40, 50, 51]. Fasting typically activates the pathway in most tissues, leading to a rapid increase in the number of autophagosomes [30, 34, 52]. However, there have been limited in vivo analyses of autophagy profiles and responses under different physiological conditions in neuronal tissues, especially in Drosophila [40, 53–55]. Atg8a (red) staining profiles were especially enriched in cortical areas that were positive for the Elav protein, a neuronal soma marker (green, Fig 1A) [47]. At higher magnifications Atg8a-positive punctae (yellow arrows) were detected in neuronal cell bodies (soma, also see S1A and S1B Fig), as well as in regions that primarily consisted of Drosophila neuropil or axonal tracts (blue arrows, Fig 1B and S1C Fig). In contrast, relatively few glial cells (Repo-positive) contained Atg8a-positive punctae (yellow arrow, S1D Fig) [48]. Non-fasted control flies demonstrated similar staining patterns in the CNS cortex regions, albeit at lower levels than fasted flies (yellow arrows, Fig 1C) [30, 40, 52]. Quantification of Atg8a-positive autophagosomes showed that following a brief 4-hour fast there was a rapid increase in vesicle numbers (68%) in adult male neural tissues (Fig 1D) [35, 53–55]. Therefore, confocal imaging studies indicate that Drosophila neural tissues have high levels of basal autophagy that can be further activated by fasting [35, 36].
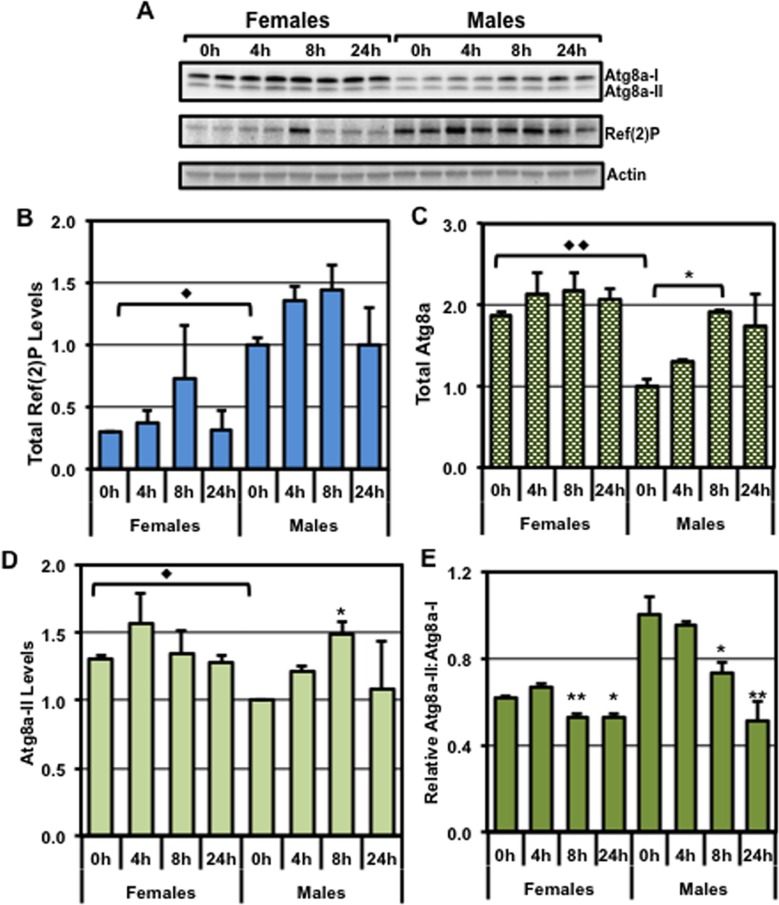
To further assess the impact of fasting on autophagic profiles, total head protein extracts from young (1-week) female and male flies were examined for Atg8a and Ref(2)P proteins (Fig 2) [40]. Under basal conditions (0-hour fast), there were distinct gender-specific differences in basal autophagic profiles (Fig 2A). Female flies showed significantly lower Ref(2)P levels than their age-matched male counterparts (Fig 2A and 2B), indicating they have higher basal levels of autophagy in neuronal tissues [56–60]. Coincidently, female flies also exhibited higher Atg8a-II and total Atg8a (I+II) levels (Fig 2A, 2C and 2D) [30, 34, 36, 59]. Brief periods of fasting (on 1% agar) did not significantly alter the neural Ref(2)P profiles in female or male flies (Fig 2A and 2B). This indicates that changes to total Ref(2)P levels do not necessarily reflect the acute induction of the pathway and in this context would be a more appropriate marker to assess basal autophagy levels. However, male flies that were fasted for 8 hours had a significant increase in both Atg8a-II and total (I+II) profiles (Fig 2C and 2D). These protein changes were independent of alterations in Atg8a mRNA expression (S2 Fig), indicating that the alterations to this key autophagic marker most likely reflect an acute change at the protein level. Female flies, on the other hand, demonstrated more modest changes to these autophagy markers (Fig 2A–2D). Interestingly, the parameter that showed the most consistent fasting-induced change for both genders was the reduction in the Atg8a-II:Atg8a-I ratios (Fig 2E). Combined, these data suggests that the fasting induced activation of neuronal autophagy can be assessed by the rapid decrease in Atg8a-II:Atg8a-I ratios, regardless of whether an individual group of flies started with higher (female) or lower (male) basal pathway levels [1, 56–59].
Fig 2. Influence of gender and fasting on autophagic responses and longevity profiles.
Young WT female and male flies (w1118/+, 1-week) were subjected to 0, 4, 8 or 24 hours of fasting (1% agar). (A) Total protein extracts from adult heads were prepared for each condition (n = 3) and used to generate Western blots that were sequentially probed for the Atg8a, Ref(2)P, and Actin proteins. Quantification of the (B) Ref(2)P and (C) total Atg8a (I+II), and (D) Atg8a-II values normalized to Actin loading controls. (E) The relative ratio between the Atg8a-II and Atg8a-I proteins. (◆) represents significant differences between genders and (*) represents a difference within a gender specific group. *, ◆P≤ 0.05, **, ◆◆P ≤ 0.01, ***P ≤ 0.001.
Longevity and autophagic changes associated with IF treatment
Dietary modifications, such as time-restricted feeding and caloric restriction, have been used to modify aging and longevity profiles in various model systems [30, 34, 44, 61–65]. Recently, select intermittent fasting (IF) protocols have been shown to positively impact the physiology and metabolic profiles of aging humans and model organisms [61, 65–68]. Since fasted flies demonstrated rapid activation of neuronal autophagy (Figs 1 and 2), it suggested that a potential benefit associated with IF-treatment could involve the periodic induction of the pathway [45, 62, 66–70]. However, the long-term impact of IF on the health and neural function of adult Drosophila has remained unclear. Previous studies have shown that exposing female flies to short daily fasts (4 or 6-hours) had a negligible effect on longevity profiles [44]. In contrast, flies exposed to a daily time-restricted feeding protocol (12-hour daily fast) maintained more youthful circadian-based behaviors [45, 61, 68, 71].
Therefore, to establish an optimal Drosophila intermittent-fasting protocol, male and female flies (1-week) were fasted and weighed at various time points [45, 72]. Flies from both genders exhibited significant weight loss after only 4-hours of fasting and by 24-hours had lost on average ~10% of their total body mass (S3A and S3B Fig and S1A and S1B Table) [45, 61, 65]. In rodent models, beneficial IF treatments typically involve a modest weight loss, followed by rapid weight recovery once food is reintroduced [45, 61, 65]. Flies that were allowed to recover on ad libitum conditions for 16-hours, following an 8-hour fast, quickly regained their original weight (S3A and S3B Fig and S1 Table). Previous human and rodent studies have also demonstrated that daily fasts are not essential to have a beneficial impact on metabolic rates and neural function [61, 65, 68, 70, 71, 73, 74]. Based on the rapid formation of autophagosomes (Fig 1D) and the reduction of Atg8a-II:Atg8a-I ratios following a fast (Fig 2E), an IF treatment regimen that exposed young adult flies (1-week) to three weekly, 8-hour fasts was established.
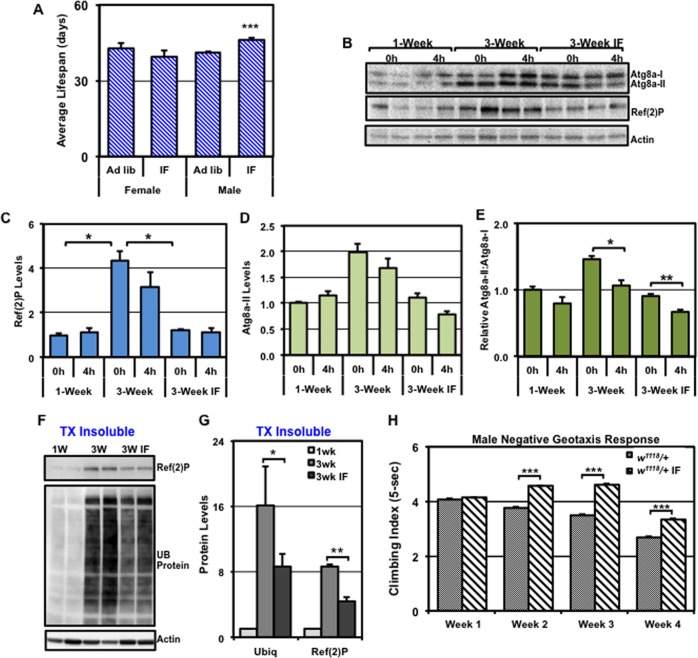
We examined the impact that IF-treatment has on adult longevity profiles by maintaining 1-week old WT female and male flies (w1118/+) on standard ad libitum or IF-treatment conditions (three weekly, 8-hour fasts) throughout their entire lifespan. Female longevity was largely unaffected by IF-treatment, while male flies showed a significant increase in average lifespan (12%, Fig 3A and S2 Table) [44]. The ability of IF-treatment to promote male longevity may reflect the sex-specific differences that acute fasting has on autophagic responses in neural tissues (Fig 2A–2E) [44]. Of note, the weights of IF-treated middle-aged (4-weeks) male flies were not significantly different from age-matched ad libitum controls (S1C Table). Therefore, the modest IF protocol appears to be well tolerated by adult flies and, unlike caloric restriction, does not cause flies to become underweight [44, 45, 61, 64].
Fig 3. The impact of aging and IF treatment on longevity, neural autophagy, aggregates and behaviors.
WT flies (w1118/+) were maintained using standard ad libitum or IF treatment conditions beginning at 1-week of age. (A) The average lifespan profiles obtained from WT female and male flies. (B) Western blots of total protein extracts from control (0h) or fasted (4h) male fly heads at 1-week, 3-week or 3-weeks IF-treated of age (25 per condition, n = 3), were probed for Atg8a, Ref(2)P, and Actin proteins. Quantification of (C) Ref(2)P and (D) Atg8a-II that were normalized using Actin. (E) The relative ratio of the Atg8a-II to Atg8a-I proteins. (F) Triton X-100 insoluble protein extracts were prepared from WT male fly at 1-week, 3-week or IF-treated 3-weeks and Western blots probed for Ref(2)P, ubiquitin (UB), and Actin proteins. (G) Quantification of Ref(2)P and UB-proteins, normalized using Actin. (H) WT (w1118/+) male flies were maintained on ad libitum or IF conditions and allowed 2 days of recovery on standard food before the initiation of the NGR assay. The climbing indexes (5-sec) of freely responding ad libitum or IF treated flies were performed at weekly intervals starting at 1-week and continuing until 4-weeks of age. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
To determine whether IF-treatment could also alter long-term autophagic profiles in the aging CNS, we assessed changes in Atg8a and Ref(2)P profiles from neural tissues of young (1-week) and 3-week old ad libitum and IF-treated WT male fly cohorts (basal profiles). Non-fasted (0h), ad libitum treated middle-aged males (3-weeks) showed an age-dependent build-up of total Ref(2)P (Fig 3B–3C) [1, 18, 32]. In contrast, IF-treatment blunted the build-up of Ref(2)P under basal conditions (0h), strongly suggesting that older IF-treated flies maintained more youthful basal autophagy profiles (Fig 3C).
Following a fast, Atg8a-II levels did not significantly change in the 3-week old ad libitum flies or the age-matched IF-treated flies (Fig 3D). In contrast, both 3-week old ad libitum and IF-treated flies exhibited lower Atg8a-II:Atg8a-I ratios in the head lysates following a 4h fast (Fig 3E), again suggesting this Atg8a-based parameter could be used to assess pathway induction due to fasting [30, 40]. Consistent with improved basal autophagy profiles, IF-treated flies also had a significant reduction in the build-up of Triton X-100 insoluble Ref(2)P and UB-proteins, which naturally accumulates in the neural tissues of middle-aged Drosophila (Fig 3F and 3G) [1, 5, 18]. Combined, these findings indicate that older flies exposed to a modest IF-treatment schedule maintained more youthful autophagy profiles and potentially neural-based capacities.
Behavioral changes associated with intermittent fasting
Since IF treatment improved the molecular markers associated with neural aging, we also examined whether IF-treatment could slow the decline of behaviors in aging Drosophila [1, 44, 45]. Innate adult fly behaviors are routinely used to examine genetic and age-related changes to neuronal responses [8, 49, 69, 75]. To assess the impact of IF treatment, we examined the negative geotaxis response (NGR) of male flies using a customized rapid iterative negative geotaxis (RING) assay system [1, 16, 49, 75, 76]. The fly NGR is associated with a robust climbing behavior, which can be influenced by environmental manipulations such as diet and exercise training [16, 75]. In addition, the NGR behavior starts to degenerate at a time (3 to 4-weeks) that coincides with the natural accumulation of neural aggregates [1, 5, 18]. Consistent with previous studies, ad libitum flies showed a rapid decrease in climbing abilities starting at 2-weeks, which becomes pronounced by 4-weeks of age (Fig 3H) [1, 49]. In contrast, IF-treated males maintained more youthful climbing profiles and continued to outperform control flies for up to 4-weeks (Fig 3H) [1].
The impact of genetics on neuronal aging and autophagy responses
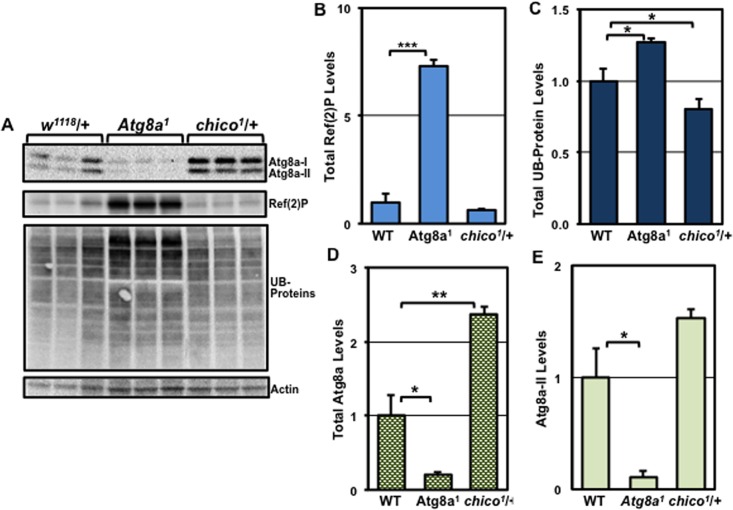
Our studies have demonstrated that age- and gender-induced alterations to neuronal autophagy profiles can be quantified by Western analysis of key pathway markers (Figs 2 and 3). To determine if this technique could also be used to detect genetic-based differences to pathway responses, we assessed the neural profiles of young Atg8a1 (autophagy-impaired) [5, 18] and heterozygous chico1/+ (autophagy-enhanced) male flies [18, 43, 77] Previous studies have demonstrated these fly genotypes produce normal appearing adults that have divergent lifespan and neural aggregate profiles [5, 18, 25, 43]. Indeed, young males (1-week) from each genotype showed unique protein patterns that reflect the endogenous basal autophagic profiles in the nervous system (Fig 4A–4E). When compared to WT males, young Atg8a1 mutants exhibited a robust accumulation of total Ref(2)P indicating diminished basal autophagy profiles in these flies, as well as a buildup of UB-proteins (Fig 4A–4C). As expected, Atg8a1 mutants also showed reduced Atg8a protein levels (Fig 4A and 4D and 4E). In contrast, chico1/+ flies exhibited lower UB-protein profiles and Ref(2)P levels also trended lower, further suggesting this genotype starts with elevated pathway activity or capacity (Fig 4A–4C). In addition, under basal conditions the chico1/+ males had elevated total Atg8a and Atg8a-II levels (Fig 4A and 4D and 4E). Combined, these results demonstrate that additional considerations should be made when comparing different fly genotypes with regards to their basal autophagy profiles.
Fig 4. The influence of genetics on basal autophagy profiles occurring in the fly CNS.
Age-matched WT (w1118/+), Atg8a1 and chico1/+ male flies (1-week) were collected and heads used to prepare total protein extracts. (A) Western blots were probed for Atg8a, Ref(2)P, ubiquitin (UB), and Actin proteins (n = 3). Quantification of total (B) Ref(2)P, (C) UB-proteins, (D) total Atg8a (I+II), and (E) Atg8a-II proteins, normalized using Actin. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Neuronal responses of flies with insulin signaling defects (chico1/+)
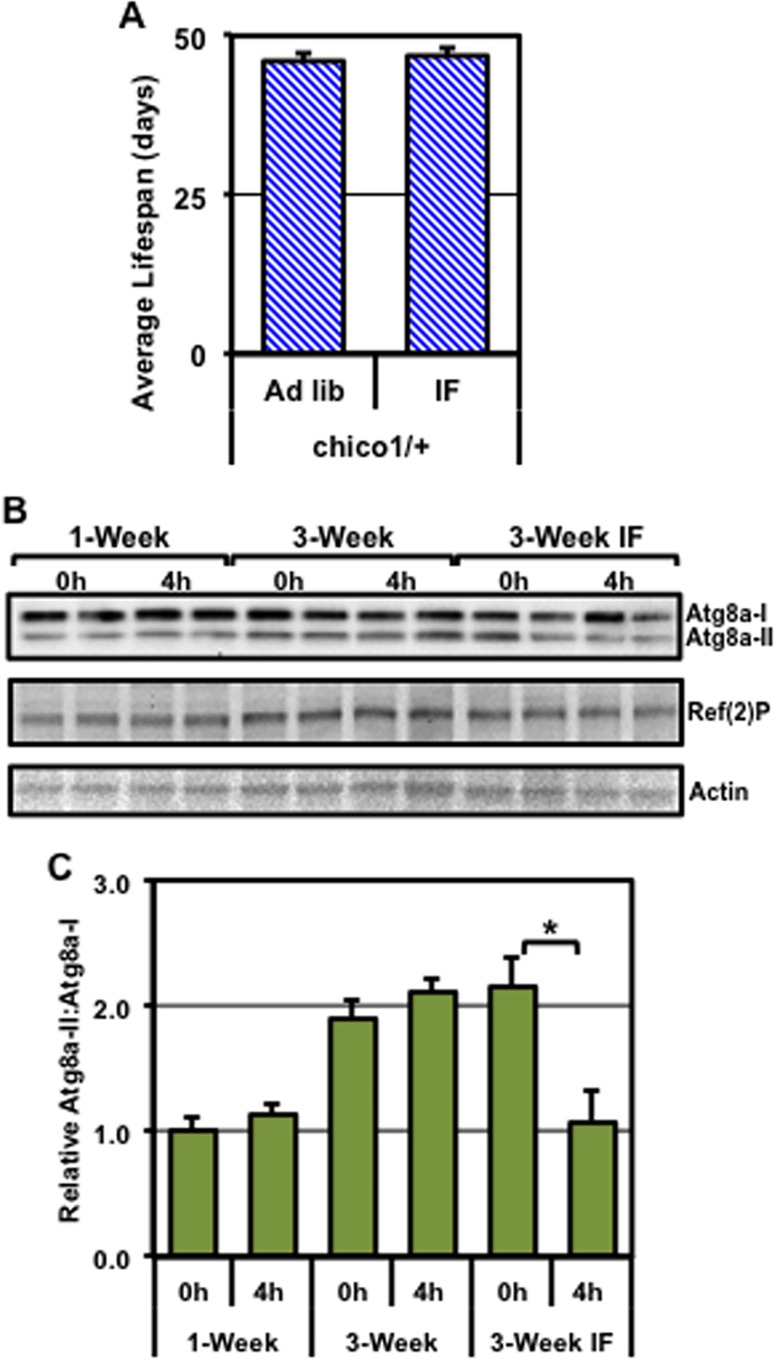
Given the positive effects that IF treatment had on the lifespan and autophagic induction in WT flies, we tested whether our protocol could benefit flies that have enhanced basal autophagy. Unlike WT flies, IF-treatment did not improve the average lifespan profiles of male chico1/+ flies (Fig 5A and S2 Table) or improve their NGR (S4A Fig). Furthermore, total Ref(2)P levels were largely unchanged in neural samples from young, 3-week ad libitum, and 3-week IF-treated chico1/+ fly cohorts (Fig 5B and S4B Fig). This was consistent with young chico1/+ flies having elevated basal levels of autophagy (Fig 4), which was largely maintained in middle-aged adults (Fig 5and S4 Fig) [42, 43]. Again, total Atg8a and Atg8a-II levels failed to accumulate in young or 3-week old chico1/+ flies following a fast (S4C and S4D Fig). Interestingly, IF-treated chico1/+ flies exposed to a 4-hour fast showed a significant reduction in Atg8a-II:Atg8a-I ratios, while young and 3-week old ad libitum controls did not (Fig 5C). These data demonstrate that, although IF treatment likely promoted the acute fasting-induced activation of autophagy, the overall benefit to chico1/+ flies is modest, which is consistent with the results from other dietary studies for this fly genotype [43].
Fig 5. Changes to the lifespan and autophagy profiles of chico1/+ male flies.
(A) The average lifespan profiles of chico1/+ mutant male flies exposed to ad libitum or IF treatment conditions, starting at 1-week of age. (B) Total head protein extracts from control (0h) or fasted (4h) male flies at 1-week, 3-week or IF-treated 3-week of age (n = 3), were used for Western blot analysis of the Atg8a, Ref(2)P, and Actin proteins. (C) The relative ratio of Atg8a-II to Atg8a-I proteins. *P ≤ 0.05.
Neuronal responses of adult autophagy mutants (Atg8a1)
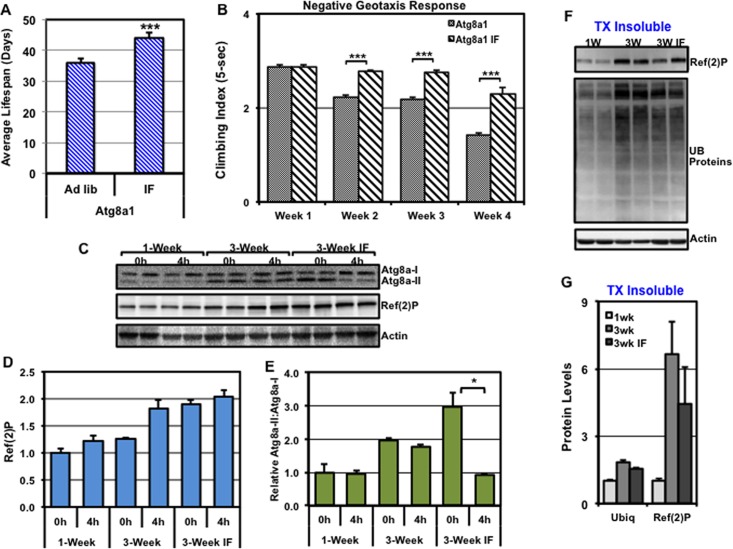
To determine whether IF-treatment could improve global physiological profiles of flies that have impaired autophagy, the lifespan profiles of ad libitum and IF-treated Atg8a1 were examined [5, 18, 25]. Atg8a1 mutant fly strains exhibited a significant increase in average longevity following IF-treatment, with a 22% increase in median lifespan extension (Fig 6A and S2 Table). Additional IF studies using a second stronger mutant strain, Atg8a2, resulted in a similar 27% increase in lifespan (S2 Table). Correlating with improved lifespans, IF-treated Atg8a1 males also showed more youthful NGR profiles, as compared to age-matched ad libitum controls (2 to 4-weeks, Fig 6B).
Fig 6. IF-dependent changes to the lifespan and autophagy profiles of Atg8a1 mutant flies.
(A) The average lifespan profiles of Atg8a1 mutant male flies were exposed to ad libitum or IF treatment conditions starting at 1-week of age. (B) The climbing indexes (5-sec) of freely responding ad libitum or IF treated Atg8a1 male flies, which were performed at weekly intervals starting at 1-week and continuing until 4-weeks of age. (C) Western blots containing total protein extracts prepared from control (0h) or fasted (4h) Atg8a1 male fly heads taken at 1-week, 3-week or IF-treated 3-week of age (n = 3) were sequentially probed for Atg8a, Ref(2)P, and Actin proteins. (D) Quantification of Ref(2)P protein levels, normalized using Actin. (E) The relative ratio of Atg8a-II to Atg8a-I protein levels. (F) Western blots of Triton X-100 insoluble head extracts from 1-week, 3-week or IF-treated 3-week old of Atg8a1 male flies that were sequentially probed for the Ref(2)P, ubiquitin (UB), and Actin proteins. (G) Quantification of Ref(2)P and UB-proteins in the Triton X-100 insoluble fraction, normalized using Actin. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Interestingly, IF-treatment led to a build-up of Ref(2)P in the basal state in total neural extracts from Atg8a1 flies (Fig 6C and 6D). However, IF-treated flies showed a fasting-induced decrease in Atg8a-II:Atg8a-I ratios (Fig 6E) and both UB-protein and Ref(2)P levels trended lower in the Triton X-100 insoluble fraction (Fig 6F and 6G), which was consistent with improved basal and activated pathway profiles [1, 18]. Combined, these data indicate that IF-treatment does not significantly change basal autophagy levels in Atg8a1 mutants, but in aged cohorts may improve pathway activation following a fast.
Discussion
The systematic analysis of endogenous autophagic responses occurring in the nervous system has been limited, especially in a genetically tractable system such as Drosophila [34–36, 39]. Alirezaei et al. demonstrated that GFP-tagged-LC3 expressed in the nervous system of transgenic mice could be used to detect autophagosome formation following a fast [34, 41]. However, our group has previously shown that either transgenic overexpression of endogenous Atg8a or a UAS-GFP-Atg8a construct within the fly CNS can alter pathway dynamics, which is reflected in lower neural aggregate levels, the maintenance of behaviors and enhanced adult longevity [1, 5, 18]. Therefore, using the GFP-Atg8a protein to mark and follow autophagic profiles (autophagosome formation) in aging neural tissues may complicate the assessment of endogenous basal and acute pathway responses. In this study, we utilized a commercially available antibody for imaging and Western based studies to directly detect the fly Atg8a protein and characterize endogenous pathway responses. We identified regions of the adult Drosophila CNS, primarily in areas containing neural cell bodies or soma, which were highly enriched in Atg8a-positive punctae (Fig 1and S1 Fig) [1]. Interestingly, we also observed a significant increase in the number of endogenous autophagosomes (~70%) following even a brief 4h fast, indicating that fly neurons can respond quickly to a fasting stimulus and further induce autophagy (Fig 1D).
Fasting is known to activate the autophagy pathway in many tissues and changes in Atg8a-II levels are often used to assess the acute induction of the pathway [30, 34]. However, the assessment and the detection of endogenous Atg8a-II or LC3-II changes in the nervous system has not been routinely employed as a method to detect pathway activation [30]. In young (1-week) female flies, we did not observe a rapid build-up in Atg8a-II levels following 4 or 8 hours of fasting, although male flies showed an increase after an 8-hour fast (Fig 2D). In contrast, flies from both genders exhibited a significant reduction in Atg8a-II:Atg8a-I ratios following an 8 or 24 hour fast (Fig 2E). Combined, these data further underscore the use of Atg8a-II:Atg8a-I ratios to assess changing pathway profiles, such as in times of fasting, in the adult Drosophila neural tissues. As with other Western-based studies, we propose that comparisons of Atg8a-II:Atg8a-I ratios from various treatment conditions must be performed on the same blot. The concern is that slight differences in transfer, hybridization and exposure conditions on different blots could complicate the assessment of dynamic changes in protein ratios. These studies are the first to our knowledge that have directly examined acute changes to the endogenous autophagy pathway within the adult fly CNS.
By assessing Atg8a-II:Atg8a-I ratios, as well as Ref(2)P levels, we demonstrate that gender, aging, dietary changes and genetic differences have an impact on the autophagic responses that occur in Drosophila neurons. One of the more unexpected findings from this study was the dramatic gender-based difference in autophagic profiles [56, 57, 60]. Young male flies started with lower basal autophagy levels, but demonstrated a rapid induction of the pathway following fasting (Fig 2). Conversely, females started with higher autophagic profiles, but had a more limited fasting response (Fig 2). These data highlight that gender should also be taken into consideration when performing detailed analysis of autophagic profiles in flies or other model organisms.
Drosophila genetic approaches have been used extensively to study complex in vivo autophagic responses, especially those occurring in the larval fat body [29, 30, 52]. For this study the Atg8a1 and chico1/+ fly strains were selected for the characterization of adult neuronal autophagic responses partly due to these genotypes having established neural aggregate profiles that are consistent with suppressed (Atg8a1) or enhanced (chico1/+) autophagy [5, 18, 42, 78]. These genetic backgrounds also produce viable, normal appearing adult flies (body size, fecundity) and have well-defined longevity profiles [5, 42, 77, 78]. In regards to Atg8a1 flies, this hypomorphic Atg8a allele results in only modest autophagy pathway impairments; however, these flies still maintain the potential ability to up-regulate the pathway [5, 18]. In this study, we confirmed that young Atg8a1 mutant males have suppressed neuronal autophagy (elevated Ref(2)P protein levels, Fig 4) and exhibited reduced NGR profiles that are consistent with modest behavioral impairments (Fig 6) [1, 40]. Unlike WT flies, the Atg8a1 mutants (3-weeks) did not exhibit a decrease in Atg8a-II:Atg8a-I ratios following an acute fast (Fig 6), which suggests that autophagic responses are further impaired. However, IF treatment led to a fasting-induced reduction in the Atg8a-II:Atg8a-I ratios in Atg8a1 flies indicating improved autophagic responses. Further, IF treatment in aged Atg8a1 mutants maintained NGR profiles (Fig 6) and extended the lifespans of both Atg8a1 and Atg8a2 mutant males (Fig 6and S2 Table). These data demonstrate that flies that start with impaired autophagic responses can exhibit aging-associated benefits following IF treatment (Fig 6). Although, markers of neural health were improved in IF-treated Atg8a1 flies, an unanticipated increase in whole tissue lysate Ref(2)P levels was detected, which is normally used as an in vivo marker of impaired autophagic flux [18, 30, 40]. Although not common, there are certain conditions where the build-up of Ref(2)P/p62 does not closely correlate with impaired autophagic flux [30, 79]. Whether the maintenance of Ref(2)P levels is dissociated from the enhanced autophagy observed in IF-treated Atg8a1 flies remains to be determined.
The chico1/+ flies represent a genotype that has a modest impairment of insulin-signaling, which does not impact growth-development processes or lead to insulin-resistance [42, 43, 77]. Consistent with enhanced neural autophagy, chico1/+ flies showed reduced Ref(2)P protein levels and had extended lifespans (Fig 5 and S2 Table). Similarly, modest insulin-signaling pathway impairment in heterozygous Irs2 knockout mice (mammalian chico homolog) promotes longevity and upregulates the autophagy pathway [80, 81]. Although previous studies have highlighted that the dysregulation of insulin-signaling in obesity or Type-II diabetes is a risk factor for the development of neurological diseases [17, 65, 82], these studies suggest that simply reducing insulin signaling in the adult CNS is not a causative factor for neural decline [80, 81]. In contrast to WT and Atg8a1 flies, chico1/+ flies were minimally impacted by IF treatment. IF-treated chico1/+ flies did not display improved behavioral responses or improved longevity and several key autophagic markers were largely unchanged (Fig 5, S4 Fig and S2 Table). This is consistent with previous studies showing dietary modifications, such as caloric restriction, did not extend longevity in these flies [42, 77]. Combined, these data suggest that chico1/+ flies may be at near maximal levels of autophagic capacity, which limits IF-induced autophagy pathway upregulation to extend lifespan further.
Our findings in aged Drosophila underscore how environmental factors, such as IF-treatment or other modest dietary manipulations could influence the long-term maintenance of the nervous system. Different IF protocols have been shown to stabilize weight profiles, improve insulin sensitivity and lower markers associated with age-related stress and inflammation [61–64, 73, 82, 83]. The cellular mechanisms that mediate the beneficial effects of IF-based interventions in humans are not yet fully characterized, but activation of autophagy has been implicated in facilitating at least some of the beneficial effects of limited diets [45, 61, 63, 73, 74]. Our studies highlight that IF treatment does improve the long-term in vivo autophagic responses in the aging Drosophila CNS across diverse genotypes [5, 42, 43]. In summary, our studies highlight novel methods (imaging and Western blot-based) to examine the induction of the autophagy pathway in the adult fly CNS. In addition, IF treatment improved the neuronal autophagic response of Drosophila, as well as promoted the long-term maintenance of behaviors and longevity profiles.
Supporting Information
The *N values represent weights of different fly cohorts (25 per conditions) following a fast and re-feeding At least 125 individual flies were used for each study. A. Average adult female fly (w1118/+) weights (mg), SEM and percentage weight change that occurred following fasting and an overnight re-feeding. B. Average adult male fly (w1118/+) weights (mg), SEM and the percentage weight change that occurred following fasting and an overnight re-feeding. C. The average weights (mg) of WT male flies (w1118/+) at 1-week and 4-weeks of age maintained using ad librium conditions or after 3-weeks of IF-treatment (4-weeks of age).
(PDF)
Statistical description of the average lifespan (days), SEM, N and P values determined between ad libitum and IF-treatment gender and genotype fly cohorts. The percentage change in average longevity is also presented.
(PDF)
Confocal images adult fly brains co-stained for autophagic vesicle marker (Atg8a) and neuronal (Elav) or glial (Repo) markers. (A-B) Higher magnification images of neural soma (anti-Elav, green) and Atg8a positive punctae (anti-Atg8a, red) taken from CNS regions highlighted from Fig 1B (see yellow arrows in Fig 1B). (C) Representative confocal images of a female fly CNS (8 hour fast) show similar staining patterns to those of male flies seen in Fig 1(n = 10 brains). The location of neuronal soma (cell bodies, yellow arrows) and regions primarily consisting of neuropil are indicated (blue arrows). (D) Representative confocal images of a male CNS (8h fast) co-stained for glial (anti-Repo, green) and autophagy (anti-Atg8a, red) markers in the adult CNS (n = 15 brains). Magnified images (inset) show a single Repo-positive glial cell that contains Atg8a positive punctae (yellow arrow). Most glial cells showed limited Atg8a staining (yellow circles).
(TIFF)
Triplicate cohorts of young (w1118/+) adult WT male flies were fasted for 0, 4 or 8-hours, flash frozen and total RNA isolated and used for qRT-PCR analysis of Atg8a message levels. Values were normalized using Cyp1 as a reference gene.
(TIF)
Starting at 9:00 am, triplicate weights of (A) female and (B) male fly cohorts (w1118/+, 25 flies per group) were obtained following 0, 4, 8 or 24-hour fast (125 total flies). Fly groups fasted for 8h were placed back onto standard media starting at 5:00 pm and allowed to re-feed overnight (16h) before being re-weighed again the following day at 9:00 am (red columns and arrows). *** P ≤ 0.001.
(TIF)
Young outcrossed chico1/+ male flies (1-week) were maintained using ad libitum or IF-treatment conditions. (A) The climbing indexes (3-sec) of freely responding ad libitum or IF-treated chico1/+ male flies that were performed at weekly intervals starting at 1-week and continuing until 4-weeks of age. (B) Quantified Ref(2)P, (C) total Atg8a (I+II), and (D) Atg8a-II protein profiles protein of chico1/+ neural samples illustrated in Fig 5B, were corrected using Actin as a loading control.
(TIF)
Acknowledgments
We would like to thank Dr. Mark Kern (SDSU), Dr. William Joiner (UCSD), and Dr. Cynthia Hughes (NSI) for the helpful discussions regarding Drosophila diet, behavior and aging studies. We would also like to thank Dr. Mark Sussman and Dr. Natalie Gude for their confocal microscopy expertise (SDSU). This work was supported by grants from NIH/NIA and include: R21AG030187 and R01AG039628.
Abbreviations
- Atg
autophagy-specific gene
- CNS
central nervous system
- IF
intermittent fasting
- NGR
negative geotaxis response
- Ref(2)P
refractory to sigma P
- RING
rapid iterative negative geotaxis
- ROS
reactive oxygen species
- SQSTM1
Sequestosome-1
- IRS
Insulin receptor substrate
- TOR
target of rapamycin
- UB-proteins
ubiquitinated proteins
- WT
wild-type
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
We received the following funding: R21AG030187 and R01AG039628 grants from the NIA (National Institute on Aging) division at NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ratliff EP, Mauntz RE, Kotzebue RW, Gonzalez A, Achal M, Barekat A, et al. Aging and Autophagic Function Influences the Progressive Decline of Adult Drosophila Behaviors. PloS one. 2015;10(7):e0132768 10.1371/journal.pone.0132768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wesson DW, Borkowski AH, Landreth GE, Nixon RA, Levy E, Wilson DA. Sensory network dysfunction, behavioral impairments, and their reversibility in an Alzheimer's beta-amyloidosis mouse model. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(44):15962–71. 10.1523/JNEUROSCI.2085-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, et al. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer's disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28(27):6926–37. Epub 2008/07/04. 28/27/6926 [pii] 10.1523/JNEUROSCI.0800-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang DS, Stavrides P, Saito M, Kumar A, Rodriguez-Navarro JA, Pawlik M, et al. Defective macroautophagic turnover of brain lipids in the TgCRND8 Alzheimer mouse model: prevention by correcting lysosomal proteolytic deficits. Brain: a journal of neurology. 2014;137(Pt 12):3300–18. 10.1093/brain/awu278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simonsen A, Cumming RC, Brech A, Isakson P, Schubert DR, Finley KD. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy. 2008;4(2):176–84. Epub 2007/12/07. 5269 [pii]. 10.4161/auto.5269 . [DOI] [PubMed] [Google Scholar]
- 6.Yang DS, Lee JH, Nixon RA. Monitoring autophagy in Alzheimer's disease and related neurodegenerative diseases. Methods Enzymol. 2009;453:111–44. Epub 2009/02/17. S0076-6879(08)04006-8 [pii] 10.1016/S0076-6879(08)04006-8 . [DOI] [PubMed] [Google Scholar]
- 7.Kett LR, Stiller B, Bernath MM, Tasset I, Blesa J, Jackson-Lewis V, et al. alpha-Synuclein-independent histopathological and motor deficits in mice lacking the endolysosomal Parkinsonism protein Atp13a2. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35(14):5724–42. 10.1523/JNEUROSCI.0632-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long DM, Blake MR, Dutta S, Holbrook SD, Kotwica-Rolinska J, Kretzschmar D, et al. Relationships between the circadian system and Alzheimer's disease-like symptoms in Drosophila. PloS one. 2014;9(8):e106068 Epub 2014/08/30. 10.1371/journal.pone.0106068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauw JJ, Hausser-Hauw C, De Girolami U, Hasboun D, Seilhean D. Neuropathology of sleep disorders: a review. J Neuropathol Exp Neurol. 2011;70(4):243–52. 10.1097/NEN.0b013e318211488e . [DOI] [PubMed] [Google Scholar]
- 10.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. The Journal of cell biology. 2008;183(5):795–803. Epub 2008/11/26. jcb.200809125 [pii] 10.1083/jcb.200809125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto A, Cremona ML, Rothman JE. Autophagy-mediated clearance of huntingtin aggregates triggered by the insulin-signaling pathway. The Journal of cell biology. 2006;172(5):719–31. Epub 2006/03/01. jcb.200510065 [pii] 10.1083/jcb.200510065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36(6):585–95. Epub 2004/05/18. 10.1038/ng1362ng1362 [pii]. . [DOI] [PubMed] [Google Scholar]
- 13.Kim B, Feldman EL. Insulin resistance as a key link for the increased risk of cognitive impairment in the metabolic syndrome. Experimental & molecular medicine. 2015;47:e149 10.1038/emm.2015.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nixon RA. Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci. 2007;120(Pt 23):4081–91. Epub 2007/11/23. 120/23/4081 [pii] 10.1242/jcs.019265 . [DOI] [PubMed] [Google Scholar]
- 15.Stratman NC, Castle CK, Taylor BM, Epps DE, Melchior GW, Carter DB. Isoform-specific interactions of human apolipoprotein E to an intermediate conformation of human Alzheimer amyloid-beta peptide. Chem Phys Lipids. 2005;137(1–2):52–61. Epub 2005/09/06. S0009-3084(05)00107-6 [pii] 10.1016/j.chemphyslip.2005.06.005 . [DOI] [PubMed] [Google Scholar]
- 16.Rogers I, Kerr F, Martinez P, Hardy J, Lovestone S, Partridge L. Ageing increases vulnerability to abeta42 toxicity in Drosophila. PloS one. 2012;7(7):e40569 10.1371/journal.pone.0040569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naderali EK, Ratcliffe SH, Dale MC. Obesity and Alzheimer's disease: a link between body weight and cognitive function in old age. Am J Alzheimers Dis Other Demen. 2009;24(6):445–9. Epub 2009/10/06. 1533317509348208 [pii] 10.1177/1533317509348208 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartlett BJ, Isakson P, Lewerenz J, Sanchez H, Kotzebue RW, Cumming RC, et al. p62, Ref(2)P and ubiquitinated proteins are conserved markers of neuronal aging, aggregate formation and progressive autophagic defects. Autophagy. 2011;7(6). Epub 2011/02/18. 14943 [pii]. 10.4161/auto.7.6.14943 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining "clean" cells. Autophagy. 2005;1(3):131–40. Epub 2006/07/29. 2017 [pii]. 10.4161/auto.1.3.2017 . [DOI] [PubMed] [Google Scholar]
- 20.Klionsky DJ, Cuervo AM, Dunn WA Jr., Levine B, van der Klei I, Seglen PO. How shall I eat thee? Autophagy. 2007;3(5):413–6. Epub 2007/06/15. 4377 [pii]. 10.4161/auto.4377 . [DOI] [PubMed] [Google Scholar]
- 21.Reggiori F, Klionsky DJ. Autophagosomes: biogenesis from scratch? Curr Opin Cell Biol. 2005;17(4):415–22. Epub 2005/06/28. S0955-0674(05)00077-3 [pii] 10.1016/j.ceb.2005.06.007 . [DOI] [PubMed] [Google Scholar]
- 22.Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, et al. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64(2):113–22. Epub 2005/03/09. 10.1093/jnen/64.2.113 . [DOI] [PubMed] [Google Scholar]
- 23.Reggiori F, Komatsu M, Finley K, Simonsen A. Autophagy: more than a nonselective pathway. International journal of cell biology. 2012;2012:219625 10.1155/2012/219625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipinski MM, Zheng B, Lu T, Yan Z, Py BF, Ng A, et al. Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(32):14164–9. 10.1073/pnas.1009485107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simonsen A, Cumming RC, Finley KD. Linking lysosomal trafficking defects with changes in aging and stress response in Drosophila. Autophagy. 2007;3(5):499–501. Epub 2007/07/10. 4604 [pii]. 10.4161/auto.4604 . [DOI] [PubMed] [Google Scholar]
- 26.Clausen TH, Lamark T, Isakson P, Finley K, Larsen KB, Brech A, et al. p62/SQSTM1 and ALFY interact to facilitate the formation of p62 bodies/ALIS and their degradation by autophagy. Autophagy. 2010;6(3). Epub 2010/02/20. 11226 [pii]. 10.4161/auto.6.3.11226 . [DOI] [PubMed] [Google Scholar]
- 27.Moscat J, Diaz-Meco MT. To aggregate or not to aggregate? A new role for p62. EMBO Rep. 2009;10(8):804 Epub 2009/08/04. embor2009172 [pii] 10.1038/embor.2009.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rui YN, Xu Z, Patel B, Chen Z, Chen D, Tito A, et al. Huntingtin functions as a scaffold for selective macroautophagy. Nat Cell Biol. 2015;17(3):262–75. 10.1038/ncb3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagy P, Karpati M, Varga A, Pircs K, Venkei Z, Takats S, et al. Atg17/FIP200 localizes to perilysosomal Ref(2)P aggregates and promotes autophagy by activation of Atg1 in Drosophila. Autophagy. 2014;10(3):453–67. 10.4161/auto.27442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8(4):445–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282(33):24131–45. Epub 2007/06/21. M702824200 [pii] 10.1074/jbc.M702824200 . [DOI] [PubMed] [Google Scholar]
- 32.Finley KD, Edeen PT, Cumming RC, Mardahl-Dumesnil MD, Taylor BJ, Rodriguez MH, et al. blue cheese mutations define a novel, conserved gene involved in progressive neural degeneration. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23(4):1254–64. Epub 2003/02/25. 23/4/1254 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young AR, Chan EY, Hu XW, Kochl R, Crawshaw SG, High S, et al. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J Cell Sci. 2006;119(Pt 18):3888–900. Epub 2006/08/31. jcs.03172 [pii] 10.1242/jcs.03172 . [DOI] [PubMed] [Google Scholar]
- 34.Alirezaei M, Kemball CC, Flynn CT, Wood MR, Whitton JL, Kiosses WB. Short-term fasting induces profound neuronal autophagy. Autophagy. 2010;6(6):702–10. 10.4161/auto.6.6.12376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takats S, Nagy P, Varga A, Pircs K, Karpati M, Varga K, et al. Autophagosomal Syntaxin17-dependent lysosomal degradation maintains neuronal function in Drosophila. The Journal of cell biology. 2013;201(4):531–9. 10.1083/jcb.201211160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagy P, Varga A, Kovacs AL, Takats S, Juhasz G. How and why to study autophagy in Drosophila: it's more than just a garbage chute. Methods. 2015;75:151–61. 10.1016/j.ymeth.2014.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441(7095):885–9. Epub 2006/04/21. nature04724 [pii] 10.1038/nature04724 . [DOI] [PubMed] [Google Scholar]
- 38.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441(7095):880–4. Epub 2006/04/21. nature04723 [pii] 10.1038/nature04723 . [DOI] [PubMed] [Google Scholar]
- 39.Young JE, Martinez RA, La Spada AR. Nutrient deprivation induces neuronal autophagy and implicates reduced insulin signaling in neuroprotective autophagy activation. J Biol Chem. 2009;284(4):2363–73. Epub 2008/11/20. M806088200 [pii] 10.1074/jbc.M806088200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barekat A, Gonzalez A, Mauntz RE, Kotzebue RW, Molina B, El-Mecharrafie N, et al. Using Drosophila as an integrated model to study mild repetitive traumatic brain injury. Sci Rep. 2016;6:25252 10.1038/srep25252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarkar C, Zhao Z, Aungst S, Sabirzhanov B, Faden AI, Lipinski MM. Impaired autophagy flux is associated with neuronal cell death after traumatic brain injury. Autophagy. 2014;10(12):2208–22. 10.4161/15548627.2014.981787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292(5514):104–6. Epub 2001/04/09. 10.1126/science.1057991292/5514/104 [pii]. . [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto R, Tatar M. Insulin receptor substrate chico acts with the transcription factor FOXO to extend Drosophila lifespan. Aging cell. 2011;10(4):729–32. 10.1111/j.1474-9726.2011.00716.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grandison RC, Wong R, Bass TM, Partridge L, Piper MD. Effect of a standardised dietary restriction protocol on multiple laboratory strains of Drosophila melanogaster. PloS one. 2009;4(1):e4067 10.1371/journal.pone.0004067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gill S, Le HD, Melkani GC, Panda S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science. 2015;347(6227):1265–9. 10.1126/science.1256682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaninovich OA, Kim SM, Root CR, Green DS, Ko KI, Wang JW. A single-fly assay for foraging behavior in Drosophila. Journal of visualized experiments: JoVE. 2013;(81):e50801 10.3791/50801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yannoni YM, White K. Association of the neuron-specific RNA binding domain-containing protein ELAV with the coiled body in Drosophila neurons. Chromosoma. 1997;105(6):332–41. 10.1007/s004120050192 . [DOI] [PubMed] [Google Scholar]
- 48.Halter DA, Urban J, Rickert C, Ner SS, Ito K, Travers AA, et al. The homeobox gene repo is required for the differentiation and maintenance of glia function in the embryonic nervous system of Drosophila melanogaster. Development. 1995;121(2):317–32. . [DOI] [PubMed] [Google Scholar]
- 49.Gargano JW, Martin I, Bhandari P, Grotewiel MS. Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Experimental gerontology. 2005;40(5):386–95. Epub 2005/05/28. S0531-5565(05)00034-3 [pii] 10.1016/j.exger.2005.02.005 . [DOI] [PubMed] [Google Scholar]
- 50.Hegedus K, Nagy P, Gaspari Z, Juhasz G. The putative HORMA domain protein Atg101 dimerizes and is required for starvation-induced and selective autophagy in Drosophila. BioMed research international. 2014;2014:470482 10.1155/2014/470482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jain A, Rusten TE, Katheder N, Elvenes J, Bruun JA, Sjottem E, et al. p62/Sequestosome-1, Autophagy-related Gene 8, and Autophagy in Drosophila Are Regulated by Nuclear Factor Erythroid 2-related Factor 2 (NRF2), Independent of Transcription Factor TFEB. J Biol Chem. 2015;290(24):14945–62. 10.1074/jbc.M115.656116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Juhasz G, Neufeld TP. Experimental control and characterization of autophagy in Drosophila. Methods Mol Biol. 2008;445:125–33. Epub 2008/04/22. 10.1007/978-1-59745-157-4_8 . [DOI] [PubMed] [Google Scholar]
- 53.Juhasz G, Neufeld TP. Drosophila Atg7: required for stress resistance, longevity and neuronal homeostasis, but not for metamorphosis. Autophagy. 2008;4(3):357–8. Epub 2008/01/25. 5572 [pii]. 10.4161/auto.5572 . [DOI] [PubMed] [Google Scholar]
- 54.Juhasz G, Erdi B, Sass M, Neufeld TP. Atg7-dependent autophagy promotes neuronal health, stress tolerance, and longevity but is dispensable for metamorphosis in Drosophila. Genes Dev. 2007;21(23):3061–6. Epub 2007/12/07. 21/23/3061 [pii] 10.1101/gad.1600707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hegedus K, Takats S, Kovacs AL, Juhasz G. Evolutionarily conserved role and physiological relevance of a STX17/Syx17 (syntaxin 17)-containing SNARE complex in autophagosome fusion with endosomes and lysosomes. Autophagy. 2013;9(10):1642–6. 10.4161/auto.25684 . [DOI] [PubMed] [Google Scholar]
- 56.Tower J. Mitochondrial maintenance failure in aging and role of sexual dimorphism. Arch Biochem Biophys. 2015;576:17–31. 10.1016/j.abb.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weis SN, Toniazzo AP, Ander BP, Zhan X, Careaga M, Ashwood P, et al. Autophagy in the brain of neonates following hypoxia-ischemia shows sex- and region-specific effects. Neuroscience. 2014;256:201–9. 10.1016/j.neuroscience.2013.10.046 . [DOI] [PubMed] [Google Scholar]
- 58.Au AK, Chen Y, Du L, Smith CM, Manole MD, Baltagi SA, et al. Ischemia-induced autophagy contributes to neurodegeneration in cerebellar Purkinje cells in the developing rat brain and in primary cortical neurons in vitro. Biochim Biophys Acta. 2015;1852(9):1902–11. 10.1016/j.bbadis.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du L, Hickey RW, Bayir H, Watkins SC, Tyurin VA, Guo F, et al. Starving neurons show sex difference in autophagy. J Biol Chem. 2009;284(4):2383–96. 10.1074/jbc.M804396200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herrera VL, Decano JL, Bagamasbad P, Kufahl T, Steffen M, Ruiz-Opazo N. Sex-specific hippocampus-dependent cognitive deficits and increased neuronal autophagy in DEspR haploinsufficiency in mice. Physiol Genomics. 2008;35(3):316–29. 10.1152/physiolgenomics.00044.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015;22(1):86–99. 10.1016/j.cmet.2015.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hansen M, Chandra A, Mitic LL, Onken B, Driscoll M, Kenyon C. A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS genetics. 2008;4(2):e24 Epub 2008/02/20. 07-PLGE-RA-0723 [pii] 10.1371/journal.pgen.0040024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jia K, Levine B. Autophagy is required for dietary restriction-mediated life span extension in C. elegans. Autophagy. 2007;3(6):597–9. Epub 2007/10/04. 4989 [pii]. 10.4161/auto.4989 . [DOI] [PubMed] [Google Scholar]
- 64.Min KJ, Yamamoto R, Buch S, Pankratz M, Tatar M. Drosophila lifespan control by dietary restriction independent of insulin-like signaling. Aging cell. 2008;7(2):199–206. 10.1111/j.1474-9726.2008.00373.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, et al. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer's disease. Neurobiology of disease. 2007;26(1):212–20. 10.1016/j.nbd.2006.12.019 . [DOI] [PubMed] [Google Scholar]
- 66.Lee S, Notterpek L. Dietary restriction supports peripheral nerve health by enhancing endogenous protein quality control mechanisms. Experimental gerontology. 2013;48(10):1085–90. 10.1016/j.exger.2012.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mattson MP. Lifelong brain health is a lifelong challenge: from evolutionary principles to empirical evidence. Ageing research reviews. 2015;20:37–45. 10.1016/j.arr.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murphy T, Dias GP, Thuret S. Effects of diet on brain plasticity in animal and human studies: mind the gap. Neural plasticity. 2014;2014:563160 10.1155/2014/563160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma D, Panda S, Lin JD. Temporal orchestration of circadian autophagy rhythm by C/EBPbeta. EMBO J. 2011;30(22):4642–51. 10.1038/emboj.2011.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse WJ, et al. Meal frequency and timing in health and disease. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(47):16647–53. 10.1073/pnas.1413965111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Praag H, Fleshner M, Schwartz MW, Mattson MP. Exercise, energy intake, glucose homeostasis, and the brain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34(46):15139–49. 10.1523/JNEUROSCI.2814-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walls SM Jr., Attle SJ, Brulte GB, Walls ML, Finley KD, Chatfield DA, et al. Identification of sphingolipid metabolites that induce obesity via misregulation of appetite, caloric intake and fat storage in Drosophila. PLoS genetics. 2013;9(12):e1003970 10.1371/journal.pgen.1003970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, et al. Interventions to Slow Aging in Humans: Are We Ready? Aging cell. 2015;14(4):497–510. 10.1111/acel.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Longo VD, Panda S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016;23(6):1048–59. 10.1016/j.cmet.2016.06.001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Piazza N, Gosangi B, Devilla S, Arking R, Wessells R. Exercise-training in young Drosophila melanogaster reduces age-related decline in mobility and cardiac performance. PloS one. 2009;4(6):e5886 Epub 2009/06/12. 10.1371/journal.pone.0005886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marcora MS, Fernandez-Gamba AC, Avendano LA, Rotondaro C, Podhajcer OL, Vidal R, et al. Amyloid peptides ABri and ADan show differential neurotoxicity in transgenic Drosophila models of familial British and Danish dementia. Molecular neurodegeneration. 2014;9:5 10.1186/1750-1326-9-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429(6991):562–6. Epub 2004/06/04. 10.1038/nature02549nature02549 [pii]. . [DOI] [PubMed] [Google Scholar]
- 78.Simonsen A, Cumming RC, Lindmo K, Galaviz V, Cheng S, Rusten TE, et al. Genetic modifiers of the Drosophila blue cheese gene link defects in lysosomal transport with decreased life span and altered ubiquitinated-protein profiles. Genetics. 2007;176(2):1283–97. Epub 2007/04/17. genetics.106.065011 [pii] 10.1534/genetics.106.065011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sahani MH, Itakura E, Mizushima N. Expression of the autophagy substrate SQSTM1/p62 is restored during prolonged starvation depending on transcriptional upregulation and autophagy-derived amino acids. Autophagy. 2014;10(3):431–41. 10.4161/auto.27344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sadagurski M, Cheng Z, Rozzo A, Palazzolo I, Kelley GR, Dong X, et al. IRS2 increases mitochondrial dysfunction and oxidative stress in a mouse model of Huntington disease. J Clin Invest. 2011;121(10):4070–81. 10.1172/JCI46305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317(5836):369–72. 10.1126/science.1142179 . [DOI] [PubMed] [Google Scholar]
- 82.Maher PA, Schubert DR. Metabolic links between diabetes and Alzheimer's disease. Expert Rev Neurother. 2009;9(5):617–30. Epub 2009/05/01. 10.1586/ern.09.18 . [DOI] [PubMed] [Google Scholar]
- 83.Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, et al. Neuroinflammation in Alzheimer's disease. The Lancet Neurology. 2015;14(4):388–405. 10.1016/S1474-4422(15)70016-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The *N values represent weights of different fly cohorts (25 per conditions) following a fast and re-feeding At least 125 individual flies were used for each study. A. Average adult female fly (w1118/+) weights (mg), SEM and percentage weight change that occurred following fasting and an overnight re-feeding. B. Average adult male fly (w1118/+) weights (mg), SEM and the percentage weight change that occurred following fasting and an overnight re-feeding. C. The average weights (mg) of WT male flies (w1118/+) at 1-week and 4-weeks of age maintained using ad librium conditions or after 3-weeks of IF-treatment (4-weeks of age).
(PDF)
Statistical description of the average lifespan (days), SEM, N and P values determined between ad libitum and IF-treatment gender and genotype fly cohorts. The percentage change in average longevity is also presented.
(PDF)
Confocal images adult fly brains co-stained for autophagic vesicle marker (Atg8a) and neuronal (Elav) or glial (Repo) markers. (A-B) Higher magnification images of neural soma (anti-Elav, green) and Atg8a positive punctae (anti-Atg8a, red) taken from CNS regions highlighted from Fig 1B (see yellow arrows in Fig 1B). (C) Representative confocal images of a female fly CNS (8 hour fast) show similar staining patterns to those of male flies seen in Fig 1(n = 10 brains). The location of neuronal soma (cell bodies, yellow arrows) and regions primarily consisting of neuropil are indicated (blue arrows). (D) Representative confocal images of a male CNS (8h fast) co-stained for glial (anti-Repo, green) and autophagy (anti-Atg8a, red) markers in the adult CNS (n = 15 brains). Magnified images (inset) show a single Repo-positive glial cell that contains Atg8a positive punctae (yellow arrow). Most glial cells showed limited Atg8a staining (yellow circles).
(TIFF)
Triplicate cohorts of young (w1118/+) adult WT male flies were fasted for 0, 4 or 8-hours, flash frozen and total RNA isolated and used for qRT-PCR analysis of Atg8a message levels. Values were normalized using Cyp1 as a reference gene.
(TIF)
Starting at 9:00 am, triplicate weights of (A) female and (B) male fly cohorts (w1118/+, 25 flies per group) were obtained following 0, 4, 8 or 24-hour fast (125 total flies). Fly groups fasted for 8h were placed back onto standard media starting at 5:00 pm and allowed to re-feed overnight (16h) before being re-weighed again the following day at 9:00 am (red columns and arrows). *** P ≤ 0.001.
(TIF)
Young outcrossed chico1/+ male flies (1-week) were maintained using ad libitum or IF-treatment conditions. (A) The climbing indexes (3-sec) of freely responding ad libitum or IF-treated chico1/+ male flies that were performed at weekly intervals starting at 1-week and continuing until 4-weeks of age. (B) Quantified Ref(2)P, (C) total Atg8a (I+II), and (D) Atg8a-II protein profiles protein of chico1/+ neural samples illustrated in Fig 5B, were corrected using Actin as a loading control.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.